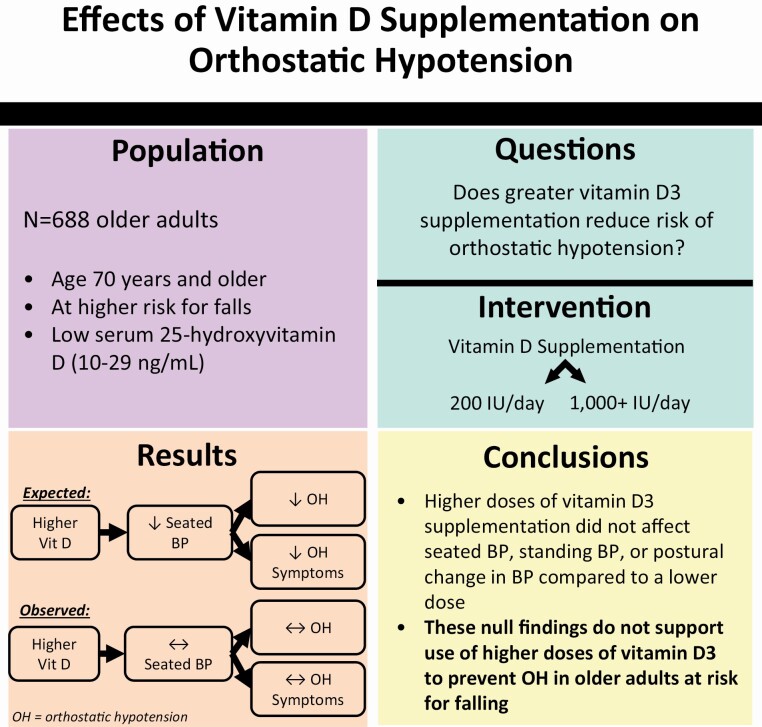
Abstract
Background
Vitamin D3 supplementation is considered a potential intervention to prevent orthostatic hypotension (OH) based on observational evidence that vitamin D levels are inversely associated with OH. With data from The Study to Understand Fall Reduction and Vitamin D in You (STURDY), a double-blind, randomized, response-adaptive trial, we determined if higher doses of vitamin D3 reduced risk of OH.
Methods
STURDY tested the effects of higher (1,000+ IU/day, i.e., 1,000, 2,000, and 4,000 IU/day combined) vs. lower-dose vitamin D3 (200 IU/day, comparison) on fall risk in adults ages 70 years and older with low serum 25-hydroxyvitamin D (25(OH)D, 10–29 ng/ml). OH was determined at baseline, 3, 12, and 24 months by taking the difference between seated and standing blood pressure (BP). OH was defined as a drop in systolic or diastolic BP of at least 20 or 10 mm Hg after 1 minute of standing. Participants were also asked about OH symptoms during the assessment and the preceding month.
Results
Among 688 participants (mean age 77 [SD, 5] years; 44% women; 18% Black), the mean baseline systolic/diastolic BP was 130 (19)/67 (11) mm Hg, serum 25(OH)D was 22.1 (5.1) ng/ml, and 2.8% had OH. There were 2,136 OH assessments over the maximum 2-year follow-up period. Compared with 200 IU/day, 1,000+ IU/day was not associated with seated, standing, or orthostatic BP, and it did not lower risk of OH or orthostatic symptoms.
Conclusions
These findings do not support use of higher doses of vitamin D3 supplementation as an intervention to prevent OH.
Clinical trials registration
Trial Number NCT02166333.
Keywords: blood pressure, hypertension, orthostatic hypotension, trial, vitamin D3
Graphical Abstract
Orthostatic hypotension (OH) is prevalent among older adults1,2 and strongly associated with risk of falls.3 Vitamin D blood concentrations are inversely associated with OH in observational studies4–8 and implicated in blood pressure (BP) regulation through interactions with the renin–angiotensin9,10 and autonomic nervous systems.11,12 However, trial evidence has been inconclusive with one of the only studies of intermittent, high-dose oral vitamin D3 showing a temporary reduction in OH at 3 months that was not sustained over time.13 Whether higher doses of vitamin D3 supplementation lower the risk of OH in older adults at risk for falls remains unclear.
The Study to Understand Fall Reduction and Vitamin D in You (STURDY) was a double-blind, randomized, response-adaptive trial that used a dose-finding phase to test the effects of 4 doses of vitamin D3 (200, 1,000, 2,000, and 4,000 IU/day) on fall risk in adults aged 70 years and older with low serum 25-hydroxyvitamin D [25(OH)D] levels (10–29 ng/ml).14,15 STURDY did not find that higher doses of vitamin D3 supplements lowered the risk of falls and thus participants were transitioned to a confirmatory phase where all the participants on 2,000 or 4,000 IU/day were switched to the 1,000 IU/day dose. Ultimately, those randomized to the 1,000+ IU/day doses did not have a lower incidence of falls compared with those assigned the 200 IU/day dose during the up to 2-year follow-up period. However, the impact of vitamin D3 supplementation on OH risk was not investigated as a primary outcome.
