Abstract
Although mRNA vaccines against SARS-CoV-2 were highly efficacious against severe illness and hospitalization, they seem to be less effective in preventing infection months after vaccination, especially with the Delta variant. Breakthrough infections might be due to higher infectivity of the variants, relaxed protective measures by the general public in “COVID-19 fatigue”, and/or waning immunity post-vaccination. Determining the neutralizing antibody levels in a longitudinal manner may address this issue, but technical complexity of classic assays precludes easy detection and quick answers. We developed a lateral flow immunoassay NeutraXpress™ (commercial name of the test kit by Antagen Diagnostics, Inc.) and tested fingertip blood samples of subjects receiving either Moderna or Pfizer vaccines at various time points. With this device, we confirmed the reported clinical findings that mRNA vaccine-induced neutralizing antibodies quickly wane after 3–6 months. Thus, using rapid tests to monitor neutralizing antibody status could help identify individuals at risk, prevent breakthrough infections, and guide social behavior to curtail the spread of COVID-19.
Keywords: SARS-CoV-2, mRNA vaccine, neutralizing antibody, LFIA, rapid test
Statement of Significance
Mounting evidence suggests that mRNA vaccine-induced neutralizing antibody titres against SARS-CoV-2 wane in 3–6 months. Quick identification of fully vaccinated persons with high risk of breakthrough infections is key to control the COVID-19 pandemic. The described LFIA device having a control/sample dual-lane design serves this purpose with successful field-test data.
INTRODUCTION
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the etiological agent that caused the COVID-19 pandemic has infected over 250 million people and claimed the lives of more than 5 million worldwide. Starting from December 2020 in the UK and followed by other parts of the world in early 2021, the vaccination campaign, together with social measures, has led to a sharp curtailing of the outbreak. However, the arrival of the Delta variant (B.1.617.2), coincident with the waning immunity post-vaccination contributed to the surges of viral transmission in many places. Breakthrough infections are not uncommon in fully vaccinated subjects and can sometimes be severe. For example, as of October 21, 2021, 35% of the 519 patients hospitalized with COVID-19 in Massachusetts had been fully vaccinated [1]. In another study by Yale University, among the fully vaccinated breakthrough patients admitted to the New Heaven Hospital between March 23 and July 1, 2021, 20% had moderate disease and 26% had severe or critical illness [2]. Furthermore, fully vaccinated individuals with breakthrough infections can have peak viral loads similar to unvaccinated subjects and are able to efficiently transmit infection in household settings, including to fully vaccinated contacts [3]. Even though the viral loads in breakthrough vaccinated individuals may be as high as in unvaccinated individuals, the former clear the virus faster and remain contagious for shorter periods than the latter [4]. Clearly, even in breakthrough infections, vaccination shortens the time window of high transmission potential, minimizes symptom severity and duration, and may restrict tissue dissemination of the virus [5].
Protective immunity induced by natural infection or vaccination includes both humoral immunity and cellular immunity, the latter of which involves cytotoxic lymphocytes to clear virus-infected host cells. Although status of neutralizing antibodies (NAbs) as part of the humoral response does not reflect the overall immunity against the virus, it does correlate with the susceptibility of the host to the initial infection, especially when the current muscle-injected vaccines fall short of inducing strong mucosal NAb response. Thus, from an epidemiology and public health point of view, it is absolutely necessary to monitor the humoral protection by vaccines and evaluate breakthrough infections in the context of waning NAb titres vs. higher infectivity of the variants.
Many studies have reported that vaccine-induced antibody response against SARS-CoV-2 quickly wanes and is less effective in preventing infection after 6 months [6–10]. For example, in a study with 620,000 U.S. veterans, vaccine protection declined significantly over time. In March 2021, protection against infection was: 88% for J&J/Janssen; 91% for Pfizer-BioNTech; and 92% for Moderna. Five months later, protection against infection declined to: 3% for J&J/Janssen; 50% for Pfizer-BioNTech; and 64% for Moderna [8]. In another study, Israeli researchers found that NAb levels among 4,800 health care workers who had received two doses of Pfizer vaccine fell rapidly in the first 3 months, especially among men, among individuals 65 years of age or older, and among individuals with weak immune systems [10].
