Abstract
Background
Whether long-term blood pressure variability (BPV) predicts kidney function decline in generally healthy older adults is unknown. We investigated this association in ASPirin in Reducing Events in the Elderly (ASPREE) trial participants.
Methods
Between 2010 and 2014, Australian and US individuals aged ≥70 years (≥65 if US minority) were recruited and followed with annual study visits for a median of 4.7 years. Time-to-event analyses and linear mixed effects models were used to examine associations between incident chronic kidney disease (CKD), and trajectories of estimated glomerular filtration rate (eGFR) and log albumin–creatinine ratio (log ACR) with systolic BPV as a continuous measure, and, by tertile of SD of systolic blood pressure (BP). BPV was estimated using systolic BP measures from baseline through the second annual visit, and kidney outcomes were assessed following this period.
Results
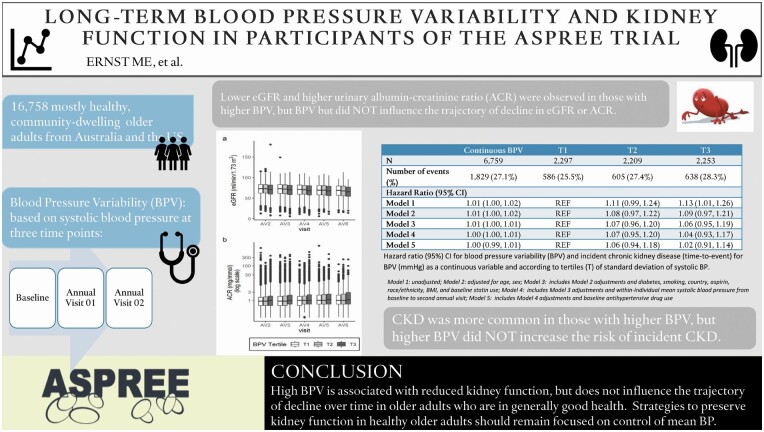
Incident CKD occurred in 1,829 of 6,759 participants (27.2%), and more commonly in BPV tertiles 2 (27.4%) and 3 (28.3%) than tertile 1 (25.5%); however, the risk was not significantly increased after covariate adjustment (tertile 3 hazard ratio = 1.02; 95% confidence interval: 0.91–1.14). Analysis of eGFR (n = 16,193) and log ACR trajectories (n = 15,213) showed individuals in the highest BPV tertile having the lowest eGFR and highest log ACR, cross-sectionally. However, the trajectories of eGFR and log ACR did not differ across BPV tertiles.
Conclusions
CKD and markers of reduced kidney function occur more commonly in individuals with higher BPV; however, BPV does not influence trajectory of decline in kidney function over time in older adults who are in generally good health.
Clinical trials registration
Trial Number NCT01038583 and ISRCTN83772183.
Keywords: aging, blood pressure, blood pressure variability, chronic kidney disease, geriatric, hypertension, long-term follow-up
Graphical Abstract
Graphical Abstract.
Chronic kidney disease (CKD) is a public health concern of global importance.1 An estimated 1.7 million (10%) Australian adults have biochemical signs of CKD,2 while an estimated 15% of the US population is affected.3,4 CKD amplifies the risk of cardiovascular disease events, and lowering this risk and slowing CKD progression traditionally focuses on treating underlying comorbidities such as hypertension and diabetes. Control of mean blood pressure (BP) is essential, given the robust association of high mean BP with adverse kidney outcomes.5
However, BP is not a static parameter, fluctuating within-day (short-term, or 24-hour) and across days (long-term, or “visit-to-visit”) in response to interactions between intrinsic cardiovascular regulatory mechanisms and extrinsic environmental and behavioral factors.6 Accumulating evidence indicates that high degrees of BP variability (BPV), relative to those with less variability, are associated with higher risk of cardiovascular disease events and dementia,7,8 and that this risk manifests independently of mean BP. A recent systematic review and meta-analysis found that increased visit-to-visit BPV might also be a risk factor for CKD, but noted significant heterogeneity in multiple characteristics of the studies evaluated, concluding that more research was needed.9
Most investigations of BPV and kidney outcomes have been conducted in relatively uniform populations sharing specific comorbidities such as hypertension,10,11 diabetes,12 or CKD.13,14 The relevance of BPV in adults who have reached their seventh, eighth, and ninth decades of life, in relatively good health, is less certain. If high BPV independently predicts CKD in older persons, a group at high risk for CKD and its complications, it could possibly become a novel clinical target. To address this gap, we examined the risk of CKD and deterioration of kidney function associated with long-term, visit-to-visit BPV in participants of the ASPirin in Reducing Events in the Elderly (ASPREE) study.15 We hypothesized that higher BPV would be associated with increased risk of CKD and decline in kidney function during follow-up.
METHODS
Study design and participants
This was a post hoc exploratory analysis of data from ASPREE,15 a multicenter, randomized, placebo-controlled trial of 100 mg daily aspirin conducted in Australia and the United States. The trial was conducted in accordance with the principles of the Declaration of Helsinki and approved by local institutional review boards at each site, with all participants providing written informed consent. The trial was registered with ClinicalTrials.gov (NCT01038583) and ISRCTN83772183. The data (version 3.0) supporting the findings of this study are available from the ASPREE Data Coordinating Center, Monash University School of Public Health (Aspree.AMS@monash.edu) upon qualified request.