In this secondary analysis, our objective was to determine the effects of vitamin D3 supplementation at doses of 1,000 IU/day or greater (compared with 200 IU/day) on OH and its associated symptoms in older adults at risk for falls. We hypothesized that higher doses of vitamin D3 would lower risk of OH and its associated symptoms during the study follow-up period.
METHODS
STURDY was a NIA-sponsored trial conducted between July 2015 and March 2019 at 2 community-based research units (Hagerstown, MD; Woodlawn, MD), each at ~39° latitude. A description of the trial’s design as well as its main results and protocol have been published.14,15 In brief, STURDY examined the effect of vitamin D3 supplementation dose on the occurrence of falls among older adults at risk for falls. The Johns Hopkins University Institutional Review Board approved the core study and the OH ancillary proposal. All participants provided written, informed consent.
Participants
STURDY enrolled community-dwelling, older adults, aged 70 years and older with an elevated fall risk (i.e., 2 or more falls or 1 injurious fall in the past year, fear of falling, difficulty maintaining balance, or use of an assistive device) and a low serum 25(OH)D level between 10 and 29 ng/ml. Excluded were adults with cognitive impairment, hypercalcemia, and kidney stones. Persons consuming ≤1,000 IU/day of vitamin D3 supplements or ≤1,200 mg/day of calcium supplements were eligible as long as they met the other criteria of the trial.14,15
Participants were followed up to 24 months or until the study ended. The primary endpoint of the study was time to first fall or death over 2 years; however, participants continued to be followed even after fall events. Principal study visits occurred at baseline, 3, 12, and 24 months.
Vitamin D3 supplementation
STURDY was a response-adaptive trial with 2 phases: a dose-finding phase and confirmatory phase. Initially, participants were randomized to 1 of 4 doses of vitamin D3: 200, 1,000, 2,000, or 4,000 IU/day. The probability of assignment to 200 IU/day (the reference dose) was 0.50 throughout the trial. The starting probability of assignment to each of the 3 noncontrol doses was 0.1667.
Assignment probabilities to the noncontrol doses were adjusted using Bayes’ theorem at prespecified times after the 100th participant was randomized to the noncontrol group. Ultimately, STURDY found that the 1,000 IU/day dose was the best dose for preventing falls, at which point the dose-finding phase ended. All active participants assigned to noncontrol doses (i.e., 2,000 or 4,000 IU/day) were transitioned to the 1,000 IU/day dose (referred to in this study as the 1,000+ IU/day), and newly enrolled participants were randomly assigned to either 200 or 1,000 IU/day at a 0.50 probability of assignment. Randomization in the dose-finding stage began 30 October 2015, and ended 23 March 2018. The confirmatory stage began 10 April 2018, and ended 31 May 2019. As previously described, the proportion of participants who reported taking their study pill at least 80% of the expected days was 72.5% in 200 IU/day group and 75.8% in the 1,000+ IU/day group. At 3 months after randomization, the mean 25(OH)D was 67.4 nmol/l among those assigned 200 IU/day vs. 91.1 nmol/l among those assigned 1,000+ IU/day.
OH measurement and symptoms
OH was measured as part of the core STURDY protocol at the baseline, 3-, 12-, and 24-month visits by trained study staff. Participants sat for 5 minutes with their feet flat on the ground and their backs supported. Arm circumference was measured to determine the appropriate cuff size. Participants underwent 3 BP measurements separated by 30 seconds, using an Omron HEM907XL (Omron Healthcare, Lake Forest, IL). Afterward, they were asked to stand up and rest their arm on a bedside table, positioned to maintain their arm at heart level. After standing for 1 minute, they underwent 3 additional BP measurements separated by 30 seconds in the standing position. During the OH protocol, participants were asked if they felt lightheaded or dizzy immediately after standing and again after the 3 standing BP measurements were completed.
OH was defined using the consensus definition as a drop in systolic BP of at least 20 mm Hg or a drop in diastolic BP of at least 10 mm Hg.16
Historic symptoms
As part of an ancillary study that was initiated in July 2017 (about 2 years after the start of STURDY), a subset of the population (N = 589) answered questions about the frequency of symptoms experienced in the preceding 30 days during the process of standing up: lightheadedness, dizziness, fainting, blacking out, seeing spots, imbalance, room spinning, racing heart, sweating episodes, vision changes, nausea, trouble concentrating, shortness of breath, headache, muscle weakness, and fatigue. Participants answered on a 9-point Likert scale. Based on the distribution of the responses, symptoms were dichotomized as none (score 1) or any symptoms (scores 2–9). These symptoms were assessed at the randomization visit (as opposed to the baseline visit), 3-, 12-, and 24-month visits.
Other covariates
Age, sex, and race (Black or non-Black) were self-reported. Body mass index (kg/m2) was derived from measured height and weight. Participants reported medical history, including cardiovascular disease (yes or no), diabetes (yes or no), fall in the past year (yes or no) as well as use of antidepressants, beta blockers, or diuretics.