It should be noted that the initial vaccine efficacies were calculated from clinical trials conducted with healthy volunteers without underlying medical conditions. In the real world, however, some individuals might not respond to vaccinations. This includes cancer patients receiving chemotherapy, organ transplant recipients, and autoimmune patients taking certain immunosuppressant medications. There are six million such people in the U.S. alone. Therefore, when it is said that a vaccine is 95% protective, one has to consider its dynamic effectiveness, weaving in factors such as time-since-vaccination [6, 8, 9, 11], subjects’ immune status [12], and neutralization-resistant variants that escape from vaccine-elicited immunity [13–15]. Even under the best scenario, efforts are needed to identify the 5% unprotected vaccine recipients and provide them with special instruction and care. Five percent in the U.S. alone means 16 million people. With the possibility that this pandemic will transition into a potential recurrent seasonal disease [16], it is crucial to monitor the waning antibody response over time, to be able to address when and to whom to give a booster or another extra dose of COVID-19 vaccine.
SARS-CoV-2 virus invades host cells via interaction of the Spike (S) protein with its cognate receptor ACE-2 protein on host cell surface. Antibodies (Abs) against SARS-CoV-2 can be generally divided into two main categories, NAbs and non-neutralizing virus binding antibodies (BAbs). Piccoli et al. tested Abs against different SARS-CoV-2 proteins and different domains of S protein from 647 SARS-CoV-2-infected subjects and found that SARS-CoV-2 receptor binding domain (RBD)-specific Abs dominated IgG responses, whereas much lower titers were observed to the S2 subunit, and the majority of the neutralizing activity (90%) against SARS-CoV-2 is mediated by RBD-specific Abs blocking virus binding to ACE2 [17]. The most classic method to measure NAbs is the plaque reduction neutralization test (PRNT) that is considered the gold standard for NAb measurement [18, 19]. However, the assay takes several days for the virus plaques to form and to be counted. Engineered SARS-CoV-2 virus with a fluorescence reporter has been used to avoid manual counting and to improve assay throughput [20]. But this assay is quite cumbersome and requires Biosafety Level-3 lab setting to work with live and infectious SARS-CoV-2 virus. Several virus neutralization tests using pseudovirus with GFP or luciferase reporters have been developed [21–23] and can be performed at Biosafety Level-1 or -2 facility. Still, there are several common issues with virus neutralization tests regardless of adopting a pseudovirus or not. In these tests, the neutralization ability of the Abs is highly dependent on the maturation state or titer of the virus as well as the cell type and cell condition used in the assay [24]. Poor reproducibility or even false results can be generated if the virus and host cells are not at optimal assay conditions [24]. Simpler and faster ELISA-based competition assays that detect SARS-CoV-2 NAbs blocking ACE2:RBD interaction have been developed, with two from GenScript and InBios approved by the FDA for Emergency Use Authorization as of November 2021. Nevertheless, these ELISA tests still need a few hours to complete in a Biosafety Level-1 or -2 environment and are not suitable for individual at-home use.
Lateral flow immunoassay (LFIA) is the most convenient and fastest test and typically takes only 15–20 min to complete [25]. It can be performed either in a professional laboratory or as a rapid point-of-care test (POCT) used by an individual at home. So far, no LFIA-based rapid test for detecting SARS-CoV-2 NAbs has been approved by the FDA, but several products are already in late development stage. Herein, we describe an LFIA, NeutraXpress™ (www.antagendiagnostics.com), for monitoring the longitudinal changes of SARS-CoV-2 NAb levels among the same individuals using fingertip blood. Our results indicate that even in fully vaccinated subjects, mRNA vaccine-induced NAb levels quickly wane after 3–6 months. Thus, a highly portable, semi-quantitative POCT for monitoring SARS-CoV-2 NAb status could fill in the gap of post-vaccination management and could be useful in identifying high-risk individuals to prevent breakthrough infections.
MATERIALS AND METHODS
Human subjects
All the subjects are fully vaccinated U.S. residents having received two doses of mRNA vaccines from either Moderna or Pfizer and participated in this study with their full consent on the disclosed purpose and data usage. None of the subjects had a prior PCR-confirmed SARS-CoV-2 infection, nor was on active immunosuppressant medication. Subject demographics in terms of gender, age, and vaccine brand are listed in Table 1.
Table 1.
Subject demographics on gender, age, and vaccine brand
| Age | 20–30 yr | 31–40 yr | 41–50 yr | 51–60 yr | 61–70 yr | >70 yr | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 7 | 5 | 6 | 10 | 5 | 7 | 40 | |||||||
| 2a | 5b | 3a | 2b | 2a | 4b | 3a | 7b | 4a | 1b | 4a | 3b | 18a | 22b | |
| Female | 1 | 6 | 9 | 10 | 3 | 7 | 36 | |||||||
| 0a | 1b | 1a | 5b | 2a | 7b | 3a | 7b | 1a | 2b | 4a | 3b | 11a | 25b | |
aModerna mRNA-1273; bPfizer-BioNTech BNT162b2.
