Abstract
Background
The aim of this network meta‐analysis (NMA) was to evaluate the efficacy and safety of PD‐1/PD‐L1 inhibitors, alone or in combination with chemotherapy, as first‐line treatment for wild‐type advanced non‐small cell lung cancer.
Methods
We systematically searched databases, Clinical Trial.gov and included randomized clinical trials focusing on advanced NSCLC using PD‐1/PD‐L1 inhibitors as first‐line treatment. Hazard ratio for overall survival and progression‐free survival, odds ratio for any‐cause high‐adverse events (grade 3 or higher) were documented according to Bayesian NMA. Subgroup analysis was performed according to PD‐L1 level and histology.
Results
Thirteen trials including 9154 patients were included. In the PD‐L1 nonselective cohort, chemotherapy in combination with pembrolizumab and atezolizumab, respectively, were significantly better than any other treatment strategies in both OS benefit (HR = 0.63; HR = 0.85) and PFS benefit (HR = 0.52; HR = 0.63). In subgroup analysis, pembrolizumab appeared to provide the best OS benefit (HR = 0.67) as well as the best PFS benefit (HR = 0.67) in the PD‐L1 ≥ 50% cohort. In contrast, pembrolizumab combined with chemotherapy exhibited the best OS benefit in the PD‐L1 < 50% cohort. Furthermore, OS benefit from pembrolizumab plus chemotherapy was more obvious in nonsquamous patients (HR = 0.56). Additionally, pembrolizumab plus chemotherapy was associated with fewer adverse events than other chemotherapy combination strategies.
Conclusions
In the first‐line treatment, chemotherapy plus pembrolizumab or atezolizumab could enhance efficacy compared with chemotherapy alone or other PD‐1/L1‐based treatment strategies, especially in the nonsquamous population. Furthermore, pembrolizumab plus chemotherapy guarantees reliable security simultaneously, which may be the optimal treatment strategy for patients with major advanced NSCLC.
Keywords: carcinoma, immune checkpoint inhibitors, network meta‐analysis, non‐small cell lung
Bayesian network meta‐analysis showed that the combination of pembrolizumab and chemotherapy had the highest efficacy and the highest safety. This agent was suggested to be quite suitable for patients with advanced wild‐type NSCLC.

INTRODUCTION
Among all the causes of cancer deaths, lung cancer remains the first, 1 and non‐small cell lung cancer (NSCLC) accounts for approximately 85% of patients with lung primary carcinoma. NSCLC is hard to diagnose in the early stages, and the 5‐year survival of advanced or metastatic NSCLC patients is low with around 5% 2 receiving traditional treatment of chemotherapy‐based strategies. Recently, tyrosine kinase inhibitors (TKIs) are being used more for mutated advanced NSCLC patients, and have better benefits than chemotherapy in improving longer‐time‐to‐event outcomes.
However, more than 50% patients are diagnosed with wild‐type NSCLC and to date an efficient treatment strategy has been lacking in these patients. The recent introduction of immune checkpoint inhibitors (ICIs) has improved the survival results for patients with advanced wild‐type NSCLC, and improved this unacceptable situation. The programmed cell death 1/anti‐programmed death ligand 1(PD‐1/L1) pathway, previously detected in a variety of malignant tumors, plays an important role in fighting against tumors by regulating the function of autoimmunity. 3 , 4 Anti‐PD‐(L) 1 monoclonal antibodies are one kind of ICI gradually being approved for the treatment of NCSLC and have been reported to perform satisfactory clinical effects. 5 , 6 , 7
Although there are many clinical trials which have evaluated the efficacy and safety of immunotherapy or chemoimmunotherapy compared with chemotherapy, many of which have suggested a hopeful increase in overall survival (OS) and progression‐free survival (PFS) 8 , 9 , 10 , 11 (Impower130; Keynote 042; Keynote189), direct comparison evidence between immunotherapy‐based agents is insufficient. There have been some meta‐analyses which have evaluated the efficacy and safety among various anti‐PD (L) 1 drugs, 12 , 13 but some eligible trials have not been included, and data of some included individual studies are out of date.
The objective of this network meta‐analysis (NMA) was to summarize and incorporate up‐to‐date information of eligible studies using PD‐1/L1 inhibitors as front‐line treatment, evaluating the optimal treatment strategies for advanced NSCLC.
METHODS
The referred reporting items for systematic reviews and meta‐analysis (PRISMA) extension statement for network meta‐analysis 14 was followed to perform this NMA.
Data sources and searches
English and Chinese databases were systematically searched before August 17, 2020 in all languages, involving PubMed, the Cochrane Library, CBM, CNKI, Wang Fang, and VIP. We also screened ClinialTrials.gov in case of missing ongoing studies. The keywords used for searching the databases included carcinoma, non‐small cell lung, NSCLC, PD‐1, PD‐L1, checkpoint inhibitor, nivolumab, pembrolizumab, atezolizumab, ipilimumab, avelumab, tremelimumab, durvalumab, advanced, 1st‐line, first‐line, untreated, chemo‐naive, etc. In addition, we supplemented Camel study and Impower 132 study before data analysis. The detailed retrieval strategy in the Cochrane Library is presented in Table S1.
Study selection
We included phase II/III randomized controlled clinical trials which met the following criteria: (1) Advanced, metastatic or recurrent NSCLC. (2) PD‐1/L1 inhibitors alone or combined with chemotherapy as first‐line therapy. (3) Reporting survival outcomes including OS, PFS or grade ≥ 3 adverse events (the definition of OS: time from randomization to death from any cause; definition of PFS: time from randomization until disease progression or death from any cause).
The definition and grade of adverse events is on the basis of the National Cancer Institute Common Terminology Criteria for AEs (NCI‐CTCAE).
Studies not meeting the above criteria were excluded. Other exclusion criteria were: (1) Patients receiving other therapies as frontline other than PD‐1/L1 inhibitor‐based agents. (2) Patients with sensitive mutations such as EGFR or ALK mutation.Titles and abstracts were screened first followed by assessment of the full text. Durations of any follow‐up were eligible and updated data were used for analysis.
Data extraction and risk of bias assessment
Detailed information of eligible trials and characteristics of involved patients were extracted, including the study name, publication sources, year of publication, study phase, treatment regimen, stage of NSCLC, histological type, PD‐L1 level, hazard ratio (HR) with 95% confidence interval (95%CI) for OS and PFS, times of any cause high‐adverse events (≥ grade 3), sample size, patients' medium age, sex and histology distribution, smoking status, Eastern Cooperative Oncology Group (ECOG) performance‐status score, central nervous system (CNS) status, and ethnic background.
Cochrane risk of bias tools from seven perspectives were used to perform the assessment of risk of bias of individual studies: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting and (7) other bias.
Four investigators (L.W., Y.Y., X. N, X.L) extracted data and assessed the risk of bias of included studies independently. Disagreements were resolved by consulting other authors (B.A.).
Statistical analysis
The primary outcome was OS, the secondary outcomes were PFS and high‐AEs, HR with 95% CI for OS and PFS, OR with 95% CI for grade ≥ 3 AEs were synthesized by Bayesian approach, direct and indirect data were both included in this NMA.
HR was first transformed into the form of lnHR and standard error of lnHR (selnHR). As for head‐to‐head comparison in two‐arm original trials, lnHR and selnHR for OS and PFS were calculated by this formula: , referring to the previously reported methods. 15 In terms of multiarms study, for example, a three‐arm study was divided into three direct head‐to‐head comparisons. Covariance between three comparisons was deemed to be the same, lnHR and selnHR for OS and PFS in the reference group was calculated based on previous methods 15 : *(* b: Reference group; k1/k2: Intervention group).