Statistical analysis
In this analysis, we compared those assigned to pooled higher vitamin D3 doses (1,000+ IU/day) with those assigned to the lower vitamin D3 dose (200 IU/day). We described baseline population characteristics by vitamin D3 assignment using means (SD) and proportions.
Blood pressure
The distribution of seated, standing, and postural change in systolic and diastolic BP was depicted using kernel density plots at baseline and follow-up (according to randomized assignment). We used generalized estimating equations (GEEs) to estimate change in BP over time (normal family, identity link, and robust variance estimator) for seated, standing, and postural change in systolic and diastolic BP in 3 separate models. These models included values representing the month of each visit (i.e., baseline, 3-, 12-, and 24-month visits, which assumed values 0, 3, 12, and 24, respectively), the intervention (0 [200 IU/day] or 1 [1,000+ IU/day]), and the interaction between the 2 variables to determine change in BP compared with baseline and to compare across randomized assignment at specific follow-up months. The overall effect, regardless of visit, was determined using a visit covariate that did not differentiate by month (i.e., 0 [baseline] or 1 [follow-up visit]).
Orthostatic hypotension
We determined the proportion of participants with OH at each study visit according to randomization assignment, using GEE (Poisson family, log link, and robust variance estimator) which included terms for visit (values 0, 3, 12, and 24), the intervention (0 [200 IU/day] or 1 [1,000+ IU/day]), and the interaction between the 2 variables. We used GEE models (binomial family, logit link, and robust variance estimator) with the same terms above to determine the odds ratio of OH at each visit comparing the 1,000+ IU/day dose to the 200 IU/day dose. Estimates of the proportions and odds ratios were repeated combining all follow-up visits using the models above with a visit covariate that did not differentiate by month (i.e., 0 [baseline] or 1 [follow-up visit]).
Orthostatic symptoms
We used the overall follow-up GEE model described above to determine the odds ratio for a dichotomous version of each of the ancillary orthostatic symptoms (scores of 2–9 vs. a score of 1) using GEE models described above (binomial family, logit link, and robust variance estimator with a simplified follow-up visit covariate).
A 2-tailed P value <0.05 was considered statistically significant. All analyses were performed with Stata version 15.1 (Stata Corporation, College Station, TX).
RESULTS
Baseline characteristics
Baseline characteristics of the participants of STURDY are shown by randomized assignment in Table 1 and by baseline OH in Supplementary Table S1 online. There were minimal differences across the intervention assignments. Ultimately, 686 participants had standing BP measured and 589 participated in the ancillary orthostatic symptom questionnaire. See Supplementary Figure S1 online for details of exclusions and the number of OH assessments at each visit.
Table 1.
Baseline characteristics of participants
| 200 IU/day, N = 339 | 1,000+ IU/daya, N = 349 | |
|---|---|---|
| Mean (SD) or % | Mean (SD) or % | |
| Age, yr | 76.4 (5.4) | 76.5 (5.4) |
| Women, % | 41.6 | 45.6 |
| Black, %b | 16.4 | 19.9 |
| Seated blood pressure, mm Hgc | ||
| Systolic | 128.8 (18.6) | 130.5 (19.3) |
| Diastolic | 67.2 (11.1) | 67.6 (10.8) |
| Body mass index, kg/m2c | 30.4 (6.3) | 30.6 (5.6) |
| History of cardiovascular disease, % | 31.3 | 27.5 |
| History of diabetes, % | 23.9 | 28.9 |
| History of high blood pressure or hypertension, % | 59.3 | 66.2 |
| Beta blocker use, % | 28.0 | 29.2 |
| Diuretic use, % | 20.6 | 20.6 |
| Antidepressant use, % | 15.6 | 17.5 |
| Fall in the past year, % | 65.2 | 65.6 |
| 25-Hydroxyvitamin D, ng/ml | 22.1 (5.3) | 22.1 (4.9) |
aThe 1,000+ IU/day includes participants randomized to 1,000, 2,000, or 4,000 IU/day.
bBlack race was unknown for 4 participants assigned to the 200 IU/day group and 3 participants assigned the 1,000+ IU/day group.
cThere were only 348 with blood pressure and body mass index measures in the 1,000+ IU/day group.