Details of the ASPREE study protocol and its main findings have been previously published.15,16 Briefly, between 2010 and 2014, community-dwelling adults aged 70 years and older (65 years for US minorities) who were free of documented evidence of cardiovascular disease, dementia, major physical disability, or chronic illness expected to limit survival to less than 5 years, were enrolled and randomized 1:1 to enteric coated aspirin 100 mg daily, or matching placebo. The main exclusion criteria included a known high risk of bleeding, contraindication to aspirin, systolic BP ≥180 mm Hg or diastolic ≥105 mm Hg, or substantial limitation in any basic activity of daily living.
Study measurements
All consented participants completed two baseline visits to finalize eligibility, followed by a 4-week placebo run-in period. After randomization, participants were seen annually by trained study staff following standardized operating procedures, to collect comprehensive assessments of physical and cognitive function and additional health measures.
BP was measured at each study visit according to American Heart Association criteria,17 in the seated position after at least 5 minutes of rest using an automated oscillometric monitor with appropriately sized cuff. Three separate and consecutive BP readings, 1 minute apart, were performed; the mean of these measurements was recorded as the BP for the visit. The ASPREE protocol required use of a validated, automated monitor, but did not specify a particular model. Readings could be attended or unattended at the discretion of the study staff and based on the capabilities of the monitor. All study sites were monitored regularly to ensure adherence to the study protocol and standardized operating procedures.
Annual study visits included laboratory pathology collection. Serum creatinine and urine albumin–creatinine ratio (ACR) were scheduled to be obtained at baseline, years 3 and 5, and closeout (which could be year 3 or any year thereafter depending on the participant’s year of enrollment). However, based on sites’ study visit workflow, the full laboratory panel, including the kidney function measures, could be obtained at each visit. This occurred in the majority of participants. If laboratory was not collected as part of the study visit, measurements obtained through usual clinical care were accepted if they occurred within a 6-month window.
Assessment of visit-to-visit office BPV
Long-term, visit-to-visit BPV can be estimated using SD, coefficient of variation, average real variability, and variation independent of the mean, with SD and coefficient of variation most commonly used.6 In previous analyses, we examined the correlation of these different indices in our sample and found high correlation (Pearson’s R = 0.98) between SD and coefficient of variation.18 Therefore, we defined BPV according to within-individual SD of the mean systolic BP obtained from the baseline and first two annual study visits, consistent with previous ASPREE analyses.18,19
Kidney function outcomes and study population
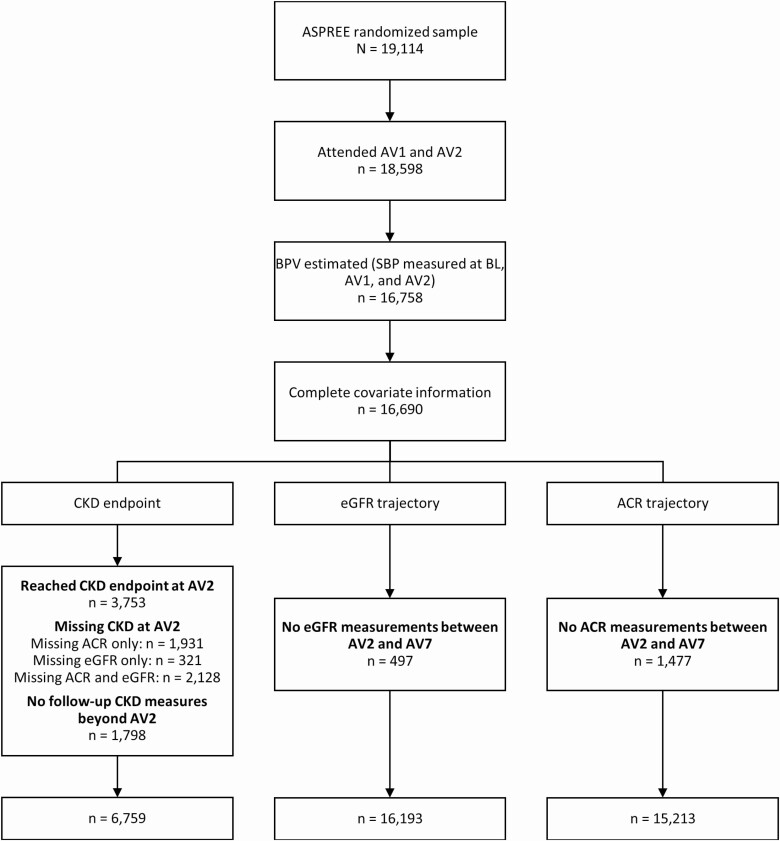
Three outcomes were used to investigate associations between BPV and kidney function: (i) incident CKD, (ii) estimated glomerular filtration rate (eGFR) trajectory, and (iii) log ACR trajectory. To minimize potential for immortal time bias, only those outcomes occurring after the BPV estimation period, from the second annual visit through the seventh annual visit (or end of follow-up for a participant), were considered. Figure 1 shows the flow of participants through the analyses of the three outcomes.
Figure 1.