Lateral flow NeutraXpress™ kit and NAb assay
The detailed procedure for constructing the NeutraXpress™ test cassette is provided in the Supplementary Methods. In brief, NeutraXpress™ is a double lane cassette (Supplementary Fig. 1). Both nitrocellulose membranes are equally striped with two test lines, T1 and T2, coated with His-tagged human ACE2 at T1 and a mixture of monoclonal mouse anti-human IgM and IgG Abs at T2, respectively. The control C line is coated with polyclonal goat anti-chicken IgY Abs (Fig. 1A). Upon adding the specimen onto the sample pad, the analyte migrates through the nitrocellulose membrane. The conjugate pad is impregnated with colloidal gold-labeled recombinant SARS-CoV-2 antigen (RBD) and colloidal gold-labeled chicken IgY as a tracer. When performing the LFIA NAb assay, the cassettes were placed on a level surface. Only fresh fingertip blood was used, per instructions of the NeutraXpress™ kit. The subjects were advised to drip one drop of fingertip blood and one drop of diluent into the right sample well, wait for 1 min, and then hold the diluent vial vertically and add two to three drops of diluent into the right and the left sample wells, respectively (see Supplementary Fig. 1 for Quick Reference Guide of the kit). Smartphone images were taken 30 min after the start of the assay. One should always compare the intensities of T1 or T2 lines across the lane between the specimen and the diluent in the same cassette. For inhibition of ACE2:RBD interaction by NAbs present in the blood samples, inhibition % = (1-T1sample/T1diluent) × 100% (for instructions for use and troubleshooting, visit www.antagendiagnostics.com or see Supplementary Materials).
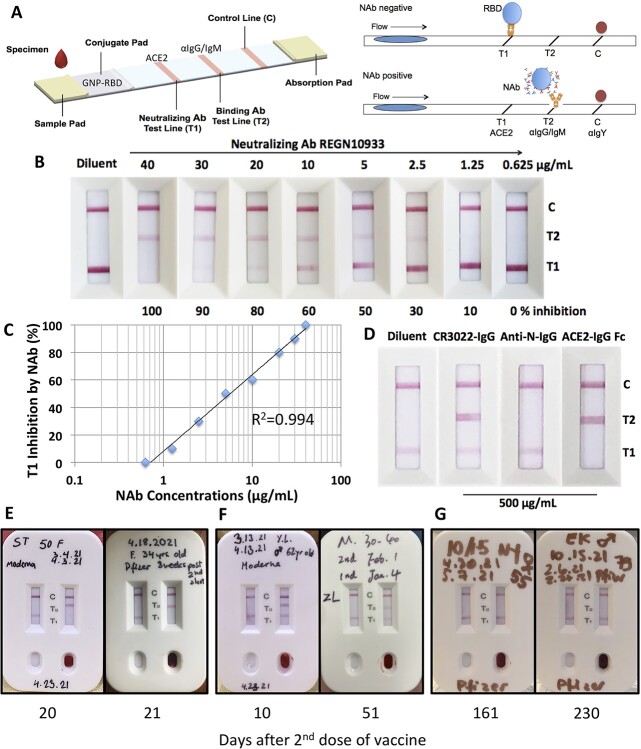
Figure 1.
