Abstract
Some reproductive-aged individuals remain unvaccinated against coronavirus disease 2019 (COVID-19) because of concerns about potential adverse effects on fertility. Using data from an internet-based preconception cohort study, we examined the associations of COVID-19 vaccination and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with fertility among couples trying to conceive spontaneously. We enrolled 2,126 self-identified female participants aged 21–45 year residing in the United States or Canada during December 2020–September 2021 and followed them through November 2021. Participants completed questionnaires every 8 weeks on sociodemographics, lifestyle, medical factors, and partner information. We fit proportional probabilities regression models to estimate associations between self-reported COVID-19 vaccination and SARS-CoV-2 infection in both partners with fecundability (i.e., the per-cycle probability of conception), adjusting for potential confounders. COVID-19 vaccination was not appreciably associated with fecundability in either partner (female fecundability ratio (FR) = 1.08, 95% confidence interval (CI): 0.95, 1.23; male FR = 0.95, 95% CI: 0.83, 1.10). Female SARS-CoV-2 infection was not strongly associated with fecundability (FR = 1.07, 95% CI: 0.87, 1.31). Male infection was associated with a transient reduction in fecundability (for infection within 60 days, FR = 0.82, 95% CI: 0.47, 1.45; for infection after 60 days, FR = 1.16, 95% CI: 0.92, 1.47). These findings indicate that male SARS-CoV-2 infection may be associated with a short-term decline in fertility and that COVID-19 vaccination does not impair fertility in either partner.
Keywords: COVID-19, fecundability, fertility, preconception, prospective cohort, SARS-CoV-2, vaccination
Abbreviations
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- FR
fecundability ratio
- LMP
last menstrual period
- PRESTO
Pregnancy Study Online
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
The 3 vaccines for coronavirus disease 2019 (COVID-19) approved by the US Food and Drug Administration have shown high efficacy in reducing the occurrence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe COVID-19 disease (1–3). As of November 20, 2021, 71% of US adults had received 2 doses of the Pfizer-BioNTech (BioNTech, Mainz, Germany; Fosun Pharma, Shanghai, China; Pfizer, New York, NY) or Moderna (Moderna Therapeutics, Cambridge, MA) vaccines or 1 dose of the Johnson & Johnson (Janssen Pharmaceutical Companies, Beerse, Belgium) vaccine, with 82% having received at least 1 dose of any vaccine (4). Vaccination rates were lower among reproductive-aged adults, with approximately 60% of adults aged 18–39 years fully vaccinated (4). Safety is an important factor in individual decision-making. Concern about possible side effects is a top reported reason for remaining unvaccinated (5) and, among reproductive-aged adults, there is particular concern about the potential effects of vaccination on fertility (6–8).
The hypothesis that COVID-19 vaccination may impair female fertility originated with a blog post that claimed the similarity between a SARS-CoV-2 surface glycoprotein and syncytin-1 (an envelope protein essential for formation of the placenta (9)) could lead to development of anti–syncitin-1antibodies that would impair placental function. However, in 3 studies, researchers have demonstrated the absence of anti–syncitin-1 antibodies after messenger RNA (mRNA) vaccination (10–12). Anecdotal reports of menstrual cycle irregularities after vaccination have also contributed to concerns about the vaccine’s potential effect on fertility (13). Data on the association between COVID-19 vaccination and fertility are still limited but do not indicate a harmful association. Although pregnant individuals were ineligible for the initial COVID-19 vaccine trials, the rate of unintended pregnancies occurring during the trials did not differ substantially between vaccinated and control groups (14–16). In clinical trials for the AstraZeneca vaccine (ChAdOx1 nCoV-19), fertility rates were similar in participants who received the vaccine (n = 50 pregnancies) versus the placebo (n = 43 pregnancies) (17). In 3 separate studies of female patients undergoing in vitro fertilization, no meaningful association was found between COVID-19 vaccination status and implantation rates (18), stimulation characteristics (19), embryological outcomes (19), or ovarian follicular function (20).
Likewise, in a limited number of studies, researchers have evaluated the association of COVID-19 vaccination with male fertility. No appreciable differences in semen volume, sperm concentration, or motility measures before and after COVID-19 vaccination were found in 2 studies of couples undergoing fertility treatments (19, 21) and 1 conducted in the general population (22).
In contrast to data on COVID-19 vaccination, which do not indicate adverse associations with fertility, infection with SARS-CoV-2 has been associated with reproductive dysfunction (23). Recent SARS-CoV-2 infection has been associated with poor sperm quality, including abnormal morphology, decreased concentration, lower motility, and increased DNA fragmentation (24–31); these findings may result from COVID-19–associated fever and inflammation (32, 33). SARS-CoV-2 infection has also been associated with impaired Leydig cell function (34) and dysregulation of the hypothalamic-pituitary-gonadal axis (35). Some reports suggest that female patients with SARS-CoV-2 infection experience menstrual cycle changes, including irregular cycles, decreased menstrual volume, and prolonged menstrual cycles, (36, 37) although these studies lacked an uninfected comparison group. In studies of patients undergoing fertility treatment, authors report that SARS-CoV-2 infection is largely unrelated to treatment outcomes (38, 39). However, in an observational study among reproductive-aged women, recent SARS-CoV-2 infection was associated with lower concentrations of anti-Müllerian hormone and higher concentrations of testosterone and prolactin (40).
Here, we examine the associations of fecundability, the per-cycle probability of conception, with COVID-19 vaccination and SARS-CoV-2 infection in female and male participants in a North American prospective cohort study of couples trying to conceive.
METHODS
Study design and participants
Pregnancy Study Online (PRESTO) is an internet-based, prospective, preconception cohort study of couples residing in the United States and Canada (41). Enrollment began in June 2013 and is ongoing. Eligible participants identified as female, were aged 21–45 years, and were trying to conceive without fertility treatment. Participation involved completion of a baseline questionnaire on sociodemographics, lifestyle, and reproductive and medical histories; follow-up questionnaires every 8 weeks for up to 12 months; and additional questionnaires during pregnancy and postpartum. Female participants were given the option to invite their male partners to complete a baseline questionnaire; eligible partners were aged ≥21 years. The institutional review board at Boston University Medical Campus approved the study. All participants provided informed consent.
Assessment of COVID-19 vaccination
On female and male baseline questionnaires and female follow-up and early pregnancy questionnaires, we asked, “Have you ever received a COVID-19 vaccination?” If the answer was “yes,” participants reported the vaccine brand (“Moderna,” “Pfizer,” “Johnson & Johnson,” or “Other,” with a text box to enter the brand) and dates of first and second doses. Beginning in June 2021, we also asked female participants on all questionnaires if their partners had received a COVID-19 vaccination, as well as the dates of vaccination and vaccine brand.