Flow diagram of participants included in the primary analyses. Abbreviations: ACR, urine albumin–creatinine ratio; ASPREE, ASPirin in Reducing Events in the Elderly; AV, annual visit year; BL, baseline; BPV, blood pressure variability; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Outcome 1: incident CKD
Incident CKD was defined as a binary outcome at each annual visit where both eGFR and ACR measurements were available within a participant, and derived using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria20: eGFR <60 ml/min/1.73 m2 or spot urine ACR ≥3 mg/mmol, or both. While the diagnosis of CKD requires that abnormalities of eGFR or ACR persist for ≥3 months, ASPREE was designed around annual participant visits; as such, CKD was operationally designated on the basis of a single visit but requiring both eGFR and ACR measures to make the determination. Participants (n = 6,759) were included in this analysis if they did not have CKD at the second annual visit and had at least one observation of the derived CKD outcome during the follow-up period. If either eGFR or uACR was missing at a follow-up visit, the CKD outcome was missing for that visit. Both measures were required at the visit to avoid potentially misclassifying subjects with CKD as normal, which could happen if eGFR ≥60 ml/min/1.73 m2 but ACR was missing at the same visit, or conversely, if ACR <3 mg/mmol but eGFR was missing at the same visit. For participants missing CKD outcome for an interval of one visit, the observed CKD status from the prior visit was substituted. If missing values persisted for more than one visit, participants were censored at their last observed visit before the missing value occurred.
Outcome 2: eGFR trajectory
eGFR was calculated in each participant using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation21 at annual visits where serum creatinine was available. Participants (n = 16,193) were included in this analysis if they had at least one eGFR value during the follow-up period after the second annual visit.
Outcome 3: ACR trajectory
ACR was calculated at each annual visit where urine creatinine and albumin were available. Because the distribution of ACR values is skewed, the natural logarithm of ACR was used as the outcome measure. Participants (n = 15,213) were included in this analysis if they had at least one ACR value during the follow-up period after BPV estimation. Exact values were used when reported; for measures reported categorically (e.g., <0.11, <3.39, 3.39–33.9, and >33.9 mg/mmol), 0.11, 2.99, 10.7, and 34 were substituted, respectively.
Statistical analysis
Time-to-event and linear mixed effects models were used to explore the association between BPV and kidney function, for the three outcomes described above. BPV was treated as a continuous variable and, separately, was divided into tertiles of BPV in which the lowest tertile was used as the reference. Tertiles were recalculated for each outcome to account for the differing sample sizes and analysis inclusion criteria. An initial unadjusted model (Model 1) was followed by adjustment for age and sex (Model 2), demographic variables (Model 3), mean systolic BP during the BPV estimation period (Model 4), and baseline antihypertensive drug use (Model 5).
Discrete time Cox proportional hazards regression was used to calculate hazard ratios and 95% confidence intervals for time to incident CKD. For eGFR trajectory, linear mixed effects models with a random intercept were used to explore the association between BPV and eGFR in the follow-up period. The models included fixed effects for time, BPV, and a time–BPV interaction term, and a random intercept for each individual. Model 1 included only these terms, and Models 2–5 included the additional covariates as fixed effects. The coefficients for BPV and the time–BPV interaction terms were reported. The BPV coefficients estimated the cross-sectional association of BPV with eGFR at baseline, while the interaction term tested whether the eGFR trajectory varied across BPV levels. This same method was used for ACR trajectory to assess the association between BPV and log ACR during the follow-up period. P values <0.05 were considered statistically significant. Statistical analyses were performed using R version 3.6.1.22
Sensitivity analyses
Several sensitivity analyses were performed to assess the robustness of the results; the inclusion of participants into these analyses is shown in Supplementary Figure S1 online. For time-to-event analysis, inclusion criteria were adjusted to require a second visit without CKD during the BPV estimation period. This was done attempting to ensure participants were free of CKD at the start of follow-up, and lessen the chance that occult CKD was present earlier. For the same conditions in which last observation carried forward was used in the main analysis, ACR and serum creatinine values were carried forward separately, to allow an updated calculation of eGFR based on a participant’s age and determination of CKD status at that visit. For the trajectory analyses, we increased the minimum requirement of follow-up measures from one to two visits between the second annual visit and end of follow-up. Analyses were also repeated stratifying participants by eGFR <60 ml/min/1.73 m2 at the second annual visit (or earlier in the BPV estimation period if annual visit 2 was missing). Finally, we repeated our primary analysis after expanding the BPV estimation period from three to four measurements (baseline, years 1, 2, and 3), which then shifted year 3 to the new baseline (and after excluding individuals with events prior to year 3).
RESULTS
Of the 19,114 individuals randomized into ASPREE, 16,758 attended through their second annual visit and had BPV estimated (Figure 1). Complete covariate information was available for 99.6% of these participants. For analysis of incident CKD, 8,557 individuals had eGFR ≥60 ml/min/1.73 m2 and ACR <3 mg/mmol at their second annual visit, but 1,798 of these individuals did not have any kidney measures after this visit, leading to a final analyzed sample of 6,759 participants. For eGFR trajectory, 497 individuals did not have eGFR during the follow-up, resulting in a final analyzed sample of 16,193. For ACR trajectory, ACR was missing during follow-up in 1,477 participants, for a final analyzed sample of 15,213 participants.
Table 1 shows the baseline demographics of participants included in each of the three outcomes. The mean (SD) age of participants in the incident CKD analysis was 74.5 (3.8) years, 54.3% were female, 95.7% were White, and their mean (SD) systolic BP during the BPV estimation period was 137.8 (13.4) mm Hg. Hypertension was observed in 71.1% of participants, while 8.6% had diabetes. The mean (SD) eGFR was 77.4 (10.4) ml/min/1.73 m2, and the mean (SD) ACR was 1.13 (2.05) mg/mmol. For the eGFR and log ACR trajectory analyses, a generally similar pattern of demographics was observed. Supplementary Table S1 online shows demographics of participants in each outcome analysis, further stratified according to the BPV tertiles. For each of the three outcomes, participants from lowest to highest tertile of BPV had progressively higher baseline and average systolic BP, higher rates of hypertension, and lower eGFR and higher ACR.
Table 1.
