Abstract
Background
The modulating effect of vitamin D on cytokine concentrations in severe coronavirus disease 2019 (COVID-19) remains unknown.
Objectives
We aimed to investigate the effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in hospitalized patients with moderate to severe COVID-19.
Methods
This is a post hoc, ancillary, and exploratory analysis from a multicenter, double-blind, placebo-controlled, randomized clinical trial. Patients with moderate to severe COVID-19 were recruited from 2 hospitals in São Paulo, Brazil. Of 240 randomly assigned patients, 200 were assessed in this study and randomly assigned to receive a single oral dose of 200,000 IU vitamin D3 (n = 101) or placebo (n = 99). The primary outcome was hospital length of stay, which has been published in our previous study. The prespecified secondary outcomes were serum concentrations of IL-1β, IL-6, IL-10, TNF-α, and 25-hydroxyvitamin D. The post hoc exploratory secondary outcomes were IL-4, IL-12p70, IL-17A, IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-8, IFN-inducible protein-10 (IP-10), macrophage inflammatory protein-1β (MIP-1β), monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF), and leukocyte count. Generalized estimating equations for repeated measures, with Bonferroni’s adjustment, were used for testing all outcomes.
Results
The study included 200 patients with a mean ± SD age of 55.5 ± 14.3 y and BMI of 32.2 ± 7.1 kg/m2, of which 109 (54.5%) were male. GM-CSF concentrations showed a significant group-by-time interaction effect (P = 0.04), although the between-group difference at postintervention after Bonferroni’s adjustment was not significant. No significant effects were observed for the other outcomes.
Conclusions
The findings do not support the use of a single dose of 200,000 IU vitamin D3, compared with placebo, for the improvement of cytokines, chemokines, and growth factor in hospitalized patients with moderate to severe COVID-19.
This trial was registered at clinicaltrials.gov as NCT04449718.
Keywords: immune response, SARS-CoV-2, inflammation, acute-phase reactants, vitamin D
Introduction
Vitamin D has arisen as a mediator of the innate (1, 2, 3) and adaptive immune responses (4, 5). The active form of vitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D], could contribute to the induction of viral neutralization and recruitment of neutrophils, monocytes, macrophages, and dendritic cells (6). Furthermore, 1,25(OH)2D may avoid chronic activation of the innate immune response by limiting maturation of dendritic cells, inducing immune tolerance, downregulating toll-like receptors, and adjusting both the TNF-α/NF-κB and IFN-γ signaling pathways. By these means, it has been hypothesized that sufficient vitamin D concentrations could prevent a cytokine storm while promoting an adequate adaptive immune response in patients with coronavirus disease 2019 (COVID-19) (6, 7, 8, 9, 10).
Evidence suggests that COVID-19 may promote hyperactivation of neutrophils, monocytes, and macrophages resulting in a dysregulated immune inflammatory response and possible cytokine storm (11). These changes have been associated with increased concentrations of IL-1β, IL-6, IFN-inducible protein-10 (IP-10), TNF, IFN-γ, macrophage inflammatory protein-1β (MIP-1β), vascular endothelial growth factor (VEGF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with COVID-19 (12, 13). In a comprehensive review, Christakos et al. (14) presented important mechanisms of action of vitamin D in the immune system, such as regulation of activated T cells, inhibition of the adaptative immune response, promotion of the innate immune response, and an immunosuppressive effect associated with the decrease of inflammatory cytokines (for example, IL-2 and IFN-γ) and production of IL-4 and IL-10. It also targets dendritic cells and interacts with multiple cell types and activation states related to the immune cascade.
In view of the possible anti-inflammatory effect of vitamin D regulating the innate and adaptative immune responses, vitamin D3 supplementation could be a relevant therapeutic strategy to manage hyperinflammation in patients with COVID-19. However, the presumed benefit of vitamin D in improving the cytokine storm remains supported only by review (6, 7, 8, 15, 16, 17) and few observational (18, 19, 20) studies. Herein, we report on a post hoc, ancillary, and exploratory analysis from our randomized clinical trial (21) to investigate the effect of a single high dose of vitamin D3 on systemic inflammatory cytokines, chemokines, and growth factor in hospitalized patients with moderate to severe COVID-19.
Methods
Study design and participants
This is a post hoc, ancillary, and exploratory analysis of secondary outcomes from a multicenter, double-blind, placebo-controlled, randomized clinical trial (NCT04449718). The study was approved by the ethical committees of both Clinical Hospital (School of Medicine of the University of São Paulo) and Ibirapuera Field Hospital (Ethics Committee Approval Number 30959620.4.0000.0068), in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent before being enrolled in the study. The trial protocol and statistical analysis plan were previously published (21).
Hospitalized patients were recruited from the Clinical Hospital of the School of Medicine of the University of São Paulo, and Ibirapuera Field Hospital. Patients were enrolled from 2 June, 2020, to 27 August, 2020. The screening criteria assumed were identical for both centers and the final follow-up occurred on 7 October, 2020. All patients had a positive COVID-19 diagnosis confirmed by PCR testing at time of randomization or by serology assay (ELISA) to detect IgG against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) throughout the study.
