Abstract
Objective
The role of health care worker hand hygiene in preventing health care associated infections (HCAI) is well-established. There is less emphasis on the hand hygiene (HH) of hospitalized patients; in the context of COVID-19 mechanisms to support it are particularly important. The purpose of this study was to establish if providing patient hand wipes, and a defined protocol for encouraging their use, was effective in improving the frequency of patient HH (PHH).
Design
Before and after study.
Settin
General Hospital, United Kingdom.
Participants
All adult patients admitted to 6 acute elderly care/rehabilitation hospital wards between July and October 2018.
Methods
Baseline audit of PHH opportunities conducted over 6 weeks. Focus group with staff and survey of the public informed the development of a PHH bundle. Effect of bundle on PHH monitored by structured observation of HH opportunities over 12 weeks.
Results
During baseline 303 opportunities for PHH were observed; compliance with PHH was 13.2% (40/303; 95% confidence interval 9.9-7.5). In the evaluation of PHH bundle, 526 PHH opportunities were observed with HH occurring in 58.9% (310/526); an increase of 45.7% versus baseline (95% confidence interval 39.7%-51.0%; P < .001).
Conclusion
Providing patients with multiwipe packs of handwipes is a simple, cost-effective approach to increasing PHH and reducing the risk of HCAI in hospital. Health care workers play an essential role in encouraging PHH.
Key Words: Patient's hands, Care bundle, Hand sanitizing wipe, Compliance, Before and after study, Feedback
INTRODUCTION
The role of the hands of health care workers (HCW) in the transmission of health care associated infections (HCAI) is well-established and multimodal strategies are recommended to support effective hand hygiene among HCW.1 In contrast, there are few studies on the role of patients’ hands in the transmission of HCAI or mechanisms to support patient hand hygiene (PHH) in health care settings.2 Laboratory-based studies have demonstrated the ability of a range of pathogenic microorganism to be acquired from the environment and survive for prolonged periods on hands, including rhinoviruses, Gram negative and positive bacteria, gastrointestinal viruses such as hepatitis A and multiresistant pathogens.2, 3, 4, 5, 6
Significant levels of carriage are also found on the hands of patients, including coliforms7 and multidrug resistant pathogens,8 and a higher prevalence of carriage of pathogens on patient hands than staff hands.8 , 9 A study that sampled the hands of 100 patients after 48 hours spent in an acute care setting found that 39% were contaminated with at least one pathogen, including Clostridium difficile, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococcus, and Gram-negative organisms.10 It has been suggested that pathogens are transferred either directly onto the skin of patients or their immediate environment by HCW ungloved or gloved hands.11 Once contaminated, patients’ hands may contribute to HCAI by contaminating susceptible sites such as intravenous devices, urinary catheters, or wounds.
The acquisition of gastrointestinal pathogens such as C. difficile or norovirus is dependent on ingestion and patients’ hands are likely to be a significant means of transmission.12 , 13 Improving PHH has been shown to reduce the transmission of methicillin-resistant Staphylococcus aureus and respiratory viruses.14 , 15 Mechanisms to support PHH have taken on increased significance since the emergence of the COVID-19 pandemic given the role that HH plays in disseminating respiratory viruses.
Studies conducted on whether patients wash their hands while in hospital suggest that whilst HCW believe that they offer patients the opportunity to wash their hands, both patient reports and direct observation suggest that this rarely happens.16, 17, 18 Unsurprisingly, patients who require assistance to clean their hands are more likely to have their hands contaminated with pathogens than those that can manage without assistance.10
Studies investigating strategies to improve PHH have focused on a range of interventions, including education, adaptation of the World Health Organization (WHO) 5 moments to fit PHH moments and electronic reminders to improve self-initiated HH.18, 19, 20, 21, 22, 23
Key considerations for promoting PHH including timing and technique, product design and placement, and education and training for patients, their families and HCWs.24 Furthermore, a different approach to HCW HH is indicated because the most critical moments for PHH will not match the 5 moments recommended for staff (WHO 2009); mobility and confinement affect the patients’ ability to perform HH without assistance, and the product formulations that are most appropriate and acceptable for patient hand cleansing are likely to be different to those of staff.24 Patient handwipes had been evaluated in a previous study and an antimicrobial handwipe applied for 60 seconds was found to be as good as soap and water in removing microbial contamination from hands.25
There are a lack of studies on the efficacy and feasibility of PHH strategies to enhance patient safety in acute health care settings. The aim of this study, which was conducted prior to the COVID-19 pandemic, was to establish if the introduction of patient hand wipes in conjunction with a defined protocol for encouraging patients to use them, provided a feasible approach to improving the frequency of PHH.