Baseline characteristics of the ASPREE participants by outcome analyzed
| Model outcome | |||
|---|---|---|---|
| CKD endpoint | eGFR trajectory | ACR trajectory | |
| Number of individuals, n | 6,759 | 16,193 | 15,213 |
| Within-individual SD of SBP (BL-AV2), mean (SD) | 9.8 (5.7) | 10.2 (6.0) | 10.1 (6.0) |
| Within-individual SD of SBP by tertile, mean (SD) | |||
| T1 | 4.2 (1.6) | 4.3 (1.6) | 4.3 (1.6) |
| T2 | 8.9 (1.3) | 9.2 (1.4) | 9.2 (1.4) |
| T3 | 16.3 (4.5) | 17.0 (4.7) | 17.0 (4.7) |
| Baseline SBP, mm Hg, mean (SD) | 139.0 (16.2) | 139.1 (16.4) | 139.1 (16.4) |
| Average SBP (BL-AV2), mean (SD) | 137.8 (13.4) | 137.7 (13.8) | 137.8 (13.7) |
| Heart rate, mean (SD) | 70.8 (10.5) | 70.6 (10.6) | 70.6 (10.6) |
| Aspirin treatment assignment, n (%) | 3,352 (49.6) | 8,052 (49.7) | 7,529 (49.5) |
| Age (years), mean (SD) | 74.5 (3.8) | 75.0 (4.4) | 75.0 (4.4) |
| Male, n (%) | 3,090 (45.7) | 7,166 (44.3) | 6,801 (44.7) |
| Race/ethnicity, n (%) | |||
| White | 6,471 (95.7) | 15,054 (93.0) | 14,170 (93.1) |
| Black | 135 (2.0) | 595 (3.7) | 544 (3.6) |
| Hispanic | 73 (1.1) | 318 (2.0) | 291 (1.9) |
| Asian | 48 (0.7) | 135 (0.8) | 121 (0.8) |
| Other | 32 (0.5) | 91 (0.6) | 87 (0.6) |
| Pulse pressure, mean (SD) | 61.4 (13.4) | 61.9 (13.7) | 61.9 (13.7) |
| BMI, kg/m2, mean (SD) | 27.7 (4.4) | 28.1 (4.7) | 28.1 (4.6) |
| Comorbidities, n (%) | |||
| Hypertension | 4,807 (71.1) | 11,986 (74.0) | 11,240 (73.9) |
| Diabetes | 579 (8.6) | 1,665 (10.3) | 1,584 (10.4) |
| Smoking (ever) | 2,934 (43.4) | 7,108 (43.9) | 6,668 (43.8) |
| Alcohol (current use) | 5,420 (80.2) | 12,589 (77.7) | 11,847 (77.9) |
| Medication, n (%) | |||
| Statins | 2,003 (29.6) | 5,046 (31.2) | 4,754 (31.2) |
| Antihypertensives | 3,170 (46.9) | 8,456 (52.2) | 7,907 (52.0) |
| Baseline kidney function, mean (SD) | |||
| eGFR mg/min/1.73 m2 | 77.36 (10.40) | 72.93 (13.77) | 73.01 (13.70) |
| ACR mg/mmol | 1.13 (2.05) | 2.06 (8.40) | 2.06 (8.49) |
Hypertension defined as SBP ≥140 mm Hg, DBP ≥90 mm Hg, or receiving treatment for high BP (regardless of BP level). Diabetes defined as a self-report, fasting glucose ≥126 mg/dl, or receiving pharmacologic treatment for diabetes (regardless of fasting glucose level). Abbreviations: ACR, albumin–creatinine ratio; ASPREE, ASPirin in Reducing Events in the Elderly; AV, annual visit year; BL, baseline; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
During an average follow-up of 2.02 years after the second annual visit, 1,829 of 6,759 (27.1%) participants developed CKD (Table 2). This endpoint occurred more frequently for participants in the second and third BPV tertiles (27.4% and 28.3%, respectively) compared with the reference tertile (25.5%). Although this difference was statistically significant in the unadjusted model treating BPV as a continuous variable, it was not significant in the unadjusted model by tertile or in any of the adjusted models (Model 5, continuous hazard ratio = 1.00, 95% confidence interval: 0.99–1.01; tertile 3 hazard ratio = 1.02, 95% confidence interval: 0.91, 1.14).
Table 2.
Hazard ratio (95%) CI for blood pressure variability (BPV) and incident chronic kidney disease (time-to-event) for BPV (mm Hg) as a continuous variable and according to tertiles (T) of SD of systolic BP
| Continuous BPV | T1 | T2 | T3 | |
|---|---|---|---|---|
| N | 6,759 | 2,297 | 2,209 | 2,253 |
| Number of events (%) | 1,829 (27.1%) | 586 (25.5%) | 605 (27.4%) | 638 (28.3%) |
| Hazard ratio (95% CI) | ||||
| Model 1 | 1.01 (1.00, 1.02) | REF | 1.11 (0.99, 1.24) | 1.13 (1.01, 1.26) |
| Model 2 | 1.01 (1.00, 1.02) | REF | 1.08 (0.97, 1.22) | 1.09 (0.97, 1.21) |
| Model 3 | 1.01 (1.00, 1.01) | REF | 1.07 (0.96, 1.20) | 1.06 (0.95, 1.19) |
| Model 4 | 1.00 (1.00, 1.01) | REF | 1.07 (0.95, 1.20) | 1.04 (0.93, 1.17) |
| Model 5 | 1.00 (0.99, 1.01) | REF | 1.06 (0.94, 1.18) | 1.02 (0.91, 1.14) |
CKD defined as eGFR <60 ml/min/1.73 m2 or spot urine ACR ≥3 mg/mmol. Abbreviations: ACR, albumin–creatinine ratio; BMI, body mass index; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; REF, reference. Model 1: unadjusted. Model 2: adjusted for age and sex. Model 3: includes Model 2 adjustments and diabetes, smoking, country, aspirin, race/ethnicity, BMI, and baseline statin use. Model 4: includes Model 3 adjustments and within-individual mean systolic blood pressure from baseline to second annual visit. Model 5: includes Model 4 adjustments and baseline antihypertensive drug use.