Patients were eligible for enrollment if they were age 18 y or older and had positive SARS-CoV-2 infection diagnosis by either nasopharyngeal swab PCR or chest computed tomography scan with compatible findings (bilateral multifocal ground-glass opacities with ≥50% lung involvement). Patients should have had diagnosis of flu syndrome with hospitalization criteria on hospital admission and presented a respiratory rate >24 breaths/min, oxygen saturation <93% on room air, or risk factors for complications (e.g., heart disease, diabetes, systemic arterial hypertension, neoplasms, immunosuppression, pulmonary tuberculosis, obesity), followed by COVID-19 confirmation. Patients who met these criteria were considered to have moderate to severe COVID-19. Patients were excluded if they were unable to read and sign the written informed consent; they had already been admitted under invasive mechanical ventilation; they had recently received a vitamin D3 supplementation (>1000 IU/d or weekly equivalent); they had renal failure requiring dialysis or creatinine concentrations >2.0 mg/dL; they had hypercalcemia defined by total calcium >10.5 mg/dL; they were pregnant or lactating women; or if they were expecting hospital discharge in <24 h. The criteria used for hospital discharge were absence of fever in the previous 72 h, no need for supplemental oxygen in the previous 48 h, and oxygen saturation >93% on room air without respiratory distress.
Randomization and masking
Eligible patients were assigned in a 1:1 ratio into either the vitamin D3 group or the placebo group. The randomization list was created using a computer-generated code, in block sizes of 20 participants, which was managed by a staff member who had no other role in the study.
The vitamin D3 group received on the same day of randomization a single oral dose of 200,000 IU vitamin D3 diluted in vehicle (10 mL of a peanut oil solution). This selected dose is within the range indicated to effectively increase serum/plasma 25-hydroxyvitamin D [25(OH)D] concentrations in several vitamin D–sufficient and –deficient populations (22). Patients enrolled in the placebo group received only vehicle. The vitamin D3 and placebo solutions were identical (in color, taste, smell, consistency, and container) and prepared by the pharmacy unit of the Clinical Hospital. Both were labeled by a staff member who did not otherwise participate in the study, and allocation blindness was maintained until the final statistical analysis.
Procedures
Self-reported anthropometric characteristics (weight and height) and coexisting chronic diseases, acute COVID-19 symptoms, patients’ concomitant medications during hospitalization, oxygen supplementation requirement, and imaging features were assessed upon hospital admission. Subsequently, self-reported coexisting chronic diseases and previous medications were checked according to the medical records for each patient. To provide a comprehensive demographic characterization, self-reported race/ethnicity data were also collected based on the following fixed categories: white, black, Asian, and Pardo [the latter refers to people of mixed race/ethnicities, according to the Brazilian Institute of Geography and Statistics (IBGE)].
Serum concentrations of 25(OH)D were assessed by a chemiluminescent immunoassay (ARCHITECT 25-OH Vitamin D 5P02; Abbott Diagnostics). All cytokines, chemokines, and growth factor were analyzed by the Luminex® xMAP (Multiple Analyte Profiling) assay, a Multiplex technique using a commercial Milliplex MAP kit (Millipore Corp.), at the same time by a blinded technician, following the manufacturer’s recommendations. Leukocyte count was assessed by automated assay. All the assessments described were performed on the day of randomization and upon hospital discharge. Importantly, only patients who had blood samples collected on the day of randomization and upon hospital discharge were assessed in this study. Therefore, patients who died during follow-up were not included owing to the absence of blood samples.
Outcomes
The primary outcome, hospital length of stay, was not significantly different between the vitamin D3 and placebo groups as previously published (21). The prespecified secondary outcomes were serum concentrations of IL-1β, IL-6, IL-10, TNF-α, and 25(OH)D.
In order to provide a broader understanding of vitamin D3 effects on COVID-19-related hyperinflammation and immunomodulation, the following exploratory secondary outcomes were included as post hoc analyses: serum concentrations of cytokines (IL-4, IL-12p70, IL-17A, IFN-γ, and GM-CSF), chemokines [IL-8, IP-10, MIP-1β, and monocyte chemoattractant protein-1 (MCP-1)], growth factor (VEGF), and white blood cells (leukocyte count).
The intra-assay and interassay CVs were as follows: IL-1β, 2.3% and 6.7%; IL-6, 2.0% and 18.3%; IL-10, 1.6% and 16.8%; TNF-α, 2.6% and 13.0%; IL-4, 2.9% and 14.2%; IL-12p70, 2.2% and 16.7%; IL-17A, 2.2% and 7.9%; IFN-γ, 1.6% and 12.0%; GM-CSF, 3.1% and 10.1%; IL-8, 1.9% and 3.5%; IP-10, 2.6% and 15.3%; MIP-1β, 2.4% and 8.8%; MCP-1, 1.5% and 7.9%; and VEGF, 3.7% and 10.4%, respectively, according to the manufacturer’s specifications.
