Abstract
Background
Studies on the application of targeted therapies for patients with non‐small cell lung cancer (NSCLC) who harbor rare genetic mutations are ongoing. In the present study, we investigated the real‐world data of NSCLC patients who harbor rare mutations.
Methods
We retrospectively analyzed patients with advanced or metastatic nonsquamous NSCLC aged >20 years with confirmed rare mutations (BRAF, ROS1, MET, RET, HER2, FGFR, and NTRK) from January 2015 to September 2020 at nine tertiary hospitals. In addition, we validated the lung cancer PCR panel kit in patients with confirmed mutations by NGS.
Results
Among 118 patients included, 88 received platinum‐based chemotherapy as first‐line chemotherapy. The progression‐free survival of patients with BRAF, ERBB2, MET, RET, and ROS1 mutations was 10.9 months (95% confidence interval [CI]: 1.3–20.5), 5.3 months (95% CI: 3.0–7.5), 7.2 months (95% CI: 3.6–10.9), 11.4 months (95% CI: 9.2–13.6), and 10.0 months (95% CI: 3.7–16.4) respectively (p = 0.041). The median overall survival (OS) was not reached in patients with ROS1 mutations; however, in BRAF, ERBB2, MET, and RET mutant patients, median OS was 14.1 months (95% CI: 10.1–14.1), 34.5 months (95% CI: 13.2–36.9), 22.7 months (95% CI: 1.7–24.0), and 29.8 months (95% CI: 28.9–61.3), respectively (p = 0.006). Of the 27 tissue samples, 26 (96.3%) showed the same PCR panel kit result with NGS.
Conclusions
First‐line platinum‐based chemotherapy showed durable benefit in patients with advanced or metastatic nonsquamous NSCLC harboring rare genetic mutation other than EGFR or ALK.
Keywords: chemotherapy, non‐small cell lung cancer, oncogene, platinum
This study shows real‐world data of NSCLC patients with rare genetic mutations, and the majority of patients were treated primarily with platinum‐based chemotherapy. First‐line platinum‐based chemotherapy showed durable benefit in patients with advanced or metastatic nonsquamous NSCLC harboring rare genetic mutations other than EGFR or ALK.

INTRODUCTION
In the treatment of non‐small cell lung cancer (NSCLC), several targetable oncogenes such as EGFR mutation, ALK translocation, ROS1 rearrangement, and BRAF mutations have been identified to date. In particular, about 60% of patients with lung adenocarcinoma have genetic mutations that can be targeted by specific targeted agents. 1 The EGFR mutation is found in 15%–20%, while the ALK translocation is found in 3%–7% of patients with lung adenocarcinoma. 2 Many studies have shown that tyrosine kinase inhibitors, which target EGFR or ALK mutations, have remarkable effectiveness in the treatment of lung adenocarcinoma. 3 Therefore, studies on the application of targeted therapies for patients with rare genetic mutations and also to find novel oncogenes that are potential therapeutic targets in NSCLC are ongoing. Consequently, in the latest treatment trend for patients with NSCLC who harbor ROS1, BRAF, NTRK1/2/3, MET, and RET mutations, the preferred first‐line therapy was changed to targeted therapy for advanced or metastatic disease. 4 , 5 Unlike systemic chemotherapy, targeted therapy is even recommended or selected by physicians for patients with poor performance status and for elderly patients in hospitals. Therefore, it is now crucial to investigate other rare mutations in addition to EGFR and ALK in NSCLC patients. 6 As per the guidelines, testing for EGFR, ALK, ROS1, and BRAF mutations is mandatory in newly diagnosed patients with metastatic NSCLC in most countries, and it has evolved to test RET, MET, HER2, and KRAS if possible. 4 To date, next generation sequencing (NGS) is used for testing rare mutations such as RET, MET, HER2, and KRAS, albeit it has the disadvantage of being time‐consuming, burdensome, and expensive. 7 Therefore, in the present study, we investigated the therapeutic response of conventional chemotherapy for patients with advanced or metastatic NSCLC with rare mutations in Korea, and we examined and validated lung cancer polymerase chain reaction (PCR) panel kit that confirms the various genetic mutations of NSCLC at once.