R software (version 3.5.3) and Stata (version 13.0) were applied to this NMA. In the R software, we established a consistency model in random effect with four Markov chains with package “gemtc” and package “ggplot”. For each chain, we used 50 000 iterations and 20 000 sample burnins. By this process, the model was initialized, and subsequent pooled analysis and mixed analysis were performed on the basis of this model. The pooled analysis included direct evidence and showed the relative effects of each intervention compared with chemotherapy, which was visualized by forest plots. The mixed analysis included both direct and indirect evidence and showed the relative effects between any two interventions, which ranked each regimen from best to worst according to outcome measurement and was visualized by the surface under the cumulative ranking curve (SUCRA) values. Stata in random model was used to assess the publication bias by funnel plots and generate network evidence plots according to the number of trials and sample size.
Heterogeneity was evaluated by I‐square visualized by forest plots. I2 value <50% was considered low probability of heterogeneity. In addition, subgroup analysis was carried out according to PD‐L1 and histology.
RESULTS
Study selection and characteristics
A total of 1931 records were identified from six online databases (PubMed, the Cochrane library, CBM, CNKI, VIP, Wan Fang) and ClinicalTrials.gov. A total of 185 records were reviewed for full‐text assessment after excluding duplicates and screening for titles and abstracts (Figure 1). Eventually, 13 trials were included in the NMA. 8 , 9 , 10 , 11 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 The detailed information on patients involved in the study are shown in Tables 1 and 2. Overall, 9154 patients were enrolled in 11 different treatment strategies: chemotherapy (Chemo), nivolumab (Nivo), pembrolizumab plus chemotherapy (Pembro+Chemo), nivolumab plus ipilimumab (Nivo+Ipi), nivolumab plus chemotherapy (Nivo+Chemo), atezolizumab plus chemotherapy (Atez+Chemo), pembrolizumab (Pembro), durvalumab (Durva), durvalumab plus tremelimumab (Durva+Treme), atezolizumab (Atez), camrelizumab plus chemotherapy (Camre+Chemo).
FIGURE 1.
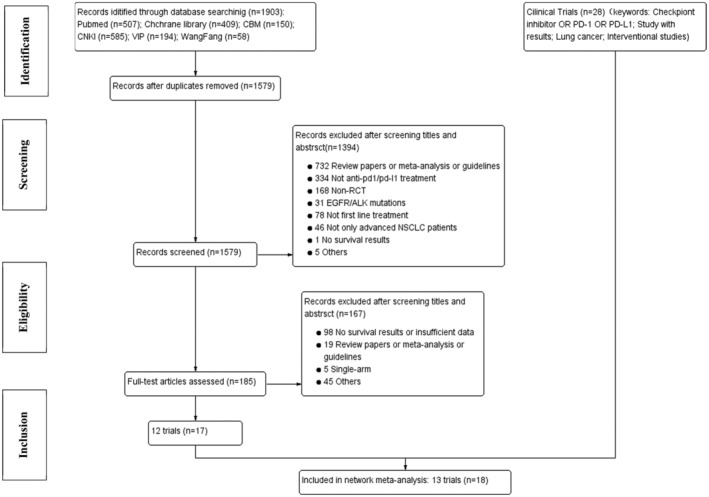
Flow diagram of study selection
TABLE 1.
Characteristics of eligible studies
| Study name | Publication | Year | Phase | Stage | Regimen | OS HR (95%CI) | PFS HR (95% CI) | Adverse events (> = 3 grade) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histology | PD‐L1 expression | Experimental arm | Control arm | Overall | Overall | Experiment/control | ||||||
| Checkmate 026 | NEJM | 2017 | III | IV/recurrent | Mix | > = 1% | Nivo 271 | Chemo 270 | 1.17 (0.95–1.43) | 1.17 (0.9–1.43) | 18% /51% | |
| Keynote 189 | JCO | 2020 | III | IV | NSq | Nonselective | Pembro+Chemo 410 | Chemo 206 | 0.56 (0.45–0.7) | 0.48 (0.4–0.58) | 71.9%/66.8% | |
| Keynote 407 | JCO | 2020 | III | IV | Sq | Nonselective | Pembro+Chemo 278 | Chemo 281 | 0.71 (0.58–0.88) | 0.57 (0.47–0.69) | 69.8%/68.2% | |
| Checkmate 227 | NEJM | 2019 | III | IV/recurrent | Mix | Nonselective |
1.Nivo+Ipi/Nivo 396/396 2.Nivol+Ipili/Nivol+Chemo 187/177 |
1.Chemo 397 2.Chemo 186 |
0.73 (0.64–0.84)(Nivo+ipi vs Chemo) | 0.83(0.72–0.96)(Nivo+ipi vs Chemo) | 132.8%/36% (Nivo+ipi vs Chemo) | |
| IMpower 130 | Lan Onco | 2019 | III | IV | NSq | Nonselective | Atez+Chemo 451 | Chemo 228 | 0·79 (0·64–0·98) | 0·64 (0·54–0·77) | 81%/71% | |
| IMpower 131 | JTO | 2020 | III | IV | Sq | Nonselective | Atez+Chemo 343 | Chemo 340 | 0.88 (0.73–1.05) | 0.71 (0.60–0.85) | 68.0%/57.5% | |
| IMpower 132 | JTO | 2020 | III | IV | NSq | Nonselective | Atez+Chemo 292 | Chemo 286 | 0.81 (0.64–1.03) | 0.60 (0.49–0.72) | 71.5%/60.6% | |
| Keynote 021G | JTO | 2019 | II | IIIB/IV | NSq | Nonselective | Pembro+Chemo 60 | Chemo 63 | 0.56 (0.32–0.95 | 0.53 (0.33–0.86) | 40.7%/27.4% | |
| Keynote 024 | JCO | 2019 | III | IV | Mix | > = 50% | Pembro 154 | Chemo 151 | 0.63 (0.47–0.86) | 0.50 (0.37–0.68) | 31.2%/53.3% | |
| Keynote 042 | Lan Onco | 2019 | III | IV | Mix | > = 1% | Pembro 637 | Chemo 636 | 0·81 (0·71–0·93) | 1·07 (0·94–1·21). | 18%/41% | |
| MYSTIC | JAMA Onco | 2020 | III | IV | Mix | Nonselective | Durva/Durva+Treme 369/371 | Chemo 352 | 0.96 (0.81–1.13)(Durva/Chemo); 0.94 (0.79–1.10)(Durva+Treme/Chemo) | 1.05 (0.72–1.53)(Durva+Treme/Chemo) | 14.9%/22.9%/33.8% | |
| IMpower 110 | NEJM | 2020 | III | IV | Mix | > = 1% | Atez 277 | Chemo 277 | 0.83 (0.65–1.07) | 0.77 (0.63–0.94) | 30.1%/52.5% | |
| Camel | Lan Resp Med | 2021 | III | IIIB/IV | NSq | Nonselective | Camre+Chemo 205 | Chemo 207 | 0.73 (0.53–1.02) | 0·60 (0·45–0·79) | 69%/98% | |
Abbreviations: Atez, atezolizumab; Camre, camrelizumab; Chemo, chemotherapy; Durva, durvalumab; Ipi, ipilimumab; JAMA Onco, JAMA Oncology; JCO, journal of clinical oncology; JTO, Journal of Thoracic Oncology; Lan Onco, Lancet Oncology; Lan Resp Med, Lancet Respiratory Medicine; Lan, Lancet; Mix, nonsquamous and squamous; NEJM, New England Journal of Medicine; Nivo, nivolumab; NSq, nonsquamous; Pembro, pembrolizumab; Sq, squamous; Treme, tremelimumab.
TABLE 2.