Statistical analysis
The sample size was chosen based on feasibility and resources, as described in detail in a previous study (21). Our study was powered considering a repeated-measure ANOVA, within-between interaction, a 2-sided significance level of 5% (α = 0.05), an assumed partial eta squared (η2 = 0.04), effect size (f = 0.20), and total sample of 200 patients, achieving post hoc power (1 − ß) >99% as performed (G*Power software, version 3.1.9.4). Generalized estimating equations (GEEs) for repeated measures were used for testing possible differences in all outcomes assuming group and time as fixed factors, with marginal distribution, and a first-order autoregressive correlation matrix to test the main and interaction effects. Bonferroni’s adjustment was performed in GEE analyses to maintain a family-wise 2-sided significance threshold of 0.05, considering 6 pairwise comparisons for all outcomes. Proportions were compared between groups using χ2 and Fisher’s exact tests. In order to handle potential confounders, the GEEs were adjusted by 4 models: cumulative glucocorticoid doses; cumulative glucocorticoid doses and time from symptom onset to randomization; cumulative glucocorticoid doses and baseline 25(OH)D concentrations; and cumulative glucocorticoid doses and hospitals from which patients were recruited, using a per protocol approach.
There were no missing data for cytokines, chemokines, growth factor, and serum 25(OH)D. Missingness for leukocyte count (2 patients in the vitamin D3 group) was at random and handled by GEE models, with no imputation for missing data. Statistical analyses were performed with IBM SPSS software, version 20.0. The significance level was set at a 2-sided P value ≤ 0.05.
Results
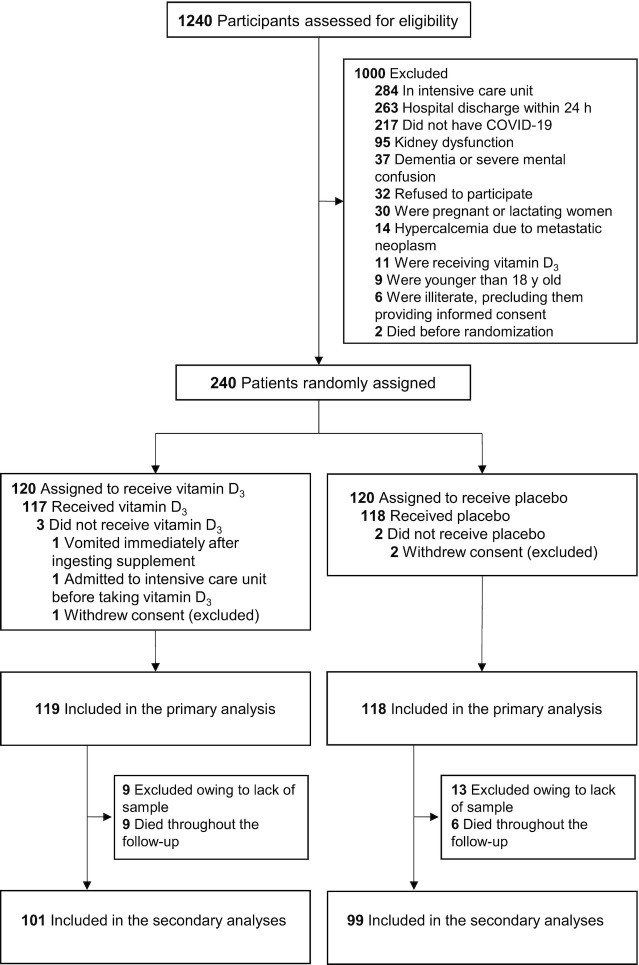
Of the 1240 patients assessed for eligibility, 240 underwent random assignment: 122 patients from the Clinical Hospital of the School of Medicine and 118 patients from the Ibirapuera Field Hospital. Of the 120 patients assigned to the vitamin D3 group, 1 withdrew their consent, 9 were excluded owing to lack of blood sample, and 9 died throughout the follow-up. Of the 120 patients assigned to the placebo group, 2 withdrew consent, 13 were excluded owing to lack of blood sample, and 6 died throughout the follow-up ( Figure 1). The mean ± SD age was 55.5 ± 14.3 y, the mean ± SD BMI was 32.2 ± 7.1 kg/m2, and 109 (54.5%) were male. Regarding ethnicity, 111 (55.5%) patients were white, 62 (31.0%) were Pardo, 26 (13.0%) were black, and 1 (0.5%) was Asian ( Table 1).
FIGURE 1.
Trial Consolidated Standards of Reporting Trials (CONSORT) diagram. All analyses were performed according to the patient’s randomization group using an intention-to-treat approach. There were no missing data for cytokines, chemokines, growth factor, and serum 25-hydroxyvitamin D concentrations. Missingness for leukocyte count (2 patients in the vitamin D3 group) was random and handled by generalized estimating equation models. For patients who died throughout the follow-up, blood samples were not collected at postintervention. COVID-19, coronavirus disease 2019.
TABLE 1.