METHODS
Study design
A before and after design was used to support the implementation of the PHH strategy in the acute care setting. As PHH is a fundamental element of patient care it would be ethically unacceptable to use a quasiexperimental control design. The study was conducted in four phases (Fig 1 ).
Fig 1.
Diagram of study phases.
Setting
The study took place between July and October 2018 in 6 implementation wards in the care of older people specialty at a large District General Hospital in England. Three wards admitted acutely ill patients, one was a neurorehabilitation unit, and 2 were for step down care. The median number of beds was 28 and average bed occupancy ranged from 95.2% to 99.8%. Patients on these wards were likely to have physical and/or cognitive impairments that contributed to increased dependency, and a longer than average length of hospital stay.
Ethical approval
Ethical approval was given by the University of West London, College of Nursing, Midwifery & Health Research Ethics Committee and permission given by the NHS Trust Research and Development and Quality Governance Departments.
Phase 1: Baseline audit of HH practice
Data was captured on the number of PHH opportunities (defined as before meal, before touching and after touching an invasive device, after using toilet, after sneezing/coughing, and after vomiting) and the proportion where HH was completed across the 6 participating wards. For each opportunity information was recorded on patient dependency and cognitive status, the type of opportunity, who was present, whether the patient had access to HH, specifying the method available/used. Access to HH was defined as at least one option (soap and water, blue wipes or patient own wipes) available to the patient at the point-of-care, and which they would be able to reach independently.
Observations were undertaken in 3-hour periods between 7 AM and 7 PM over a 6-week period. Data were captured by 3 researchers using a standardized observation schedule and entered into IBM SPSS 24. All data were binary/categorical and were analyzed using Chi-squared and binary logistic regression as appropriate.
Phase 2: Co-design of PHH bundle
A focus group of staff from on the participating units was conducted to explore opinions about the importance of PHH and strategies to support PHH. A nominal group technique26 was used to identify the most important points during the patients’ day when PHH should occur and. Participants were also asked to evaluate the preferred hand-wipe pack design and information for patients about HH. Members of public were asked to complete a brief questionnaire on each of the wipes being considered for inclusion in the bundle to obtain their views on ease of removal, effectiveness in cleaning hands, smell and feel, attractiveness of the packaging. The information captured from this phase was used to inform the development the Patient Hand-Hygiene Bundle’ (see Box 1 ).
Box 1. Patient hand hygiene bundle.
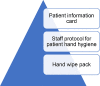
Patient information card: encourages patients to use the hand wipe to clean their hands after using the toilet, before eating food, after sneezing, coughing, blowing their nose
Staff protocol for patient hand hygiene: Prompt patients to clean their hands by offering them a hand wipe before meals, after using the toilet, bedpan, commode, after coughing, or sneezing. Other times when hand hygiene could be prompted include: after vomiting, if touching an invasive device, before taking medication
Hand wipe pack: Pack of 40 wipes issued to each patient, replace with new pack when used
Alt-text: Unlabelled box
Phase 3: Implementation of the PHH bundle
Ward staff were inducted to the PHH bundle by face-to-face meetings and by distribution of a written protocol. All patients on the participating wards at the beginning of the implementation phase received an individual pack of hand wipes and information about PHH. New packs of hand wipes and PHH information were issued as required and to new patients admitted to the wards. All hand wipe packs were provided by GAMA Healthcare. The intervention was commenced in June 2018 and continued to October 2018. An initial period of 3 weeks enabled staff to become familiar with the PHH bundle and ensure that patients were provided with wipes and information on admission. Twelve observation periods by 2 researchers were undertaken over a subsequent 14-week period in the 6 participating wards to measure compliance with the bundle. Data on the number of patients on the ward and the number with hand wipes, PHH opportunity, whether PHH occurred, the type of staff present, who initiated and completed HH. These data were recorded onto standardized data collection forms at each observation session. Data on the availability of wipes and compliance with PHH was feedback to ward staff on weeks 5 and 11.
Interrupted Time Series regression was used to estimate the size and significance of the intercept shift by creating a dummy variable D set to zero before week 4 and to 1 afterward.
