Abstract
Objective
Small cell lung cancer (SCLC) is one of the most aggressive malignancies characterized by neuroendocrine (NE) differentiation. The Delta‐like protein 3 (DLL3), as a direct downstream target of ASCL1, is involved in NE differentiation and carcinogenesis of SCLC. This study aims to investigate the relationship between ASCL1 and DLL3 expressions and their clinicopathological implications in SCLC.
Methods
A total of 247 surgically resected pure SCLC samples with limited clinical stage and follow‐up data were retrieved in this retrospective study. ASCL1 and DLL3 protein expression was detected by immunohistochemistry staining. The correlations between ASCL1 and DLL3 expressions, as well as their clinicopathological features, were analyzed by χ2 tests. Disease‐free survival (DFS) and overall survival (OS) in SCLC patients with ASCL1/DLL3 low and high expressions were compared by the Kaplan‐Meier method and log‐rank tests.
Results
ASCL1 high expression was detected in 105 (42.5%) patients. Its expression was positively correlated with the clinical stage (p = 0.02) and nerve invasion (p = 0.03). DLL3 high expression was observed in 188 (72.8%) patients and was correlated with vascular invasion (p = 0.04). ASCL1 expression was positively associated with DLL3 expression (p = 0.03). In addition, DLL3 expression has a strong correlation with the expression of thyroid transcription factor‐1 (TTF1) and conventional NE markers.
Conclusion
ASCL1 and DLL3 were highly expressed in SCLC tumor samples, and a positive correlation between these two markers was observed. Co‐analysis of ASCL1 and DLL3 may identify a distinct SCLC subgroup benefit from targeted therapy. Therefore, ASCL1 and DLL3 could be potential biomarkers served for the selection of related patients.
Keywords: ASCL1, DLL3, SCLC small cell lung cancer
We detected ASCL1 and DLL3 protein expression in 247 small cell lung cancer patients by immunohistochemistry staining, both the two markers were highly expressed in tumors. ASCL1 expression was positively associated with DLL3 expression, and DLL3 expression has a strong correlation with expression of TTF1, Syn, CgA and CD56.
INTRODUCTION
Small cell lung cancer (SCLC) is one of the most aggressive malignancies with neuroendocrine (NE) differentiation and poor prognosis, accounting for 15% of all lung cancers. 1 For decades, traditional chemotherapy and localized radiotherapy have been the predominant therapies for SCLC. Although SCLC is sensitive to the initial chemotherapy and radiotherapy, it has a high rate of relapse and resistance to traditional drugs. Therefore, seeking other forms of treatment modalities is desperately necessary.
Emerging data revealed that SCLC was a considerable heterogeneous tumor in terms of histomorphology, molecular changes, and growth features. Several recent studies applying SCLC primary human tumors, xenografts, and cell lines have attempted to divide SCLC into distinct molecular subtypes, including ASCL1‐high, NEUROD1‐high, POU2F3, and YAP1 subtypes. 2 , 3 The most well‐established subgroup is the ASCL1‐high group, representing the predominant subgroup of SCLC with high expression of NE markers. 4 , 5 , 6 ASCL1 is expressed in most SCLC tumors and supposed to be closely correlated with NE differentiation. 7
Notch‐ASCL1 signaling pathway plays an important role in the maintenance of NE phenotype and carcinogenesis of SCLC. DLL3 is an inhibitory ligand of the Notch pathway and results in the inactivation of Notch pathway. As a regulator, ASCL1 can enhance the expression of its downstream target Delta‐like protein 3 (DLL3). 8 Recently, DLL3‐targeted antibody‐drugs, rovalpituzumab tesirine (Rova‐T), bispecific antibody, and chimeric antigen receptor‐modified T cells (CAR‐T) have been used in several clinical trials, 9 , 10 , 11 demonstrating a potential prospect in SCLC treatment. Combined investigation of ASCL1 and DLL3 expression could be helpful for a selection of subgroup of patients who would benefit from these targeted therapies.
Analysis of both ASCL1 and DLL3 in SCLC patient samples has been only reported in one recent study, 12 which investigated 95 surgically resected SCLC samples, including combined SCLC. However, our recently published studies revealed distinct prognostic impact factors 13 and YAP1 protein expression between pure SCLC and combined SCLC. 14 This suggests that combined SCLC and pure SCLC might be different entities and present different targetable oncogenic pathways. Therefore, in this study, we enrolled a large scale of 247 surgically resected pure SCLC samples (without combined non‐small cell lung cancer component). All the samples were pure SCLC cases with limited clinical stages, and we aimed to clarify the relationship between ASCL1 and DLL3 expressions and their clinicopathological implications for SCLC patients.
