Abstract
BACKGROUND
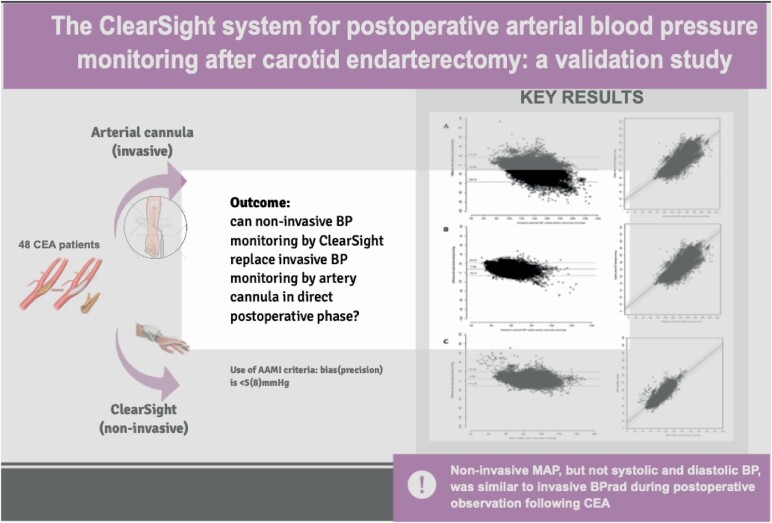
The majority of postoperative events in patients undergoing carotid endarterectomy (CEA) are of hemodynamic origin, requiring preventive strict postoperative arterial blood pressure (BP) control. This study aimed to assess whether BP monitoring with noninvasive beat-to-beat ClearSight finger BP (BPCS) can replace invasive beat-to-beat radial artery BP (BPRAD) in the postoperative phase.
METHODS
This study was a single-center clinical validation study using a prespecified study protocol. In 48 patients with symptomatic carotid artery stenosis, BPCS and BPRAD were monitored ipsilateral in a simultaneous manner during a 6-hour period on the recovery unit following CEA. Primary endpoints were accuracy and precision of BP derived by ClearSight (Edward Lifesciences, Irvine, CA) vs. the reference standard (Arbocath 20 G, Hospira, Lake Forest, IL) to investigate if BPCS is a reliable noninvasive alternative for BP monitoring postoperatively in CEA patients. Validation was guided by the standard set by the Association for Advancement of Medical Instrumentation (AAMI), considering a BP-monitor adequate when bias (precision) is <5 (8) mm Hg. Secondary endpoint was percentage under- and overtreatment, defined as exceedance of individual postoperative systolic BP threshold by BPRAD or BPCS in contrast to BPCS or BPRAD, respectively.
RESULTS
The bias (precision) of BPCS compared to BPRAD was −10 (13.6), 8 (7.2) and 4 (7.8) mm Hg for systolic, diastolic and mean arterial pressure (MAP), respectively. Based on BPCS, undertreatment was 5.6% and overtreatment was 2.4%; however, percentages of undertreatment quadrupled for lower systolic BP thresholds.
CONCLUSIONS
Noninvasive MAP, but not systolic and diastolic BP, was similar to invasive BPRAD during postoperative observation following CEA, based on AAMI criteria. However, as systolic BP is currently leading in postoperative monitoring to adjust BP therapy on, BPCS is not a reliable alternative for BPRAD.
Keywords: arterial pressure, blood pressure, carotid endarterectomy, ClearSight, hypertension, noninvasive continuous arterial pressure measurement, postoperative monitoring
Graphical Abstract
Graphical Abstract.
The benefit of carotid revascularization for severe symptomatic carotid artery stenosis is offset by stroke due to the intervention itself.1–3 Hemodynamic disturbances are held accountable for half of the periprocedural strokes following carotid endarterectomy (CEA) and postoperative events.4–6 Intraoperative hypotension may cause cerebral hypoperfusion which can result in hypoxia and subsequently cerebral ischemia, whereas postoperative hypertension may lead to ipsilateral cerebral hyperperfusion with hemorrhagic stroke when not timely recognized and treated.7 Therefore, to reduce the incidence of periprocedural stroke in patients undergoing CEA and to preserve adequate cerebral perfusion on the other hand, blood pressure (BP) has to remain between strict thresholds.
Today, intraoperative beat-to-beat invasive radial artery BP monitoring continues on the postoperative recovery unit for 3 to 6 hours following surgery. However, besides that invasive BP monitoring has a complication risk (i.e., failure of cannulation despite the use of ultrasound, bleedings, pseudo-aneurysm, thrombotic embolization or occlusion), it is invasive since monitoring by radial artery cannula requires admission on a recovery or medium care unit.8,9
Recent data revealed that Nexfin/ClearSight (Edwards Lifesciences, Irvine, CA), a noninvasive beat-to-beat BP device on the finger, could be used as an alternative for intraoperative invasive BP monitoring in the radial artery in patients undergoing CEA, both under local and general anesthesia.10,11 However, replacement of the radial artery line by a noninvasive monitoring device can only be of added value and beneficial for the patient when it applies both the intraoperative as well postoperative phase. To our knowledge, beat-to-beat finger BP was not validated during the 3–6 hours lasting postoperative admission on a postoperative recovery unit in awake patients.