Validation of NeutraXpress™ for detecting SARS-CoV-2 NAbs in vaccinated subjects. (A) Illustration of cassette design. T1 is striped with recombinant His-tagged human ACE2 protein. T2 is striped with anti-human IgM + IgG Abs. The conjugate pad is impregnated with colloidal GNPs-labeled recombinant RBD from Spike protein of SARS-CoV-2, as well as GNP-labeled chicken IgY used as a tracer to indicate the completion of the lateral flow when it is captured by goat anti-chicken antibody at the C line. If there is no NAb in the specimen, GNP-RBD is captured by ACE2 at T1 line and T2 line should not appear. If the specimen contains NAbs, the interaction between GNP-RBD with ACE2 at T1 line is blocked, T1 disappears or shows reduced intensity, in comparison with T1 from the control well with added diluent only. The appearance of T2 indicates the presence of IgM and/or IgG Abs specific for RBD, i.e., T2 shows the totality of both neutralizing and non-neutralizing RBD-binding IgM and IgG Abs. The stronger T2 is, the higher titers for RBD-binding IgM + IgG Abs, but T2 does not provide information on NAb. (B) Sensitivity of NeutraXpress™. About 15 μL of serially diluted CHO cell expressed recombinant NAb REGN10933-hIgG1 in normal human serum (MilliporeSigma, Cat. S1-100ML) was added to the wells of NeutraXpress™ per kit instructions. (C). The % inhibition of T1 signal in (B) was estimated based on diluent only control and plotted against the concentrations of REGN10933 in serum. (D) Specificity of NeutraXpress™. RBD-binding but non-neutralizing CR3022-hIgG1, and a nonrelevant anti-nucleocapsid (N)-hIgG1, as well as recombinant ACE2-hIgG1 Fc were added to the sample wells at 500 μg/mL in PBS. (E) Fingertip blood samples from subjects at peak time (about 3 weeks) post 2nd dose mRNA vaccines showed complete disappearance of T1 and strong appearance of T2 on NeutraXpress™. (F) Samples before or after the peak time showed no difference in T1 lines but strong signals of T2 lines, indicating little NAb activity and that not all the RBD BAbs are neutralizing. (G) Samples after 5–7 months no longer showed any presence of RBD BAbs (positive T2), nor any NAb activity (negative T1). De-identified information on subject gender and age, vaccine brands, vaccination dates, and testing date was recorded on NeutraXpress™ cassettes.
Chinese hamster ovary cell expression of recombinant Abs and Fc fusion proteins
For testing the sensitivity and specificity of NeutraXpress™, we used Chinese hamster ovary (CHO) cells to express positive and negative control reagents, with detailed procedures described in the Supplementary Methods. In brief, we expressed a neutralizing human IgG1 antibody REGN10933 as a positive standard for analytical control, and an RBD-binding but non-neutralizing human IgG1 antibody CR3022 as a negative control. The anti-SARS nucleocapsid (N) human IgG1 antibody (TJ21) was expressed as a nonrelevant control antibody. Finally, we expressed by CHO cells the hACE2-hIgG1 Fc fusion protein as a specificity control.
RESULTS AND DISCUSSION
In response to the unmet needs to monitor the levels of NAbs after infection or vaccination, the FDA issued on March 17, 2021 a guideline for “Developers of Serology Tests that Detect or Correlate to Neutralizing Antibodies”, in which it stipulates that the neutralization comparator method should be a test that directly measures NAbs against the live real SARS-CoV-2 virus. In other words, the neutralization comparator assay has to be PRNT or the functionally equivalent microneutralization assay or Focus Reduction Neutralization Testing, explicitly excluding ELISA or pseudovirus-based neutralization assay. In sharp contrast to the swift development of hundreds of anti-SARS-CoV-2 IgM/IgG rapid tests to assist the diagnosis of COVID-19 by mid-2020 since the pandemic, as of November 2021, not a single LFIA rapid test for detecting SARS-CoV-2 NAbs has been approved. Nevertheless, the general public as well as health professionals do want to know the status of NAbs in subjects previously infected by the virus or received full vaccination, especially in the wake of waning immunity, to guide individual or social behaviors. As a result of the lack of approved products as examples, some unscrupulous vendors claimed that their anti-SARS-CoV-2 IgM/IgG rapid tests can detect NAbs, causing unnecessary confusion to the already misinformed general public.
We emphasize here that a test that detects NAbs against SARS-CoV-2 must demonstrate the principle of blocking the interaction between ACE2 and Spike protein or its RBD domain (Fig. 1A). Hence, it has to be an inhibition assay. Unlike other popular single-lane designs, the NeutraXpress™ cassette has two identical lanes, one for the specimen and the other for the “diluent only” negative control. In other LFIA products for SARS-CoV-2 NAb detection, it is very difficult to judge how much reduction of the test line signal is real reduction, as there is no control provided. Some product instructions even erroneously direct the users to compare the intensity of T line with that of the irrelevant C line. Our design provides a real-time internal control and eliminates ambiguity when performing the test. The contrast in line patterns, i.e., disappearance of T1 and appearance of T2 (Fig. 1E), is very obvious and easy to interpret. Our test is also sensitive and specific. Using REGN10933, a recombinant human IgG1 NAb developed by Regeneron Pharmaceuticals, we showed that NeutraXpress™ can detect neutralization activity brought by > 2.5 μg/mL NAb in undiluted serum at T1 line (about 30% inhibition) and can also detect RBD-binding hIgG1 > 2.5 μg/mL in undiluted serum at T2 line (Fig. 1B). The strong correlation of logarithmic NAb concentrations in serum with the reduction of T1 line intensity (R2 = 0.994) suggests that NeutraXpress™ can be used to accurately monitor the changes of serum NAb levels over a wide range (Fig. 1C). The disappearance of T1 is specific for NAb, as an RBD-binding but non-neutralizing hIgG1 CR3022 as well as a nonrelevant anti-N-hIgG1 failed to dissipate T1 line even at a concentration as high as 500 μg/mL (Fig. 1D). The specificity of T1 line was also shown by the complete competition of interaction between membrane-striped ACE2 and gold nanoparticle (GNP)-labeled RBD by soluble ACE2-hIgG1 Fc (Fig. 1D). Moreover, the appearance of T2 line is also highly specific for RBD-binding moieties, shown by the signals with REGN10933-hIgG1, CR3022-hIgG1, and ACE2-hIgG1 Fc but no signal with anti-N-hIgG1 (Fig. 1B and D). As for the clinical performance, when 194 pre-historic serum samples (collected before December 2019 at Beth Israel Deaconess Medical Center/Harvard Medical School) were tested, no dissipation of T1 and no appearance of T2 were observed, indicating very high specificity. More testing of NeutraXpress™ with PCR-confirmed serum samples from COVID-19 patients at BIDMC and serum samples from New York State Department of Health with live SARS-CoV-2 neutralization PRNT numbers is currently underway to establish the false-negative and false-positive rates for this rapid test (manuscript in preparation).