Patient characteristics of the included studies
| Study name | Total N | PD‐L1 level (experiment/control) | Age (median) (experiment/Control) | Male (n,%) (experiment/control) | ||
|---|---|---|---|---|---|---|
| ≥50% | 1%–50% | ≤1% | ||||
| Checkmate 026 | 541 | 88/126 | 208/210 | 0 | 63.0/65.0 | 184(67.9)/148(54.8) |
| Keynote 189 | 616 | 132/70 | 128/58 | 127/63 | 65.0/63.5 | 254(62.0)/109(52.9) |
| Keynote 407 | 559 | 73/73 | 103/104 | 95/99 | 65.5/65.0 | 220(79.1)/235(83.6) |
| Checkmate 227 | 1739 | 205/214/192 (Nivo+Ipi/Nivo/Chemo) | 191/182/205 (Nivo+Ipi/Nivo/Chemo) | 187/177/186 (Nivo+Ipi/Nivo+Chemo/Chemo) |
64.0/64.0/64.0 (Nivo+Ipili/Nivo/Chemo) 63.0/64.0/64.0 (Nivo+Ipi/Nivo+Chemo/Chemo) |
255 (64.4)/272 (68.7)/260 (65.5) (Nivo+Ipili/Nivo/Chemo) 138(73.8)/130(73.4)/125(67.2) (Nivo+Ipili/Nivo+Chemo/Chemo) |
| IMpower 130 | 679 | 88/42 | 128/65 | 235/121 | 64.0/65.0 | 266(59.0)/134(58.8) |
| IMpower 131 | 683 | 47/44 | 136/125 | 160/171 | 65.5/65.0 | 279(81.0)/278(82.0) |
| IMpower 132 | 578 | 25/20 | 63/73 | 88/75 | 64.0/63.0 | 192(65.8)/192(67.1) |
| Keynote 021G | 123 | 20/17 | 19/23 | 21/23 | 62.5/63.2 | 22(37.0)/26(41.0) |
| Keynote 024 | 305 | 125/124 | 0 | 0 | 64.5/66.0 | 92(59.7)/95(62.9) |
| Keynote 042 | 1273 | 299/300 | 338/337 | 0 | 63.0/63.0 | 450(70.6)/452(71.0) |
| MYSTIC | 1092 | 118/108/107 (Durva/Durva+Treme/Chemo) | NA | 95/76/83 (Durva/Durva+Treme/Chemo) | 64.0/65.0/64.5 | 113 (69.3) /118 (72.4) /106 (65.4) |
| IMpower 110 | 554 | 107/98 | 170/179 | 0 | 64.0/65.0 | 196(70.8)/196(69.7) |
| Camel | 412 | 49/69 | 108/97 | 30/20 | 59.0/61.0 | 146(71.2)/149(72) |
| Study name | Nonsquamous (n,%)(Experiment/Control) | Never Smoked (n,%)(Experiment/Control) | ECOG 0(n,%) | CNS metastasis (n,%) | Asia | |
|---|---|---|---|---|---|---|
| Checkmate 026 | 205 (76)/206(76) | 30 (11)/29 (11) | 85 (31)/93 (34) | 33 (12)/36 (13) | 30(11)/17 (6.3) | |
| Keynote 189 | 410 (100)/206(100) | 48 (11.7)/25 (12.1) | 186 (45.4)/80 (38.8) | 73 (17.8)/35 (17) | 4 (1)/6 (2.9) | |
| Keynote 407 | 0/0 | 22 (7.9)/19 (6.8) | 73 (26.3)/90 (32.0) | 20 (7.2)/24 (8.5) | 54 (19.4)/52 (18.5) | |
| Checkmate 227 |
279 (70.5)/279 (70.5)/281 (70.8) (Nivo+Ipi/Nivo/Chemo) 140 (74.9)/134 (75.7)/140 (75.3) (Nivo+Ipi/Nivo+Chemo/Chemo) |
56 (14.1)/50 (12.6)/51 (12.8) (Nivo+Ipi/Nivo/Chemo) 23 (12.3)/27 (15.3)/27 (14.5) (Nivo+Ipi/Nivo+Chemo/Chemo) |
135 (34.1)/142 (35.9)/134 (33.8) (Nivo+Ipi/Nivo/Chemo) 69 (36.9)/59 (33.3)/57 (30.6) (Nivo+Ipi/Nivo+Chemo/Chemo) |
41 (10.4)/42 (10.6)/40 (10.1) (Nivo+Ipi/Nivo/Chemo) 23 (12.3)/16 (9.0)/11 (5.9) (Nivo+Ipi/Nivo+Chemo/ Chemo) |
81 (20.5)/66 (16.7)/81 (20.4) (Nivo+Ipi/Nivo/Chemo) 40 (21.4)/36 (20.3)/43 (23.1) (Nivo+Ipi/Nivo+Chemo/Chemo) |
|
| IMpower 130 | 451 (100)/228 (100) | 48 (11)/17(7) | 189 (42%)/91 (40%) | NG | 12 (3)/3(1) | |
| IMpower 131 | 0/0 | 32 (9.3)/23(6.8) | 115 (33.5)/110 (32.4) | NG | 41 (12.0)/37 (10.9) | |
| IMpower 132 | 292 (100)/286 (100) | 37 (12.7)/30(10.5) | 126 (43)/114 (40) | NG | 71 (24.3)/65 (22.7) | |
| Keynote 021G | 60 (100)/63 (100) | 15 (25)/9(14) | 24 (40) /29 (46) | 9 (15)/6 (10) | 5 (8)/5 (8) | |
| Keynote 024 | 125 (81.2)/124 (82.1) | 5 (3.2) /19(12.6) | 54 (35.1)/53 (35.1) | 18 (11.7)/10 (6.6) | 21(13.6) 19 (12.6) | |
| Keynote 042 | 394 (62)/388(61) | 142 (22)/140 (22) | 198 (31)/192 (30) | 35 (5)/35 (5) | 85 (29)/185 (29) | |
| MYSTIC |
111 (68.1)/ 110 (67.5)/110 (67.9) (Durva/Durva+Treme/Chemo) |
24 (14.7)/ 25 (15.3)/21 (13.0) (Durva/Durva+Treme/Chemo) |
57 (35.0)/65 (39.9)/70 (43.2) (Durva/Durva+Treme/Chemo) |
NG |
59 (36.2)/50 (30.7) /47 (29.0) (Durva/Durva+Treme/Chemo) |
|
| IMpower 110 | 277 (100)/277 (100) | 37 (13.4)/35 (12.6) | 97 (35.0)/102 (36.8) | NG | 45 (16.2)/30 (10.8) | |
| Camel | 205 (100)/207 (100) | NA/NA | 48 (23.4)/36 (17.5) | 10 (5)/5 (2) | NG | |
Abbreviations: Camre, camrelizumab; Chemo, chemotherapy; Durva, durvalumab; Ipi, ipilimumab; Nivo, nivolumab; Treme, tremelimumab.
In general, all studies were phase III random clinical control trials apart from Keynote 021G. 16 Most of the studies included were head‐to‐head trials apart from Checkmate 227 24 , 25 and MYSTIC. 29 Six trials enrolled both nonsquamous and squamous NSCLC patients: Checkmate 026, 20 Checkmate 227, Keynote 024, 18 , 19 Keynote 042, 10 MYSTIC, IMpower 110 21 ; five trials focused only on nonsquamous NSCLC patients: Keynote 189, 8 , 9 IMpower 130, 11 Keynote 021G, IMpower 132, 23 Camel, 28 with two trials focusing on only squamous NSCLC patients. PFS was final analysis in all included studies, most OS analysis was final except Keynote 042, IMpower130 and Camel. Bias assessment is shown in Figure S1.