Based on the earlier validation studies,10,11 we hypothesized that also during a prolonged monitoring period noninvasive beat-to-beat finger mean BP by ClearSight can be considered as a reliable and easy-to-assess alternative for invasive radial artery mean BP in awake moving patients on the postoperative recovery unit after a CEA procedure. Since, besides clinical symptoms, the systolic BP is the leading BP parameter to detect or prevent cerebral reperfusion and hyperperfusion syndromes. We determined the accuracy and precision of diastolic, mean, and systolic BP monitored by ClearSight (BPCS) compared to radial artery BP (BPRAD), in line with the standard of the Association for the Advancement of Medical Instrumentation (AAMI) criteria. We also quantified the frequency of potential over- and undertreatment when invasive BP monitoring would be replaced by noninvasive BP (NIBP) monitoring.
METHODS
Subjects
This study was approved by the local medical ethics committee on 26 April 2017 (Medical Research Ethics Committee UMC Utrecht, protocol number 17-2573/C). Written informed consent in accordance with the Helsinki Declaration for participation in the ClearSight study was obtained from patients undergoing CEA between July 2017 and June 2018 at a tertiary referral vascular surgery centre at the University Medical Center Utrecht in the Netherlands. Exclusion criteria were: refusal to participate and patients who underwent CEA for asymptomatic carotid artery stenosis.
Study design
All patients received standard monitoring, i.e., electrocardiography, end-tidal carbon dioxide, oscillometric NIBP with an upper arm cuff and pulse oximetry via a Datex Ohmeda S/5 anesthesia monitor (GE, Healthcare, Waukesha, WI). Invasive continuous BP was monitored with an artery cannula (Arbocath 20 G, Hospira, Lake Forest, IL) placed preoperatively in the radial artery ipsilateral to the upper arm cuff. Standard monitoring data and invasive BPRAD data of the radial artery cannula are stored by monitoring program Anstat (Carepoint Nederland B.V., Ede, the Netherlands). Postoperatively, patients were connected to the ClearSight system (Edwards Lifesciences). A noninvasive beat-to-beat finger BP device was applied to the index finger, ipsilateral to the upper arm NIBP and radial artery cannula. The size of the cuff was selected to fit the size of the mid-phalanx of the index finger. The ClearSight system is based on a photoplethysmograph in a cuff and integrated into a simplified clinical platform (EV1000). The finger cuff will be inflated and deflated through the cardiac cycle in a way that the blood flow in the finger becomes constant instead of pulsatile as monitored with the photoplethysmograph. The pressure in the cuff to create a continuous finger blood flow is the inverse of the BP, a technique proposed by Peňáz. A detailed description has been previously published.12 Calibration of the ClearSight system was performed by a built-in expert system (Physiocal), detecting changes in finger arterial diameter to establish and adjust the arterial unloaded volume at least once every 70 heartbeats.13 According to ClearSight operator’s manual, a Physiocal interval >30 beats indicates stable and reliable pressure measurements.14 A heart reference sensor (HRS) was used to compensate for hydrostatic pressure changes due to height differences between the finger and the right atrium. Therefore, the reference sensor was connected to the finger cuff and the other site was taped on the lateral side of the thorax, on the position of the right atrium. Inflation of oscillometric NIBP was used as a marker at the start and during monitoring to calibrate BPCS in time with BPRAD measurements.
CEA protocol
The CEA procedure is described in detail earlier.10 All patients underwent CEA under volatile anesthesia performed by an experienced vascular surgeon or a vascular trainee under the supervision of a vascular surgeon. Patients were monitored neurologically during surgery by electroencephalogram and transcranial Doppler (TCD). Postoperatively, an individual systolic BP restriction was determined for each patient based on the intraoperative increase of mean velocity measured in the middle cerebral artery (MCAVmean) after clamping assessed by TCD. This maximum systolic BP threshold could be adjusted in response to an increase of MCAVmean determined by a standard 2-hour postoperative TCD measurement. All patients were admitted to the recovery unit for hemodynamic and neurological monitoring for 3–6 hours.
Data collection
Both BPRAD measurements (112 Hz) derived from the Datex Ohmeda S/5 monitoring system (GE Healthcare) and BPCS measurements (200 Hz) derived from the ClearSight system were stored on a hard disk for offline analysis. ClearSight used an algorithm to reconstruct a finger BP measurement to a brachial artery waveform. The algorithm corrects for a decrease in diastolic pressure and an increase in systolic pressure due to a pressure gradient in the smaller arteries a pressure wave reflection.14,15 For each patient, the systolic BP threshold and BP medication (either vasopressor agents or antihypertensive agents) were registered. Beat-to-beat data of the first 6 hours on the recovery unit were used in this study. BPCS and BPRAD were aligned in time. Data were visually inspected for any artifacts such as arterial flushing, episodes where the Physiocal frequency was more than once every 30 heartbeats, the inability for BPRAD and BPCS to measure due to occlusion of the arteries during oscillometric NIBP measurement. The beat-to-beat data were averaged over slots of 20 seconds. All timeslots in which the previously mentioned artifacts occurred in either the BPCS and BPRAD were excluded from further analysis.
Sample size calculation
The sample size for this study was calculated based on AAMI criteria and previous studies validating finger BP devices with invasive intra-arterial BP device as a reference.10,11,15 In accordance with the AAMI criteria, a minimum of 15 patients was recommended. However, AAMI criteria do not specify for continuous noninvasive sphygmomanometers.16 Previous studies for intraoperative validation had a sample size of 25–30 patients. Due to expected dropouts, we believed a larger sample size was needed. Therefore, we aimed to include 50 patients.