With this device, we tested fingertip blood samples from 76 healthy subjects at various time points after receiving two doses of mRNA vaccines from either Moderna or Pfizer, to generate a longitudinal snapshot of a well-represented population (Table 1). We also followed certain individuals with > 60 years of age for kinetics study, as this population may have a faster turnover of their NAbs. Throughout the study, the reproducibility and repeatability of the LFIA test were very well. In general, we observed strong dissipation of T1 line and appearance of T2 line around 3 weeks after the 2nd doses for both brands of mRNA vaccines (Fig. 1E), suggesting that the mRNA vaccines are highly effective in inducing NAbs in the circulation. If tested only 7–10 days after the 2nd dose, the body may not have developed high enough NAb titers to dissipate the T1 line, although T2 line could be strongly positive, indicative of the induction of RBD BAbs (Fig. 1F).
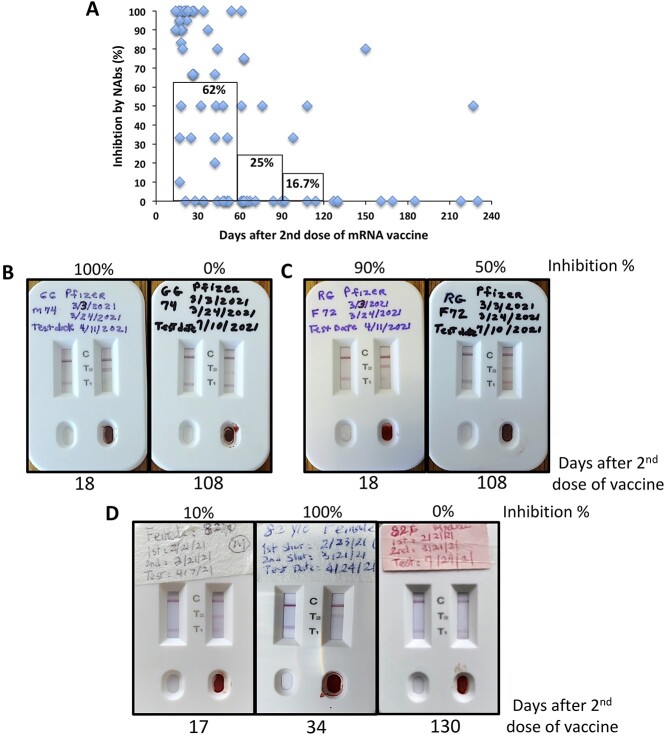
Throughout this project, we were quite surprised that mRNA vaccine-induced NAb activities quickly waned after 3–4 months post the 2nd vaccination (Fig. 2A). That is, when tested between 15 and 60 days post the 2nd doses, the group of subjects had on average 62% inhibition of ACE2:RBD interaction by NAbs present in their blood, whereas this inhibition reduced to 25% when tested between 2 and 3 months, and further down to 16.7% when tested between 3 and 4 months (Fig. 2A). In fact, most subjects did not even show T2 lines after 4–6 months (Figs 1G and 2D and Supplementary Fig. 2). Thus, our NeutraXpress™ rapid test confirms the clinical findings that mRNA vaccine-induced antibody responses against SARS-CoV-2 infection quickly waned after 3–6 months [6, 8, 9, 11], which formed the basis for the recent government campaign for booster shots.