Consumption of wipes
Data on the number of packs issued, number of packs, and wipes used was captured during the implementation period to provide evidence of wipe usage and estimate the costs of the PHH bundle.
Phase 4: User acceptably
The user experience of the PHH bundle was evaluated through a patient questionnaire administered to patients on the study wards who volunteered to complete the survey during the last 4 weeks of the implementation period. Staff acceptability was evaluated in a focus group with staff at the end of the study.
Baseline data were entered into IBM SPSS 2 binary/categorical and analyzed using Chi-squared and binary logistic regression as appropriate. Data from the implementation period were entered into Microsoft ExcelTM and analyzed using frequencies and descriptive statistics. The rate of patient hand wipes usage during the study period was calculated using the number of packs (or wipes included in the pack) distributed the wards as the numerator and the number of bed-days on the participating wards as the denominator.
RESULTS
Audit of PHH practice
A total of 43 hours of structured observations of PHH were conducted. Complete data were collected on 303 of 325 HH opportunities observed and were included in the analysis. PHH occurred on only 13.2% (40/303) of opportunities (Table 1 ). A mechanism to enable patients to clean their hands at the point-of-care was available on 31.4% (93/303; 95% confidence interval [CI] 26.4-36.8) of opportunities. PHH was more likely to occur when a HH mechanism was available (odds ratio [CI 95% 10.6-91.8]; P = .000).
Table 1.
Baseline data: Frequency of patient HH by type of opportunity and access to mechanism of undertaking HH
| Hand hygiene opportunity | No. (%) opportunities | No. (%) of opportunities with access to mechanism of HH* | No. (%) of opportunities where HH occurred |
|---|---|---|---|
| Before food/drink | 191 (63.0%) | 56 (29.3%) | 9 (4.7%) |
| After using the toilet | 66 (21.8%) | 38 (57.6%) | 29 (43.9%) |
| Toilet | 33 (10.9%) | 28.0 (84.8%) | 22 (66.7%) |
| Commode/bedpan/urinal | 33 (10.9%) | 10 (30.3%) | 7 (21.2%) |
| Touching nose/mouth | 36 (11.9%) | - | - |
| Touching invasive devices | 1 (0.3%) | - | - |
| Exposure to body fluids | 5 (1.7%) | - | - |
| During personal hygiene | 4 (1.3%) | 3 (75%) | 3 (75.0%) |
| Total | 303 (100%) | 95 (31.4%) | 40 (13.2%) |
HH, hand hygiene.
Alcohol hand cleanser, soap and water, hand cleansing wipe within reach of the patient.
Staff were present at 76% (230/303) of PHH opportunities but the presence of staff did not significantly affect the likelihood of PHH occurring (31/230 vs 9/73; odds ratio 1.1 [CI95% 0.50-2.45]; P = .8). In terms of access to a mechanism to perform patient HH, soap and water was available for 13% (39/303) of opportunities and 75% (27/39) of these opportunities were when using the toilet. Single-use wrapped patient hand wipes were available for 16% (47/303) of opportunities, but 95% (45/47) of these were before eating or drinking as this wipe was commonly place on patient meal trays. These wipes were only used to clean hands for 13% (3/45) of these occasions and the wipes were otherwise discarded unopened with the contents of the meal tray.
Implementation of PHH bundle
In total, 68 periods of observation of PHH were conducted across the 6 wards. During these periods, 526 opportunities for PHH were identified and HH occurred in 58.9% (310/526). This reflected an increase of 45.7% compared to the compliance of 13.2% (40/303) observed prior to the intervention (95% CI 39.7-51.0%; P< .001; Table 2 ).
Table 2.