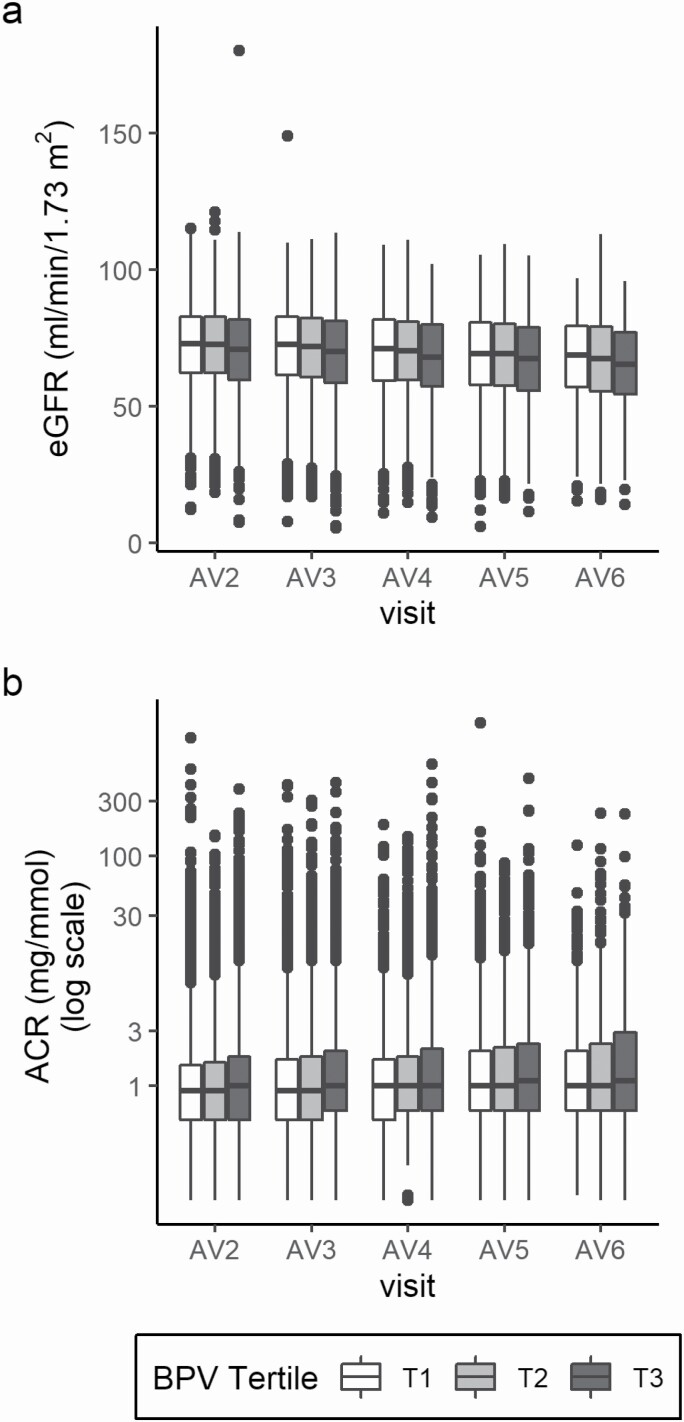
The relationships between BPV and eGFR and log ACR trajectories are depicted in Figure 2. For the analysis of eGFR trajectory, the estimate coefficients in each model in tertile 3 were negative and statistically significant, indicating that individuals with higher BPV had lower eGFR at baseline, cross-sectionally. When eGFR was considered as a continuous variable, each one SD unit increase in BPV was associated with an average decrease of 0.08 ml/min/1.73 m2 in eGFR (Model 5, Table 3). When considered categorically, there was a mean difference of 0.90 ml/min/1.73 m2 in eGFR from the reference to the third tertile (Model 5, Table 3). However, the interaction terms between BPV and eGFR were not significant, indicating that the trajectory of eGFR from baseline did not differ by BPV tertile or the continuous BPV measure. A similar pattern, of higher BPV with lower kidney function (i.e., higher ACR), was observed for the relationship between BPV and log ACR trajectory (Table 4). Individual model estimates in the continuous model and in the third tertile were statistically significant (with the exception of Model 5 for tertile 3), but the interaction terms were not.
Figure 2.
Distribution of available renal measures at each annual visit by BPV tertile: (a) eGFR ml/min/1.73 m2 and (b) ACR mg/mmol. 25th, 50th, 75th percentiles shown; outliers ≥ or ≤1.5 * IQR. Abbreviations: ACR, albumin–creatinine ratio; AV, annual visit year; BPV, blood pressure variability; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Table 3.