Statistical analysis
Baseline characteristics that are normally distributed continuous data are presented as mean (±SD). Non-normally distributed continuous data are provided as median (interquartile) and categorical variables as n (percentage).
To assess whether there was sufficient agreement between the 2 continuous methods of measurements (i.e., ClearSight finger BP vs. radial arterial BP), the Bland–Altman method and plot were used.17,18 Herein, the mean difference and the range of these differences (in which 95% of measurements fall) are calculated using the SD and graphically depicted. Whether this 95% interval (limits of agreement (LOA)) meaningfully affect the interpretation of the results, is subject to the clinical context.19 In accordance with AAMI criteria, a mean error of <5 mm Hg and SD <8 mm Hg compared to the reference method (i.e., radial artery BP) are considered clinically acceptable.16
However, the original Bland–Altman method was developed for independent data, i.e., one measurement of both methods per patient. Therefore, it is not suitable for situations in which there are multiple measurements per patient as it underestimates the true variation of the difference, although it is often misused for this purpose.19,20 Therefore, mixed effects LOA, obtained through the use of random effects models in which is accounted for repeated measurements through the use of a different intercept per subject, were performed.21 The derived total SD is used to obtain the LOA, whereas the between-subject and within-subject SD are reported and used to obtain the intra-class correlation coefficient (ICC), a measure to assess the degree of total variation explained by the between-subject variation.20
To evaluate if baseline characteristics could partly explain the variation, the variables sex, age, body mass index, diabetes mellitus, smoking, hypertension, peripheral and central arterial disease, and pulse pressure were added in another model. Pulse pressure was added to the model to correct for arterial stiffness of the smaller arteries. Assumptions checked were constant variances, residuals vs. fitted values plots, q–q plots of the residuals, and random effects predictions. None were substantially violated. Results were graphically visualized using Bland–Altman plots, with arterial pressure at the x-axis, as this is the reference standard.22
Potential undertreatment and overtreatment were calculated per measurement time (20-second window) and per 5-minute window (mean of 15 measurements). Individual systolic BP thresholds determined for each patient-based increase of MCAVmean assessed by TCD were used. Undertreatment was defined as when systolic radial arterial pressure exceeded this predetermined systolic BP threshold, while BPCS was lower than this threshold. Overtreatment was defined as when systolic radial arterial BP was below the predetermined systolic BP threshold, while BPCS was exceeding this threshold.
RESULTS
Written informed consent was obtained from 52 patients. In one patient, no measurements could be performed due to logistic reasons (directly postoperative admission to a high care unit). The measurements of 2 other patients were excluded for analysis due to technical problems (error of pump unit of ClearSight system, erroneous time registration of ClearSight). In one patient, only 45 minutes were recorded and therefore not representative for a prolonged measurement. Of 48 patients (75% males, 71.5 years (50–93)), both BPCS and BPRAD data were analyzed. Baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics
| All patients (n = 48) | |
|---|---|
| Age | 71.5 (50–93) |
| Sex, male | 36 (75%) |
| Risk factors | |
| Hypertension | 34 (71%) |
| Hyperlipidemia | 35 (73%) |
| Diabetes mellitus | 13 (27%) |
| Coronary artery disease | 20 (42%) |
| Peripheral arterial disease | 11 (23%) |
| Smoker (current/ex) | 36 (76%) |
| Symptomatic | 48 (100%) |
| Ipsilateral stenosis | |
| 50–70% | 5 (10%) |
| >70% | 42 (87%) |
| Contralateral stenosis | |
| Occlusion | 10 (21%) |
| >70% | 5 (10%) |
| 50–70% | 5 (10%) |
| <50% | 28 (58%) |
| Shunt use | 5 (10%) |
| Medication | |
| Statins | 39 (81%) |
| Antiplatelets | 41 (85%) |
| Anti-coagulants | 5 (10%) |
| Diuretics | 12 (25%) |
| BP-lowering drugs | 32 (67%) |
| Preoperative systolic BP, mm Hg (SD) | 147 (17) |
| Preoperative diastolic BP, mm Hg (SD) | 77 (12) |
| Preoperative MAP, mm Hg (SD) | 101 (11) |
| Postoperative events | |
| Total events | 7 (15%) |
| Cerebral hyperperfusion | 3 (6%) |
| Bleeding requiring surgery | 1 (2%) |
| Stroke | 1 (2%) |
| TIA | 2 (4%) |
| Medium care admission | 8 (17%) |
| Extended recovery admission | 11 (23%) |
| Labetalol use | 11 (23%) |
| Norephedrine use | 4 (8%) |
| Clonidine use | 6 (13%) |
Abbreviations: BP, blood pressure; MAP, mean arterial pressure. Data in median (range) or number (%); TIA, trans ichemic attack.