Implementation phase: Patient hand hygiene opportunities observed and proportion where patient hand hygiene occurred
| Ward (no. beds) | No. of patient hand hygiene opportunities | No. where patient hand hygiene occurred | % Where patient hand hygiene occurred | No. of patients on ward | % Patients with wipes available | % Patients with wipes availability |
|---|---|---|---|---|---|---|
| 3B (28) | 103 | 70 | 68 | 292 | 176 | 60 |
| 1C (12) | 64 | 39 | 61 | 115 | 100 | 87 |
| 6H (21) | 104 | 58 | 56 | 169 | 135 | 80 |
| 5L (26) | 105 | 45 | 43 | 256 | 175 | 68 |
| 2M (28) | 71 | 49 | 69 | 243 | 191 | 79 |
| 4W (28) | 79 | 49 | 62 | 285 | 210 | 74 |
| Total | 526 | 310 | 59 | 1360 | 987 | 73 |
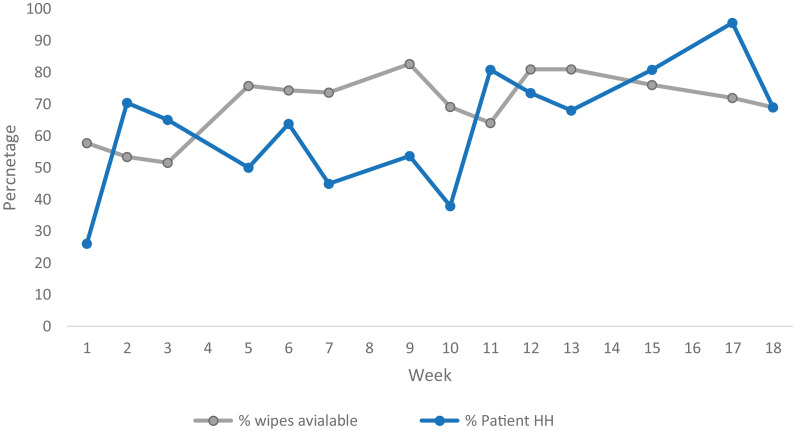
Over the period as a whole, compliance with availability of patient hand wipes was 73% (987/1,360). Figure 2 illustrates the trend in proportion of patients who had wipes available and the proportion of PHH opportunities where HH occurred over the 16-week intervention period. The proportion of patients with wipes available increased over the intervention period from 57.75% (67/116) in week 1 to 68.8% (86/125) in week 16 (95% CI1.1%- 22.8%; P= .076).
Fig 2.
Trend in compliance with patient hand hygiene during the 18-week intervention period (June to October 2018). Familiarization period week 1-3; feedback reports given in week 5 and 11
The proportion of HH opportunities when PHH occurred significantly increased between the beginning and the end of the intervention period from 22.5% (16/71) in week 1 to 69% (29/42) in week 16 (95%CI 27.85-60.91%; P< .001).
All wards increased both the availability of patient hand wipes and the proportion of opportunities where PHH occurred. However, the proportion of opportunities where patient HH occurred varied between wards with an overall compliance on the best ward of 68% (70/103) compared to 43% (45/105) on the poorest performing ward. All the wards made the patient hand wipes available for most patients with the best performing ward having wipes available for 87% (100/115) at the point of observation and even the most poorly performing ward had wipes available for 60% (176/292) of patients (Table 2).
The ITS regression estimated that after the familiarization period the availability of wipes increased by 11% (P< .01) and an average weekly increase in compliance of 3% (P < .001).
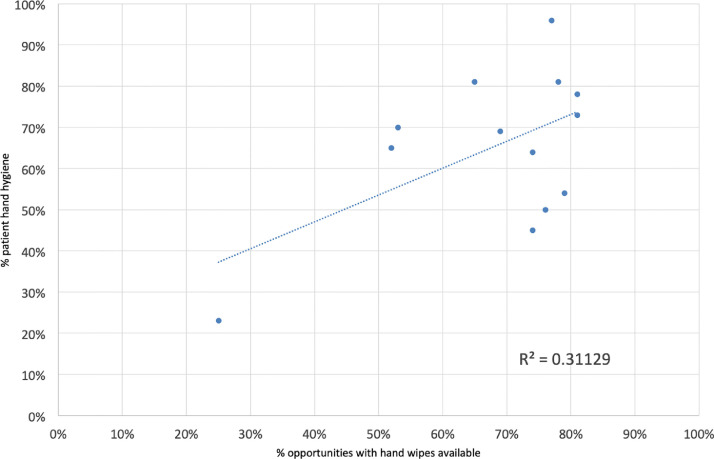
Figure 3 shows the correlation between the availability of wipes and compliance with PHH (R2 0.311) which suggests a moderate correlation between compliance of PHH and the availability of wipes.
Fig 3.
Correlation between wipe availability and proportion of opportunities where patient hand hygiene occurred.
There was a total of 66,232 wipes used in 6 wards over 5 months, corresponding to an average usage of 2,207 wipes per ward per month. The average number of wipes used was 3,308 wipes per 1,000 bed-days, ranging between wards from 2,872 to 4,653 wipes per 1,000 bed-days. The overall costs for these wipes was £2186 (wipes = £1,973.71) or an average of £73 per ward per month, or £3 per bed per month.