Primary analysis for OS and PFS in PD‐L1 nonselective NSCLC patients
Seven treatments were included for OS and PFS in patients with any PD‐L1 level (Figure 2). Pooled direct comparison results between immunotherapy or immunotherapy‐combination therapy and standard chemotherapy are shown as forest plots (Figure 3). Pooled mixed comparison results involving all included treatment agents are shown in ranking plots (Figure 4) and league‐tables (Figure 5).
FIGURE 2.
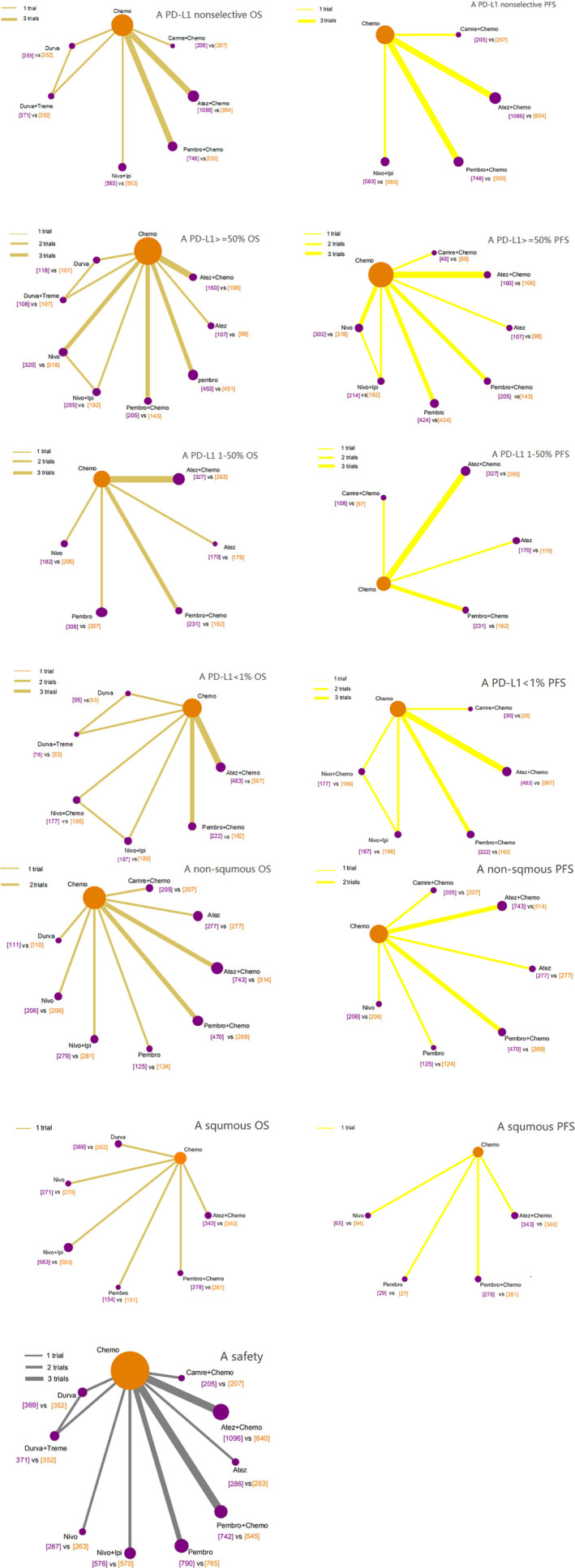
Network evidence plots. All groups were included (for example, “A PD‐L1 nonselective OS” meant the OS analysis in all patients without PD‐L1 section; “A PD‐L1 nonselective PFS” meant the PFS analysis in all patients without PD‐L1 section; “A PD‐L1 >= 50% OS” meant the OS analysis in PD‐L1 > = 50% cohort; so as others)
FIGURE 3.
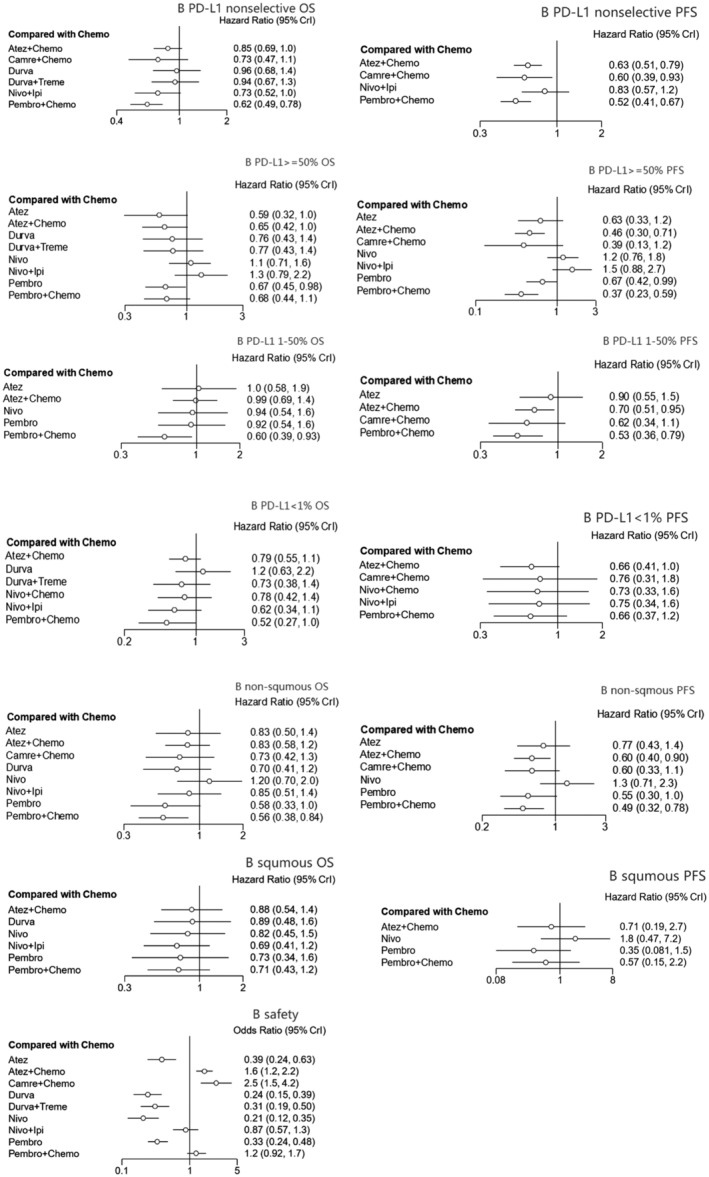
Forest plots of pooled direct comparison. “Chemotherapy” was the reference group, the hazards ratio/odds ratio of “intervention group” to “chemotherapy” were provided. (all groups were included, for example, “B PD‐L1 nonselective OS” meant the OS analysis in all patients without PD‐L1 section, so as others)
FIGURE 4.