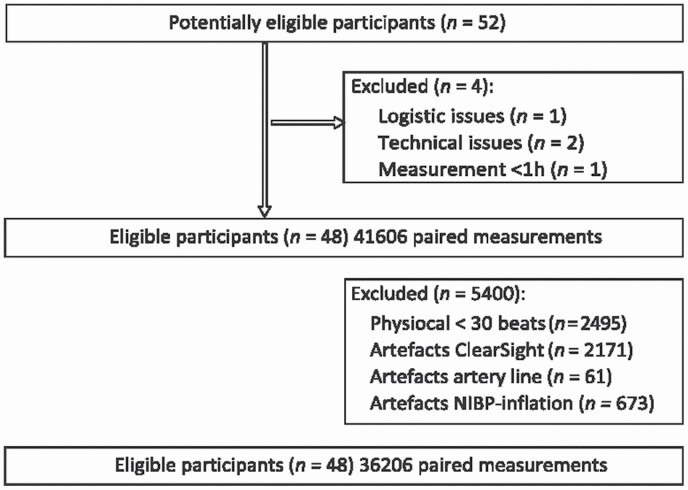
The median duration of BP monitoring was 5.7 hours (range 178 minutes–6 hours). Of 41,606 (20 seconds averaged) paired samples gathered from 48 patients, 5,400 samples (13%) were excluded from analyses. Of these 5,400 excluded samples, 2,495 (6%) were excluded due to ClearSight Physiocal frequency greater than once per 30 heartbeats, and 2,171 (5%) samples were excluded due to artifacts of ClearSight. In 673 (2%) samples, both BPCS and BPRAD measurements were unreliable due to inflation of ipsilateral noninvasive upper arm BP cuff. Lastly, 61 samples (0.2%) were excluded due to an unreliable arterial BP curve or flushing of the arterial line. The majority of the excluded samples were 1–3 sequenced BP samples and not long sequenced measurements (70%). A total of 36,206 (87%) paired samples of BPCS and BPRAD measurements collected from 48 patients were suitable for analysis. This was a median of 812 samples per patient (range 249–982) (Figure 1).
Figure 1.
Study flowchart. Flow of participants through the study. The study time frame is from the first 6 hours postoperative on the recovery unit.
AAMI validation
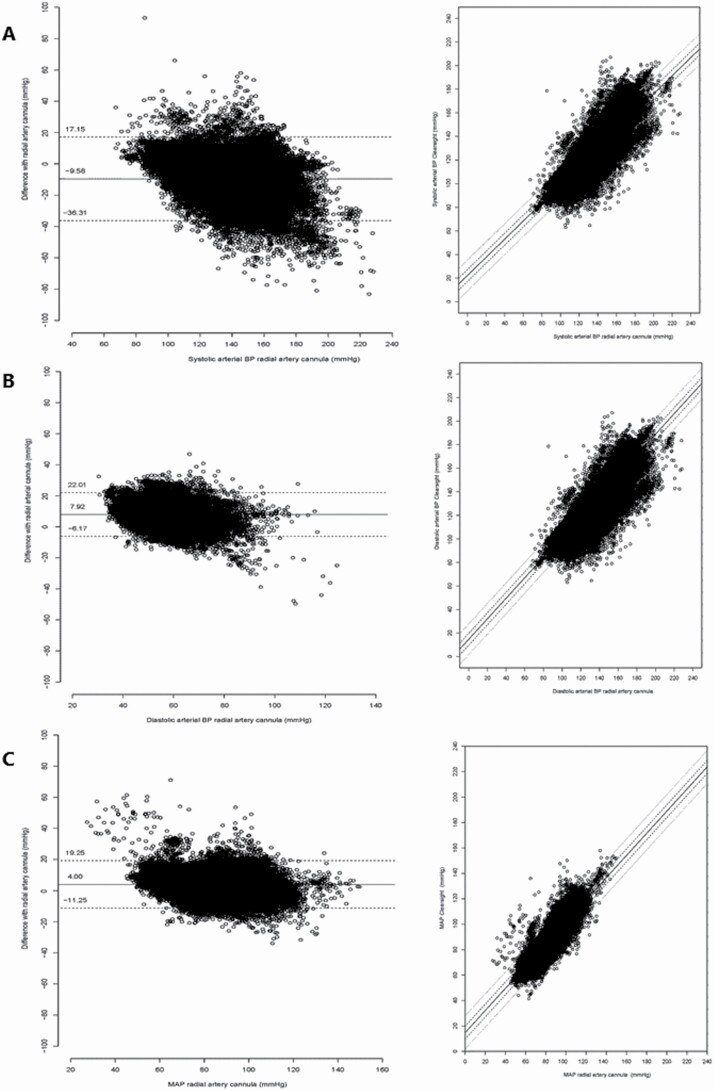
The levels of agreement for systolic BP, diastolic BP, and mean arterial pressure (MAP) by Clearsight (BPCS) compared to BP measured by the reference radial artery cannula (BPRAD) are presented in Figure 2. From this, it follows that the systolic BP shows a trend bias shape, with on average lower systolic arterial pressure as measured by Clearsight (larger negative difference) when invasive arterial pressure increases. The biases, precisions, LOA, and ICC are presented in Table 2. The mean bias of systolic BP was −10 mm Hg, of diastolic BP 8 mm Hg, and MAP 4 mm Hg.
Figure 2.
Bland–Altman plots systolic, diastolic, and mean arterial pressure—BPCS and BPRAD and corresponding scatterplots. Left: Bland–Altman plot of all 20-second systolic (a), diastolic (b), and mean arterial pressure (c) data points (n = 36 290). Solid line indicates mean difference (bias) and dashed lines are the upper and lower 95% limits of agreement. Right: Corresponding scatterplots of systolic, diastolic, and mean arterial pressure of BPCS vs. BPRAD with lines of identity (solid line is slope, dashed lines is ±5 mm Hg, and dotted lines ±13 mm Hg indicating the AAMI validation borders). Abbreviations: BP, blood pressure; AAMI, Advancement of Medical Instrumentation.
Table 2.