Assessment of SARS-CoV-2 infection
On female and male baseline questionnaires and female follow-up and early pregnancy questionnaires, we asked participants if they had ever tested positive for SARS-CoV-2 and, if so, the date they tested positive. On female questionnaires, we asked if their partners had ever tested positive for SARS-CoV-2 and, if so, the date they tested positive. For both vaccination and infection, we prioritized male partner data from the male baseline questionnaire (available for 25% of couples); otherwise, we relied on female report of male exposures.
Assessment of fecundability
We collected menstrual cycle information on the baseline and follow-up questionnaires. At baseline, participants reported how long they had been trying to conceive (in terms of number of menstrual cycles), their last menstrual period (LMP) date, typical menstrual cycle length, and whether their cycles were regular (i.e., can usually predict date of next period within a few days). On follow-up questionnaires, we asked for the number of cycles the respondent had had since completing the previous questionnaire, LMP dates for each cycle, and length of the most recent cycle. On follow-up questionnaires, participants also reported whether they were currently pregnant, had initiated fertility treatment, or had experienced any pregnancy losses since completing their previous questionnaire. Those who conceived reported how the pregnancy was confirmed (e.g., urine test, blood test, ultrasound). We asked nonpregnant participants if they were still trying to conceive.
For each menstrual cycle during follow-up, we identified the first day of menses. If participants did not provide information on the number and dates of cycles since completing the previous questionnaire, we estimated LMP date(s) that occurred between questionnaires, using information on time between reported LMP dates, length of the most recent menstrual cycle, and typical cycle length (42).
Exclusions
In this analysis, we included PRESTO participants who enrolled between December 14, 2020 (when COVID-19 vaccines first became available in the United States), and September 22, 2021 (n = 2,679) (Web Figure 1, available at https://doi.org/10.1093/aje/kwac011). We followed participants through November 11, 2021. We excluded 91 individuals with implausible baseline dates for LMP. We restricted the analysis to those who had been trying to conceive for ≤ 6 cycles at enrollment, to reduce the potential for reverse causation, which could occur if fertility concerns influence decisions about vaccination. The final analytic sample included 2,126 couples. Analyses of male partner vaccination and fecundability were restricted to the 1,369 couples for whom these data were available from either partner.
Statistical analysis
We used the Andersen–Gill data structure, with 1 observation per menstrual cycle, to account for left truncation due to delayed entry and to update exposure status over time. For analyses of vaccination, we compared participants who had received at least 1 dose of vaccine by the first day of each menstrual cycle with participants who had not received any vaccine doses. In secondary analyses, we compared participants who had received a full vaccine regimen (defined as 2 doses of the Pfizer-BioNTech or Moderna vaccines or 1 dose of the Johnson & Johnson vaccine) with participants who had not received any vaccine doses. For analysis of SARS-CoV-2 infection, we compared participants who had ever tested positive for SARS-CoV-2 by the first day of the menstrual cycle with those who had never tested positive. We fit proportional probabilities regression models (i.e., log-binomial models in which we adjusted for cycle number at risk) to estimate fecundability ratios (FRs) and 95% confidence intervals (CIs). The FR represents the per-cycle probability of conception comparing exposed and unexposed individuals. We followed couples until pregnancy (regardless of outcome) or the occurrence of a censoring event (i.e., initiation of fertility treatment, cessation of pregnancy attempt, loss to follow-up, or 12 cycles of pregnancy attempt), whichever came first. To examine the association between time since vaccination or infection with fecundability, we fit restricted cubic splines.
In multivariable-adjusted models, we adjusted for the following female baseline variables: age (years); educational attainment (high school or less, some college, college degree, graduate school); household income (<US $50,000, 50,000–99,999, 100,000–149,999, ≥150,000); current smoker; private health insurance; hours/week of work; rotating shift work; night shift work; body mass index; intercourse frequency (<1, 1–3, ≥4 times/week); doing something to improve chances of conception (e.g., timing intercourse, measuring basal body temperature); sleep duration (<6, 6–8, ≥9 hours/night); 10-item Perceived Stress Scale score (43); Major Depression Inventory score (44); having had a Pap smear in past 3 years; history of self-reported infertility; parity (parous vs. nulliparous); irregular menstrual cycles; menstrual cycle length (<25, 25–31, ≥32 days); geographic region of residence (northeastern, southern, midwestern, and western United States; Canada); last method of contraception (oral contraceptive pills, other hormonal methods, barrier or natural methods); occupation in the health-care industry (defined on the basis of the following US Census Industry codes: 8190 (hospitals); 8180 (other health-care services); 8170 (home health-care services); 8080 (offices of other health practitioners); 8070 (offices of optometrists); 8090 (outpatient care centers); 8270 (nursing care facilities); 8290 (residential care facilities, without nurses); 7970 (offices of physicians); and 7980 (offices of dentists)) and race/ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, non-Hispanic other race, Hispanic). To account for expanding vaccine eligibility over time, we also adjusted for time since December 14, 2020 (days), as well as time squared and cubed. For analyses of vaccination, we adjusted for history of SARS-CoV-2 infection; for analyses of SARS-CoV-2 infection, we adjusted for history of COVID-19 vaccination.
We also fit models adjusting for confounding using fine stratification by propensity score (45, 46). Use of propensity scores to control confounding is as effective as stratification or regression modeling and offers the ability to improve validity by excluding individuals who are outside the mutual range of propensity scores for exposed and unexposed (47). We fit a logistic regression model of cycle-specific vaccination status (or infection status) regressed on covariates to calculate propensity scores (i.e., predicted probabilities of exposure). The propensity score models included the following variables that are either associated with both exposure and outcome or outcome only: age; educational attainment; household income; current smoker; private health insurance; rotating shift work; night shift work; body mass index; intercourse frequency; doing something to improve chances of conception; sleep duration; 10-item Perceived Stress Scale score; Major Depression Inventory score; having had a Pap smear in the past 3 years; history of infertility; parity; irregular menstrual cycles; menstrual cycle length; geographic region of residence; last method of contraception; occupation in health-care industry; race/ethnicity; time since December 14, 2020; time squared and time cubed; and tested positive for SARS-CoV-2 (or COVID-19 vaccination, as appropriate).
After developing the propensity score model, we excluded individuals who were outside the overlapping range of propensity scores for exposed and unexposed. We then divided the data set into 50 strata of propensity scores on the basis of the distribution of propensity scores in exposed individuals and developed weighted regression models to derive an adjusted exposure association. Exposed individuals were assigned weights of 1; unexposed individuals were assigned weights as follows:
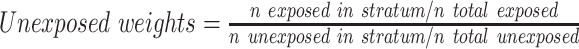
This weighting scheme generates a pseudopopulation in which confounder balance is achieved within each stratum and, thus, in the population overall. We then calculated the marginal measures of association in the weighted population to estimate the average treatment effect among the treated.