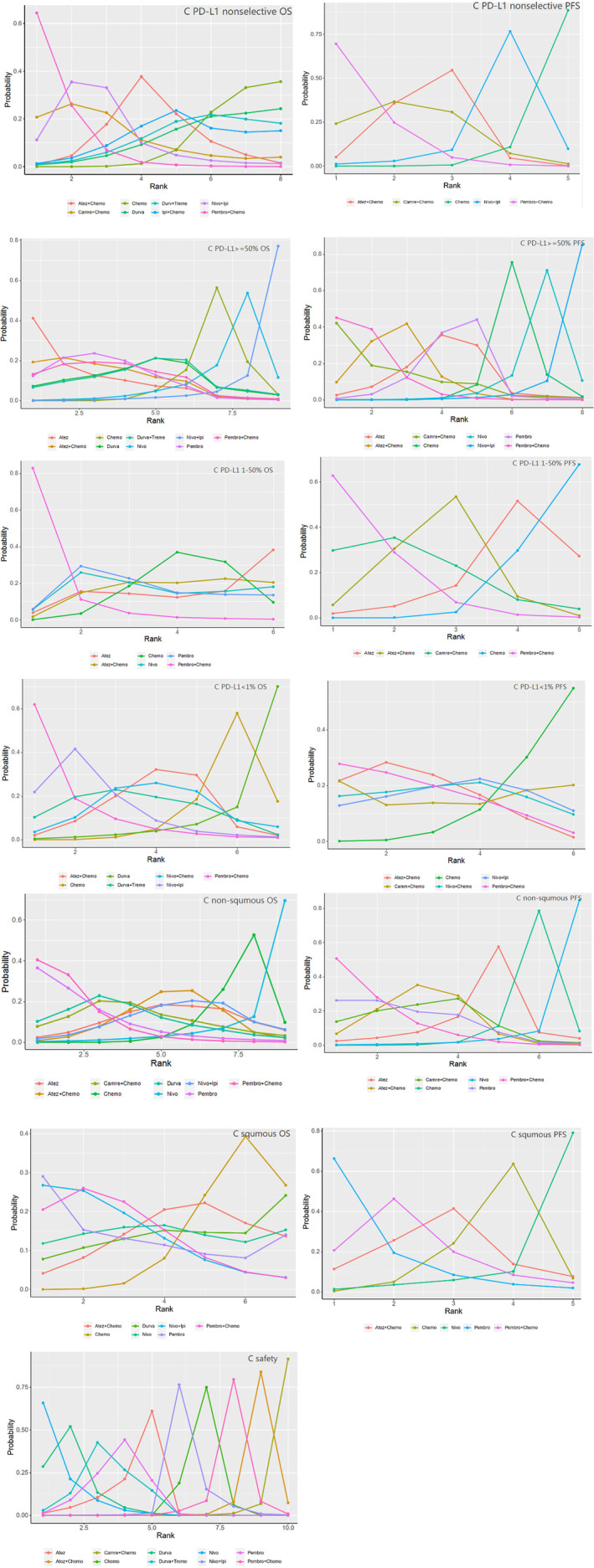
Ranking plots of pooled mixed comparison, which show the probability of treatment agents to be ranked at first, second, third … and the last (all groups were included, for example, “C PD‐L1 nonselective OS” meant the OS analysis in all patients without PD‐L1 section, so as others)
FIGURE 5.
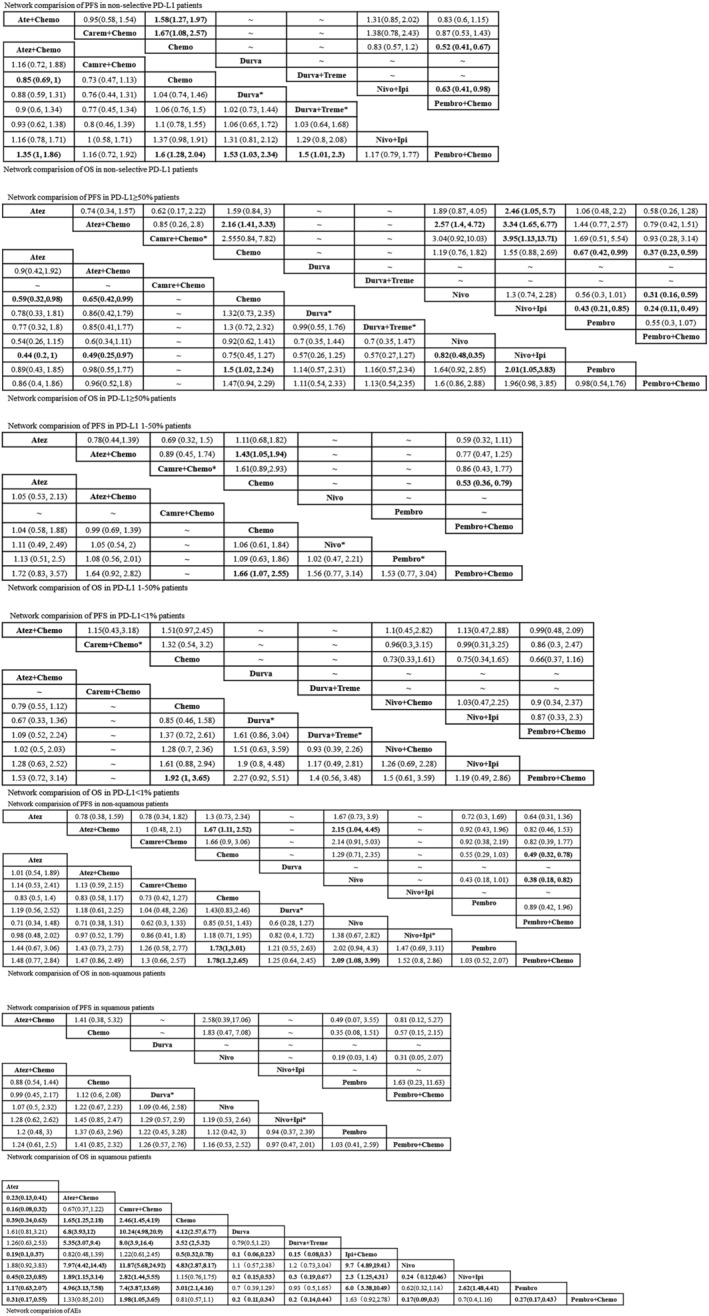
League tables of pooled mixed comparison, which show the value of HR/OR with 95% CI between two random treatment agents (all groups were included, for example, “Network comparison of OS in nonselective PD‐L1 patients” meant the OS analysis in all patients without PD‐L1 section, so as others. * meant that only one survival index was accessed and included: OS or PFS. Durva* and Durva+Treme* were from MYSTIC, a three‐arm study; Nivo+Ipi* was from checkmate 227, a three‐arm study; Camre+Chemo was from Camel, an ongoing head‐to‐head study; Pembro* was from Keynote 024 and Keynote 042, both focusing on PD‐L1 positive NSCLC)
In terms of OS, immunotherapy combination therapy seemed to exhibit better benefit than chemotherapy alone. Pembro+Chemo (HR = 0.62, 95% CI: 0.49 to 0.78) and Atez+Chemo (HR = 0.85, 95% CI: 0.69 to 1) yielded superior OS benefit over traditional chemotherapy. Furthermore Pembro+Chemo obtained greater OS benefit than Atez+Chemo (HR = 0.74, 95% CI: 0.54 to 1). In addition, Pembro+Chemo showed the best OS benefit versus other included treatment agents including Durva (HR = 0.65, 95% CI: 0.43 to 0.97), Durva+Treme (HR = 0.67, 95%CI: 0.43 to 0.99). Bayesian ranking profiles (Figure 4) suggested that Pembro+Chemo was most likely to be ranked first for prolonging OS (probability = 65%) in PD‐L1 expression nonselective NSCLC patients.
As for PFS, immunotherapy combination therapy was also perceived to obtain greater benefit than chemotherapy. Pembro+Chemo (HR = 0.52, 95% CI: 0.41 to 0.67), Atez+Chemo (HR = 0.63, 95% CI: 0.51 to 0.79), Camre+Chemo (HR = 0.6, 95% CI: 0.39 to 0.93) were significantly better than chemotherapy alone in improving PFS. No significant differences were noted between these three advantageous strategies. Pembro+Chemo also showed better PFS upon comparison with Nivo+Ipi (HR = 0.63, 95% CI: 0.41,0.98). However, Bayesian ranking profiles suggested that the pembrolizumab combination strategy should possibly be ranked first to offer best PFS (probability = 69%).