Baseline demographic and clinical characteristics1
| Characteristic | Vitamin D3 group (n = 101) | Placebo group (n = 99) |
|---|---|---|
| Age, y | 55.3 ± 14.2 | 55.7 ± 14.5 |
| Sex | ||
| Male | 58 (57.4) | 51 (51.5) |
| Female | 43 (42.6) | 48 (48.5) |
| Race or ethnicity | ||
| White | 52 (51.5) | 59 (59.6) |
| Pardo2 | 32 (31.7) | 30 (30.3) |
| Black | 16 (15.8) | 10 (10.1) |
| Asian | 1 (1.0) | 0 (0) |
| Time from symptom onset to randomization, d | 10.0 [7.0–12.5] | 10.0 [7.0–14.0] |
| Time from symptom onset to hospital discharge, d | 17.0 [13.0–21.0] | 18.0 [15.0–22.0] |
| Time from hospital admission to randomization, d | 1.0 [1.0–2.0] | 1.0 [1.0–2.0] |
| Time for hospital length of stay, d | 6.0 [4.0–8.0] | 7.0 [5.0–10.0] |
| BMI,3 kg/m2 | 32.2 ± 6.7 | 32.1 ± 7.5 |
| BMI category, kg/m2 | ||
| <18.5 | 0 (0) | 1 (1.1) |
| 18.5–24.9 | 8 (8.6) | 13 (14.4) |
| 25.0–29.9 | 29 (31.2) | 24 (26.7) |
| ≥30 | 56 (60.2) | 52 (57.8) |
| Acute COVID-19 symptoms | ||
| Cough | 87 (86.1) | 82 (82.8) |
| Fatigue | 81 (80.2) | 86 (86.9) |
| Fever | 73 (72.3) | 69 (69.7) |
| Myalgia | 61 (60.4) | 60 (60.6) |
| Joint pain | 42 (41.6) | 33 (33.3) |
| Runny nose | 36 (35.6) | 37 (37.4) |
| Diarrhea | 33 (32.7) | 40 (40.4) |
| Nasal congestion | 34 (33.7) | 34 (34.3) |
| Sore throat | 36 (35.6) | 23 (23.2) |
| Coexisting diseases | ||
| Hypertension | 54 (53.5) | 49 (49.5) |
| Diabetes | 39 (38.6) | 29 (29.3) |
| Cardiovascular disease | 14 (13.9) | 13 (13.1) |
| Rheumatic disease | 10 (9.9) | 10 (10.1) |
| Asthma | 6 (5.9) | 7 (7.1) |
| Chronic obstructive pulmonary disease | 5 (5.0) | 5 (5.1) |
| Chronic kidney disease | 2 (2.0) | 0 (0) |
| Concomitant medications | ||
| Anticoagulant | 94 (93.1) | 85 (85.9) |
| Antibiotic | 85 (84.2) | 86 (86.9) |
| Glucocorticoid | 67 (66.3) | 63 (63.6) |
| Antihypertensive | 55 (54.5) | 46 (46.5) |
| Proton pump inhibitor | 40 (39.6) | 41 (41.4) |
| Antiemetic | 39 (38.6) | 49 (49.5) |
| Analgesic | 39 (38.6) | 46 (46.9) |
| Hypoglycemic | 23 (22.8) | 20 (20.2) |
| Hypolipidemic | 14 (13.9) | 14 (14.1) |
| Thyroid | 8 (7.9) | 8 (8.1) |
| Antiviral4 | 4 (4.0) | 3 (3.0) |
| Dose of glucocorticoid at randomization,5 mg | 5.1 ± 10.3 | 4.2 ± 4.3 |
| Cumulative dose of glucocorticoid,5 mg | 45.7 ± 81.4 | 33.6 ± 33.0 |
| Oxygen supplementation | ||
| Oxygen therapy | 72 (71.3) | 82 (82.8) |
| Noninvasive ventilation | 13 (12.9) | 12 (12.1) |
| No oxygen therapy | 16 (15.8) | 5 (5.1) |
| Computed tomography findings | ||
| Ground-glass opacities ≥50% | 48 (53.3) | 53 (62.4) |
| Ground-glass opacities <50% | 42 (46.7) | 32 (37.6) |
Values are mean ± SD, median [IQR], or n (%). Continuous variables were analyzed by independent t test. Percentages were analyzed by chi-square or Fisher’s exact test. COVID-19, coronavirus disease 2019.
Pardo is the exact term used in Brazilian Portuguese, meaning “mixed ethnicity,” according to the Brazilian Institute of Geography and Statistics.
BMI data were missing for 8.5% of patients (n = 17; 8 in the vitamin D3 group and 9 in the placebo group).
Included 3 patients from the vitamin D3 group and 3 patients from the placebo group receiving 75 mg oseltamivir twice per day for 5 d, and 1 patient from the vitamin D3 group receiving 400 mg acyclovir twice per day for herpes zoster prophylaxis.
Glucocorticoid information was standardized in dexamethasone doses.