In sensitivity analyses, we defined vaccination date as dose date plus 14 days to assess the association with a full immune response to the dose. We also stratified by vaccination brand, country of residence (United States vs. Canada), occupation in health-care industry, and calendar time at risk (December 2020–March 2021 vs. April 2021–November 2021). To assess potential for reverse causation, we stratified by attempt time at study entry (<3 vs. 3–6 cycles) and restricted the analysis to participants without a history of infertility. Finally, for vaccination analyses, we restricted the data to that of participants who never tested positive for SARS-CoV-2 to control for potential confounding by infection.
We used multiple imputation with fully conditional specification to impute missing data. We generated 20 imputed data sets and combined estimates across analytic data sets. Missingness was generally low: no participants were missing vaccination status or brand, and covariate missingness ranged from 0% (age) to 2% (household income).
RESULTS
Most female participants in our analysis had high educational attainment (83% with ≥16 years), high household income (57% with income ≥US $100,000/year), and private health insurance (employment based or purchased privately; 86%). Most participants self-identified as non-Hispanic White (85%). A large proportion worked in the health-care industry (25%). Approximately 37% had a previous live birth, and 9% reported a history of infertility.
Vaccination prevalence was similar among female and male participants. Respectively, 73% and 74% had received at least 1 dose of COVID-19 vaccine by the LMP date of the final observed cycle. Vaccinated individuals were more likely to have higher education and income, reside in the United States, work in the health-care industry, and perform night or rotating shift work, and were less likely to be parous, report history of infertility, and have irregular menstrual cycles than were unvaccinated individuals (Table 1). We observed few differences in participant characteristics by vaccine brand (Web Table 1).
Table 1.
Distribution of Female Baseline Characteristics by Coronavirus Disease 2019 Vaccination Status and History of Infection With Severe Acute Respiratory Syndrome Coronavirus 2,a Pregnancy Study Online, December 2020–November 2021
| COVID-19 Vaccination | SARS-CoV-2 Infection | |||||||
|---|---|---|---|---|---|---|---|---|
|
No
(n = 897) |
Yes
(n = 1,229) |
No
(n = 1,963) |
Yes
(n = 163) |
|||||
| Characteristic | % | Mean | % | Mean | % | Mean | % | Mean |
| Age, years | 30.2 | 31.2 | 30.8 | 30.5 | ||||
| Attempt time at study entry, no. of cycles | 2.2 | 1.8 | 2.0 | 1.9 | ||||
| Educational attainment, years | ||||||||
| ≤12 | 5.6 | 1.4 | 3.2 | 4.0 | ||||
| 13–15 | 20.1 | 8.8 | 13.5 | 14.1 | ||||
| 16 | 33.2 | 31.4 | 32.3 | 29.1 | ||||
| ≥17 | 41.2 | 58.5 | 51.0 | 52.8 | ||||
| Household income, USD/year | ||||||||
| <50,000 | 17.0 | 9.1 | 12.5 | 13.0 | ||||
| 50,000–99,999 | 35.4 | 27.9 | 30.3 | 37.1 | ||||
| 100,000–149,999 | 26.9 | 30.6 | 29.3 | 23.1 | ||||
| ≥150,000 | 20.7 | 32.4 | 27.9 | 26.8 | ||||
| Race/ethnicity | ||||||||
| Hispanic | 7.4 | 6.8 | 6.7 | 8.8 | ||||
| Non-Hispanic White | 83.5 | 85.6 | 84.9 | 83.1 | ||||
| Non-Hispanic Black | 4.0 | 1.8 | 2.6 | 4.9 | ||||
| Non-Hispanic Asian | 2.8 | 3.0 | 3.1 | 1.4 | ||||
| Non-Hispanic mixed or other race | 2.3 | 2.8 | 2.8 | 1.8 | ||||
| Geographic region of residence | ||||||||
| Northeastern United States | 14.5 | 18.3 | 16.5 | 18.5 | ||||
| Southern United States | 25.2 | 23.0 | 23.5 | 28.9 | ||||
| Midwestern United States | 19.6 | 21.7 | 20.3 | 27.9 | ||||
| Western United States | 15.4 | 21.4 | 18.7 | 20.4 | ||||
| Canada | 25.3 | 15.7 | 20.9 | 4.3 | ||||
| Current smoker | 5.3 | 3.2 | 4.1 | 3.8 | ||||
| Received ≥1 dose of COVID-19 vaccine | 57.8 | 57.8 | ||||||
| Ever tested positive for SARS-CoV-2 | 7.7 | 7.8 | ||||||
| Partner received ≥1 dose of COVID-19 vaccine | 8.7 | 77.7 | 56.5 | 61.0 | ||||
| Partner ever tested positive for SARS-CoV-2 | 8.2 | 8.9 | 4.1 | 62.0 | ||||
| Private health insurance | 81.1 | 89.9 | 85.4 | 93.3 | ||||
| Work duration, hours/week | 31.5 | 36.1 | 34.0 | 35.7 | ||||
| Rotating shift work | 11.0 | 13.3 | 12.3 | 13.4 | ||||
| Night shift work | 10.2 | 11.6 | 11.1 | 10.5 | ||||
| Occupation in health-care industryb | 16.8 | 30.4 | 24.2 | 34.3 | ||||
| Body mass indexc | ||||||||
| <25 | 43.8 | 48.5 | 46.5 | 46.1 | ||||
| 25–29 | 24.6 | 23.5 | 24.2 | 20.9 | ||||
| ≥30 | 31.6 | 28.0 | 29.3 | 33.1 | ||||
| Intercourse <1 time/week | 24.1 | 28.0 | 26.4 | 24.7 | ||||
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a Vaccination status defined by at least 1 dose of vaccine by the last menstrual period (LMP) date of the final observed cycle. Infection history defined as self-report of testing positive for SARS-CoV-2 by the LMP date of the final observed cycle.
b Occupation in health-care industry defined on the basis of the US Census Industry codes 8190 (hospitals); 8180 (other health-care services); 8170 (home health-care services); 8080 (offices of other health practitioners); 8070 (offices of optometrists); 8090 (outpatient care centers); 8270 (nursing care facilities); 8290 (residential care facilities, without nurses); 7970 (offices of physicians); and 7980 (offices of dentists).
c Weight (kg)/height (m)2.
COVID-19 vaccination was not appreciably associated with fecundability in either partner (Table 2). Female participants who received at least 1 dose of vaccine before a given menstrual cycle had 1.08 times the probability of conceiving during that cycle compared with unvaccinated participants (95% CI: 0.95, 1.23). The corresponding adjusted FR for female receipt of a full vaccine regimen (i.e., 2 doses of the Pfizer-BioNTech or Moderna vaccines, or 1 dose of the Johnson & Johnson vaccine) before a given menstrual cycle was 1.07 (95% CI: 0.93, 1.23). For male partners, the adjusted FR for at least 1 dose was 0.95 (95% CI: 0.83, 1.10) and for a full vaccine regimen was 1.00 (95% CI: 0.86, 1.17). The FR for couples in which both partners had received at least 1 dose compared with couples in which neither partner had received any doses was 0.97 (95% CI: 0.82, 1.16).