Subgroup analysis for OS and PFS according to PD‐L1 level and histology
PD‐L1 ≥ 50%
Ten treatments were included in this cohort. As for OS, monoimmunotherapy and immunotherapy combination treatment strategies were discerned to provide better OS benefit. Pembro (HR = 0.67, 95% CI: 0.45 to 0.98) and Atez (HR = 0.59, 95% CI: 0.32 to 0.98) showed a significant benefit compared with Chemo in OS comparison. In addition, Atez+Chemo (HR = 0.65, 95% CI = 0.42 to 0.99) was better than Chemo alone in prolonging OS. No significant differences were found between these three superior agents. Bayesian ranking profiles (Figure 4) suggested that Atez was most likely to be ranked first (probability = 41%) for OS benefit in PD‐L1 ≥ 50% advanced NSCLC patients, followed by Atez+Chemo (probability = 19%) and Pembro alone (probability = 12%).
When it came to PFS, monoimmunotherapy and immunotherapy combination treatment strategies were also perceived to provide better PFS benefit. Pembro (HR = 0.67, 95% CI: 0.42 to 0.99) exhibited superior PFS benefit compared with Chemo alone; Pembro+Chemo (HR = 0.36, 95% CI: 0.23 to 0.59) was similar to Atez+Chemo (HR = 0.46, 95% CI: 0.3 to 0.71) in providing better PFS versus Chemo. There was no apparent benefit difference between these three advantageous strategies. Bayesian ranking profiles indicated that Pembro+Chemo was most likely to be ranked first to offer the best PFS (probability = 45%).
PD‐L1 1%–50%
In total, seven treatments were included. In terms of OS, among all the regimens, only Pembro+Chemo (HR = 0.60, 95% CI: 0.39 to 0.93) was significantly better than Chemo in OS comparison. Bayesian ranking profiles suggested that Pembro+Chemo was most likely to be ranked first (probability = 83%) for OS benefit.
As for PFS, Pembro+Chemo (HR = 0.53, 95% CI: 0.36 to 0.79), Atez+Chemo (HR = 0.7, 95%CI: 0.51 to 0.95) was significantly better than Chemo in improving PFS, and no significant difference was found between these two regimens (HR = 1.3, 95%CI: 0.8 to 2.14). Bayesian ranking plots suggested that Pembro+Chemo was most likely to be the best regimen for increasing PFS (probability = 63%) compared with any other included treatment agents.
PD‐L1 < 1%
Eight treatments were included. Being similar to the results of OS analysis in PD‐L1 1%–50% cohort, Pembro+Chemo was also the only strategy superior to Chemo in prolonging OS in the PD‐L1 negative cohort. The remainder of the regimens including monoimmunotherapy and doublet immunotherapy agents exhibited similar benefit with chemotherapy alone in OS comparison. Bayesian ranking plots suggested that Pembro+Chemo was most likely to be ranked first for best OS (probability = 62%).
With regard to PFS, all strategies included showed similar benefit. Bayesian ranking plots also suggested that Pembro+Chemo appeared to be ranked first to improve PFS (probability = 28%).
Nonsquamous NSCLC
For nonsquamous advanced NSCLC patients, nine treatments were included. With regard to OS, all included regimens showed similar efficacy except Pembro+Chemo (HR = 0.56, 95% CI: 0.38 to 0.84) and Pembro alone (HR = 0.58, 95% CI: 0.33 to 1). No significant difference was noted between these two strategies. Pembro+Chemo and Pembro alone appeared to be ranked in the top two for OS benefit, with overall probability at 40% and 36%, respectively.
As for PFS, among all included strategies, Pembro+Chemo (HR = 0.56, 95% CI: 0.38 to 0.84), Atez+Chemo (HR = 0.6, 95% CI: 0.4 to 0.9), Pembro (HR = 0.58, 95 CI: 0.33 to 1) were superior to Chemo alone in PFS improvement. Bayesian profiles suggested Pembro+Chemo was mostly likely to be the best regimen for increasing PFS (probability = 51%).
Squamous NSCLC
Seven treatments were included in squamous NSCLC patients. All treatment strategies included showed no significant difference according to OS benefit and PFS benefit. Bayesian ranking profiles indicated that Pembro was most likely to be the best regimen for prolonging both OS (probability = 28%) and PFS (probability = 65%).
Safety analysis
Ten treatments in 13 trials were included. Monoimmunotherapy and dual‐immunotherapy appeared to show a significantly lower hazard ratio than Chemo alone in terms of the incidence of any cause grade ≥ 3 adverse events, including Atez (OR = 0.39, 95% CI: 0.24, 0.63), Durva (OR = 0.24, 95% CI: 0.15, 0.39), Nivo (OR = 0.21, 95% CI: 0.12, 0.35), Pembro (OR = 0.33, 95% CI: 0.24, 0.47), Durva+Treme (OR = 0.31, 95% CI: 0.19 to 0.5), and there was no significant difference between these agents. The immunochemo combination strategy appeared to show a higher risk of causing adverse events apart from Pembro+Chemo (OR = 0.81, 95% CI: 0.57 to 1.1). Bayesian ranking plots suggested that Nivo was most likely to be ranked first not to cause adverse events (probability = 67%); Additionally, Pembro+Chemo was most likely to be ranked first to show the least probability of causing adverse events among all immunochemotherapy agents.
Heterogeneity analysis and publication bias
The result of heterogeneity analysis was shown by I‐square value and forest (Figure S2). Generally, no obvious heterogeneity (I2 < 50%) was found in the primary OS analysis and other subgroup analysis. In addition, funnel plots provided in the appendix (Figure S3) suggested that no obvious publication bias existed.
DISCUSSION
In this NMA, we provide up‐to‐date information of first‐line phase II/III randomized studies evaluating immunotherapy alone or combination regimens. The patients included in the analysis had advanced/metastatic NSCLC, without ALK/EGFR mutation. The endpoints were OS and PFS, as well as the incidence rate of any cause high‐AEs. Based on 13 well‐controlled randomized clinical trials, our results may provide evidence for clinical practice as follows: (1) The addition of PD‐1/L1 inhibitors to chemotherapy may provide more survival benefits compared with chemotherapy according to OS and PFS. (2) Pembro+Chemo and Atez+Chemo were superior over Chemo and any other included treatment agents in OS and PFS benefit irrespective of PD‐L1 level. (3) In consideration of PD‐L1 level, Pembro and Atez+Chemo were most likely to improve survival profiles including both OS and PFS in the PD‐L1 ≥ 50% cohort. However, in the PD‐L1 < 50% cohort, Pembro+Chemo was more likely to exhibit superior survival benefit in terms of both OS and PFS. (4) According to histology, nonsquamous patients were likely to gain more survival benefit than squamous patients by using the advantageous Pembro+Chemo agents. (5) The addition of PD‐1/L1 inhibitors to Chemo appeared to increase the toxicity over Chemo alone except Pembro. (6) Pembro+Chemo could balance efficacy and safety well, which ranked first for both OS and PFS for PD‐L1 nonselective NSCLC and last for high‐AEs across all immunochemo combination strategies.In the last 10 years, the promising results of ICI treatments from randomized clinical exploration for many kinds of tumors have been given extensive attention, including in NSCLC patients. Since 2015, when the second‐line ICI agents for NSCLC showed satisfactory results, a series of PD‐1/L1 inhibitors, such as pembrolizumab, nivolumab, atezolizumab, have been approved. 30 , 31 , 32 , 33 Pembrolizumab was initially approved as a first‐line treatment strategy for advanced/metastatic NSCLC patients with high PD‐L1 expression level (KN024), then pembrolizumab plus chemotherapy and atezolizumab plus chemotherapy were gradually applied to clinical practice for any PD‐L1 expression NCSLC patients under the approval of EMA (European Medicines Agency) and the guidance of ESMO (European Society for Medical Oncology) guidelines. 12 More and more randomized clinical trials focusing on frontline PD‐1/L1 treatments for advanced NCSLC are being performed at a rapid pace.