Association between blood pressure variability (BPV) and eGFR trajectory for BPV (mm Hg) as a continuous variable and according to tertiles (T) of SD of systolic BP
| Continuous BPV | T1 | T2 | T3 | ||||
|---|---|---|---|---|---|---|---|
| N | 16,193 | 5,398 | 5,430 | 5,365 | |||
| Estimatea | Interactionb | Estimatea | Interactionb | Estimatea | Interactionb | ||
| Model 1 | −0.18 (P < 0.01) |
−0.01 (P = 0.08) |
REF | −0.34 (P = 0.21) |
0.02 (P = 0.73) |
−2.23 (P < 0.01) |
−0.04 (P = 0.54) |
| Model 2 | −0.12 (P < 0.01) |
−0.01 (P = 0.09) |
REF | −0.05 (P = 0.85) |
0.02 (P = 0.75) |
−1.44 (P < 0.01) |
−0.04 (P = 0.55) |
| Model 3 | −0.11 (P < 0.01) |
−0.01 (P = 0.10) |
REF | −0.02 (P = 0.94) |
0.02 (P = 0.76) |
−1.29 (P < 0.01) |
−0.04 (P = 0.56) |
| Model 4 | −0.10 (P < 0.01) |
−0.01 (P = 0.10) |
REF | 0.01 (P = 0.96) |
0.02 (P = 0.76) |
−1.21 (P < 0.01) |
−0.04 (P = 0.56) |
| Model 5 | −0.08 (P < 0.01) |
−0.01 (P = 0.11) |
REF | 0.11 (P = 0.68) |
0.02 (P = 0.75) |
−0.90 (P < 0.01) |
−0.04 (P = 0.57) |
Abbreviations: BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; REF, reference. Model 1: unadjusted. Model 2: adjusted for age and sex. Model 3: includes Model 2 adjustments and diabetes, smoking, country, aspirin, race/ethnicity, BMI, and baseline statin use. Model 4: includes Model 3 adjustments and within-individual mean systolic blood pressure from baseline to second annual visit. Model 5: includes Model 4 adjustments and baseline antihypertensive drug use.
aEstimate of the cross-sectional association of BPV with eGFR at baseline.
bTime–BPV interaction term to test variation of the eGFR trajectory among BPV levels.
Table 4.
Association between blood pressure variability (BPV) and log ACR trajectory for BPV (mm Hg) as a continuous variable and according to tertiles (T) of SD of systolic BP
| Continuous BPV | T1 | T2 | T3 | ||||
|---|---|---|---|---|---|---|---|
| N | 15,213 | 5,102 | 5,053 | 5,058 | |||
| Estimatea | Interactionb | Estimatea | Interactionb | Estimatea | Interactionb | ||
| Model 1 | 0.01 (P < 0.01) |
0.00 (P = 0.18) |
REF | 0.04 (P = 0.05) |
0.00 (P = 0.66) |
0.13 (P < 0.01) |
0.01 (P = 0.18) |
| Model 2 | 0.01 (P < 0.01) |
0.00 (P = 0.19) |
REF | 0.03 (P = 0.17) |
0.00 (P = 0.65) |
0.10 (P < 0.01) |
0.01 (P = 0.18) |
| Model 3 | 0.01 (P < 0.01) |
0.00 (P = 0.21) |
REF | 0.03 (P = 0.18) |
0.00 (P = 0.63) |
0.09 (P < 0.01) |
0.01 (P = 0.20) |
| Model 4 | 0.00 (P < 0.01) |
0.00 (P = 0.21) |
REF | 0.01 (P = 0.48) |
0.00 (P = 0.64) |
0.06 (P = 0.01) |
0.01 (P = 0.20) |
| Model 5 | 0.00 (P = 0.04) |
0.00 (P = 0.22) |
REF | 0.01 (P = 0.67) |
0.00 (P = 0.64) |
0.04 (P = 0.06) |
0.01 (P = 0.21) |
Abbreviations: ACR, albumin–creatinine ratio; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; REF, reference. Model 1: unadjusted. Model 2: adjusted for age and sex. Model 3: includes Model 2 adjustments and diabetes, smoking, country, aspirin, race/ethnicity, BMI, and baseline statin use. Model 4: includes Model 3 adjustments and within-individual mean systolic blood pressure from baseline to second annual visit. Model 5: includes Model 4 adjustments and baseline antihypertensive drug use.
aEstimate of the cross-sectional association of BPV with eGFR at baseline.
bTime–BPV interaction term to test variation of the eGFR trajectory among BPV levels.
Several sensitivity analyses demonstrated similar findings. For incident CKD, modifying the inclusion criteria to require two visits without CKD during the BPV estimation period did not change the findings (Supplementary Table S2 online), nor were conclusions different when last observation of ACR and serum creatinine were carried forward separately for missing variables (Supplementary Table S3 online). Findings for eGFR trajectory (Supplementary Table S4 online) and log ACR (Supplementary Table S5 online) requiring an additional measurement during the follow-up period yielded results unchanged from our main findings. Repeating our analyses stratifying participants based on baseline eGFR <60 ml/min/1.73 m2 also found no significant interaction between BPV tertile and trajectory of eGFR (Supplementary Table S6 online) or log ACR (Supplementary Table S7 online). Lastly, increasing our BPV estimation period to four measures, and repeating the primary analyses with year 3 as the new baseline did not change the results (Supplementary Tables S8 and S9 online).
Discussion
In prior ASPREE analyses, high visit-to-visit BPV was associated with increased risk of cardiovascular disease events, as well as dementia and cognitive decline, independent of mean BP.18,19 Expanding upon this work, the current analysis examined the relationship between BPV and kidney function in this cohort of older adults who were mostly healthy at baseline. We found that higher BPV was associated with higher rates of CKD, lower eGFR, and higher ACR cross-sectionally, but it was not associated with greater decline in kidney function as characterized by eGFR and log ACR trajectories.