METHODS
Study design and patients
In this retrospective multicenter cohort study, we included patients aged over 20 years who were diagnosed or treated for nonsquamous NSCLC from January 2015 to September 2020 at nine tertiary hospitals in Korea. The patients had advanced or metastatic stage cancer with EGFR/ALK‐negative and positive for any other mutations; BRAF, ROS1, c‐MET, RET, HER2, FGFR, and NTRK by NGS or the PCR method. Patient characteristics, date of diagnosis, diagnostic methods, histological type, mutation type, presence of cerebral metastasis, types of chemotherapy regimens, response to chemotherapy, and survival status of patients were collected. We tested the lung cancer PCR panel kit in patients with confirmed mutations by NGS and had previously agreed to secondary use of remaining tissue samples. The results of the PCR panel were collected.
Pan lung cancer PCR panel kit
The 9‐in‐1 Lung Cancer PCR Panel (AmoyDx, Xiamen, China) is a quantitative real‐time PCR assay for detection of 167 hot spot alterations in EGFR, ALK, ROS1, KRAS, BRAF, HER2, RET, MET, NTRK1, NTRK2, and NTRK3. This kit contains a detection system of RNA gene fusion and DNA gene mutations. The RNA gene fusion detection includes two processes: (1) Reverse transcription: extracted RNA from formalin‐fixed paraffin‐embedded tissue or fresh tumor tissue is employed in this step. Reverse transcription of target RNA enables complementary DNA (cDNA) synthesis with the action of reverse transcriptase and specific primers. (2) PCR amplification: the specific primers are designed for amplification of cDNA, and ALK, ROS1, RET, MET, NTRK1, NTRK2, and NTRK3 variant amplicon is detected by fluorescent probes; the DNA gene mutation detection system uses specific primers and fluorescent probes to detect gene mutations. During amplification, the target mutant DNA is matched with the bases at the 3′ end of the primer, and amplified efficiently. Subsequently, the mutant amplicon is detected by fluorescent‐labeled probes. If the wild‐type DNA cannot be matched with specific primers, amplification does not occur. It is designed for detection of hotspot mutations in EGFR, HER2, KRAS, and BRAF genes. The turnaround time of the lung cancer panel PCR kit is 7–8 h.
Statistical analysis
Variables are presented as either the mean with standard deviation or median with an interquartile range (IQR), as appropriate. Survival analyses were performed using the Kaplan–Meier method and the log‐rank test. All p‐values were two‐tailed, and statistical significance was set at a p < 0.05. Statistical Package for the Social Sciences (version 25.0; IBM Corp.) and MedCalc (version 20.0; MedCalc Software Inc.) were used for all statistical analyses.
RESULTS
In the present study, we analyzed the data of 118 patients diagnosed with advanced or metastatic nonsquamous NSCLC and harboring rare mutations confirmed by PCR or NGS were analyzed. The median age was 61 years, the proportion of male patients was 44.1%, and 39% of patients had a smoking history (Table 1). Seventeen patients (14.4%) had cerebral metastasis at initial diagnosis. Among the 118 included patients, 46 (39%) had ROS1, 27 (22.9%) had RET, 14 (11.9%) had MET, 14 (11.9%) had ERBB2, 10 (8.5%) had BRAF, five (4.2%) had FGFR and two (1.7%) had NTRK mutations. Eighty‐one patients (68.6%) were diagnosed by NGS, while 37 (37%) were diagnosed by RT‐PCR. All the patients diagnosed by RT‐PCR had ROS1 mutations. A total of 107 patients received palliative chemotherapy, and 88 received platinum‐based chemotherapy as first‐line chemotherapy. Among them, 58 patients received platinum‐pemetrexed. Thirteen patients received crizotinib as first‐line chemotherapy and all patients had ROS 1 mutation. Table S1 shows the baseline characteristics of patients stratified by mutation type. In patients with the ERBB2 mutation, the median age was 48 years, which was younger than that of other mutations; however, in patients with the MET mutation, the median age was 71.5 years, which was older than that of other mutations (p < 0.001). No significant difference was observed in terms of sex, state of cerebral metastasis, and PD‐L1 expression at the initial diagnosis between mutation types, but there was a higher proportion of female patients with the ERBB2 mutation (78.6%).
TABLE 1.