The mean ± SD serum GM-CSF concentrations demonstrated a significant group-by-time interaction (P < 0.05 for all models), with a decrease from baseline to post after a single dose of vitamin D3 (from 3.4 ± 5.2 pg/mL to 2.9 ± 4.8 pg/mL) compared with an increase in the placebo group (from 3.0 ± 4.1 pg/mL to 4.4 ± 9.7 pg/mL), although no significant difference after Bonferroni’s adjustment was observed (between-group difference at postintervention: −1.5 pg/mL; 95% CI: −4.4, −1.3 pg/mL; P > 0.05 for all models) ( Table 2). The mean ± SD 25(OH)D concentrations significantly increased from baseline after a single high dose of vitamin D3 (from 21.1 ± 10.1 ng/mL to 44.6 ± 14.7 ng/mL) compared with placebo (from 20.2 ± 8.1 ng/mL to 19.8 ± 10.5 ng/mL) (between-group difference at postintervention: 24.9 ng/mL; 95% CI: 20.2, 29.6 ng/mL; P < 0.001 for all models) (Table 2). No significant differences between the vitamin D3 and placebo groups for serum concentrations of IL-1β, IL-4, IL-6, IL-10, IL-12p70, IL-17A, IFN-γ, TNF-α, IL-8, IP-10, MIP-1β, MCP-1, and VEGF, and for leukocyte counts, were observed (Table 2).
TABLE 2.
Cytokines, chemokines, growth factor, and laboratory outcomes1
| Vitamin D3 group (n = 101) | Placebo group (n = 99) | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Baseline | Post | Baseline | Post | P2 | P3 | P4 | P5 |
| Cytokines | ||||||||
| IL-1β, pg/mL | 1.9 ± 2.2 | 1.8 ± 2.4 | 2.2 ± 3.7 | 2.7 ± 5.5 | 0.39 | 0.44 | 0.39 | 0.39 |
| IL-4, pg/mL | 263.4 ± 1072.8 | 236.6 ± 988.7 | 220.2 ± 783.6 | 213.5 ± 780.9 | 0.33 | 0.25 | 0.33 | 0.33 |
| IL-6,* pg/mL | 26.8 ± 48.7 | 16.4 ± 41.2 | 25.3 ± 40.5 | 17.4 ± 38.6 | 0.42 | 0.45 | 0.42 | 0.42 |
| IL-10,* pg/mL | 28.0 ± 21.7 | 14.2 ± 10.3 | 33.6 ± 40.1 | 17.5 ± 18.9 | 0.62 | 0.59 | 0.62 | 0.62 |
| IL-12p70, pg/mL | 6.9 ± 25.6 | 4.2 ± 8.2 | 3.8 ± 7.2 | 4.7 ± 11.5 | 0.10 | 0.10 | 0.10 | 0.10 |
| IL-17A, pg/mL | 12.8 ± 78.0 | 12.4 ± 70.8 | 7.6 ± 20.2 | 6.9 ± 18.5 | 0.88 | 0.87 | 0.88 | 0.88 |
| IFN-γ,* pg/mL | 11.3 ± 17.8 | 7.7 ± 12.7 | 9.1 ± 13.2 | 7.3 ± 10.0 | 0.40 | 0.71 | 0.40 | 0.40 |
| TNF-α,* pg/mL | 29.5 ± 14.2 | 25.9 ± 12.1 | 28.1 ± 17.3 | 26.7 ± 13.9 | 0.11 | 0.14 | 0.11 | 0.11 |
| GM-CSF, pg/mL | 3.4 ± 5.2 | 2.9 ± 4.8 | 3.0 ± 4.1 | 4.4 ± 9.7 | 0.04 | 0.05 | 0.04 | 0.04 |
| Chemokines | ||||||||
| IL-8,* pg/mL | 22.9 ± 22.9 | 18.3 ± 22.0 | 22.0 ± 21.6 | 19.1 ± 20.5 | 0.31 | 0.40 | 0.31 | 0.31 |
| IP-10,* pg/mL | 3283.0 ± 3340.3 | 861.9 ± 972.5 | 3747.2 ± 3702.0 | 747.8 ± 1337.4 | 0.24 | 0.27 | 0.24 | 0.24 |
| MIP-1β, pg/mL | 12.4 ± 59.3 | 18.0 ± 102.8 | 21.5 ± 73.4 | 50.4 ± 233.4 | 0.21 | 0.21 | 0.21 | 0.21 |
| MCP-1,* pg/mL | 1172.2 ± 1208.2 | 754.0 ± 594.0 | 1159.7 ± 961.0 | 870.2 ± 564.9 | 0.33 | 0.38 | 0.33 | 0.33 |
| Growth factor | ||||||||
| VEGF,* pg/mL | 278.8 ± 399.9 | 221.3 ± 297.5 | 391.7 ± 1242.8 | 271.4 ± 868.6 | 0.20 | 0.19 | 0.20 | 0.20 |
| Laboratory | ||||||||
| 25(OH)D,*,** ng/mL | 21.1 ± 10.1a | 44.6 ± 14.7b | 20.2 ± 8.1a | 19.8 ± 10.5a | <0.001 | <0.001 | <0.001 | <0.001 |
| C-reactive protein,6* mg/L | 77.5 ± 70.5 | 21.8 ± 41.6 | 82.9 ± 72.6 | 20.5 ± 35.3 | 0.47 | 0.34 | 0.47 | 0.47 |
| Leukocyte count,6* ×103/mm3 | 8.4 ± 4.2 | 9.3 ± 3.9 | 8.9 ± 3.6 | 9.4 ± 3.9 | 0.30 | 0.26 | 0.30 | 0.31 |
Values are mean ± SD. Data were analyzed by generalized estimating equations with normal distribution and an identity link function with a first-order autoregressive correlation matrix. SI conversion factors: to convert 25(OH)D to nmol/L, multiply values by 2.496. GM-CSF, granulocyte-macrophage colony-stimulating factor; IP-10, IFN-inducible protein-10; MCP-1, monocyte chemoattractant protein-1; MIP-1β, macrophage inflammatory protein-1β; Post, postintervention; VEGF, vascular endothelial growth factor; 25(OH)D, 25-hydroxyvitamin D.