Based on nine randomized clinical trials (RCTs), we found that Pembro+Chemo and Atez+Chemo were superior to Chemo and any other included treatment agents in OS benefit. With a significant difference between Pembro+Chemo and Atez+Chemo, the former agents brought higher benefit than the latter one in OS comparison. As for PFS comparison, three combination strategies including Pembro+Chemo, Carem+Chemo, Atez+Chemo were superior to Chemo alone, and there was no PFS benefit difference among these three regimens. Pembro+Chemo was probably the best regimen to offer both OS and PFS benefit, irrespective of PD‐L1 status, and this was in accordance with previous analyses. 12 , 34
As for PD‐L1 high subgroup analysis, the OS benefit of Pembro, Atez+Chemo were significantly higher than other treatments examined, with no difference between them. As for PFS comparison, there was a better response to Pembro, Atez+Chemo, Pembro+Chemo than the other regimens, which is in line with the previous NMA conclusions. 12 , 13 There was no significant difference among these three regimens. Hence, Pembro was suggested to be the best treatment strategy to offer both better OS benefit and PFS benefit for PD‐L1 > =50% NSCLC patients.
When examining the PD‐L1 intermediate subgroup, Pembro+Chemo and Atez+Chemo provided significant PFS benefit compared to Chemo, which were consistent with previous studies. In the PD‐L1 negative cohort, pembrolizumab combination agents were indicated to possibly show the best benefit for both OS and PFS. Therefore, Pembro+Chemo was more likely to be the better treatment strategy for PD‐L1 < 50% NSCLC.
When it came to subpopulation by histology, again Pembro+Chemo was the better strategy in prolonging OS in nonsquamous NSCLC patients, followed by Pembro alone, and there was no difference between these two agents. Pembro+Chemo also showed more benefit in PFS improvement. In contrast, in terms of squamous patients, all the strategies included were equal and no superior treatment agent was found with regard to OS or PFS. Thus, Pembro+Chemo may be suggested to be the optimal agent for nonsquamous NSCLC.
As for the safety analysis cohort, monoimmunotherapy strategies were less likely to cause high‐AEs and be safer than immunochemo combination agents. Notably, most immunochemo combination regimens increased toxicity upon comparison with traditional chemotherapy, but Pembro+Chemo did not increase the risk of causing grade ≥ 3 adverse events compared with Chemo, meaning reliable safety as first‐line treatment.
A previous study indicated that PD‐L1 level is associated with a different prognosis, in particular that a high PD‐L1 level tends to indicate a poor prognosis, 35 but that PD‐L1 positive patients are more sensitive to anti‐PD1/L1 drugs and thereby obtain greater relief. 36 In accordance with previous studies, our analysis focused on subgroup analysis according to PD‐L1 level, which suggests that NSCLC with different PD‐L1 expression level should be treated with different treatment agents for better survival benefits, with a notable PD‐L1 expression threshold at 50%.
In addition, histological types have also been deemed to be associated with obtaining different clinical therapeutic effects. 36 , 37 , 38 Similar to previous results, we found that nonsquamous NSCLC and squamous NSCLC were suitable for different treatment agents, with Pembro+Atez better for nonsquamous NSCLC and Pembro alone better for squamous NSCLC in our study.
In comparison with previous conclusions, 13 we also found different treatment agents exhibited different efficacy benefits in this study. Several reasons are given from the perspective of signal pathways including PD‐1/L1 pathway, PD‐1/L2 pathway, PD‐L1/B7‐1(CD80) pathway and the heterogeneous combination treatment regimens. For example, apart from blocking the binding of PD‐1 to PD‐L1, PD‐1 inhibitors and PD‐L1 inhibitors can additionally block different pathways independently, including PD‐L1/B7‐1 pathway and PD‐1/PD‐L2 pathway39, 40 leading to different clinical effects between anti‐PD‐1 and anti‐PD‐L1 drugs. In addition, as previously described, 41 we found that anti‐PD‐1 drugs were superior than anti‐PD‐L1 drugs with regard to survival benefit; Pembro+Chemo exhibited higher OS benefit than Atez+Chemo. Furthermore, chemotherapy can aid in enhancing the antigenicity and immunogenicity of malignant cells, resulting in stronger immune attacks by ICIs, 13 which have been shown as better survival benefits when adding PD‐1/L1 inhibitors to chemotherapy.
A strength of this NMA was that the search was thorough and included the latest information on RCT. In addition, by assessing the quality of eligible studies, we found that the trials included in our research were relatively well‐controlled. Additionally, this NMA provides evidence for the effectiveness and safety between various treatment regimens including monoimmunotherapy, dual‐immunotherapy, chemotherapy alone and chemoimmunotherapy, which still lacks sufficient evidential support from clinical trials.
However, we acknowledge that there are several limitations in our analysis. First, the benefit effects of Pembro alone according to OS and PFS were not available in the PD‐L1 nonselective cohort due to the design of the original studies included (Keynote 042, 10 Keynote024 18 , 19 ), but the evaluation of pembro monotherapy can be found in other cohorts. Second, our study was based on published results rather than original individual patient data, which may have led to some discount in credibility, but this is an unavoidable problem with a meta‐analysis.
In conclusion, subject to the limitations described above, this NMA indicates that the combination of Pembro/Atez and Chemo are preferred first‐line treatments for most patients with wild‐type NSCLC with regard to efficacy especially for non‐squamous NSCLC. Of note, Pembro + Chemo, which exhibited the most reliable safety simultaneously, are most likely to be optimal agents according to both high efficacy and high safety. In addition, this study suggests that PD‐L1 status may affect the clinical selection of treatment agents with a threshold at 50%. However, more well‐controlled randomized clinical trials are urgently needed in order to further research.
CONFLICT OF INTEREST
There are no conflicts of interest.
Supporting information
Figure S1. The bias assessment of eligible trials.
Figure S2. Forest plots of heterogeneity analysis. Atez, Atezolizumab; Camre, Camrelizumab; Chemo, Chemotherapy; Durva, Durvalumab; Nivo, Nivolumab; Pembro, Pembrolizumab; Treme, Tremelimumab; Ipi, Ipilimumab
Figure S3. Funnel plots of publication bias. A, Atezolizumab; C/Ca, Camrelizumab; Ch, Chemotherapy; Du, Durvalumab; N/Nivo, Nivolumab; P, Pembrolizumab; T, Tremelimumab; I, Ipilimumab
Table S1. Retrieval strategy in Cochrane Library
Wang L, Yang Y, Yu J, Zhang S, Li X, Wu X, et al. Efficacy and safety of anti‐PD‐1/PD‐L1 in combination with chemotherapy or not as first‐line treatment for advanced non‐small cell lung cancer: A systematic review and network meta‐analysis. Thorac Cancer. 2022;13:322–337. 10.1111/1759-7714.14244
Funding information Beijing Hospital 121 Project, Grant/Award Number: 121‐2016007; Chinese National Major Project for New Drug Innovation, Grant/Award Number: 2017ZX09304026
Contributor Information
Ailing Li, Email: lialwork@imc.pumc.edu.cn.