Bias, precision, and ICC of BPCS compared to BPRAD
| Bias | Precision | Between-subject variability | Within-subject precision | 95% Limits of agreement | ICC | |
|---|---|---|---|---|---|---|
| Systolic BP (mm Hg) | −9.58 | 13.64 | 10.43 | 8.79 | −36, 17 | 0.58 |
| Diastolic BP (mm Hg) | 7.92 | 7.19 | 5.97 | 4.00 | −6, 22 | 0.69 |
| MAP (mm Hg) | 4.00 | 7.78 | 5.81 | 5.18 | −11, 19 | 0.56 |
| Systolic BP (mm Hg) (corrected) | −9.58 | 14.05 | 10.97 | 8.79 | −37, 18 | 0.61 |
| Diastolic BP (mm Hg) (corrected) | 7.92 | 6.47 | 5.09 | 4.00 | −5, 21 | 0.62 |
| MAP (mm Hg) (corrected) | 4.00 | 7.74 | 5.75 | 5.18 | −11, 19 | 0.55 |
| Systolic BP (mm Hg) (corrected+) | −9.58 | 8.35 | 6.98 | 4.58 | −26, 7 | 0.70 |
| Diastolic BP (mm Hg) (corrected+) | 7.92 | 6.53 | 5.22 | 3.93 | −5, 21 | 0.64 |
| MAP (mm Hg) (corrected+) | 4.00 | 7.52 | 5.86 | 4.72 | −11, 19 | 0.61 |
Abbreviations: BP, blood pressure; ICC, intra-class correlation coefficient; MAP, mean arterial pressure. Data are presented in mm Hg.
Only for the MAP data, 95% of measurements met the criteria prescribed by the AAMI. Correction for baseline characteristics and pulse pressure did not substantially change bias and precision of the measurements (Table 2; Figure 2).
Clinical decision making
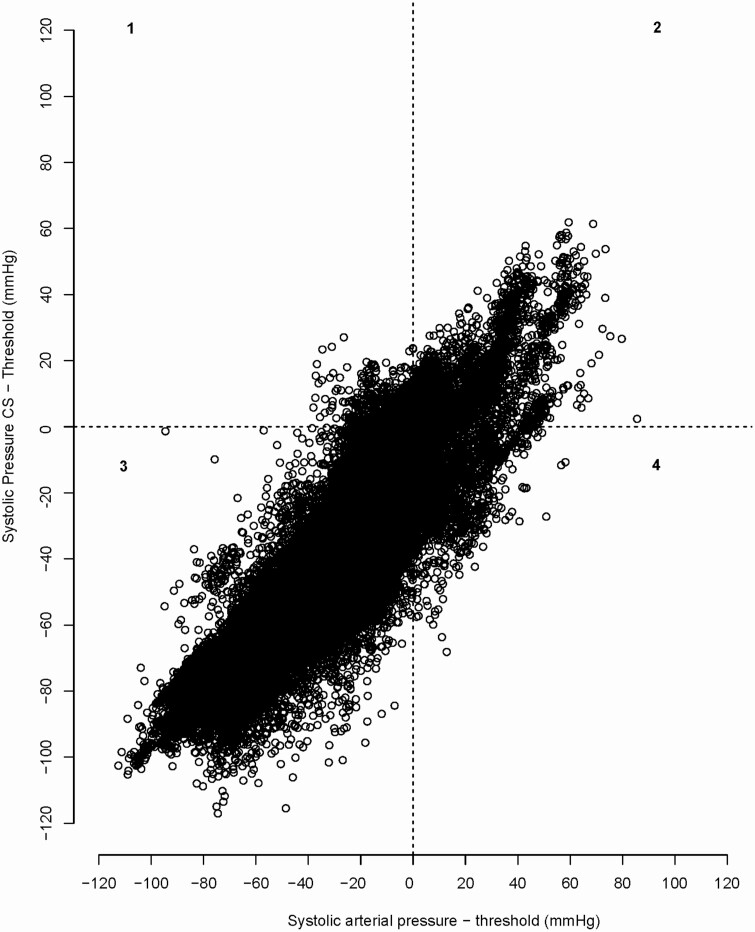
The overall percentage of overtreatment was 2.38%. The overall percentage of undertreatment was 5.56%. When specified per postoperative systolic threshold, undertreatment was almost 4 times as high in patients with a systolic threshold of 120 or 140 mm Hg (Table 3; Figure 3).
Table 3.
Clinical decision making
| All | 180 mm Hg | 160 mm Hg | 140 mm Hg | 120 mm Hg | |
|---|---|---|---|---|---|
| 20-second samples | |||||
| Overtreatment, n (%) | 860 (2.38%) | 197 (0.72%) | 114 (9.30%) | 83 (2.07%) | 466 (15.24%) |
| Undertreatment, n (%) | 2,103 (5.81%) | 447 (1.63%) | 15 (1.22%) | 816 (20.33%) | 640 (20.93%) |
| 5-minute samples | |||||
| Overtreatment, n (%) | 69 (2.38%) | 10 (0.46%) | 6 (7.41%) | 6 (1.91%) | 45 (17.79%) |
| Undertreatment, n (%) | 161 (5.55%) | 31 (1.42%) | 2 (1.85%) | 61 (19.43%) | 53 (20.95%) |
Figure 3.
Bland–Altman plot systolic arterial pressure BPCS and BPRAD with over- and undertreatment. Scatter plot with on the y-axis the difference between systolic BP by ClearSight compared to individual systolic threshold of patient (mm Hg). x-axis: the difference between systolic BP by radial artery cannula and individual systolic threshold. All potentially overtreatment when using Clearsight is plotted in upper left quadrant (1), and all potentially undertreatment when using ClearSight is presented in lower right square (4). Abbreviation: BP, blood pressure.