P value represents group-by-time interaction adjusted by cumulative glucocorticoid doses (39.7 mg).
P value represents group-by-time interaction adjusted by cumulative glucocorticoid doses and time from symptom onset to enrollment.
P value represents group-by-time interaction adjusted by cumulative glucocorticoid doses and baseline 25(OH)D concentrations.
P value represents group-by-time interaction adjusted by cumulative glucocorticoid doses and hospitals from which patients were recruited.
Data were missing for 1.0% (n = 2) of the patients in the vitamin D3 group at post.
*P < 0.05 for main effect of time; **P < 0.05 for main effect of group. Values without a common superscript letter significantly differ (P < 0.05).
Discussion
In this post hoc, ancillary, and exploratory analysis from a multicenter, double-blind, placebo-controlled, randomized clinical trial, a single high dose of vitamin D3 did not significantly change systemic inflammatory cytokines, chemokines, and growth factor, compared with placebo, among hospitalized patients with moderate to severe COVID-19. To our knowledge, this is the first randomized clinical trial to report the effects of a single high dose of vitamin D3 on cytokine-related inflammation in this population.
To gather knowledge on the effects of a single high dose of vitamin D3 on systemic inflammation, we broadly assessed serum concentrations of cytokines, chemokines, and growth factor. However, the current trial demonstrated that administration of a single dose of 200,000 IU vitamin D3 did not result in any effect on these inflammatory markers, except for an interaction effect on GM-CSF.
GM-CSF is an immunoregulatory cytokine with a pivotal role in inflammation, and its overexpression may be associated with the cytokine storm in severe COVID-19 (13). GM-CSF-blockade therapies have been proposed to mitigate hyperimmunoinflammation in severe COVID-19 (23). The present findings suggest that vitamin D could have a slight effect on GM-CSF in COVID-19 patients, although no significant differences between vitamin D3 and placebo were observed.
Severe inflammatory states of COVID-19 are associated with GM-CSF overexpression by autocrine response and a positive feedback loop (23). In this sense, the presumed therapeutic effect of vitamin D could modulate an adequate innate immune response while decreasing the GM-CSF upregulation, although, to date, this has not been reported. This study found reduced concentrations of GM-CSF from baseline to post in the vitamin D3 group compared with increased concentrations in the placebo group, although this does not rule out the possibility that glucocorticoid use influences the finding of no significant difference in the multiple comparison test (24).
Regarding timing, the recent study of Liu et al. (25) suggests that the most critical patients with COVID-19 demonstrated a late immune response with mild and delayed performance of the immediate first line of defense to suppress viral replication/spread, and a peak in proinflammatory cytokines. In these more critical patients, an inflammatory peak that characterizes a second wave in the cytokine storm is expected by days 17–23 from symptom onset.
In our study, the median time of 18 d from onset of symptoms to hospital discharge suggests that cytokine assessments were clinically timely to detect changes in the target outcomes if they had occurred. Notably, the relatively long time from symptom onset to vitamin D3 administration (i.e., median of 10 d), in addition to the time required for the active form of vitamin D to act on the expected immune function, may have blunted the effect of vitamin D3 on clinical and biochemical outcomes.
Glucocorticoids, such as dexamethasone, have been adopted to attenuate COVID-19-related inflammatory injury (26) and reduce mortality (27). In the present findings, 65.0% of patients (67 in the vitamin D3 and 63 in the placebo group) received glucocorticoids with a mean dexamethasone cumulative dose of 39.7 mg, leading to the assumption of a possible mitigating effect on acute-phase reactants (28) such as cytokines, chemokines, and growth factor that overlaps with the purported immunomodulatory effect of high-dose vitamin D3 in the presence (29, 30) or absence (31, 32) of acute inflammation. However, we observed that concentrations of C-reactive protein, an important systemic inflammatory marker, remained elevated after a mean of 7 d of glucocorticoid treatment, suggesting that vitamin D3 supplementation could play a role as an additional therapeutic agent in this disease (33, 34, 35).
It is important to note that circulating concentration of 25(OH)D is the best clinical indicator of vitamin D nutritional status, and its greater amount comes from dermal production in response to UV-B sunlight exposure, whereas the least part comes from diet (36). Regarding diagnosis, there is a lack of international consensus on the definition of vitamin D deficiency and sufficiency (37), which hinders the classification of sufficiency status as >20 ng/mL (37, 38) or >30 ng/mL (39). The European Calcified Tissue Society position statement estimates that vitamin D deficiency [serum 25(OH)D <20 ng/mL] occurs in ≤20% of the population in Northern Europe, between 30% and 60% in Western, Southern, and Eastern Europe, and ≤80% in Middle Eastern countries (37), a difference proportional to the seasonality of exposure to sunlight in these regions (40). Because the best response to sun exposure is elevation of vitamin D status and patients hospitalized with COVID-19 are deprived of it, a higher prevalence of vitamin D deficiency (<50 nmol/L or <20 ng/mL) is observed while increasing the chance of hospitalization and death in patients with COVID-19 (41).