Use acceptance
A total of 53 questionnaires were completed by patients during the intervention period; 79% (42/53) indicated that they had used hand wipes and 87% (37/42) agreed or strongly agreed that the wipes were a good idea. In particular, they agreed that the wipes enabled them to clean their hands themselves (86%; 36/42) and that they helped staff to clean their hands (76%; 32/42). The majority of the patients found the wipes made their hands feel clean and was easy to remove from the pack. Some patients indicated that they preferred soap and water, 1 patient did not realize they were hand wipes not “general cleans” and 1 patient said she was allergic to them. Only 66% (35/53) of patients said they would usually wash their hands after using the toilet when at home and 57% (30/53) before a meal. Feedback from staff was positive with ward managers highlighting that patient experience was improved and patient awareness of the need for had hygiene increased “patients started to ask for their wipes” with the possibility that HH behavior would “be taken home” on discharge. In terms of acceptability for staff, the opinion was that ease of access was important and that being able to secure them to the bed table was an advantage, as this made the packs readily accessible and staff did not “have to hunt for them.” Overall the wards wanted to continue to promote PHH and felt that patient wipes was a more efficient and practical means of enabling patients to clean their hand with assistance or independently.
DISCUSSION
Our results highlight the importance of multimodal approaches to improve PHH. This study has confirmed the findings of other studies that patients rarely decontaminate their hands while in hospital.17 , 18 and although nursing staff recognize PHH as an important infection control measure, they rarely make HH available for patients.16 Prior to implementing the PHH bundle, we found that overall patient compliance with HH was only 13% and although more likely to occur after using the toilet, less than 5% of patients performed HH before eating. This was considerably lower than that suggested in a recent study by Srigley et al27 where compliance was 30% after using the bathroom and 39% at mealtimes, although in this study the use of an electronic monitoring may overestimate HH among a more mobile patient population. PHH prior to eating is particularly important given the risk of transferring pathogens acquired through touching the environment or staff onto mucous membranes or their ingesting when handling and consuming food in a hospital ward. In addition, given that the predominant route of transmission of SARS-CoV-2 virus is via respiratory droplets which will contaminate infected patients’ hands and elements of their immediate environment that they touch, mechanisms that support regular PHH should be a key component of strategies to minimize transmission in health care settings.
The approach taken by our study attempted to create a multimodal strategy to improve PHH by introducing a bundle comprising individual patient hand wipe packs, a PHH protocol for staff, information about PHH for patients and monitoring and feedback of rates of compliance. The pack of hand wipes proved popular with both patients and staff and markedly improved the availability of options for HH for this group of elderly patients, who predominantly had limited mobility. Results from another study on improving PHH highlighted the importance of having products for PHH available at the bedside particularly for patients who are bed bound.28
The PHH bundle was associated with a significant increase in compliance with PHH; patient hand wipes were observed to be available at the point of use on three-quarters of occasions and patient compliance with hand hygiene increased from 13% preintervention to 60% after the intervention. However, compliance with both availability of wipes and PHH varied between wards and over time; the availability of wipes at the point of use inevitably had an impact on whether PHH occurred. Sunkesula et al18 also used staff to encourage PHH in combination with canisters of hand wipes and posters. They found a similar increase in compliance with hand hygiene from an overall 10% before the intervention to 79% before meals after the intervention. Our study also found that the HCW was critical in determining whether PHH occurred as they prompted almost 80% of the PHH events. Given that only about half of this patient group reported that they would wash their hands prior to eating and only two-thirds after using the toilet, the healthcare worker needs to be proactive in supporting the best interests of their patients.
The limitations of this study include the potential Hawthorne effect by using direct observations.29 We attempted to mitigate this by undertaking very short periods of observation of compliance with PHH, before staff became overtly aware of our presence. The study was undertaken in a single NHS trust and was restricted to 6 medical wards over an 17-week period and the number of opportunities observed was concentrated around specific times during the day. However, baseline observations suggested that these were the most appropriate periods, given the ward activity. In addition, the hand wipes used for this study had not been designed specifically for patient use, and therefore, did not have intentionally patient-focused packaging which may have been helpful in encouraging patients to use the wipes.