Figure 2.
Longitudinal monitoring of SARS-CoV-2 NAbs post mRNA vaccination using NeutraXpress™. (A) Subjects fully vaccinated with mRNA vaccines from Moderna or Pfizer were asked with consent to provide fingertip blood samples to test on NeutraXpress™. Photos were taken at 30 min after the start of the test, and intensities of T1 lines between sample and diluent lanes were compared and estimated to calculate the inhibition of ACE2-RBD interaction by NAbs present in the blood samples as follows: inhibition % = (1-T1sample/T1diluent) × 100%. The inhibition numbers were plotted against the days post 2nd vaccination. The bars indicate mean inhibition % of samples collected at 15–60 days, 61–90 days, and 91–120 days post 2nd vaccination, respectively. (B–D) Longitudinal images of SARS-CoV-2 NAb status of the same individuals, respectively, indicative of kinetic changes of NAb levels over time. De-identified information on subject gender and age, vaccine brands, vaccination dates, and testing date was recorded on NeutraXpress™ cassettes.
It should be noted that after antigen stimulation, there is a natural contraction phase for Ag-specific B cells and the majority of these B cells undergo apoptosis with only a small fraction of them develop into memory B cells. If those primed B/plasma cells did not die and maintained their secretion of Abs at peak levels, all the Abs from the past antigen exposure would have made our blood too viscous to flow. But still, such quick waning of mRNA vaccine-induced NAb titers is somewhat unpredicted. It might be due to: (1) the mRNA vaccination schemes may not be optimal, i.e., the 2nd dose is given 3–4 weeks after the 1st dose. This may not be ideal when compared with spacing doses more apart, like the practice in the UK and (2) perhaps for coronavirus, three doses of vaccine should be considered full vaccination.
Whether previously infected or vaccinated, it is informative for individuals to learn if they generated high levels of NAbs and how long the NAbs can last so that they can resume normal activities without fear of reinfection and transmitting the virus. However, as the pandemic unfolded, such a concept of “immunity passport” based on having Abs did not pan out. As we showed here (Fig. 1F), having S/RBD BAbs (positive T2 line) does not necessarily correlate with NAb activities (negative T1 line). The efforts in this field to extrapolate NAb activities (regardless of demonstration by classical assays or POCT) to the protection against infection were also not successful, partly due to the difficulty of establishing such a correlation (i.e., how much NAb is good enough), but more because of the advent of the variants and the rapid waning of NAb activities as we showed here. Understandably, the regulatory agency is reluctant to give a green light for such a proposition, for fear of premature laying down guard by the public already in COVID-19 fatigue. Then, what is the application of a POCT for SARS-CoV-2 NAb? We advocate the following:
For the general public at individual level, one can use NeutraXpress™ or alike to test his/her NAb status at “prime time” post-vaccination, i.e., 3–4 weeks after the 2nd dose or 1–2 weeks after the booster shot. Then, one could monitor his/her NAb status bimonthly afterwards, to get a longitudinal sense of how long the NAbs last in his/her own body. When it is said that a vaccine is 95% protective, people normally assume they belong to that 95%, not the 5%. In fact, it is just an assumption unless they test themselves at the individual level, especially for subjects with weak immune systems taking immunosuppressive medications and/or with old ages. In addition, since the majority of SARS-CoV-2-induced Abs in COVID-19 patients with obesity are autoimmune and not neutralizing [26], it is possible that vaccinated obese people may not have a good NAb response even at their “prime time” post-vaccination. In the U.S. and many western countries, there is a large population of obese people, who usually have underlying medical conditions and are more susceptible to severe illness of COVID-19. Instead of comparing to some standards derived from studies at population level, such personalized testing at individual level can eliminate complex confounding factors and focus only on his/her kinetic changes of NAb levels. When a person sees his/her NAb level is down from the peak to undetectable (no difference between the two T1 lines from the sample and diluent lanes), this person should be on alert and take measures to prevent possible breakthrough infection, especially if community transmission of the Delta variant is high. Besides Delta, other variants-of-concern such as Omicron (B.1.1.529) from South Africa that has 32 mutations in the Spike protein, could potentially blunt NAb protection induced by vaccines. Active monitoring programs of NAb status to evaluate vaccine effectiveness are urgently needed, especially when Omicron may be at the doorsteps.