Patient characteristics
| Total (n = 118) | |
|---|---|
| Age, median (IQR) | 61 (54–71.25) |
| Male sex, n (%) | 52 (44.1) |
| Ever smoking, n (%) | 46 (39) |
| Histopathological type, n (%) | |
| Adenocarcinoma | 112 (94.9) |
| Others | 6 (5.1) |
| Mutation type, n (%) | |
| ROS1 | 46 (39) |
| RET | 27 (22.9) |
| MET | 14 (11.9) |
| ERBB2 | 14 (11.9) |
| BRAF | 10 (8.5) |
| FGFR | 5 (4.2) |
| NTRK | 2 (1.7) |
| Diagnostic method, n (%) | |
| NGS | 81 (68.6) |
| PCR | 37 (31.4) |
| Brain metastasis at diagnosis, n (%) | 17 (14.4) |
| Palliative first‐line chemotherapy | n = 107 |
| Platinum based therapy, n (%) | 88 (82.2) |
| Platinum + pemetrexed | 58 (54.2) |
| Platinum + others | 30 (28.4) |
| Crizotinib, n (%) | 13 (12.1) |
| Others, n (%) | 6 (5.6) |
Abbreviations: IQR, interquartile range; NGS, next‐generation sequencing; PCR, polymerase chain reaction.
Median progression‐free survival (PFS) and overall survival (OS) were compared in patients with the ROS1, RET, MET, ERBB2, and BRAF mutations who received palliative first‐line platinum‐based chemotherapy. The median follow‐up duration was 15.8 months (IQR 7.5–27.8). The median PFS and OS of five mutations treated with first‐line platinum base chemotherapy was 9.6 months (95% confidence interval [CI]: 7.7–11.5) and 36.9 months (95% CI: 11.3–62.6). The median PFS of patients with BRAF, ERBB2, MET, RET, ROS1 mutations was 10.9 months (95% CI: 1.3–20.5), 5.3 months (95% CI: 3.0–7.5), 7.2 months (95% CI: 3.6–10.9), 11.4 months (95% CI: 9.2–13.6), and 10.0 months (95% CI: 3.7–16.4), respectively (p = 0.041) (Figure 1). Patients with the ROS1 mutation did not attain the median OS; however, in patients with BRAF, ERBB2, MET, and RET mutations, median OS was 14.1 months (95% CI: 10.1–14.1), 34.5 months (95% CI: 13.2–36.9), 22.7 months (95% CI: 1.7–24.0), and 29.8 months (95% CI: 28.9–61.3), respectively (p = 0.006) (Figure 2). The median PFS of patients with ROS1 who received first‐line platinum‐based chemotherapy or crizotinib was 10.0 months (95% CI: 3.7–16.4) versus not reached, respectively (p = 0.399) (Figure S1). The total objective response rate for first‐line platinum‐based chemotherapy was 37.0% (95% CI: 26.6–48.5) and the disease control rate was 77.8% (95% CI: 67.2–86.3) (Table 2).
FIGURE 1.
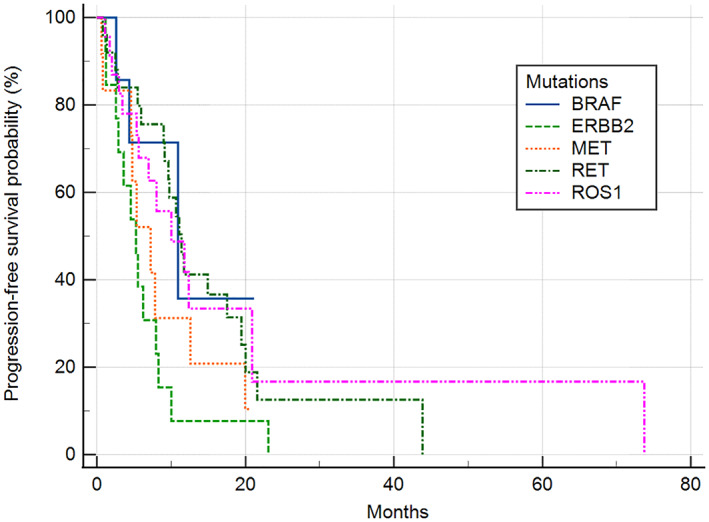
Progression‐free survival of patients harboring BRAF, ERBB2, MET, RET or ROS1 treated with palliative first‐line platinum‐based chemotherapy. The median PFS of patients with each mutation was 10.9 months (95% CI: 1.3–20.5) in BRAF, 5.3 months (95% CI: 3.0–7.5) in ERBB2, 7.2 months (95% CI: 3.6–10.9) in MET, 11.4 months in RET (95% CI: 9.2–13.6), and 10.0 months (95% CI: 3.7–16.4) in ROS1 (p = 0.041). PFS, progression‐free survival
FIGURE 2.