Making individual multiwipe packs available to patients is a simple, cost-effective approach to increasing PHH and reducing the risk of them acquiring HCAI while in hospital. In addition, hand wipe packs may provide a more practical, low cost, means of enabling staff to support PHH than attempting to access soap and water, particularly important for older patients who may have limited mobility. However, staff needs to be educated about the importance of PHH, be encouraged to ensure hand wipes are readily available and to actively prompt and support patients to use them.
Acknowledgments
The authors acknowledge Simon Wells, Susan Burch and the other members of the infection prevention and control team, staff and patients on the six wards that participated in the study at the Royal Berkshire Hospital NHS Trust. Authors also thank Dr Andrew Dunnett for statistical advice.
Footnotes
Conflicts of interest: None to report.
Funding: This study was partially funded by a grant from GAMA Healthcare Ltd and they provided the hand hygiene wipes. GAMA Healthcare Ltd had no involvement in the study design, implementation, data collection, analysis or reporting.
References
- 1.WHO Guidelines on hand hygiene in healthcare. World Health Organisation website. Available at: www.who.int/publications/i/item/9789241597906. Accessed April 21, 2020.
- 2.Knighton SC, Richmond M, Zabarsky T, Dolansky M, Rai H, Donskey CJ. Patients’ capability, opportunity, motivation, and perception of inpatient hand hygiene. Am J Infect Control 2020;48:157–161. [DOI] [PMC free article] [PubMed]
- 3.Hendley JO, Edmondson WP, Gwaltney JM., Jr. Relation between naturally cquired immunity and infectivity of two Rhinoviruses in Volunteers. J Infect Dis. 1972;125:243–248. doi: 10.1093/infdis/125.3.243. Casewell M, Phillips I. Hands as route of transmission for Klebsiella species. Br Med J. 1977;2:1315-1317. [DOI] [PubMed] [Google Scholar]
- 4.Mackintosh CA, Hoffman PN. An extended model for transfer of micro-organisms via the hands: differences between organisms and the effect of alcohol disinfection. J Hyg (Lond) 1984:345–355. doi: 10.1017/s0022172400064561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mbithi JN, Springthorpe VS, Boulet JR, Sattar SA. Survival of hepatitis a virus on human hands and its transfer on contact with animate and inanimate surfaces. J Clin Microbiol. 1992;30:757–763. doi: 10.1128/jcm.30.4.757-763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noskin GA, Stosor V, Cooper I, Peterson LR. Recovery of vancomycin-resistant enterococci on fingertips and environmental surfaces. Infect Control Hosp Epidemiol. 1995;16:577–581. doi: 10.1086/647011. [DOI] [PubMed] [Google Scholar]
- 7.Sanderson PJ, Weissler S. Recovery of coliforms from the hands of nurses and patients: activities leading to contamination. J Hosp Infect. 1992;21:85–93. doi: 10.1016/0195-6701(92)90027-j. [DOI] [PubMed] [Google Scholar]
- 8.Lemmen SW, Hafner H, Zolldann D, Stanzel S, Lutticken R. Distribution of multi-resistant Gram-negative versus Gram-positive bacteria in the hospital inanimate environment. J Hosp Infect. 2004;56:191–197. doi: 10.1016/j.jhin.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Bayuga S, Zeana C, Sahni J, Della-Latta P, El-Sadr W, Larson E. Prevalence and antimicrobial patterns of Acinetobacter baumannii on hands and nares of hospital personnel and patients: the iceberg phenomenon again. Heart Lung. 2002;31:382–390. doi: 10.1067/mhl.2002.126103. [DOI] [PubMed] [Google Scholar]
- 10.Istenes N, Bingham J, Hazelett S, Fleming E, Kirk J. Patients' potential role in the transmission of health care-associated infections: prevalence of contamination with bacterial pathogens and patient attitudes toward hand hygiene. Am J Infect Control. 2013;41:793–798. doi: 10.1016/j.ajic.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Banfield KR, Kerr KG. Could hospital patients' hands constitute a missing link? J Hosp Infect. 2005;61:183–188. doi: 10.1016/j.jhin.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Borriello SP, Barclay FE, Welch AR, et al. Epidemiology of diarrhoea caused by enterotoxigenic clostridium perfringens. J Med Microbiol. 1985;20:363–372. doi: 10.1099/00222615-20-3-363. [DOI] [PubMed] [Google Scholar]