Of note, the current version of NeutraXpress™ uses RBD from the original L strain of SARS-CoV-2 to label with GNP. In response to the variants, our future improved version would use S/RBD proteins from the variants. More preferably, S trimer protein should be used to label with GNP, as there are NAbs that can bind outside the ACE2/RBD binding site. For example, a supersite within the N-terminal domain (NTD) recognized by multiple potent NAbs has been discovered [27]. Liu et al. reported that they did not isolate any RBD BAbs from one of the five COVID-19 patients with high NAb titers [28]. Seven of the 13 non-RBD binders from this patient were NAbs and two of them were potent NAbs targeting NTD [28]. Thus, our current format could generate false-negative result for this type of individuals. By using S trimer, we probably will not be able to increase the sensitivity to detect those non-RBD binding NAbs, as NAbs against NTD, such as 4A8 [29], do not directly block S/RBD:ACE2 interaction, and will not dissipate T1 line in our assay. Nevertheless, using S trimer for GNP labeling will increase the sensitivity of T2 line.
Undoubtedly, COVID-19 vaccines have been highly effective in preventing severe symptomatic disease and death [30, 31]. Even though protection against SARS-CoV-2 infection appeared to wane rapidly following its peak after the 2nd dose, protection against hospitalization and death persisted at a robust level for 6 months after the 2nd dose, thanks to the elicited cellular immunity [9]. Although fully vaccinated people are eager to resume their normal social activities, it is an urgent call for researchers to find out whether there is a strong correlation of breakthrough infections with low NAb levels detectable by a POCT like NeutraXpress™. In other words, it will be crucial to investigate whether individuals with low NAb levels have higher risks of breakthrough infections, or conversely, whether breakthrough infections happened at a time when individuals had low NAb levels. If that is the case, then wide monitoring of post-vaccination NAb status over time at individual levels, especially in high-risk populations, and advising them for continued anti-measures (e.g., masking and social distancing) can be an efficient strategy to minimize breakthrough transmission. With the society embracing for booster shots and the possible evolution of this pandemic into a seasonal disease, POCT for monitoring post-vaccination SARS-CoV-2 NAb status could play an important role in the control of SARS-CoV-2 infection.
FUNDING
This study was supported by Massachusetts Life Science Center’s Accelerating Coronavirus Testing Solutions (ACTS) grant awarded to Antagen Diagnostics, Inc.
AUTHOR CONTRIBUTIONS
Q.W. optimized conditions and produced LFIA tests. C.M. generated recombinant antibody and ACE2-Fc reagents. L.F., H.Z., J.G., E.L.B., and G.M. collected samples to test. J.M.L. did literature search and provided insights. W.Z., S.H., and W.G. designed the experiments, monitored and financially supported the study. W.G. wrote the manuscript. All authors critically reviewed the drafts of the manuscript and approved the final version.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
CONFLICTS OF INTEREST STATEMENT
C.M. and W.G. are employees of Antagen Diagnostics, Inc. that produces NeutraXpress™. Q.W., W.Z. and S.H. are employees of Shijiazhuang Hipro Biotechnology Co., Ltd., a contract manufacturer for Antagen Diagnostics, Inc., which owns the IP and trademark of NeutraXpress™ and is seeking its EUA approval by the FDA.
ANIMAL RESEARCH STATEMENT
No animal is used in the present study.
ETHICS AND CONSENT STATEMENT
Consent was obtained from all the participants in this study on the purpose and data usage. Ethics approval was received from the Institutional Review Board of Antagen Diagnostics, Inc.