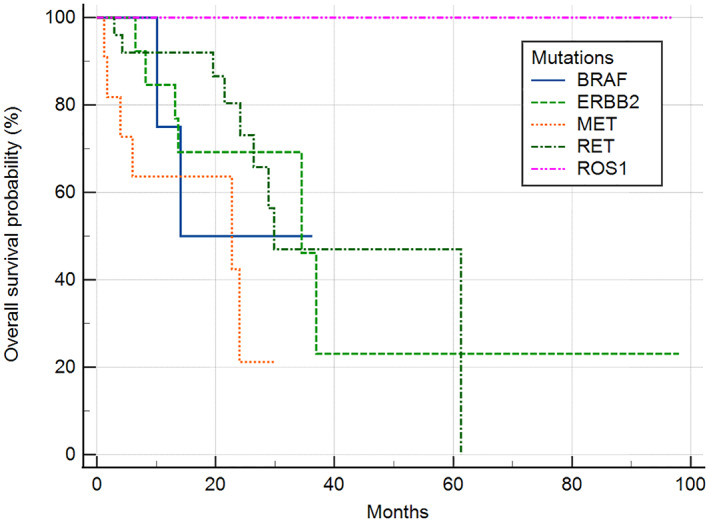
Overall survival of patients harboring BRAF, ERBB2, MET, RET, or ROS1 who were treated with palliative first‐line platinum‐based chemotherapy. The median OS was not reached in patients with ROS1 mutations. In patients with BRAF, ERBB2, MET, and RET mutations, median OS was 14.1, 34.5, 22.7, and 29.8 months, respectively (p = 0.006). OS, overall survival
TABLE 2.
Response to first‐line platinum‐based chemotherapy
| ORR (CR + PR) | DCR (CR + PR + SD) | |
|---|---|---|
| BRAF | 0% (n = 0/8) | 75% (n = 6/8) |
| ERBB2 | 53.8% (n = 7/13) | 76.9% (n = 10/13) |
| MET | 25% (n = 3/12) | 66.7% (n = 8/12) |
| RET | 52% (n = 13/25) | 80% (n = 20/25) |
| ROS1 | 30.4% (n = 7/23) | 82.6% (n = 19/23) |
| Total | 37.0% (30/81) | 77.8% (n = 63/81) |
Abbreviations: CR, complete response; DCR, disease control rate; ORR, objective response rate; PR, partial response; SD, stable disease.
In total, the tumor samples of 35 patients were tested with the lung cancer PCR panel kit and among them, the samples of eight patients were substandard because of concentrations that were too low or because the samples had been collected too long ago (Table S2). Of the 27 samples, 26 showed the same result with NGS (96.3%). One tumor sample with ERBB2 mutations did not show the result despite a sufficient concentration.
DISCUSSION
For patients who lacked targetable genetic alteration like EGFR and ALK mutations, platinum‐based chemotherapy was the recommended first‐line chemotherapy before the introduction of pembrolizumab. 8 Likewise, patients with rare mutations other than EGFR and ALK, who do not have options for available targeted drugs, were commonly treated with platinum‐based chemotherapy in clinics. However, to date, few studies have reported the efficacy of this treatment in these patients. In this study, we evaluated the PFS and OS of patients with five rare mutations who received platinum‐based chemotherapy, and apart from patients with an ERBB2 alteration, the patients with the BRAF, MET, RET, and ROS1 alteration had a median PFS of more than 6 months. In a previous retrospective study of 104 patients treated with pemetrexed‐based chemotherapy including 79.1% of pemetrexed‐platinum combination, the median PFS was reported to be 19 months in RET, 23 months in ROS1, 19 months in ALK, and 6 months in patients with the KRAS mutation. 9 In another clinical study comparing crizotinib and platinum‐pemetrexed in ROS1, the median PFS of patients on platinum‐pemetrexed was 8.6 months. 10 Patients with V600E BRAF and non‐V600E BRAF mutations who received platinum‐based chemotherapy showed 4.1 and 8.9 months of median PFS, respectively, in one observational study. 11 The total objective response rate (ORR) was comparable to the reported value of a systematic meta‐analysis of first‐line platinum‐pemetrexed chemotherapy in advanced NSCLC. 12 Therefore, studies on rare mutations including our cohort showed some durable benefit of platinum‐based chemotherapy, so this regimen can be considered first for patients with advanced or metastatic NSCLC harboring rare mutations but who do not have options of available targeted drugs.