- 13.Rusin P, Maxwell S, Gerba C. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J Appl Microbiol. 2002;93:585–592. doi: 10.1046/j.1365-2672.2002.01734.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheng VCC, Wu AKL, Cheung CHY, et al. Outbreak of human metapneumovirus infection in psychiatric inpatients: implications for directly observed use of alcohol hand rub in prevention of nosocomial outbreaks. J Hosp Infect. 2007;67:336–343. doi: 10.1016/j.jhin.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Gagne D, Bedard G, Maziade PJ. Systematic patients' hand disinfection: impact on meticillin-resistant Staphylococcus aureus infection rates in a community hospital. J Hosp Infect. 2010;75:269–272. doi: 10.1016/j.jhin.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Burnett E. Perceptions, attitudes, and behavior towards patient hand hygiene. AJIC: Am J Infect Control. 2009;37:638–642. doi: 10.1016/j.ajic.2009.04.281. [DOI] [PubMed] [Google Scholar]
- 17.Ardizzone LL, Smolowitz J, Kline N, Thom B, Larson EL. Patient hand hygiene practices in surgical patients. Am J Infect Control. 2013;41:487–491. doi: 10.1016/j.ajic.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Sunkesula VCK, Knighton S, Zabarsky TF, Kundrapu S, Higgins PA, Donskey CJ. Four moments for patient hand hygiene: a patient-centered, provider-facilitated model to improve patient hand hygiene. Infect Control Hosp Epidemiol. 2015;36:986–989. doi: 10.1017/ice.2015.78. [DOI] [PubMed] [Google Scholar]
- 19.Pokrywka M, Feigel J, Douglas B, Grossberger S, Hensler A, Weber D. A bundle strategy including patient hand hygiene to decrease clostridium difficile infections. Medsurg Nursing. 2014;23:145. [PubMed] [Google Scholar]
- 20.Whiller J, Cooper T. Clean hands: how to encourage good hygiene by patients. Nurs Times. 2000;96:37–38. [Google Scholar]
- 21.Cheng VCC, Tai JWM, Li WS, et al. Implementation of directly observed patient hand hygiene for hospitalized patients by hand hygiene ambassadors in Hong Kong. Am J Infect Control. 2016;44:621–624. doi: 10.1016/j.ajic.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Rai H, Knighton S, Zabarsky TF, Donskey CJ. A randomized trial to determine the impact of a 5 moments for patient hand hygiene educational intervention on patient hand hygiene. Am J Infect Control. 2017;45:551–553. doi: 10.1016/j.ajic.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Knighton SC, Dolansky M, Donskey C, Warner C, Rai H, Higgins PA. Use of a verbal electronic audio reminder with a patient hand hygiene bundle to increase independent patient hand hygiene practices of older adults in an acute care setting. Am J Infect Control. 2018;46:610–616. doi: 10.1016/j.ajic.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Landers T, Abusalem S, Coty M, Bingham J. Patient-centered hand hygiene: the next step in infection prevention. Am J Infect Control. 2012;40(4 suppl 1):S11. doi: 10.1016/j.ajic.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Wilkinson MAC, Kiernan MA, Wilson JA, Loveday HP, Bradley SR. Assessment of the efficacy of a patient hand wipe: development of a test method. J Hosp Infect. 2018;98:339–344. doi: 10.1016/j.jhin.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Harvey N, Holmes CA. Nominal group technique: an effective method for obtaining group consensus. Int J Nurs Pract. 2012;18:188–194. doi: 10.1111/j.1440-172X.2012.02017.x. [DOI] [PubMed] [Google Scholar]
- 27.Srigley JA, Furness CD, Gardam M. Measurement of patient hand hygiene in multiorgan transplant units using a novel technology: an observational study. Infect Control Hosp Epidemiol. 2014;35:1336–1341. doi: 10.1086/678419. [DOI] [PubMed] [Google Scholar]
- 28.Manresa Y, Abbo L, Sposato K, de Pascale D, Jiminez A. Improving patients’ hand hygiene in the acute care setting: is staff education enough? Am J Infect Control. 2020;48:1100–1101. doi: 10.1016/j.ajic.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Chang NN, Reisinger HS, Jesson AR, et al. Feasibility of monitoring compliance to the my 5 moments and entry/exit hand hygiene methods in US hospitals. Am J Infect Control. 2016;44:938–940. doi: 10.1016/j.ajic.2016.02.007. [DOI] [PubMed] [Google Scholar]