Aside from experimental design, the strengths of this trial include the enrollment of hospitalized patients with moderate to severe COVID-19; and the adequate timing of collection of diverse acute-phase reactants and cell-signaling molecules regarding the peak of immune-cell signaling and hyperinflammation. This study also has limitations. First, patients showed heterogeneous medication regimens for pre-existing diseases which may have contributed to the results. Second, the study may have had inadequate power to detect small between-group differences, particularly considering the known variability inherent to the reactants. This study does not rule out the possibility that early vitamin D treatment could improve clinical status and cytokine-related inflammation in patients with less severe COVID-19, so further randomized clinical trials that consider this perspective would be critical.
In summary, a single high dose of 200,000 IU vitamin D3 compared with placebo did not elicit meaningful changes in systemic inflammatory cytokines, chemokines, and growth factor among hospitalized patients with COVID-19 who already had sufficient vitamin D. The findings do not support the use of high-dose vitamin D3 in the modulation of cytokine-related inflammation of moderate to severe COVID-19 in cases of advanced onset of symptoms.
Acknowledgments
We are thankful to Monica Pinheiro and Roberta Costa (Ibirapuera Field Hospital) for assistance with the study; Cleuber Esteves Chaves (pharmacy unit of the Clinical Hospital) for the vitamin D3 and placebo solution preparation; Rogério Ruscitto do Prado (Albert Einstein Hospital) for conducting statistical analyses; Caroline C dos Santos (Rheumatology Division, School of Medicine of University of São Paulo) and Caroline S Faria (Clinical Hospital of the School of Medicine of University of São Paulo) for technical support; and all of the staff members from both centers. None of these individuals received compensation for their participation.
The authors’ responsibilities were as follows—RMRP: had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; ALF, IHM, AJP, KFG, BG, and RMRP: conceived and designed the study; ALF, IHM, BZR, BG, and RMRP: drafted the manuscript; ALF, IHM, BZR, AJP, BG, and RMRP: performed statistical analysis; BG and RMRP: obtained funding and provided supervision; LPS, MDS, LA, and VFC: provided administrative, technical, or material support; and all authors performed data acquisition, analysis, interpretation and critically revised the manuscript for important intellectual content. All authors read and approve the manuscript as submitted.
Data Availability
Deidentified participant data of this study must be requested from the corresponding author (rosamariarp@yahoo.com) upon publication. The codebook of this study will be made available upon request by qualified clinical researchers for specified purposes dependent on the nature of the request and the intended use of the data, with investigator support. The request must include a statistician. The authors report no conflicts of interest.
Footnotes
Supported Supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grants 2020/11102-2 (to ALF), 2019/24782-4 (to IHM), 2019/18039-7 (to KFG), and 2020/05752-4 (to RMRP), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant 305556/2017-7 (to RMRP). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ALF and IHM contributed equally to this work.
References
- 1.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 2.Aglipay M, Birken CS, Parkin PC, Loeb MB, Thorpe K, Chen Y, Laupacis A, Mamdani M, Macarthur C, Hoch JS, et al. Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA. 2017;318(3):245–254. doi: 10.1001/jama.2017.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8(10):1523–1525. doi: 10.4161/auto.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97(1–2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Laplana M, Royo JL, Fibla J. Vitamin D Receptor polymorphisms and risk of enveloped virus infection: a meta-analysis. Gene. 2018;678:384–394. doi: 10.1016/j.gene.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Boulkrane MS, Ilina V, Melchakov R, Fedotova J, Drago F, Gozzo L, Das UN, Abd El-Aty AM, Baranenko D. COVID-19 disease and vitamin D: a mini-review. Front Pharmacol. 2020;11:604579. doi: 10.3389/fphar.2020.604579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, Madhavan MV, Nair N, Babalyan V, Hutchings N, et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol. 2020;183(5):R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kloc M, Ghobrial RM, Lipińska-Opałka A, Wawrzyniak A, Zdanowski R, Kalicki B, Kubiak JZ. Effects of vitamin D on macrophages and myeloid-derived suppressor cells (MDSCs) hyperinflammatory response in the lungs of COVID-19 patients. Cell Immunol. 2021;360:104259. doi: 10.1016/j.cellimm.2020.104259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8(7):570. doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martineau AR, Forouhi NG. Vitamin D for COVID-19: a case to answer? Lancet Diabetes Endocrinol. 