Supplementary Material
REFERENCES
- 1. Klompas, M. Understanding breakthrough infections following mRNA SARS-CoV-2 vaccination. JAMA 2021; 326: 2018–20. 10.1001/jama.2021.19063. [DOI] [PubMed] [Google Scholar]
- 2. Juthani, PV, Gupta, A, Borges, KAet al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis 2021; 21: 1485–6. 10.1016/S1473-3099(21)00558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singanayagam, A, Hakki, S, Dunning, Jet al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 2021; 0. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00648-4/fulltext: 5 November 2021, last accessed. 10.1016/S1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chia, PY, Ong, SWX, Chiew, CJet al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. 2021:2021.07.28.21261295. 10.1101/2021.07.28.21261295v1 (5 November 2021, last accessed). . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ke, R, Martinez, PP, Smith, RLet al. Longitudinal analysis of SARS-CoV-2 vaccine breakthrough infections reveal limited infectious virus shedding and restricted tissue distribution. 2021:2021.08.30.21262701. 10.1101/2021.08.30.21262701v1 (5 November 2021, last accessed). . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldberg, Y, Mandel, M, Bar-On, YMet al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021; 385: e85. 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mizrahi, B, Lotan, R, Kalkstein, Net al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun 2021; 12: 6379. 10.1038/s41467-021-26672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohn, BA, Cirillo, PM, Murphy, CCet al. Breakthrough SARS-CoV-2 infections in 620,000 U.S. Veterans, February 1, 2021 to August 13, 2021. 2021:2021.10.13.21264966. 10.1101/2021.10.13.21264966v1(6 November 2021, last accessed). . [DOI] [Google Scholar]
- 9. Chemaitelly, H, Tang, P, Hasan, MRet al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021; 385: e83. 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levin, EG, Lustig, Y, Cohen, Cet al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021; 385: e84. 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fontán, MMR, Nieves, EG, Gerena, ICet al. Time-Varying Effectiveness of Three Covid-19 Vaccines in Puerto Rico. 2021:2021.10.17.21265101. 10.1101/2021.10.17.21265101v2(6 November 2021, last accessed). . [DOI] [Google Scholar]
- 12. Munro, C. Covid-19: 40% of patients with weakened immune system mount lower response to vaccines. BMJ 2021; 374: n2098. 10.1136/bmj.n2098. [DOI] [PubMed] [Google Scholar]
- 13. Chen, RE, Zhang, X, Case, JBet al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med 2021; 27: 717–26. 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang, B, Goh, YS, Fong, S-Wet al. Resistance of SARS-CoV-2 Delta variant to neutralization by BNT162b2-elicited antibodies in Asians. The Lancet Regional Health – Western Pacific 2021; 15. https://www.thelancet.com/journals/lanwpc/article/PIIS2666-6065(21)00185-1/fulltext: 6 November 2021, last accessed. 10.1016/j.lanwpc.2021.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wall, EC, Wu, M, Harvey, Ret al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. The Lancet 2021; 397: 2331–3. 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray, CJL, Piot, P. The potential future of the COVID-19 pandemic: will SARS-CoV-2 become a recurrent seasonal infection? JAMA 2021; 325: 1249–50. 10.1001/jama.2021.2828. [DOI] [PubMed] [Google Scholar]
- 17. Piccoli, L, Park, Y-J, Tortorici, MAet al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 2020; 183: 1024–1042.e21. 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas, SJ, Nisalak, A, Anderson, KBet al. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: how alterations in assay conditions impact performance. Am J Trop Med Hyg 2009; 81: 825–33. 10.4269/ajtmh.2009.08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ratnam, S, Gadag, V, West, Ret al. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J Clin Microbiol 1995; 33: 811–5. 10.1128/jcm.33.4.811-815.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muruato, AE, Fontes-Garfias, CR, Ren, Pet al. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun 2020; 11: 4059. 10.1038/s41467-020-17892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiong, H-L, Wu, Y-T, Cao, J-Let al. Robust neutralization assay based on SARS-CoV-2 S-protein-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressing BHK21 cells. Emerg Microbes Infect 2020; 9: 2105–13. 10.1080/22221751.2020.1815589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidt, F, Weisblum, Y, Muecksch, Fet al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med 2020; 217: e20201181. 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie, X, Muruato, AE, Zhang, Xet al. A nanoluciferase SARS-CoV-2 for rapid neutralization testing and screening of anti-infective drugs for COVID-19. Nat Commun 2020; 11: 5214. 10.1038/s41467-020-19055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mukherjee, S, Dowd, KA, Manhart, CJet al. Mechanism and significance of cell type-dependent neutralization of flaviviruses. J Virol 2014; 88: 7210–20. 10.1128/JVI.03690-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang, JJ, Zhang, N, Richardson, SAet al. Rapid lateral flow tests for the detection of SARS-CoV-2 neutralizing antibodies. Expert Rev Mol Diagn 2021; 21: 363–70. 10.1080/14737159.2021.1913123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frasca, D, Reidy, L, Romero, Met al. The majority of SARS-CoV-2-specific antibodies in COVID-19 patients with obesity are autoimmune and not neutralizing. Int J Obes 2021; 1–6. 10.1038/s41366-021-01016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCallum, M, Marco, AD, Lempp, Fet al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. bioRxiv. 2021:2021.01.14.426475. 10.1101/2021.01.14.426475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu, L, Wang, P, Nair, MSet al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020; 584: 450–6. 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 29. Chi, X, Yan, R, Zhang, Jet al. A neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science 2020; 369: 650–5. 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. El Sahly, HM, Baden, LR, Essink, Bet al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385: 1774–85. 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bar-On, YM, Goldberg, Y, Mandel, Met al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021; 385: 1393–400. 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.