Table 2.
Association between Coronavirus Disease 2019 Vaccination, Severe Acute Respiratory Syndrome Coronavirus 2 Infection, and Fecundability,a Pregnancy Study Online, December 2020–November 2021
| Exposure | No. of Cycles | No. of Pregnancies | Unadjusted | Adjusted b | Adjusted c | |||
|---|---|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | FR | 95% CI | |||
| Female COVID-19 vaccination status | ||||||||
| Unvaccinated | 2,844 | 539 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| First dose | 3,675 | 676 | 1.06 | 0.95, 1.18 | 1.08 | 0.95, 1.23 | 1.09 | 0.92, 1.30 |
| Second dosed | 3,144 | 565 | 1.05 | 0.94, 1.17 | 1.07 | 0.93, 1.23 | 1.13 | 0.93, 1.39 |
| Female SARS-CoV-2 infectione | ||||||||
| Never | 6,063 | 1,130 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Ever | 456 | 85 | 0.99 | 0.81, 1.22 | 1.07 | 0.87, 1.31 | 0.99 | 0.80, 1.23 |
| Male COVID-19 vaccination status | ||||||||
| Unvaccinated | 2,418 | 432 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| First dose | 2,486 | 408 | 0.98 | 0.86, 1.11 | 0.95 | 0.83, 1.10 | 1.05 | 0.87, 1.25 |
| Second dosed | 2,140 | 352 | 0.99 | 0.87, 1.13 | 1.00 | 0.86, 1.17 | 0.95 | 0.78, 1.15 |
| Male SARS-CoV-2 infectione | ||||||||
| Never | 5,977 | 1,112 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Ever | 542 | 103 | 1.03 | 0.85, 1.24 | 1.07 | 0.88, 1.31 | 1.06 | 0.84, 1.34 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; FR, fecundability ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a Fecundability is the per-cycle probability of conception. FRs >1 indicate an exposure associated with improved fecundability (or shorter time to pregnancy), whereas FRs <1 indicate an exposure associated with reduced fecundability (or longer time to pregnancy).
b Adjusted for female age; educational attainment; household income; current smoker; private health insurance; hours/week of work; rotating shift work; night shift work; body mass index; intercourse frequency; doing something to improve chances of conception; sleep duration; Perceived Stress Scale score; Major Depression Inventory score; having had a Pap smear in past 3 years; history of infertility; parity; irregular menstrual cycles; menstrual cycle length; geographic region of residence; last method of contraception; occupation in health-care industry; race/ethnicity; days since December 14, 2020. Analysis of COVID-19 vaccination status was adjusted for ever having tested positive for SARS-CoV-2, and analysis of SARS-CoV-2 infection was adjusted for COVID-19 vaccination.
c Propensity scores were developed to predict the odds of vaccination. We adjusted for propensity score using fine stratification weighting and calculated the Mantel–Haenszel summary FR.
d Individuals included in “second dose” are also included in “first dose.” Those who received the Johnson & Johnson vaccine are included in the sample of “first dose” and “second dose.”
e SARS-CoV-2 infection was defined as self-report of ever testing positive for SARS-CoV-2.
Findings were similar after adjustment for potential confounders, using fine stratification on propensity scores (Table 2). After trimming nonoverlapping propensity scores and reweighting across 50 propensity score strata, the distribution of propensity scores was similar across exposure groups (Web Figure 2), and we achieved reasonable balance of covariates by exposure status (Web Figure 3).
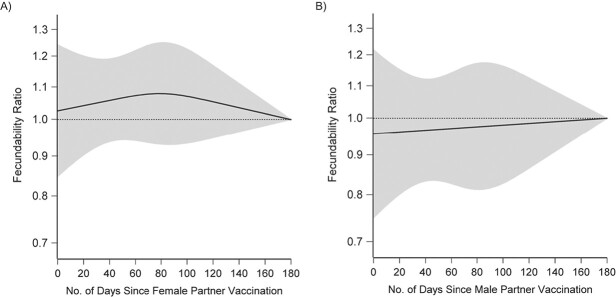
Figures 1 and 2 present FRs and 95% CIs for several sensitivity analyses comparing data of individuals who had received at least 1 dose of vaccine with data of unvaccinated individuals. For both partners, when we compared individuals who received their vaccine dose at least 14 days before the first day of their cycle with those who were unvaccinated, results were similar to the main analysis. We did not observe any substantial variation in FRs by vaccine brand, country of residence, occupation in the health-care industry, or calendar time at risk. FRs were similar when we stratified by attempt time at study entry and when we restricted analysis to individuals with no history of infertility. FRs were also similar among individuals who had never tested positive for SARS-CoV-2. We observed little variation in fecundability by time since vaccination in female or male partners (Figure 3).
Figure 1.
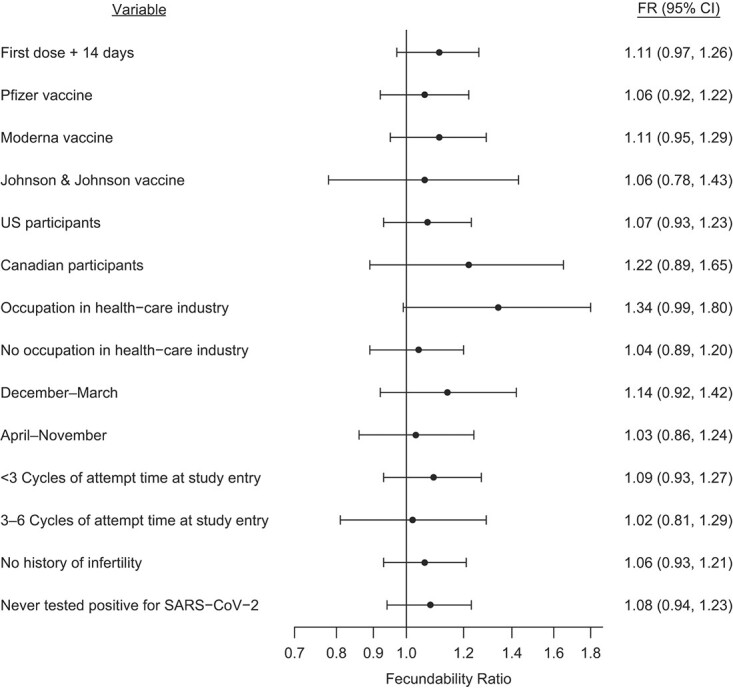
Association between female partner receipt of coronavirus disease 2019 (COVID-19) vaccine by first day of menses and fecundability, stratified by selected variables, Pregnancy Study Online, December 2020–November 2021. The reference group comprises individuals who were unvaccinated as of the first day of menses. Estimates are adjusted for age; educational attainment; household income; current smoker; private health insurance; hours/week of work; rotating shift work; night shift work; body mass index; intercourse frequency; doing something to improve chances of conception; sleep duration; Perceived Stress Scale score; Major Depression Inventory score; having had a Pap smear in past 3 years; history of infertility; parity; irregular menstrual cycles; menstrual cycle length; geographic region of residence; last method of contraception; occupation in health-care industry; race/ethnicity; days since December 14, 2020; and ever tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The x-axis is plotted on the natural log scale. CI, confidence interval.