Bin Ai, Email: docaibin@163.com.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Goldstraw P, Chansky K, Crowley J, Rami‐Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 3. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non‐small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15:504–35. 10.6004/jnccn.2017.0050 [DOI] [PubMed] [Google Scholar]
- 4. Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, et al. PD‐1/PD‐L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234:16824–37. 10.1002/jcp.28358 [DOI] [PubMed] [Google Scholar]
- 5. Ackermann CJ, Reck M, Paz‐Ares L, Barlesi F, Califano R. First‐line immune checkpoint blockade for advanced non‐small‐cell lung cancer: travelling at the speed of light. Lung Cancer. 2019;134:245–53. 10.1016/j.lungcan.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 6. Afzal MZ, Dragnev KH, Shirai K. An extended overall survival analysis of pemetrexed and carboplatin with or without pembrolizumab as first‐line therapy for advanced non‐squamous non‐small cell lung cancer. Ann Transl Med. 2019;7:S53. 10.21037/atm.2019.03.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Theelen W, Baas P. Pembrolizumab monotherapy for PD‐L1 ≥50% non‐small cell lung cancer, undisputed first choice? Ann Transl Med. 2019;7:S140. 10.21037/atm.2019.06.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gadgeel S, Rodríguez‐Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE‐189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2020;38:1505–17. 10.1200/jco.19.03136 [DOI] [PubMed] [Google Scholar]
- 9. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, Esteban E, Felip E, de Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378:2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 10. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet (London, England). 2019;393:1819–30. 10.1016/s0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 11. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20:924–37. 10.1016/s1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 12. Dafni U, Tsourti Z, Vervita K, Peters S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first‐line treatment for advanced non‐small cell lung cancer. A systematic review and network meta‐analysis. Lung Cancer. 2019;134:127–40. 10.1016/j.lungcan.2019.05.029 [DOI] [PubMed] [Google Scholar]
- 13. Liang H, Lin G, Wang W, Huang J, Yang Y, Lan Y, et al. Feasibility and safety of PD‐1/L1 inhibitors for non‐small cell lung cancer in front‐line treatment: a Bayesian network meta‐analysis. Transl Lung Cancer Res. 2020;9:188–203. 10.21037/tlcr.2020.02.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84. 10.7326/m14-2385 [DOI] [PubMed] [Google Scholar]
- 15. Woods BS, Hawkins N, Scott DA. Network meta‐analysis on the log‐hazard scale, combining count and hazard ratio statistics accounting for multi‐arm trials: a tutorial. BMC Med Res Methodol. 2010;10:54. 10.1186/1471-2288-10-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borghaei H, Langer CJ, Gadgeel S, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. 24‐month overall survival from KEYNOTE‐021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first‐line therapy for advanced nonsquamous non‐small cell lung cancer. J Thorac Oncol. 2019;14:124–9. 10.1016/j.jtho.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 17. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: a randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol. 2016;17:1497–508. 10.1016/s1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 19. Reck M, Rodríguez–Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE‐024: pembrolizumab versus platinum‐based chemotherapy for advanced non‐small‐cell lung cancer with PD‐L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–46. 10.1200/jco.18.00149 [DOI] [PubMed] [Google Scholar]
- 20. Carbone DP, Reck M, Paz‐Ares L, Creelan B, Horn L, Steins M, et al. First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med. 2017;376:2415–26. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first‐line treatment of PD‐L1‐selected patients with NSCLC. N Engl J Med. 2020;383:1328–39. 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 22. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez‐Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab‐paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15:1351–60. 10.1016/j.jtho.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 23. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first‐line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16:653–64. 10.1016/j.jtho.2020.11.025 [DOI] [PubMed] [Google Scholar]
- 24. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier‐Valette C, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–104. 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hellmann MD, Paz‐Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus Ipilimumab in advanced non‐small‐cell lung cancer. N Engl J Med. 2019;381:2020–31. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 26. Paz‐Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med. 2018;379:2040–51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 27. Paz‐Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo‐controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol‐specified final analysis of KEYNOTE‐407. J Thorac Oncol. 2020;15:1657–69. 10.1016/j.jtho.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 28. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy‐naive patients with advanced non‐squamous non‐small‐cell lung cancer (CameL): a randomised, open‐label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9:305–14. 10.1016/s2213-2600(20)30365-9 [DOI] [PubMed] [Google Scholar]
- 29. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first‐line treatment of metastatic non‐small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6:661–74. 10.1001/jamaoncol.2020.0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou GW, Xiong Y, Chen S, Xia F, Li Q, Hu J. Anti‐PD‐1/PD‐L1 antibody therapy for pretreated advanced nonsmall‐cell lung cancer: a meta‐analysis of randomized clinical trials. Medicine. 2016;95:e4611. 10.1097/md.0000000000004611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. You W, Liu M, Miao JD, Liao YQ, Song YB, Cai DK, et al. A network meta‐analysis comparing the efficacy and safety of anti‐PD‐1 with anti‐PD‐L1 in non‐small cell lung cancer. J Cancer. 2018;9:1200–6. 10.7150/jca.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan PS, Aguiar P Jr, Haaland B, Lopes G. Comparative effectiveness of immune‐checkpoint inhibitors for previously treated advanced non‐small cell lung cancer ‐ a systematic review and network meta‐analysis of 3024 participants. Lung Cancer. 2018;115:84–8. 10.1016/j.lungcan.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 33. Ramos‐Esquivel A, van der Laat A, Rojas‐Vigott R, Juárez M, Corrales‐Rodríguez L. Anti‐PD‐1/anti‐PD‐L1 immunotherapy versus docetaxel for previously treated advanced non‐small cell lung cancer: a systematic review and meta‐analysis of randomised clinical trials. ESMO Open. 2017;2:e000236. 10.1136/esmoopen-2017-000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bozcuk H, Yıldırım M, Sever Ö, Mutlu H, Artaç M. Checkpoint inhibitors in advanced nonsmall‐cell lung cancer; a Bayesian network meta‐analysis. J Cancer Res Ther. 2020;16:828–37. 10.4103/jcrt.JCRT_450_19 [DOI] [PubMed] [Google Scholar]
- 35. Ohaegbulam KC, Assal A, Lazar‐Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD‐1 and PD‐L1 pathway. Trends Mol Med. 2015;21:24–33. 10.1016/j.molmed.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gangadhar TC, Salama AK. Clinical applications of PD‐1‐based therapy: a focus on pembrolizumab (MK‐3475) in the management of melanoma and other tumor types. Onco Targets Ther. 2015;8:929–37. 10.2147/ott.S53164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami‐Porta R, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 38. Port JL, Kent MS, Korst RJ, Libby D, Pasmantier M, Altorki NK. Tumor size predicts survival within stage IA non‐small cell lung cancer. Chest. 2003;124:1828–33. 10.1378/chest.124.5.1828 [DOI] [PubMed] [Google Scholar]
- 39. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death‐1 ligand 1 interacts specifically with the B7‐1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. 10.1016/j.immuni.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Butte MJ, Peña‐Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD‐L1 and B7‐1. Mol Immunol. 2008;45:3567–72. 10.1016/j.molimm.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The bias assessment of eligible trials.
Figure S2. Forest plots of heterogeneity analysis. Atez, Atezolizumab; Camre, Camrelizumab; Chemo, Chemotherapy; Durva, Durvalumab; Nivo, Nivolumab; Pembro, Pembrolizumab; Treme, Tremelimumab; Ipi, Ipilimumab
Figure S3. Funnel plots of publication bias. A, Atezolizumab; C/Ca, Camrelizumab; Ch, Chemotherapy; Du, Durvalumab; N/Nivo, Nivolumab; P, Pembrolizumab; T, Tremelimumab; I, Ipilimumab
Table S1. Retrieval strategy in Cochrane Library