2020;8(9):735–736. doi: 10.1016/S2213-8587(20)30268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z, Gao G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalia V, Studzinski GP, Sarkar S. Role of vitamin D in regulating COVID-19 severity—an immunological perspective. J Leukocyte Biol. 2021;110(4):809–819. doi: 10.1002/JLB.4COVR1020-698R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorino S, Gallo C, Zippi M, Sabbatani S, Manfredi R, Moretti R, Fogacci E, Maggioli C, Travasoni Loffredo F, Giampieri E, et al. Cytokine storm in aged people with CoV-2: possible role of vitamins as therapy or preventive strategy. Aging Clin Exp Res. 2020;32(10):2115–2131. doi: 10.1007/s40520-020-01669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farid N, Rola N, Koch EAT, Nakhoul N. Active vitamin D supplementation and COVID-19 infections: review. Ir J Med Sci. 2021;190(4):1271–1274. doi: 10.1007/s11845-020-02452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, Silva CBR, Franco AS, Macedo MB, Dalmolin HHH, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. 2014;20(4):341–351. doi: 10.4158/EP13265.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta P, Porter JC, Manson JJ, Isaacs JD, Openshaw PJM, McInnes IB, Summers C, Chambers RC. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respir Med. 2020;8(8):822–830. doi: 10.1016/S2213-2600(20)30267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwingshackl A, Kimura D, Rovnaghi CR, Saravia JS, Cormier SA, Teng B, West AN, Meduri UG, Anand KJ. Regulation of inflammatory biomarkers by intravenous methylprednisolone in pediatric ARDS patients: results from a double-blind, placebo-controlled randomized pilot trial. Cytokine. 2016;77:63–71. doi: 10.1016/j.cyto.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Martins AJ, Lau WW, Rachmaninoff N, Chen J, Imberti L, Mostaghimi D, Fink DL, Burbelo PD, Dobbs K, et al. Time-resolved systems immunology reveals a late juncture linked to fatal COVID-19. Cell. 2021;184(7):1836–1857. doi: 10.1016/j.cell.2021.02.018. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020;80(13):1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grossmann RE, Zughaier SM, Liu S, Lyles RH, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. 2012;66(9):1072–1074. doi: 10.1038/ejcn.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Heart Lung and Blood Institute PETAL Clinical Trials Network. Ginde AA, Brower RG, Caterino JM, Finck L, Banner-Goodspeed VM, Grissom CK, Hayden D, Hough CL, Hyzy RC, et al. N Engl J Med. 2019;381(26):2529–2540. doi: 10.1056/NEJMoa1911124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha-Hikim I, Duran P, Shen R, Lee M, Friedman TC, Davidson MB. Effect of long term vitamin D supplementation on biomarkers of inflammation in Latino and African-American subjects with pre-diabetes and hypovitaminosis D. Horm Metab Res. 2015;47(4):280–283. doi: 10.1055/s-0034-1383652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Hajj C, Walrand S, Helou M, Yammine K. Effect of vitamin D supplementation on inflammatory markers in non-obese Lebanese patients with type 2 diabetes: a randomized controlled trial. Nutrients. 2020;12(7):2033. doi: 10.3390/nu12072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Bayyari N, Hailat R, Subih H, Alkhalidy H, Eaton A. Vitamin D3 reduces risk of cardiovascular and liver diseases by lowering homocysteine levels: double-blinded, randomised, placebo-controlled trial. Br J Nutr. 2021;125(2):139–146. doi: 10.1017/S0007114520001890. [DOI] [PubMed] [Google Scholar]
- 34.de Medeiros Cavalcante IG, Silva AS, Costa MJ, Persuhn DC, Issa CT, de Luna Freire TL, da Conceição Rodrigues Gonçalves M. Effect of vitamin D3 supplementation and influence of BsmI polymorphism of the VDR gene of the inflammatory profile and oxidative stress in elderly women with vitamin D insufficiency: vitamin D3 megadose reduces inflammatory markers. Exp Gerontol. 2015;66:10–16. doi: 10.1016/j.exger.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Tabatabaeizadeh SA, Avan A, Bahrami A, Khodashenas E, Esmaeili H, Ferns GA, Abdizadeh MF, Ghayour-Mobarhan M. High dose supplementation of vitamin D affects measures of systemic inflammation: reductions in high sensitivity C-reactive protein level and neutrophil to lymphocyte ratio (NLR) distribution. J Cell Biochem. 2017;118(12):4317–4322. doi: 10.1002/jcb.26084. [DOI] [PubMed] [Google Scholar]
- 36.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr. 2009;89(6):1997S–2008S. doi: 10.3945/ajcn.2009.27230D. [DOI] [PubMed] [Google Scholar]
- 37.Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 38.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for calcium and vitamin D. National Academies Press (US); Washington (DC): 2011. [PubMed] [Google Scholar]
- 39.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 40.Baggerly CA, Cuomo RE, French CB, Garland CF, Gorham ED, Grant WB, Heaney RP, Holick MF, Hollis BW, McDonnell SL, et al. Sunlight and vitamin D: necessary for public health. J Am Coll Nutr. 2015;34(4):359–365. doi: 10.1080/07315724.2015.1039866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020 doi: 10.1080/10408398.2020.1841090. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data of this study must be requested from the corresponding author (rosamariarp@yahoo.com) upon publication. The codebook of this study will be made available upon request by qualified clinical researchers for specified purposes dependent on the nature of the request and the intended use of the data, with investigator support. The request must include a statistician. The authors report no conflicts of interest.