As many recent studies showed that targeted drugs have a better survival outcome than conventional chemotherapy, or showed good outcome for patients treated after platinum‐based chemotherapy failure, the updated National Comprehensive Cancer Network guideline recommends performing molecular diagnostic studies of EGFR, KRAS, ALK, ROS1, BRAF, NTRK, MET, and RET. In case of insufficient tissue, molecular biomarker analysis should be performed even if the biopsy is repeated. 13 In the present study, there was a significant difference in median OS between patients with the ROS1 mutation and those with other mutations. All the ROS‐1 mutant patients were alive during the entire follow‐up period and among them, 47.8% with the ROS1 mutation were sequentially treated with crizotinib after platinum‐based chemotherapy failure. Therefore, the increased OS of ROS1‐mutant patients can be explained by the effect of crizotinib. In a previous study of 50 ROS1‐positive patients, crizotinib showed good antitumor activity with an ORR of 72% and a median PFS of 19.2 months. 14 Recently, new targeted drugs such as ceritinib and entreactinib have been studied, 15 because crizotinib resistance often occurs, making the treatment of ROS1‐positive lung cancer more difficult. Entrectinib is known to penetrate and remain in the central nervous system, so has good effect on intracranial metastasis. 16
RET mutations are identified in 1%–2% of NSCLC and are mainly found in young, nonsmoker patients with adenocarcinoma and are known to be at a high risk of cerebral metastasis. 17 In the present study, 27 patients with RET mutation who had been treated with first‐line platinum‐based chemotherapy showed 11.4 months of median PFS and 52% of ORR. Cerebral metastasis at initial diagnosis was reported in 11.1% but increased to 29.6% during treatment. The antitumor effects of selpercatinib and pralsetinib were demonstrated in RET fusion‐positive NSCLC. In a previous study of selpercatinib, the ORR was 64% and 85% in patients with RET mutation who had previously been treated with platinum‐based chemotherapy and treatment‐naïve patients, respectively. 18 In a study of pralsetinib, the ORR was 61% and 70% in patients who had previously been treated with platinum‐based chemotherapy and treatment‐naïve patients, respectively. 19 Both targeted drugs also have an antitumor effect on intracranial tumors because they cross the blood–brain barrier. 20 Therefore, these targeted drugs could be a good option for patients with cerebral metastasis from the initial diagnosis or during chemotherapy. ERBB2 (human epidermal growth factor receptor 2, HER2) is reported in 3% of nonsquamous NSCLC and is associated with female sex, nonsmoker, and young age. In the present cohort, patients with the ERBB2 mutation were youngest, had the highest proportion of females, and showed poor PFS than patients with other mutations. A study of 91 which previously treated HER2‐mutant NSCLC patients confirmed the antitumor activity of trastuzumab plus deruxtecan. The ORR was 55%, the median PFS and OS were 8.2 and 17.8 months, respectively. 21 Ado‐trastuzumab showed 44% of partial response in a group of 18 patients with an average of two previous treatments. 22
We performed a lung cancer PCR panel kit in 35 patients who had remaining samples after NGS testing. Except for eight cases with sample quality problems, the concordance of PCR panel results with NGS was 96.3%. In one case diagnosed with ERBB2 mutation in NGS but negative on the PCR panel, NGS was additionally performed with the same sample, and G776delinsCV mutation was confirmed. The PCR panel does not cover this gene, and also it is not identified in COSMIC. 23 It is not a functionally validated gene but may have oncogenic potential. Of the eight samples with a quality problem, three were obtained more than 2 years ago. There can be a problem with DNA/RNA degradation acquired more than 2 years prior. 24 The concentration of five samples was smaller than recommended because of exhaustion of the tumor part in the sample. NGS is used to verify the results of RT‐PCR and vice‐versa. 25 The sensitivity of RT‐PCR is higher than that of NGS in detecting target genes, and the amount of tissue required for RT‐PCR is smaller than that required for NGS. In addition, NGS is a high‐cost test and not suitable as a screening test and takes a longer time than PCR. The lung cancer PCR panel kit is a test that can make up for these shortcomings. It costs less than NGS, and most laboratories have RT‐PCR workflow; therefore, the test is less burdensome and proceeds quickly despite its high specificity and sensitivity. Therefore, the PCR panel kit can be performed preferentially in patients requiring mutation testing. This kit has previously been validated by Matsumoto et al. It showed a higher success rate and short turnaround time than NGS. 26