Our findings differ from those of other studies examining BPV and kidney outcomes. Two large epidemiologic studies of several million individuals observed that higher BPV was associated with increased incidence of end-stage kidney disease,23,24 as well as all-cause mortality, coronary heart disease, and stroke.24 Other analyses conducted within specific cohorts of hypertensives or diabetics have reported long-term visit-to-visit BPV as an independent risk factor for deterioration in kidney function.10–14 In a recent systematic review and meta-analysis which included several of these studies, each 1 mm Hg increase in systolic BP SD resulted in a combined risk ratio for CKD of 1.05 (95% confidence interval: 1.03–1.07).9 In contrast, ours was not the only study which did not detect an association between BPV and kidney outcomes. Recent analysis of combined data from the ONTARGET and TRANSCEND trials also found visit-to-visit BPV was not a major determinant for risk of adverse kidney function outcomes.25 In that analysis of 28,790 subjects, the incidence of end-stage kidney disease, doubling of serum creatinine, new microalbuminuria, and new macroalbuminuria were better predicted by mean on-treatment BP, with only a marginal increase in prediction when mean BP was combined with BPV.
Although our null findings do not preclude the possibility of a relationship between BPV and decline in kidney function, they do highlight the need for better understanding of the principal regulator of such a relationship. The associations observed in prior studies could reflect reverse causality, with increased BPV occurring as a consequence of advancing CKD, rather than being causal itself. CKD is associated with impairment of kidney-related mechanisms that control intravascular volume and sodium excretion, which may reduce homeostatic buffering of volume-related BP changes. In addition, baroreflex impairment and enhanced sympathetic activity occur in CKD which can also contribute to increased BPV.26 Two large cohorts have reported the observation of BPV increasing with worsening kidney function—the first, a cross-sectional analysis in 16,546 patients from the Spanish ABPM registry,27 and another study in 19,175 primary care patients in the Netherlands.28
Although ours was a post hoc analysis, our findings are strengthened by using both a traditional binary outcome of incident CKD and analyses of kidney function trajectory, the latter approach helping circumvent the potential impact of individuals whose eGFR reside on the border of the CKD threshold. Regardless of outcome assessed, our findings were consistent. We also acknowledge important limitations, most notably that we used a small number of BP readings to estimate BPV, which can potentially limit the ability of BPV to predict the outcome risk; however, there is currently no accepted consensus on the optimal number of BP measurements to calculate BPV.29 We also had relatively short follow-up because we considered only those outcomes occurring after the BPV estimation period. While protecting against immortal time bias, this removed from analysis a substantial number of incident CKD that may have occurred earlier. Longer follow-up may have strengthened the possibility of observing a relationship. Finally, ASPREE enrolled mostly healthy individuals, few with diabetes or low kidney function at baseline, and those with hypertension had generally well-controlled BP which included substantial use of renin–angiotensin system inhibitors as previously reported.30 These factors may have contributed to overall low risk of deterioration of kidney function in our cohort and reduced our ability to observe an association with BPV.
In adults who have reached older ages in relatively good health, CKD and markers of reduced kidney function are more common in those with high visit-to-visit BPV; however, BPV does not appear to influence the trajectory of kidney function decline. BP-focused strategies aimed at preserving kidney health in older adults should remain focused on controlling mean BP.
Supplementary Material
Contributor Information
Michael E Ernst, Department of Pharmacy Practice and Science, College of Pharmacy, The University of Iowa, Iowa City, Iowa, USA; Department of Family Medicine, Carver College of Medicine, The University of Iowa, Iowa City, Iowa, USA.
Michelle A Fravel, Department of Pharmacy Practice and Science, College of Pharmacy, The University of Iowa, Iowa City, Iowa, USA.
Katherine L Webb, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
James B Wetmore, Division of Nephrology, Department of Medicine, Hennepin Healthcare Systems, Minneapolis, Minnesota, USA; Hennepin Healthcare Research Institute, Minneapolis, Minnesota, USA.
Rory Wolfe, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Enayet Chowdhury, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia; School of Public Health, Curtin University, Perth, Western Australia, Australia.
Christopher M Reid, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia; School of Public Health, Curtin University, Perth, Western Australia, Australia.
Robyn L Woods, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia.
Lawrence Beilin, Medical School Royal Perth Hospital, University of Western Australia, Perth, Western Australia, Australia.
Karen L Margolis, HealthPartners Institute, Minneapolis, Minnesota, USA.
Anne M Murray, Division of Geriatrics, Department of Medicine, Hennepin Healthcare, Minneapolis, Minnesota, USA.
Kevan R Polkinghorne, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia; Department of Nephrology, Monash Medical Centre, Monash Health, Melbourne, Victoria, Australia; Department of Medicine, Monash University, Melbourne, Victoria, Australia.
Funding
Supported by grants (U01AG029824 and U19AG062682) from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health, by grants (334047 and 1127060) from the National Health and Medical Research Council of Australia, and by Monash University and the Victorian Cancer Agency.
DISCLOSURE
The authors declared no conflict of interest.
References
- 1. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395:709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Australian Government, Australian Institute of Health and Welfare. Chronic Kidney Disease. Web Report. https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease/data. 2020. Accessed 2 June 2021.