Effects of vitamin D dose on seated and standing BP
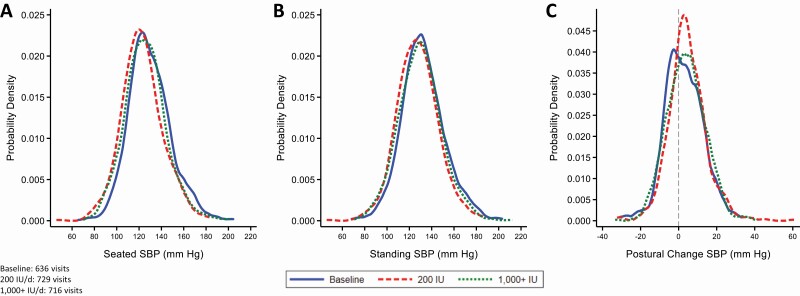
The distribution of seated BP, standing BP, and postural change in BP was similar at baseline (prerandomization) and during the follow-up visits of those assigned 200 IU/day, and the follow-up visits of those assigned 1,000+ IU/day (see Figure 1 for systolic BP and Supplementary Figure S2 online for diastolic BP). Both seated and standing BP were significantly reduced during follow-up compared with baseline for both 200 and 1,000+ IU/day (Table 2). Compared with 200 IU/day, there was no significant or consistent difference in change in seated BP, standing BP, or the postural difference in BP over time among those assigned 1,000+ IU/day.
Figure 1.
Kernel density plots of the probability density (y-axis) of (a) seated systolic blood pressure (SBP), (b) standing SBP, and (c) postural change in SBP. Curves represent blood pressure measurements (mm Hg) during baseline (solid line), the 200 IU/day vitamin D assignment follow-up visits (dashed line), and the 1,000+ IU/day vitamin D3 assignment follow-up visits (dotted line). The 1,000+ IU/day includes participants randomized to 1,000, 2,000, or 4,000 IU/day. These figures are restricted to the subpopulation with both baseline and follow-up visits (N = 679).
Table 2.
Effect of vitamin D on postural change in systolic blood pressure and diastolic blood pressure, N = 686 participants with 2,136 measurementsa
| 200 IU/day, β (95% CI) | 1,000+ IU/dayb, β (95% CI) | 1,000+ b vs. 200 IU/day | |||||
|---|---|---|---|---|---|---|---|
| Seated | Standing | Postural change (standing minus seated) | Seated | Standing | Postural change (standing minus seated) | P comparing postural changes | |
| Systolic blood pressure, mm Hg | |||||||
| Mean at baseline | 128.8 (126.9, 130.8) | 131.7 (129.7, 133.7) | 2.8 (1.7, 3.9) | 130.7 (128.6, 132.7) | 133.2 (131.1, 135.3) | 2.5 (1.4, 3.6) | 0.70 |
| Change at 3 months from baseline | −5.7 (−7.5, −4.0) | −4.5 (−6.3, −2.8) | 1.3 (0.1, 2.4) | −3.2 (−6.0, −0.3) | −2.0 (−4.8, 0.9) | 1.2 (−0.4, 2.7) | 0.80 |
| Change at 12 months from baseline | −6.1 (−8.0, −4.1)d | −5.2 (−7.1, −3.3)d | 1.0 (−0.2, 2.2) | −1.4 (−4.3, 1.4)d | −0.9 (−3.8, 2.0)d | 0.5 (−1.1, 2.0) | 0.81 |
| Change at 24 months from baseline | −7.1 (−9.6, −4.6) | −4.3 (−6.6, −2.0) | 2.9 (1.5, 4.4) | −3.3 (−6.5, −0.1) | −1.4 (−4.8, 1.9) | 1.9 (0.0, 3.8) | 0.47 |
| Overall change during follow-up visits from baselinec | −6.1 (−7.7, −4.6) | −4.7 (−6.2, −3.2) | 1.5 (0.6, 2.5) | −2.6 (−5.3, 0.0) | −1.5 (−4.2, 1.2) | 1.1 (−0.3, 2.5) | 0.84 |
| Diastolic blood pressure, mm Hg | |||||||
| Mean at baseline | 67.2 (66.0, 68.4) | 69.8 (68.5, 71.0) | 2.6 (2.0, 3.2) | 67.6 (66.5, 68.7) | 69.8 (68.6, 71.0) | 2.2 (1.6, 2.8) | 0.37 |
| Change at 3 months from baseline | −2.1 (−3.1, −1.2) | −2.2 (−3.2, −1.2) | −0.0 (−0.7, 0.7) | −2.2 (−3.8, −0.6) | −2.1 (−3.7, −0.4) | 0.1 (−0.8, 0.9) | 0.32 |
| Change at 12 months from baseline | −2.9 (−4.1, −1.7) | −2.8 (−4.0, −1.6) | 0.2 (−0.6, 1.1) | −1.8 (−3.4, −0.1) | −1.5 (−3.3, 0.3) | 0.2 (−0.6, 1.0) | 0.55 |
| Change at 24 months from baseline | −4.5 (−5.8, −3.2) | −3.3 (−4.6, −2.0) | 1.4 (0.5, 2.3) | −3.2 (−5.1, −1.3) | −2.6 (−4.5, −0.6) | 0.6 (−0.4, 1.7) | 0.58 |
| Overall change during follow-up visits from baselinec | −2.9 (−3.8, −2.0) | −2.6 (−3.5, −1.7) | 0.4 (−0.2, 1.0) | −2.2 (−3.8, −0.7) | −2.0 (−3.6, −0.3) | 0.2 (−0.5, 1.0) | 0.56 |
Abbreviation: CI, confidence interval.
aTwo participants and 33 visits were excluded due to missing standing blood pressure measurements. For details of number of participants with orthostatic hypotension measurements at each visit see the CONSORT diagram.
bThe 1,000+ IU/day includes participants randomized to 1,000, 2,000, or 4,000 IU/day.
cThese models used a dichotomous variable for study visit whereby all visits were categorized as baseline or a follow-up visit (3, 12, or 24 months).
d P comparing seated or standing blood pressure (1,000+ IU/day vs. 200 IU/day) was <0.05. All others were nonsignificant.