We retrospectively collected cases with rare mutations from nine centers; nevertheless, the number was still small, and the follow‐up duration was short, so there was a limit to compare the characteristics of each mutation. In addition, the PCR panel kit was validated by collecting only the remaining tissues of the patients who agreed to their secondary use. Consequently, PCR detection was not done for all patients and some of the tissue qualities were low. Nevertheless, the present study shows the real‐world data of patients with advanced NSCLC with rare mutations in Korea. Although various targeted therapies for NSCLC patients with rare mutations are being studied and approved, only a few of the drugs are actually used in clinical practice, and most of the patients were treated with conventional chemotherapy during enrollment. This is a meaningful study showing the durable benefit of platinum‐based chemotherapy and the importance of developing novel biomarkers and introducing targeted drugs in patients with advanced NSCLC and rare mutations.
In conclusion, first‐line platinum‐based chemotherapy showed durable benefit in patients with advanced or metastatic nonsquamous NSCLC harboring rare genetic mutations other than EGFR or ALK.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL STATEMENT
The study protocol was reviewed and approved by the Institutional Review Board at the Asan Medical Center (IRB #2020‐1516), which waived the requirement for informed consent because of the retrospective study design.
Supporting information
Table S1 Baseline characteristics of patients stratified by mutation type
Table S2. Validation of the lung cancer PCR panel kit in patients with confirmed rare mutations by next‐generation sequencing
Figure S1. Progression‐free survival of patients harboring ROS1 mutation treated with first line crizotinib or platinum‐based chemotherapy.
ACKNOWLEDGMENTS
This research was supported by Korean Association for the Study of Targeted Therapy (KASTT) affiliated with Korean Association for Lung Cancer (KASTT‐20190121).
Lee SY, Kim YC, Lee KY, Lee SY, Lee SY, Lee MK, et al. Multicenter real‐world data of patients harboring rare mutations other than EGFR or ALK in advanced or metastatic non‐small cell lung cancer. Thorac Cancer. 2022;13:380–385. 10.1111/1759-7714.14266
Funding information Korean Association for the Study of Targeted Therapy (KASTT), Grant/Award Number: KASTT‐20190121
REFERENCES
- 1. Tsao AS, Scagliotti GV, Bunn PA Jr, Carbone DP, Warren GW, Bai C, et al. Scientific advances in lung cancer 2015. J Thorac Oncol. 2016;11(5):613–38. [DOI] [PubMed] [Google Scholar]
- 2. Shea M, Costa DB, Rangachari D. Management of advanced non‐small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches. Ther Adv Respir Dis. 2016;10(2):113–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vu P, Patel SP. Non‐small cell lung cancer targetable mutations: present and future. Precis Cancer Med. 2020;3:5. [Google Scholar]
- 4. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre‐Finn C, et al. Metastatic non‐small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29(Suppl 4):iv192–237. [DOI] [PubMed] [Google Scholar]