Figure 2.
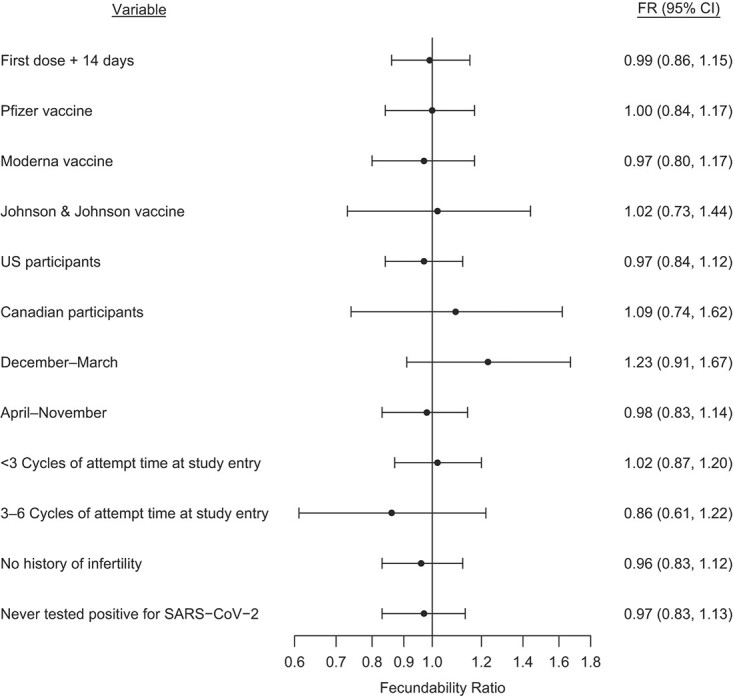
Association between male partner receipt of coronavirus disease 2019 (COVID-19) vaccine by first day of menses of the female partner and fecundability, stratified by selected variables, Pregnancy Study Online, December 2020–November 2021. The reference group comprises individuals who were unvaccinated as of the first day of menses of the female partner. Estimates are adjusted for age; educational attainment; household income; current smoker; private health insurance; hours/week of work; rotating shift work; night shift work; body mass index; intercourse frequency; doing something to improve chances of conception; sleep duration; Perceived Stress Scale score; Major Depression Inventory score; having had a Pap smear in past 3 years; history of infertility; parity; irregular menstrual cycles; menstrual cycle length; geographic region of residence; last method of contraception; occupation in health-care industry; race/ethnicity; days since December 14, 2020; and ever tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The x-axis is plotted on the natural log scale. CI, confidence interval.
Figure 3.
Association between time since female (A) and male (B) partner coronavirus disease 2019 (COVID-19) vaccination and fecundability, fit using restricted cubic splines, Pregnancy Study Online, December 2020–November 2021. The black solid line represents the fecundability ratio (FR), the gray shaded area represents the 95% confidence band, and the black dotted line represents the reference FR of 1.0. The splines have knots at 30, 60, 90, and 120 days. The reference group comprises unvaccinated individuals and individuals who were vaccinated at least 180 days ago. Splines are adjusted for age; educational attainment; household income; current smoker; private health insurance; hours/week of work; rotating shift work; night shift work; body mass index; intercourse frequency; doing something to improve chances of conception; sleep duration; Perceived Stress Scale score; Major Depression Inventory score; having had a Pap smear in past 3 years; history of infertility; parity; irregular menstrual cycles; menstrual cycle length; geographic region of residence; last method of contraception; occupation in health-care industry; race/ethnicity; days since December 14, 2020; and ever tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
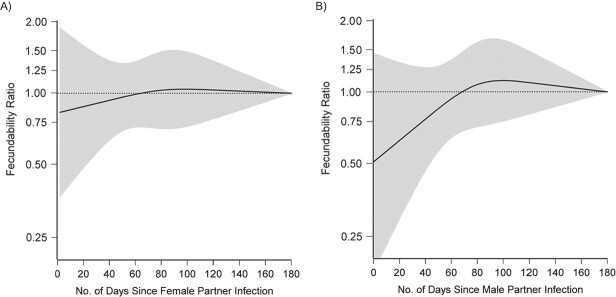
By the final observed LMP date in the study, 7.2% of female and 7.8% of male participants had a history of a positive test for SARS-CoV-2 infection. Overall, history of testing positive for SARS-CoV-2 in either partner was not strongly associated with fecundability (for female partner, adjusted FR = 1.07; 95% CI: 0.87, 1.31; for male partner, adjusted FR = 1.07; 95% CI: 0.88, 1.31) (Table 2). However, restricted cubic spline analyses showed that among male partners, recent infection was associated with a transient reduction in fecundability (Figure 4): men who reported testing positive for SARS-CoV-2 within 60 days of a given cycle had reduced fecundability compared with men who never tested positive or who tested positive at least 60 days prior. FRs for male partner infection 0–30 and 0–60 days after infection were 0.20 (95% CI: 0.03, 1.39; n = 41 exposed cycles) and 0.82 (95% CI: 0.47, 1.45; n = 99 exposed cycles), respectively. Male partner infection at least 60 days ago was not associated with reduced fecundability (FR = 1.16, 95% CI: 0.92, 1.47). Among female partners, SARS-CoV-2 infection was not appreciably associated with fecundability regardless of time since infection.
Figure 4.
Association between time since female (A) and male (B) partner severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and fecundability, fit using restricted cubic splines, Pregnancy Study Online, December 2020–November 2021. The black solid line represents the fecundability ratio, the gray shaded area represents the 95% confidence band, and the black dotted line represents the reference fecundability ratio of 1.0. The splines have knots at 30, 60, 90, and 120 days. The reference group comprises individuals who have never tested positive for SARS-CoV-2 and who tested positive at least 180 days ago. Splines are adjusted for age; educational attainment; household income; current smoker; private health insurance; hours/week of work; rotating shift work; night shift work; body mass index; intercourse frequency; doing something to improve chances of conception; sleep duration; Perceived Stress Scale score; Major Depression Inventory score; having had a Pap smear in past 3 years; history of infertility; parity; irregular menstrual cycles; menstrual cycle length; geographic region of residence; last method of contraception; occupation in health-care industry; race/ethnicity; days since December 14, 2020; and received coronavirus disease 2019 (COVID-19) vaccination.