Effects of vitamin D3 dose on OH
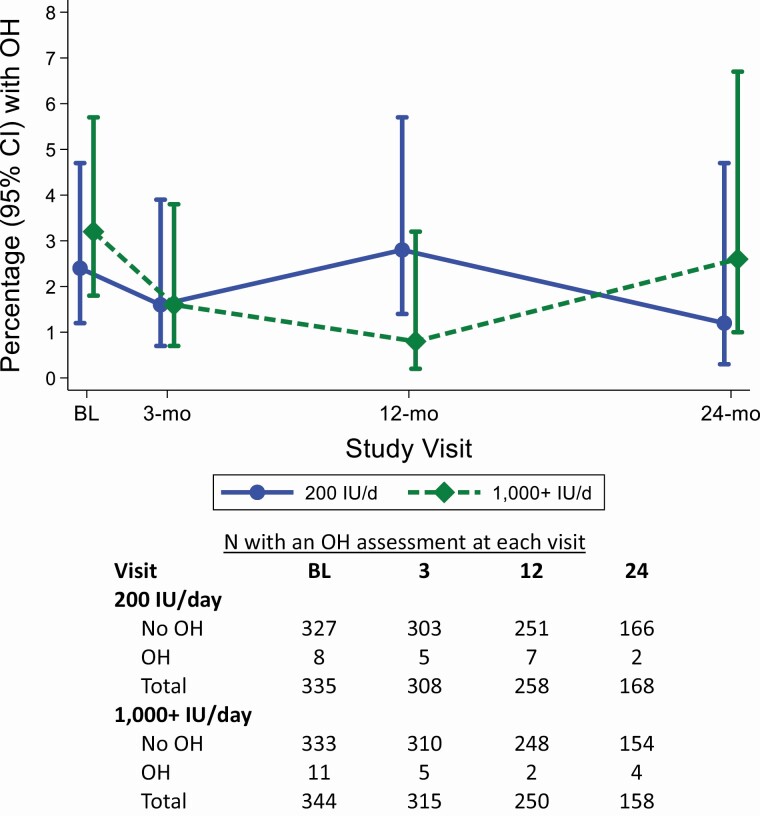
At baseline, OH was present among 2.4% of adults assigned 200 IU/day and among 3.2% of adults assigned 1,000+ IU/day of vitamin D3. There were no clear differences in OH prevalence between the 2 groups over the course of the study (Figure 2). Furthermore, 1,000+ vs. 200 IU/day was not associated with lower odds of OH (Table 3).
Figure 2.
The proportion with orthostatic hypotension during the trial and number of participants contributing to orthostatic hypotension assessments by treatment assignment (1,000+ or 200 IU/day). The 1,000+ IU/day includes participants randomized to 1,000, 2,000, or 4,000 IU/day. Point estimates and confidence intervals were estimated using a generalized estimating equation, using the Poisson family, log link, and robust variance estimator. Abbreviations: CI, confidence interval; OH, orthostatic hypotension.
Table 3.
Effects of vitamin D dose on orthostatic hypotension over time, N = 686 participants and 2,136 assessments
| % with orthostatic hypotension | 1,000+a vs. 200 IU/day | ||||
|---|---|---|---|---|---|
| 200 IU/day | 1,000+a IU/day | P interaction | OR (95% CI) | P | |
| % at baseline | 2.4 (0.8, 4.0) | 3.2 (1.4, 5.1) | 0.51 | 1.36 (0.54, 3.42) | 0.52 |
| Change at 3 months | −0.7 (−2.7, 1.2) | −0.8 (−2.9, 1.3) | 0.56 | 0.66 (0.21, 2.04) | 0.47 |
| Change at 12 months | 0.4 (−2.1, 3.0) | −1.6 (−3.6, 0.4) | 0.10 | 0.33 (0.07, 1.57) | 0.16 |
| Change at 24 months | −1.1 (−3.4, 1.1) | 0.2 (−2.7, 3.1) | 0.79 | 1.09 (0.33, 3.60) | 0.88 |
| Overall change during follow-up visits | −0.4 (−2.3, 1.4) | −0.9 (−2.7, 1.0) | 0.35 | 0.63 (0.25, 1.59) | 0.33 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aThe 1,000+ IU/day includes participants randomized to 1,000, 2,000, or 4,000 IU/day.