Discussion
In this single-center validation study, we assessed if noninvasive beat-to-beat finger BP monitoring by ClearSight can replace invasive radial artery BP monitoring for postoperative hemodynamic observation in patients who underwent CEA. Both systolic and diastolic BP monitored by ClearSight are below the clinically acceptable limits of the AAMI criteria and therefore unsuitable to replace invasive BP monitoring in this population of patients undergoing CEA for symptomatic carotid disease. Besides, replacement of invasive BPRAD by noninvasive BPCS may lead to extensive undertreatment of patients with strict low systolic BP thresholds, as the systolic BP is the leading parameter in current postoperative hemodynamic BP monitoring of CEA patients to adjust BP therapy on because of the ability to detect and prevent cerebral reperfusion and hyperperfusion syndromes. MAP determined by ClearSight meets the AAMI criteria with expected insignificant undertreatment (due to bias +4 mm Hg) and may therefore be considered as a suitable noninvasive alternative, both intraoperatively10,11 and postoperatively, for general hemodynamic monitoring of CEA patients when one uses MAP as leading hemodynamic parameter.
In the majority of CEA patients, both the baroreflex sensitivity and the cerebral autoregulation are impaired.23,24 Periprocedurally, this can lead to larger BP fluctuations that cannot be counter-regulated by the brain vasculature.4 Postoperative CEA-triggered changes in cerebral hemodynamics, i.e., hypertension, are assumed the underlying mechanism for the development of cerebral hyperperfusion syndrome (CHS).25–27 Strict BP control is therefore required to maintain adequate cerebral perfusion in effort to prevent for periprocedural strokes. Recent guidelines by the European Society for Vascular Surgery (ESVS) recommend neurologic and intra-arterial BP monitoring during the first 3-6 hours on an observed postoperative recovery unit, followed by hourly NIBP control during the first 24 hours.3 However, specific recommendations on how to determine postoperative BP thresholds are lacking. Some suggest a one-size-fits-all systolic BP policy by treating >170 or >160 mm Hg in patients with symptoms, while others advocate implementing TCD measurements to monitor the postoperative cerebral blood flow velocity of the ipsilateral MCA and adjust individual systolic BP thresholds upon this.26,28–30
In today’s clinical practice, the postoperative BP monitoring policy in patients who undergo CEA is based on the systolic BP. Specific data for systolic-controlled postoperative hemodynamic monitoring as opposed to MAP-controlled postoperative monitoring have not been reported in literature.
Although no validation criteria for continuous BP monitoring systems exists, the AAMI criteria for intermittent NIBP monitoring are frequently used in previous studies validating a finger BP device.16 Our study is in line with a literature review studying the accuracy of the finger cuff method to estimate BP (CNAP, FinaPress, Nexfin, and ClearSight) and concluded that although easy-in-use and able to measure a reasonable BP, finger cuff devices do not meet the criteria for clinical interchangeability with currently used invasive techniques.31 Up to now, there are only 2 studies which compared noninvasive finger BP devices and invasive BP in patients undergoing carotid surgery. Both studies, one in patients undergoing CEA under general anesthesia and the other in awake patients under regional anesthesia, reported that MAP, but not systolic and diastolic BP, (suboptimal) met the AAMI criteria.10,11 These results support our findings.
Our results should be put into perspective, as the patient population in the present study was vascular compromised with a high incidence of systemic atherosclerotic vascular disease and may therefore not representative for all postoperative patients. The vast majority of the patient population had a history of smoking and hyperlipidemia and one third of the patients had diabetes mellitus type 2. Reduced quality of peripheral small vessels and increased arterial stiffness in the smaller arteries due to calcification, can be expected.32,33 This may influence BPcs, making BPCS less reliable in this specific patient population and is a possible explanation for systolic and diastolic BP imprecision compared to BPRAD. Despite correction for baseline characteristics and pulse pressure, different results might be found within a healthy patient population.
Today, arterial BP measured invasively by radial artery cannula is still the accepted reference technique for clinical assessment of arterial BP in patients undergoing a CEA. The results of the current study represent an equation of how BP measured by ClearSight relates to BP measured by invasive radial artery cannula and not what technique is best in measuring arterial BP.19 To address this issue, we believe that choosing the use of plotting the differences between BPCS and BPRAD against BPRAD, the reference method, instead of the correlation coefficient (BPRAD + BPcs/2) when comparing field methods, was most appropriate.22
Furthermore, current study assesses the agreement between the methods in absolute values, as this reflects current clinical practice. However, it does not assess whether the change in arterial pressure over time is comparable between the different methods. For such purpose, the trend interchangeability method could be an interesting method in further research.34
Limitations
The results of this study should be interpreted in light of several limitations. Firstly, several technical issues of the ClearSight and EV1000 system occurred during the measurements. The pump unit of the ClearSight system and several HRS cables had to be replaced due to failure of providing adequate pressure in finger cuff or the inability to zero and thereby level the HRS to the phlebostatic axis, respectively. In addition, HRS cable secured on the level of phlebostatic axis at the thorax may have moved during nursing on the recovery. This might have influenced our results. However, all BP measurements of the patient in whom the pump unit failed to work were excluded and failure of zeroing of HRS cable impeded to start measuring BP. Secondly, the position of the invasive radial artery cannula was ipsilateral to the finger cuff, possibly leading to decreased perfusion to the finger and dampening or underestimation of BP curve measured by ClearSight. Collateral blood flow through the ulnar artery may overcome this issue. Also, in an earlier study, it was found that in about half of the cases, the noninvasive mean BP of the finger device was slightly higher than the mean BPRAD.10 Moreover, comparing BPCS to a contralaterally measured BPRAD is not an option since one out of 5 CEA patients has an inter-arm BP difference of >15 mm Hg.35 Lastly, 5 of 48 patients experienced an unconformable tingling sensation or pain in the distal end of the finger during long-term BP measurement by ClearSight. This might have led to increased movement of the finger and influenced the BP measurements.