- 5. National Comprehensive Cancer Network . Non‐small cell lung cancer (Version 5. 2021). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 6. Carmichael JA, Wing‐San Mak D, O'Brien M. A review of recent advances in the treatment of elderly and poor performance NSCLC. Cancers (Basel). 2018;10(7):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng YW, Stefaniuk C, Jakubowski MA. Real‐time PCR and targeted next‐generation sequencing in the detection of low level EGFR mutations: instructive case analyses. Respir Med Case Rep. 2019;28:100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 9. Drilon A, Bergagnini I, Delasos L, Sabari J, Woo KM, Plodkowski A, et al. Clinical outcomes with pemetrexed‐based systemic therapies in RET‐rearranged lung cancers. Ann Oncol. 2016;27(7):1286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen L, Qiang T, Li Z, Ding D, Yu Y, Lu S. First‐line crizotinib versus platinum‐pemetrexed chemotherapy in patients with advanced ROS1‐rearranged non‐small‐cell lung cancer. Cancer Med. 2020;9(10):3310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cardarella S, Ogino A, Nishino M, Butaney M, Shen J, Lydon C, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non‐small cell lung cancer. Clin Cancer Res. 2013;19(16):4532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiao HQ, Tian RH, Zhang ZH, Du KQ, Ni YM. Efficacy of pemetrexed plus platinum doublet chemotherapy as first‐line treatment for advanced nonsquamous non‐small‐cell‐lung cancer: a systematic review and meta‐analysis. Onco Targets Ther. 2016;9:1471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Non‐Small Cell Lung Cancer . NCCN National Comprehensive Cancer Network 2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 14. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371(21):1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Angelo A, Sobhani N, Chapman R, Bagby S, Bortoletti C, Traversini M, et al. Focus on ROS1‐positive non‐small cell lung cancer (NSCLC): crizotinib, resistance mechanisms and the newer generation of targeted therapies. Cancer. 2020;12(11):3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drilon A, Siena S, Dziadziuszko R, Barlesi F, Krebs MG, Shaw AT, et al. Entrectinib in ROS1 fusion‐positive non‐small‐cell lung cancer: integrated analysis of three phase 1‐2 trials. Lancet Oncol. 2020;21(2):261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stinchcombe TE. Current management of RET rearranged non‐small cell lung cancer. Ther Adv Med Oncol. 2020;12:1758835920928634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, et al. Efficacy of selpercatinib in RET fusion‐positive non‐small‐cell lung cancer. N Engl J Med. 2020;383(9):813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gainor JF, Curigliano G, Kim DW, Lee DH, Besse B, Baik CS, et al. Pralsetinib for RET fusion‐positive non‐small‐cell lung cancer (ARROW): a multi‐cohort, open‐label, phase 1/2 study. Lancet Oncol. 2021;22(7):959–69. [DOI] [PubMed] [Google Scholar]
- 20. Drusbosky LM, Rodriguez E, Dawar R, Ikpeazu CV. Therapeutic strategies in RET gene rearranged non‐small cell lung cancer. J Hematol Oncol. 2021;14(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2‐mutant non‐small‐cell lung cancer. N Engl J Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado‐trastuzumab emtansine for patients with HER2‐mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018;36(24):2532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. COSMIC 2021. http://www.sanger.ac.uk/genetics/CGP/cosmic/.
- 24. Groelz D, Viertler C, Pabst D, Dettmann N, Zatloukal K. Impact of storage conditions on the quality of nucleic acids in paraffin embedded tissues. PLoS One. 2018;13(9):e0203608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuononen K, Mäki‐Nevala S, Sarhadi VK, Wirtanen A, Rönty M, Salmenkivi K, et al. Comparison of targeted next‐generation sequencing (NGS) and real‐time PCR in the detection of EGFR, KRAS, and BRAF mutations on formalin‐fixed, paraffin‐embedded tumor material of non‐small cell lung carcinoma‐superiority of NGS. Genes Chromosomes Cancer. 2013;52(5):503–11. [DOI] [PubMed] [Google Scholar]
- 26. Matsumoto S, Ikeda T, Zenke Y, Kato T, Sugawara S, Nishino K, et al. P89.06 prospective concordance study of a multi‐gene PCR assay and NGS for the detection of targetable gene alterations in lung cancer. J Thorac Oncol. 2021;16(3):S690. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline characteristics of patients stratified by mutation type
Table S2. Validation of the lung cancer PCR panel kit in patients with confirmed rare mutations by next‐generation sequencing
Figure S1. Progression‐free survival of patients harboring ROS1 mutation treated with first line crizotinib or platinum‐based chemotherapy.