Effects of vitamin D3 dose on orthostatic symptoms
Compared with 200 IU/day, the higher vitamin D3 dose did not affect any orthostatic symptoms, including lightheadedness or dizziness after standing (Table 4). Moreover, a higher vitamin D3 dose was generally not associated with historic orthostatic symptoms or functional limitations in the preceding 30 days. A higher vitamin D3 dose was associated with a reduced need to brace oneself after standing (odds ratio 0.59; 95% confidence interval: 0.38, 0.92).
Table 4.
Effects of vitamin D assignment on orthostatic symptoms
| 1,000+a IU vs. 200 IU/day | ||||
|---|---|---|---|---|
| N of participants | N of assessments | OR (95% CI) | P | |
| During seated protocolb | ||||
| Lightheaded or dizzy immediately after standing | 688 | 2156 | 1.31 (0.60, 2.85) | 0.50 |
| Lightheaded or dizzy after 3 BP measures were performed | 686 | 2132 | 1.05 (0.43, 2.58) | 0.91 |
| Historic symptoms upon standing (past 30 days) | ||||
| Lightheadedness | 589 | 1281 | 0.85 (0.53, 1.36) | 0.50 |
| Dizziness | 589 | 1279 | 0.73 (0.45, 1.19) | 0.21 |
| Fainting | 589 | 1281 | 1.39 (0.28, 6.75) | 0.69 |
| Black out | 589 | 1281 | 0.20 (0.02, 2.47) | 0.21 |
| Seeing spots | 589 | 1277 | 1.01 (0.49, 2.10) | 0.98 |
| Imbalance | 589 | 1273 | 0.88 (0.59, 1.33) | 0.56 |
| Room spinning | 589 | 1277 | 0.81 (0.35, 1.88) | 0.63 |
| Racing heart | 589 | 1275 | 0.91 (0.39, 2.12) | 0.83 |
| Sweating episodes | 589 | 1276 | 0.94 (0.37, 2.39) | 0.90 |
| Vision changes | 589 | 1271 | 0.52 (0.26, 1.07) | 0.077 |
| Nausea | 588 | 1275 | 0.58 (0.18, 1.85) | 0.36 |
| Trouble concentrating | 587 | 1271 | 0.62 (0.30, 1.28) | 0.19 |
| Shortness of breath | 589 | 1276 | 0.97 (0.50, 1.88) | 0.94 |
| Headache | 589 | 1274 | 0.85 (0.34, 2.09) | 0.72 |
| Muscle weakness | 589 | 1275 | 0.75 (0.44, 1.27) | 0.29 |
| Fatigue | 589 | 1274 | 0.71 (0.42, 1.21) | 0.21 |
| Function limitations (past 30 days) | ||||
| Concerned about falling after standing | 587 | 1274 | 0.69 (0.44, 1.10) | 0.12 |
| Brace yourself after standing | 588 | 1273 | 0.59 (0.38, 0.92) | 0.020 |
| Pause before taking a step forward after standing | 588 | 1274 | 0.64 (0.39, 1.05) | 0.080 |
| Intentionally take additional time in the process of standing to avoid falling | 587 | 1271 | 0.74 (0.49, 1.11) | 0.15 |
Abbreviations: BP, blood pressure; CI, confidence interval; OR, odds ratio.
aThe 1,000+ IU/day includes participants randomized to 1,000, 2,000, or 4,000 IU/day.
bThese were yes or no questions vs. a 9-point Likert scale for the other symptoms.
DISCUSSION
In a population of older adults at high risk for falling and with low baseline serum 25(OH)D, higher doses of vitamin D3 supplementation had no effect on seated BP, standing BP, or the change between seated and standing BP. Moreover, higher doses were not associated with reduced odds of OH or orthostatic symptoms. These findings do not support use of higher doses of vitamin D3 to prevent OH in older adults with vitamin D insufficiency.
Higher 25(OH)D, an indicator of vitamin D storage, has been inversely associated with falls across multiple cohorts.17 It has also been inversely associated with OH in diverse observational studies8 leading many to hypothesize that BP regulation might be a mechanism by which vitamin D could prevent falls. Indeed, vitamin D has been linked to the renin–angiotensin system in mice9 and lower seated BP in select populations.18 Vitamin D deficiency has also been associated with endothelial dysfunction in animal experimental and human observational studies.19,20 In addition, serum vitamin D is positively associated with autonomic nervous system activity11 and supplementation in humans has been shown to improve cardiac autonomic function.12 However, our study did not demonstrate any benefit from higher vitamin D3 supplementation in adults who were both vitamin D insufficient and at risk for falling.
Our findings are consistent with one other trial examining the effects of vitamin D3 on OH.13 Witham et al. compared intermittently dosed, oral vitamin D3 at 100,000 IU per dose to placebo over a 1-year follow-up period following a parallel design. Their population included 159 patients, aged 70 years and older, with a serum 25(OH)D <28 ng/ml and isolated systolic hypertension (systolic BP >140 mm Hg and diastolic BP <90 mm Hg). They reported a significant drop in the postural change in systolic BP at 3 months, but this finding was not maintained over time or in their repeated measures analysis.