In conclusion, noninvasive MAP, but not systolic and diastolic BP, was similar to invasive BPRAD during postoperative observation following CEA, based on AAMI criteria. However, as systolic BP is currently leading in postoperative monitoring to adjust BP therapy on, BPcs is not a reliable alternative for BPRAD.
ACKNOWLEGDMENTS
Special thanks to Marloes Janssen and Evelien de Vries for helping with data collection during and outside office hours; Leo van Wolfswinkel for the support and help during this project; Edwards Lifesciences for providing 2 ClearSight/EV1000 systems for the duration of the study; and Wilbert Wesselink, Boris Reuderink, and Jeroen van Goudoever for their support (and patience) to this manuscript.
Author contributions: study design/planning and study conduct: L.M.M.F., R.V.I., and G.J.B.; data analysis and writing of paper: L.M.M.F. and J.D.J.P.; and revision of the paper: all authors.
DISCLOSURE
The authors declared no conflict of interest.
References
- 1. Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, Lo TH, Gaines P, Dorman PJ, MacDonald S, Lyrer PA, Hendriks JM, McCollum C, Nederkoorn PJ, Brown MM. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 2010; 375:985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Borst GJ, Naylor AR. In the end, it all comes down to the beginning! Eur J Vasc Endovasc Surg 2015; 50:271–272. [DOI] [PubMed] [Google Scholar]
- 3. Naylor AR, Ricco JB, de Borst GJ, Debus S, de Haro J, Halliday A, Hamilton G, Kakisis J, Kakkos S, Lepidi S, Markus HS, McCabe DJ, Roy J, Sillesen H, van den Berg JC, Vermassen F, Kolh P, Chakfe N, Hinchliffe RJ, Koncar I, Lindholt JS, Vega de Ceniga M, Verzini F, Archie J, Bellmunt S, Chaudhuri A, Koelemay M, Lindahl AK, Padberg F, Venermo M, ESVS Guidelines Committee, ESVS Guideline Reviewers. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55:1–79. [DOI] [PubMed] [Google Scholar]
- 4. Huibers A, Calvet D, Kennedy F, Czuriga-Kovács KR, Featherstone RL, Moll FL, Brown MM, Richards T, de Borst GJ. Mechanism of procedural stroke following carotid endarterectomy or carotid artery stenting within the International Carotid Stenting Study (ICSS) randomised trial. Eur J Vasc Endovasc Surg 2015; 50:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Borst GJ, Moll FL, van de Pavoordt HD, Mauser HW, Kelder JC, Ackerstaf RG. Stroke from carotid endarterectomy: when and how to reduce perioperative stroke rate? Eur J Vasc Endovasc Surg 2001; 21:484–489. [DOI] [PubMed] [Google Scholar]
- 6. Huibers A, de Borst GJ, Thomas DJ, Moll FL, Bulbulia R, Halliday A; ACST-1 Collaborative Group . The mechanism of procedural stroke following carotid endarterectomy within the asymptomatic carotid surgery trial 1. Cerebrovasc Dis 2016; 42:178–185. [DOI] [PubMed] [Google Scholar]
- 7. Dalman JE, Beenakkers IC, Moll FL, Leusink JA, Ackerstaff RG. Transcranial Doppler monitoring during carotid endarterectomy helps to identify patients at risk of postoperative hyperperfusion. Eur J Vasc Endovasc Surg 1999; 18:222–227. [DOI] [PubMed] [Google Scholar]
- 8. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest 2011; 139:524–529. [DOI] [PubMed] [Google Scholar]
- 9. Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg 2009; 109:1763–1781. [DOI] [PubMed] [Google Scholar]
- 10. Heusdens JF, Lof S, Pennekamp CW, Specken-Welleweerd JC, de Borst GJ, van Klei WA, van Wolfswinkel L, Immink RV. Validation of non-invasive arterial pressure monitoring during carotid endarterectomy. Br J Anaesth 2016; 117:316–323. [DOI] [PubMed] [Google Scholar]
- 11. Noto A, Sanfilippo F, De Salvo G, Crimi C, Benedetto F, Watson X, Cecconi M, David A. Noninvasive continuous arterial pressure monitoring with Clearsight during awake carotid endarterectomy: a prospective observational study. Eur J Anaesthesiol 2019; 36:144–152. [DOI] [PubMed] [Google Scholar]
- 12. Penáz J. Photoelectric measurement of blood pressure, volume and flow in the finger. In Albert A, Vogt W, Hellig W (eds), Digest of the 10th International Conference on Medical and Biological Engineering. International Federation for Medical and Biological Engineering: Dresden, Germany, 1973, pp. 104. [Google Scholar]
- 13. Wesseling K, de Wit B, van der Hoeven G, van Goudoever J, Settels J. Physiocal, calibrating finger vascular physiology for FinaPres. Homeostasis 1995; 36:67–81. [Google Scholar]
- 14. EV1000 Operator’s Manual, Version 1.2. Edwards Lifesciences LLC: Irvine, CA, 2015. [Google Scholar]