DISCUSSION
High-quality data on the risks and benefits of vaccination are essential for informed COVID-19 vaccine decision-making. In this prospective cohort study of couples trying to conceive, we found no meaningful association between COVID-19 vaccination in either partner with fecundability. This adds to the evidence from animal studies (48), studies of humans undergoing fertility treatment (18–20), and the COVID-19 vaccine trials (14–17), none of which found an association between COVID-19 vaccination and lower fertility. Similarly, in several studies, researchers have documented no appreciable association between COVID-19 vaccination and miscarriage risk (49–52). In terms of benefits, vaccination is highly effective at preventing SARS-CoV-2 infection and severe COVID-19 disease (1–3). Here, we also show that SARS-CoV-2 infection among male partners was associated with a short-term decline in fertility that may be avoidable by vaccination. Therefore, given the known risks of SARS-CoV-2 infection during pregnancy to maternal and fetal health (53–56) and the evidence presented herein of no harmful association with fertility, our results support promotion of COVID-19 vaccination during the preconception period.
One hypothesized mechanism by which COVID-19 vaccination could influence female fertility is via changes in menstrual cycles. Although we and others have found in our studies no adverse associations of female COVID-19 vaccination with fertility (14–20), anecdotal reports of menstrual changes and vaginal bleeding after vaccination have contributed to skepticism of vaccine safety and concerns about fertility. An association between COVID-19 vaccination and menstrual irregularities theoretically could arise through mechanisms involving immunological influences on hormone levels (57) or through immune cells in the lining of the uterus (58). Some previous vaccines have been associated with short-term menstrual changes, including the typhoid (59), hepatitis B (60), and human papillomavirus (61) vaccines. To date, to our knowledge, the association between COVID-19 vaccination and menstruation has not been examined in a prospective study. In 2 retrospective reports (62, 63), researchers showed that high proportions of menstruating adults reported irregular cycles and heavy bleeding after vaccination and that breakthrough bleeding was common among individuals taking gender-affirming hormones or long-acting reversible contraception, and among postmenopausal individuals. However, these studies were likely enriched with individuals who noticed a change in their cycles and so cannot be used to estimate associations between vaccination and menstruation. Results from our study indicate that even if vaccines do have short-term effects on menstruation, there is likely little or no subsequent effect on fertility.
In our study, vaccinated participants were trying to conceive between 0 and 11 months after vaccination (mean = 3.5 months). Therefore, at this time, we cannot draw conclusions about long-term effects of vaccination on fertility. There are two possible sources of long-term effects of vaccination: the components of the vaccine and the immune response to vaccination. Components of the vaccine have documented safety profiles (1–3), and any potential allergic reactions attributable to vaccine ingredients would be observed within approximately 15–30 minutes of vaccination (64). The innate (rapid, nonspecific) phase of the immune response takes place over several days and triggers the adaptive (slower, highly specific) phase, which occurs over several weeks (65). Beyond this point, antibody concentrations plateau or slowly decline, and the risk of severe immunization-related complications drops dramatically. Enrollment in PRESTO is ongoing, and we will continue to monitor long-term associations of COVID-19 vaccination and fecundability; however, it is unlikely that adverse effects on fertility could arise many months after vaccination.
Our finding of a short-term decline in fertility after male SARS-CoV-2 infection is consistent with findings of several studies indicating short-term declines in sperm quality after SARS-CoV-2 infection (24–30). Fever is a known determinant of impaired spermatogenesis, and effects on sperm concentration, motility, and morphology can persist for 3–4 months (i.e., the duration of spermatogenesis) (33). Fever is one of the most common symptoms of SARS-CoV-2 infection (32); therefore, fever could explain our finding of an acute decline in fertility among men with recent SARS-CoV-2 infection. Although fever is also a side effect of vaccination, it is much less common than fever that results from infection (14–16) The fertility decline could also be related to immune response and inflammation in the testes and epididymis, which have been observed in patients hospitalized with COVID-19 (27). Erectile dysfunction is also more common among men after SARS-CoV-2 infection (66). Because we lacked data on COVID-19 symptoms or disease severity, we could not assess this hypothesis. Regardless, we did not observe any association between SARS-CoV-2 infection and fecundability that persisted beyond 60 days.
We adjusted for a broad range of sociodemographic, lifestyle, medical, occupational, and reproductive factors that could confound the association between COVID-19 vaccination or SARS-CoV-2 infection and fecundability. We adjusted for confounding using traditional regression modeling as well as propensity score stratification. As in any nonexperimental study, uncontrolled confounding is possible.
Loss to follow-up was low in our cohort (82% completed at least 1 questionnaire, and of those, only 3% subsequently were lost to follow-up) and was similar by vaccination status. Therefore, it is unlikely that differential loss to follow-up was an important source of bias.
We relied on self-report to assess COVID-19 vaccination status, which may have resulted in some misclassification. In addition, for couples in which the male partner did not complete his questionnaire, we relied on female report of male vaccination status. We expect that any misclassification was infrequent and nondifferential with respect to fecundability. In validation studies of influenza vaccination in the past year, 97% agreement was found between vaccination status based on self-report and medical records (67). Because length of the recall interval was shorter for COVID-19 vaccination in this study and recipients received vaccination cards, we anticipate little exposure misclassification.
We assessed history of SARS-CoV-2 infection by asking participants if they had ever tested positive for SARS-CoV-2. We also relied on female report of male infection for nearly 75% of couples. Underestimation of the true incidence of SARS-CoV-2 infection is probable because most participants likely were not testing regularly throughout the follow-up period. Given the high specificity of antigen and polymerase chain reaction tests for SARS-CoV-2 (68), we anticipate that our exposure definition had very high specificity but potentially low sensitivity. If misclassification of SARS-CoV-2 infection was unrelated to fecundability, there should be minimal to no bias in relative measures of association (69).
We calculated fecundability using self-reported information on LMP dates, typical menstrual cycle length, and pregnancy status. We also estimated LMP dates that occurred between follow-up questionnaires. To the extent that any of these variables were ascertained with error, outcome misclassification may have occurred. In previous work with data from this cohort, LMP dates prospectively reported on a menstrual charting app and retrospectively reported on follow-up questionnaires were within 1 day for 93% of participants (41). Because we did not have daily urinary measures of human chorionic gonadotropin, we likely missed some conceptions ending in early loss. However, 96% of the cohort used home pregnancy tests, and the median weeks’ gestation at pregnancy detection was 4.0 (interquartile range: 3.7–4.4), indicating that participants are testing early for pregnancy.