- 3. United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, 2020. [Google Scholar]
- 4. Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2021. US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, 2021. [Google Scholar]
- 5. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021; 99:S1–S87. [DOI] [PubMed] [Google Scholar]
- 6. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol 2013; 10:143–155. [DOI] [PubMed] [Google Scholar]
- 7. Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016; 354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoo JE, Shin DW, Han K, Kim D, Lee SP, Jeong SM, Lee J, Kim S. Blood pressure variability and the risk of dementia: a nationwide cohort study. Hypertension 2020; 75:982–990. [DOI] [PubMed] [Google Scholar]
- 9. Li H, Xue J, Dai W, Chen Y, Zhou Q, Chen W. Visit-to-visit blood pressure variability and risk of chronic kidney disease: a systematic review and meta-analyses. PLoS One 2020; 15:e0233233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chia YC, Lim HM, Ching SM. Long-term visit-to-visit blood pressure variability and renal function decline in patients with hypertension over 15 years. J Am Heart Assoc 2016; 5:e003825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whittle J, Lynch AI, Tanner RM, Simpson LM, Davis BR, Rahman M, Whelton PK, Oparil S, Muntner P. Visit-to-visit variability of BP and CKD outcomes: results from the ALLHAT. Clin J Am Soc Nephrol 2016; 11:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viazzi F, Russo E, Mirijello A, Fioretto P, Giorda C, Ceriello A, Copetti M, Russo GT, Di Bartolo P, Manicardi V, Leoncini G, De Cosmo S, Pontremoli R; the AMD-Annals Study Group. Long-term blood pressure variability, incidence of hypertension and changes in renal function in type 2 diabetes. J Hypertens 2020; 38:2279–2286. [DOI] [PubMed] [Google Scholar]
- 13. Yokota K, Fukuda M, Matsui Y, Hoshide S, Shimada K, Kario K. Impact of visit-to-visit variability of blood pressure on deterioration of renal function in patients with non-diabetic chronic kidney disease. Hypertens Res 2013; 36:151–157. [DOI] [PubMed] [Google Scholar]
- 14. Mallamaci F, Minutolo R, Leonardis D, D’Arrigo G, Tripepi G, Rapisarda F, Cicchetti T, Maimone I, Enia G, Postorino M, Santoro D, Fuiano G, De Nicola L, Conte G, Zoccali C. Long-term visit-to-visit office blood pressure variability increases the risk of adverse cardiovascular outcomes in patients with chronic kidney disease. Kidney Int 2013; 84:381–389. [DOI] [PubMed] [Google Scholar]
- 15. Nelson MR; ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled clinical trial. Contemp Clin Trials 2013; 36:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, Storey E, Shah RC, Lockery JE, Tonkin AM, Newman AB, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Donnan GA, Gibbs P, Johnston CI, Ryan J, Radziszewska B, Grimm R, Murray AM; ASPREE Investigator Group. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med 2018; 379:1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005; 45:142–161. [DOI] [PubMed] [Google Scholar]
- 18. Ernst ME, Chowdhury EK, Beilin LJ, Margolis KL, Nelson MR, Wolfe R, Tonkin AM, Ryan J, Woods RL, McNeil JJ, Reid CM; ASPREE Investigator Group. Long-term blood pressure variability and risk of cardiovascular disease events among community-dwelling elderly. Hypertension 2020; 76:1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ernst ME, Ryan J, Chowdhury EK, Margolis KL, Beilin LJ, Reid CM, Nelson MR, Woods RL, Shah RC, Orchard SG, Wolfe R, Storey E, Tonkin AM, Brodtmann A, McNeil JJ, Murray AM. Long-term blood pressure variability and risk of cognitive decline and dementia among older adults. J Am Heart Assoc 2021; 10:e019613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150. [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2021. <https://www.R-project.org/>. [Google Scholar]
- 23. Bae EH, Lim SY, Han KD, Oh TR, Choi HS, Kim CS, Ma SK, Kim SW. Association between systolic and diastolic blood pressure variability and the risk of end-stage renal disease. Hypertension 2019; 74:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, Kovesdy CP. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol 2016; 68:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mancia G, Schumacher H, Böhm M, Mann JFE, Redon J, Facchetti R, Schmieder RE, Lonn EM, Teo KK, Yusuf S. Visit-to-visit blood pressure variability and renal outcomes: results from ONTARGET and TRANSCEND trials. J Hypertens 2020; 38:2050–2058. [DOI] [PubMed] [Google Scholar]
- 26. Bilo G, Parati G. Blood pressure variability and kidney disease: another vicious circle? J Hypertens 2018; 36:1019–1021. [DOI] [PubMed] [Google Scholar]
- 27. Sarafidis PA, Ruilope LM, Loutradis C, Gorostidi M, de la Sierra A, de la Cruz JJ, Vinyoles E, Divisón-Garrote JA, Segura J, Banegas JR. Blood pressure variability increases with advancing chronic kidney disease stage: a cross-sectional analysis of 16 546 hypertensive patients. J Hypertens 2018; 36:1076–1085. [DOI] [PubMed] [Google Scholar]
- 28. Lasserson DS, Scherpbier de Haan N, de Grauw W, van der Wel M, Wetzels JF, O’Callaghan CA. What is the relationship between renal function and visit-to-visit blood pressure variability in primary care? Retrospective cohort study from routinely collected healthcare data. BMJ Open 2016; 6:e010702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Blood pressure measurement device, number and timing of visits, and intra-individual visit-to-visit variability of blood pressure. J Clin Hypertens (Greenwich) 2012; 14:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ernst ME, Chowdhury EK, Nelson MR, Reid CM, Margolis KL, Beilin L, Stocks NP, Murray AM, Wolfe R, Lockery JE, Orchard SG, Woods RL, McNeil JJ; ASPREE Investigator Group. Antihypertensive medication use and blood pressure control among treated older adults. J Clin Hypertens (Greenwich) 2020; 22:1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.