We speculate that one reason our study did not observe an effect from higher vitamin D3 supplementation on OH, was that both vitamin D3 assignments had reductions in BP over the course of the study. Moreover, BP reduction was marginally greater in the low-dose vitamin D3 group. In several studies, more aggressive BP management actually reduced (rather than increased) risk of OH.21–23 Notably, reductions in seated BP were similar to standing BP. This reinforces the notion that BP reduction alone does not induce OH. Rather, an agent needs to disproportionately affect standing BP, which was not observed with vitamin D3 supplementation, similar to many other BP lowering agents.21 Our findings are consistent with 1 meta-analysis of vitamin D and BP,18 which showed that baseline serum 25(OH)D did not modify the null relationship between vitamin D supplementation and BP.
One limitation to our study was the low prevalence of OH in our population despite its older age and higher risk for falls. This was particularly problematic during later visits (e.g., the 24-month visit) with fewer participants. This may reflect the use of seated BP rather than supine BP for OH determinations. Alternatively, the low prevalence may reflect the lack of BP measurements within 1 minute of standing, which might miss early OH.24,25 The low OH prevalence may also reflect the high prevalence of hypertension treatment, which has been shown to reduce OH.23 However, it also hints at a broader challenge of OH research as adults may be less prone to present for clinical assessments when they are actively experiencing orthostatic symptoms. This was observed in a recent study of older adults, where OH was not performed among those with dizziness immediately after standing.26 We are reassured that selection played less of a role in our study given our inclusion of historic self-reported symptoms. However, these data could be affected by recall bias. While higher vitamin D3 dose was associated with a reduced practice of bracing oneself after standing, this isolated association should be viewed cautiously given the absence of associations with the many other symptoms assessed and the potential for false discovery. Another important limitation is that STURDY did not have a placebo as its control. As a result, we are unable to determine the effects of the 200 IU/day dose vs. no supplementation on OH.
Despite the limitations above, our study has strengths. STURDY included a diverse, older population with vitamin D insufficiency at risk of falling, representing a population traditionally recommended to take vitamin D3 supplements. STURDY also used a randomized design with high adherence and follow-up rates that tested both lower and higher doses of vitamin D3, allowing us to examine the role of vitamin D3 dose on OH. Finally, our assessments were standardized with high retention rates.
In conclusion, higher doses of vitamin D3 supplementation did not affect seated BP, standing BP, or postural change in BP compared with a lower dose. These null findings do not support use of higher doses of vitamin D3 to prevent OH in older adults at risk for falling.
Supplementary Material
ACKNOWLEDGMENTS
We thank participants of the STURDY study who volunteered in support of this research. STURDY is registered on clinicaltrials.gov under identifier NCT02166333.
FUNDING
S.P.J. is supported by a NIH/NHLBI (7K23HL135273). STURDY was funded by the National Institute on Aging (U01AG047837) with support from the Office of Dietary Supplements, the Mid-Atlantic Nutrition Obesity Research Center (P30DK072488), and the Johns Hopkins Institute for Clinical and Translation Research (UL1TR003098). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension 1992; 19:508–519. [DOI] [PubMed] [Google Scholar]
- 2. Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med 2000; 108:106–111. [DOI] [PubMed] [Google Scholar]
- 3. Juraschek SP, Daya N, Appel LJ, Miller ER, McEvoy JW, Matsushita K, Ballantyne CM, Selvin E. Orthostatic hypotension and risk of clinical and subclinical cardiovascular disease in middle-aged adults. J Am Heart Assoc 2018; 7:e008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soysal P, Yay A, Isik AT. Does vitamin D deficiency increase orthostatic hypotension risk in the elderly patients? Arch Gerontol Geriatr 2014; 59:74–77. [DOI] [PubMed] [Google Scholar]
- 5. Annweiler C, Schott AM, Rolland Y, Beauchet O. Vitamin D deficiency is associated with orthostatic hypotension in oldest-old women. J Intern Med 2014; 276:285–295. [DOI] [PubMed] [Google Scholar]
- 6. Gilani A, Ramsay SE, Juraschek SP, Papacosta O, Lennon LT, Whincup PH, Wannamethee SG. Associations of the systolic and diastolic components of orthostatic hypotension with markers of cardiovascular risk in older men: a cross-sectional analysis from The British Regional Heart Study. J Clin Hypertens (Greenwich) 2020; 22:1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duval GT, Paré PY, Gautier J, Walrand S, Dinomais M, Annweiler C. Vitamin D and the mechanisms, circumstances and consequences of falls in older adults: a case-control study. J Nutr Health Aging 2017; 21:1307–1313. [DOI] [PubMed] [Google Scholar]