Several features of PRESTO make it an ideal setting in which to assess the relation of COVID-19 vaccination and SARS-CoV-2 infection with fertility. Recruitment of couples trying to conceive without fertility treatment is challenging, given that individuals often do not publicize their intentions or interact with health-care providers. We have successfully recruited couples during preconception using advertising on social media, with internet-based data collection and follow-up (41). Our internet-based methods allowed us to continue enrolling couples throughout the COVID-19 pandemic because participation required no face-to-face interaction with study staff. We prospectively followed couples every 2 months and collected time-varying data on COVID-19 vaccination and SARS-CoV-2 infection. Finally, our cohort is more geographically and socioeconomically diverse than most other preconception cohorts (70) and represents the largest study on these associations to date.
Our study was limited to pregnancy planners enrolled through the internet. Although both pregnancy planning status and internet access are related to sociodemographic characteristics such as income and education, we do not expect our associations to vary by these characteristics. Thus, these results may generalize to the broader population of pregnancy planners in North America.
In summary, we found no adverse association between COVID-19 vaccination and fertility and a short-term decrease in fertility after a male partner’s SARS-CoV-2 infection. These results can be used to guide informed decision-making about COVID-19 vaccination among reproductive-aged individuals, particularly those who are trying to conceive now or in the future.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Boston University School of Public Health, Boston, Massachusetts, United States (Amelia K. Wesselink, Elizabeth E. Hatch, Kenneth J. Rothman, Tanran R. Wang, Mary D. Willis, Jennifer Yland, Holly M. Crowe, Ruth J. Geller, Sydney K. Willis, Jessica Levinson, Lauren A. Wise); Research Triangle Institute, Research Triangle Park, North Carolina, United States (Kenneth J. Rothman); School of Biological and Population Health Sciences, College of Public Health and Human Sciences, Oregon State University, Corvallis, Oregon, United States (Mary D. Willis); Department of Obstetrics & Gynecology, Boston University School of Medicine, Boston, Massachusetts, United States (Rebecca B. Perkins); School of Nursing and Health Professions, University of San Francisco, San Francisco, California, United States (Annette K. Regan); and Department of Clinical Epidemiology, Department of Clinical Medicine, Aarhus University, and Aarhus University Hospital, Aarhus, Denmark (Ellen M. Mikkelsen).This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Health (grants R01-HD086742; R01-HD086742-05S2; and R01-HD105863).
Data are not available to the public.
We thank Michael Bairos for development and maintenance of the web-based infrastructure of PRESTO.
The views expressed in this article are those of the authors and do not reflect those of the National Institutes of Health.
Conflict of interest: L.A.W. is a consultant for AbbVie, Inc. and accepts in-kind donations from Swiss Precision Technologies, Sandstone Diagnostics, FertilityFriend.com, and Kindara for primary data collection in PRESTO. The other authors report no conflicts.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. . Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sadoff J, Gray G, Vandebosch A, et al. . Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . Demographic characteristics of people receiving COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic. Published August 21, 2021. Accessed August 22, 2021.
- 5. SteelFisher GK, Blendon RJ, Caporello H. An uncertain public—encouraging acceptance of COVID-19 vaccines. N Engl J Med. 2021;384(16):1483–1487. [DOI] [PubMed] [Google Scholar]
- 6. Diaz P, Reddy P, Ramasahayam R, et al. . COVID-19 vaccine hesitancy linked to increased internet search queries for side effects on fertility potential in the initial rollout phase following emergency use authorization. Andrologia. 2021;53(9):e14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sajjadi NB, Nowlin W, Nowlin R, et al. . United States internet searches for "infertility" following COVID-19 vaccine misinformation. J Osteopath Med. 2021;121(6):583–587. [DOI] [PubMed] [Google Scholar]
- 8. Fertility and Sterility Editorial Office, American Society for Reproductive Medicine . A survey of fertility patients' attitudes towards the COVID-19 vaccine. https://www.fertstertdialog.com/posts/a-survey-of-fertility-patients-attitudes-towards-the-covid-19-vaccine. Published January 6, 2021. Accessed August 9, 2021.
- 9. Chen CP, Chen LF, Yang SR, et al. . Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol Reprod. 2008;79(5):815–823. [DOI] [PubMed] [Google Scholar]
- 10. Mattar CNZ, Koh W, Seow Y, et al. . Addressing anti-syncytin antibody levels, and fertility and breastfeeding concerns, following BNT162B2 mRNA vaccination [preprint]. medRxiv. 2021. 10.1101/2021.05.23.21257686. [DOI] [Google Scholar]
- 11. Lu-Culligan A, Tabachnikova A, Tokuyama M, et al. . No evidence of fetal defects or anti-syncytin-1 antibody induction following COVID-19 mRNA vaccination [preprint]. bioRxiv. 2021. 10.1101/2021.12.07.471539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prasad M, Lin JL, Gu Y, et al. . No crossreactivity of anti-SARS-CoV-2 spike protein antibodies with Syncytin-1. Cell Mol Immunol. 2021;18(11):2566–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Male V. Menstrual changes after COVID-19 vaccination. BMJ. 2021;374:n2211. [DOI] [PubMed] [Google Scholar]
- 14. US Food and Drug Administration . Pfizer-BioNTech COVID-19 Vaccine (BNT162, PF-07302048). Vaccines and Related Biological Products Advisory Committee Briefing Document. Washington, DC: US Food & Drug Administration; 2020. Available at: https://www.fda.gov/media/144246/download. Accessed on July 1, 2021. [Google Scholar]
- 15. US Food and Drug Administration . MRNA-1273 Sponsor Briefing Document. Vaccines and Related Biological Products Advisory Committee Briefing Document. Washington, DC: US Food & Drug Administration; 2020. Available at: https://www.fda.gov/media/144434/download. Accessed on July 1, 2021. [Google Scholar]
- 16. US Food and Drug Administration . Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee Briefing Document. Washington, DC: US Food & Drug Administration; 2021. Available at: https://www.fda.gov/media/146217/download. Accessed on September 2, 2021. [Google Scholar]
- 17. Hillson K, Clemens SC, Madhi SA, et al. . Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. Lancet. 2021;398(10312):1683–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morris RS. SARS-CoV-2 spike protein seropositivity from vaccination or infection does not cause sterility. F S Rep. 2021;2(3):253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Orvieto R, Noach-Hirsh M, Segev-Zahav A, et al. . Does mRNA SARS-CoV-2 vaccine influence patients' performance during IVF-ET cycle? Reprod Biol Endocrinol. 2021;19(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bentov Y, Beharier O, Moav-Zafrir A, et al. . Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum Reprod. 2021;36(9):2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Safrai M, Reubinoff B, Ben-Meir A. BNT162b2 mRNA COVID-19 vaccine does not impair sperm parameters [preprint]. medRxiv. 2021. 10.1101/2021.04.30.21255690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalez DC, Nassau DE, Khodamoradi K, et al. . Sperm parameters before and after COVID-19 mRNA vaccination. JAMA. 2021;326(3):273–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen F, Zhu S, Dai Z, et al. . Effects of COVID-19 and mRNA vaccines on human fertility. Hum Reprod. 2021;37(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gacci M, Coppi M, Baldi E, et al. . Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod. 2021;36(6):1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erbay G, Sanli A, Turel H, et al. . Short-term effects of COVID-19 on semen parameters: a multicenter study of 69 cases. Andrology. 2021;9(4):1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pazir Y, Eroglu T, Kose A, et al. . Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: a prospective cohort study. Andrologia. 2021;53(9):e14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li H, Xiao X, Zhang J, et al. . Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma L, Xie W, Li D, et al. . Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93(1):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Temiz MZ, Dincer MM, Hacibey I, et al. . Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia. 2021;53(2):e13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holtmann N, Edimiris P, Andree M, et al. . Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114(2):233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donders GGG, Bosmans E, Reumers J, et al. . Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test [published online ahead of print December 20, 2021]. Fertil Steril. 2021. 10.1016/j.fertnstert.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carlsen E, Andersson AM, Petersen JH, et al. . History of febrile illness and variation in semen quality. Hum Reprod. 2003;18(10):2089–2092. [DOI] [PubMed] [Google Scholar]
- 34. Ma L, Xie W, Li D, et al. . Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study [preprint]. medRxiv. 2020. 10.1101/2020.03.21.20037267. [DOI] [Google Scholar]
- 35. Selvaraj K, Ravichandran S, Krishnan S, et al. . Testicular atrophy and hypothalamic pathology in COVID-19: possibility of the incidence of male infertility and HPG Axis abnormalities. Reprod Sci. 2021;28(10):2735–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khan SM, Shilen A, Heslin KM, et al. . SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2021;S0002-9378(21):01044–01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li K, Chen G, Hou H, et al. . Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42(1):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang M, Yang Q, Ren X, et al. . Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: a retrospective cohort study. EClinicalMedicine. 2021;38:101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Orvieto R, Segev-Zahav A, Aizer A. Does COVID-19 infection influence patients' performance during IVF-ET cycle? An observational study. Gynecol Endocrinol. 2021;37(10):895–897. [DOI] [PubMed] [Google Scholar]
- 40. Ding T, Wang T, Zhang J, et al. . Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med (Lausanne). 2021;8:635255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wise LA, Rothman KJ, Mikkelsen EM, et al. . Design and conduct of an internet-based preconception cohort study in North America: Pregnancy Study Online. Paediatr Perinat Epidemiol. 2015;29(4):360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wesselink AK, Wise LA, Hatch EE, et al. . Seasonal patterns in fecundability in North America and Denmark: a preconception cohort study. Hum Reprod. 2020;35(3):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 44. Bech P. Quality of life instruments in depression. Eur Psychiatry. 1997;12(4):194–198. [DOI] [PubMed] [Google Scholar]
- 45. Desai RJ, Rothman KJ, Bateman BT, et al. . A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology. 2017;28(2):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657. [DOI] [PubMed] [Google Scholar]
- 47. Sturmer T, Joshi M, Glynn RJ, et al. . A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59(5):437.e1–437.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bowman CJ, Bouressam M, Campion SN, et al. . Lack of effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, a mRNA-based COVID-19 vaccine. Reprod Toxicol. 2021;103:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Magnus MC, Gjessing HK, Eide HN, et al. . COVID-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385(21):2008–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zauche LH, Wallace B, Smoots AN, et al. . Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385(16):1533–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kharbanda EO, Haapala J, DeSilva M, et al. . Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326(16):1629–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shimabukuro TT, Kim SY, Myers TR, et al. . Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Villar J, Ariff S, Gunier RB, et al. . Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oakes MC, Kernberg AS, Carter EB, et al. . Pregnancy as a risk factor for severe coronavirus disease 2019 using standardized clinical criteria. Am J Obstet Gynecol MFM. 2021;3(3):100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lokken EM, Huebner EM, Taylor GG, et al. . Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington state. Am J Obstet Gynecol. 2021;225(1):77.e1–77e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Allotey J, Stallings E, Bonet M, et al. . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Karagiannis A, Harsoulis F. Gonadal dysfunction in systemic diseases. Eur J Endocrinol. 2005;152(4):501–513. [DOI] [PubMed] [Google Scholar]
- 58. Monin L, Whettlock EM, Male V. Immune responses in the human female reproductive tract. Immunology. 2020;160(2):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lamb AR. Experiences with prophylactic typhoid vaccination: its effect on menstruation. Arch Intern Med (Chic). 1913;XII(5):565. [Google Scholar]
- 60. Shingu T, Uchida T, Nishi M, et al. . Menstrual abnormalities after hepatitis B vaccine. Kurume Med J. 1982;29(3):123–125. [Google Scholar]
- 61. Suzuki S, Hosono A. No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: results of the Nagoya study. Papillomavirus Res. 2018;5:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Male V. Effect of COVID-19 vaccination on menstrual periods in a retrospectively recruited cohort [preprint]. medRxiv. 2021. 10.1101/2021.11.15.21266317. [DOI] [Google Scholar]
- 63. Lee KMN, Junkins EJ, Fatima UA, et al. . Characterizing menstrual bleeding changes occurring after SARS-CoV-2 vaccination [preprint]. medRxiv. 2021. https://doi.org/10/1101/2021/10/11/21264863. [Google Scholar]
- 64. Centers for Disease Control and Prevention . Interim considerations: preparing for the potential management of anaphylaxis after COVID-19 vaccination. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html. Published on November 9, 2021. Accessed on December 1, 2021.
- 65. Murphy KM, Weaver C. Janeway's Immunobiology. Ninth ed. New York, New York: W.W. Norton & Company; 2016. [Google Scholar]
- 66. Katz J, Yue S, Xue W, et al. . Increased odds ratio for erectile dysfunction in COVID-19 patients. J Endocrinol Invest. 2021;1–6. 10.1007/s40618-021-01717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. King JP, McLean HQ, Belongia EA. Validation of self-reported influenza vaccination in the current and prior season. Influenza Other Respi Viruses. 2018;12(6):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Karon BS, Donato LJ, Bridgeman AR, et al. . Analytical sensitivity and specificity of four point of care rapid antigen diagnostic tests for SARS-CoV-2 using real-time quantitative PCR, quantitative droplet digital PCR, and a mass spectrometric antigen assay as comparator methods. Clin Chem. 2021;67(11):1545–1553. [DOI] [PubMed] [Google Scholar]
- 69. Greenland S, Rothman KJ. Introduction to stratified analysis. In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. 3rd ed.New York, NY: Lippincott Williams & Wilkins; 2008: 261–262. [Google Scholar]
- 70. Harville EW, Mishra GD, Yeung E, et al. . The Preconception Period Analysis of Risks and Exposures Influencing Health and Development (PrePARED) Consortium. Paediatr Perinat Epidemiol. 2019;33(6):490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.