- 15. Martina JR, Westerhof BE, van Goudoever J, de Beaumont EM, Truijen J, Kim YS, Immink RV, Jöbsis DA, Hollmann MW, Lahpor JR, de Mol BA, van Lieshout JJ. Noninvasive continuous arterial blood pressure monitoring with Nexfin®. Anesthesiology 2012; 116:1092–1103. [DOI] [PubMed] [Google Scholar]
- 16. Association for the Advancement of Medical Instrumentation. American National Standard for Non-Invasive Sphygmomanometers Part 2: Clinical Validation of Automated Measurement Type. ISO 81060-2. AAMI: Arlington, VA, 2009. [Google Scholar]
- 17. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–310. [PubMed] [Google Scholar]
- 18. Morgan CJ, Aban I. Methods for evaluating the agreement between diagnostic tests. J Nucl Cardiol 2016; 23:511–513. [DOI] [PubMed] [Google Scholar]
- 19. Myles PS, Cui J. Using the Bland–Altman method to measure agreement with repeated measures. Br J Anaesth 2007; 99:309–311. [DOI] [PubMed] [Google Scholar]
- 20. Parker RA, Weir CJ, Rubio N, Rabinovich R, Pinnock H, Hanley J, McCloughan L, Drost EM, Mantoani LC, MacNee W, McKinstry B. Application of mixed effects limits of agreement in the presence of multiple sources of variability: exemplar from the comparison of several devices to measure respiratory rate in COPD patients. PLoS One 2016; 11:e0168321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982; 38:963–974. [PubMed] [Google Scholar]
- 22. Krouwer JS. Why Bland–Altman plots should use X, not (Y+X)/2 when X is a reference method. Stat Med 2008; 27:778–780. [DOI] [PubMed] [Google Scholar]
- 23. Mense L, Reimann M, Rüdiger H, Gahn G, Reichmann H, Hentschel H, Ziemssen T. Autonomic function and cerebral autoregulation in patients undergoing carotid endarterectomy. Circ J 2010; 74:2139–2145. [DOI] [PubMed] [Google Scholar]
- 24. Reinhard M, Gerds TA, Grabiak D, Zimmermann PR, Roth M, Guschlbauer B, Timmer J, Czosnyka M, Weiller C, Hetzel A. Cerebral dysautoregulation and the risk of ischemic events in occlusive carotid artery disease. J Neurol 2008; 255:1182–1189. [DOI] [PubMed] [Google Scholar]
- 25. Pennekamp CW, Moll FL, De Borst GJ. Role of transcranial Doppler in cerebral hyperperfusion syndrome. J Cardiovasc Surg 2012; 53:765–771. [PubMed] [Google Scholar]
- 26. Pennekamp CW, Tromp SC, Ackerstaff RG, Bots ML, Immink RV, Spiering W, de Vries JP, Kappelle LJ, Moll FL, Buhre WF, de Borst GJ. Prediction of cerebral hyperperfusion after carotid endarterectomy with transcranial Doppler. Eur J Vasc Endovasc Surg 2012; 43:371–376. [DOI] [PubMed] [Google Scholar]
- 27. Kirchoff-Torres KF, Bakradze E. Cerebral hyperperfusion syndrome after carotid revascularization and acute ischemic stroke. Curr Pain Headache Rep 2018; 22:1–10. [DOI] [PubMed] [Google Scholar]
- 28. Newman JE, Bown MJ, Sayers RD, Thompson JP, Robinson TG, Williams B, Panerai R, Lacy P, Naylor AR. Post-carotid endarterectomy hypertension. Part 2: association with peri-operative clinical, anaesthetic, and transcranial Doppler derived parameters. Eur J Vasc Endovasc Surg 2017; 54:564–572. [DOI] [PubMed] [Google Scholar]
- 29. Fassaert LMM, Immink RV, van Vriesland DJ, de Vries JPM, Toorop RJ, Kappelle LJ, Westerink J, Tromp SC, de Borst GJ. Transcranial Doppler 24 hours after carotid endarterectomy accurately identifies patients not at risk of cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg 2019; 58:320–327. [DOI] [PubMed] [Google Scholar]
- 30. Fassaert LMM, de Borst GJ. Commentary on “Post-carotid endarterectomy hypertension. Part 2: Association with peri-operative clinical, anaesthetic, and transcranial Doppler derived parameters.” Eur J Vasc Endovasc Surg 2018; 55:593. [DOI] [PubMed] [Google Scholar]
- 31. Ameloot K, Palmers PJ, Malbrain ML. The accuracy of noninvasive cardiac output and pressure measurements with finger cuff: a concise review. Curr Opin Crit Care 2015; 21:232–239. [DOI] [PubMed] [Google Scholar]
- 32. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol 2016; 77:1–7. [DOI] [PubMed] [Google Scholar]
- 34. Fischer MO, Diouf M, Wilde RBP, Dupont H, Hanouz JL, Lorne E. Evaluation of cardiac output by 5 arterial pulse contour techniques using trend interchangeability method. Medicine 2016; 95:e3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huibers A, Hendrikse J, Brown MM, Pegge SA, Arnold M, Moll FL, Kapelle LJ, de Borst GJ. Upper extremity blood pressure difference in patients undergoing carotid revascularisation. Eur J Vasc Endovasc Surg 2017; 53:153–157. [DOI] [PubMed] [Google Scholar]