- 8. Ometto F, Stubbs B, Annweiler C, Duval GT, Jang W, Kim HT, McCarroll K, Cunningham C, Soysal P, Isik AT, Luchini C, Solmi M, Sergi G, Manzato E, Veronese N. Hypovitaminosis D and orthostatic hypotension: a systematic review and meta-analysis. J Hypertens 2016; 34:1036–1043. [DOI] [PubMed] [Google Scholar]
- 9. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 2004; 89–90:387–392. [DOI] [PubMed] [Google Scholar]
- 11. Burt MG, Mangelsdorf BL, Stranks SN, Mangoni AA. Relationship between vitamin D status and autonomic nervous system activity. Nutrients 2016; 8:E565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mann MC, Hemmelgarn BR, Exner DV, Hanley DA, Turin TC, Wheeler DC, Sola DY, Ellis L, Ahmed SB. Vitamin D supplementation is associated with stabilization of cardiac autonomic tone in IgA nephropathy. Hypertension 2015; 66:e4–e6. [DOI] [PubMed] [Google Scholar]
- 13. Witham MD, Price RJ, Struthers AD, Donnan PT, Messow M, McConnachie A, Ford I, McMurdo ME. Effect of vitamin D supplementation on orthostatic hypotension: data from the vitamin D in isolated systolic hypertension randomized controlled trial. J Hypertens 2014; 32:1693–1699; discussion 1699. [DOI] [PubMed] [Google Scholar]
- 14. Michos ED, Mitchell CM, Miller ER III, Sternberg AL, Juraschek SP, Schrack JA, Szanton SL, Walston JD, Kalyani RR, Plante TB, Christenson RH, Shade D, Tonascia J, Roth DL, Appel LJ; STURDY Collaborative Research Group . Rationale and design of the Study To Understand Fall Reduction and Vitamin D in You (STURDY): a randomized clinical trial of vitamin D supplement doses for the prevention of falls in older adults. Contemp Clin Trials 2018; 73:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Appel LJ, Michos ED, Mitchell CM, Blackford AL, Sternberg AL, Miller ER III, Juraschek SP, Schrack JA, Szanton SL, Charleston J, Minotti M, Baksh SN, Christenson RH, Coresh J, Drye LT, Guralnik JM, Kalyani RR, Plante TB, Shade DM, Roth DL, Tonascia J; STURDY Collaborative Research Group . The effects of four doses of vitamin D supplements on falls in older adults: a response-adaptive, randomized clinical trial. Ann Intern Med 2021; 174:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 1996; 6:125–126. [DOI] [PubMed] [Google Scholar]
- 17. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JPA. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014; 348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farapti F, Fadilla C, Yogiswara N, Adriani M. Effects of vitamin D supplementation on 25(OH)D concentrations and blood pressure in the elderly: a systematic review and meta-analysis. F1000Res 2020; 9:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wee CL, Mokhtar SS, Singh KKB, Yahaya S, Leung SWS, Rasool AHG. Calcitriol supplementation ameliorates microvascular endothelial dysfunction in vitamin D-deficient diabetic rats by upregulating the vascular eNOS protein expression and reducing oxidative stress. Oxid Med Cell Longev 2021; 2021:3109294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanaka K, Okada Y, Hajime M, Tanaka Y. Low vitamin D levels are associated with vascular endothelial dysfunction in patients with poorly controlled type 2 diabetes: a retrospective study. J Atheroscler Thromb 2021. PMID: 33518614. doi: 10.5551/jat.59113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juraschek SP, Appel LJ, Miller ER III, Mukamal KJ, Lipsitz LA. Hypertension treatment effects on orthostatic hypotension and its relationship with cardiovascular disease. Hypertension 2018; 72:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juraschek SP, Taylor AA, Wright JT Jr, Evans GW, Miller ER III, Plante TB, Cushman WC, Gure TR, Haley WE, Moinuddin I, Nord J, Oparil S, Pedley C, Roumie CL, Whittle J, Wiggers A, Finucane C, Anne Kenny R, Appel LJ, Townsend RR; SPRINT Research Group . Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension 2020; 75:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Juraschek SP, Hu JR, Cluett JL, Ishak A, Mita C, Lipsitz LA, Appel LJ, Beckett NS, Coleman RL, Cushman WC, Davis BR, Grandits G, Holman RR, Miller ER III, Peters R, Staessen JA, Taylor AA, Thijs L, Wright JT Jr, Mukamal KJ. Effects of intensive blood pressure treatment on orthostatic hypotension: a systematic review and individual participant-based meta-analysis. Ann Intern Med 2021; 174:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen S, He T, Chu J, He J, Chen X. Uncontrolled hypertension and orthostatic hypotension in relation to standing balance in elderly hypertensive patients. Clin Interv Aging 2015; 10:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc 2011; 59:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Juraschek SP, Longstreth WT Jr, Lopez OL, Gottdiener JS, Lipsitz LA, Kuller LH, Mukamal KJ. Orthostatic hypotension, dizziness, neurology outcomes, and death in older adults. Neurology 2020; 95:e1941–e1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.