METHODS
Sample selection
The study was approved by the ethics committee and institutional review board of Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS), and all patients were exempt from informed consent. A total of 247 surgically resected pure SCLC samples were retrieved in this retrospective study. They were primarily diagnosed in CHCAMS during the period of January 2005 and December 2016. Sample inclusion criteria were used as described in our previous study 15 : (1) surgically radical excision specimen of SCLC with or without systemic lymph node dissection; (2) histologically confirmed to be pure SCLC without any combined component; and (3) clinically proven to be pulmonary primary tumor when metastasis was excluded. Tumors were staged and accessed according to the seventh edition of the American Joint Committee on Cancer (AJCC). General clinical information and follow‐up data were obtained from the medical records system.
Reassessment of clinicopathological characteristics
All archival slides of hematoxylin and eosin (H&E) and immunohistochemical (IHC) staining from the 247 SCLC samples were retrieved and reevaluated by two senior thoracic pathologists. The diagnosis of pure SCLC was confirmed by pathological morphology and staining of several conventional NE biomarkers. Clinicopathological characteristics were also reevaluated and recorded, including invasion of bronchus, vessels, nerves and pleura, proportion of necrosis and fibrosis, regional lymph node metastasis, spread through air spaces (STAS), and tumor infiltrating lymphocytes (TILs).
IHC staining and scoring criteria
IHC staining for conventional NE markers including synaptophysin (SYN, SP11, MXB Biotechnologies), chromogranin A (CgA, LK2H10+PHE5, MXB Biotechnologies), CD56 (MX039, MXB Biotechnologies), and TTF1 (SP141, Roche) was performed on formalin‐fixed paraffin‐embedded (FFPE) tissues during routine clinical practices. ASCL1 (ab74065, Abcam) and DLL3 (E3J5R, Cell Signal Technology) staining was examined on SCLC tissue microarrays. The SCLC tissue microarrays of the 247 cases were constructed as described previously. 15 All the staining was carried out on the fully automatic Roche IHC instruments (Roche Diagnosis) according to the manufacturer's protocols.
ASCL1 expression was localized in the nucleus of SCLC tumor cells, whereas DLL3 expression was localized in the cytoplasm and membrane of tumor cells. For staining pattern of conventional NE markers, cytoplasm for SYN and CgA, membrane for CD56, and nucleus for transcription termination factor 1 (TTF1) in tumor cells were evaluated to be positive. Both the intensity and proportion of positive tumor cells were taken into account and the scoring criteria were used as described in the previous study. 16 Percentages of stained tumor cells (0%–100%) and four‐level intensity of staining (0 = negative, 1 = weak, 2 = moderate, and 3 = strong) were recorded, respectively. A histoscore (H‐Score) with a range of 0–300 was produced by multiplying the score of percentage (0–100) by the intensity of stained tumor cells (0, 1, 2, and 3). The expression level was further classified as high and low according to the best cut‐off value determined by X‐tile software.
Follow‐up data
Overall survival (OS) was calculated during the time from the first diagnosis to death regardless of any cause. Disease‐free survival (DFS) was defined during the time from start of treatment to documentation of any progression such as recurrence or metastasis. Follow‐up was completed in February 2019. A total of 212 patients had complete follow‐up data, and 35 patients (14.2%) were lost to follow‐up.
Statistical analysis
The correlation between ASCL1 and DLL3 protein expressions, their expression and clinicopathological features as well as conventional NE scores were analyzed by χ2 tests and Fisher's exact tests. OS and DFS in SCLC patients with ASCL1/DLL3 low and high expressions were compared by using the Kaplan‐Meier method and log‐rank test. Univariate and multivariate analysis was carried out in the Cox proportional hazards model. All differences were considered as statistically significant if p value <0.05. Statistical analysis was performed using SPSS version 23.0 (SPSS).
RESULTS
Patient and sample characteristics
Among the entire cohort of the 247 surgically resected pure SCLC patients, 175 (70.9%) were male, 202 (81.8%) were <65 years, and 158 (64.0%) had a history of smoking. According to the 7th edition of AJCC Cancer Staging Manual, 78 (31.6%) were stage I, 68 (27.5%) were stage II, and 101 (40.9%) were stage III. A total of 143 (57.9%) patients showed regional lymph node metastasis (Table 1).
TABLE 1.
Relationship between clinicopathological characteristics and ASCL1/DLL3 expressions
| Characteristics | Patients (N = 247) | ASCL1 expression | p value | DLL3 expression | p value | ||
|---|---|---|---|---|---|---|---|
| Low (%) | High (%) | Low (%) | High (%) | ||||
| Gender | |||||||
| Male | 175 | 101(71) | 74(70) | 1.00 | 44(75) | 131(70) | 0.52 |
| Female | 72 | 41(29) | 31(30) | 15(25) | 57(30) | ||
| Age | |||||||
| ≤65 | 202 | 115(81) | 87(83) | 0.74 | 48(81) | 154(82) | 1.00 |
| >65 | 45 | 27(19) | 18(17) | 11(19) | 34(18) | ||
| Smoking | |||||||
| Yes | 158 | 90(63) | 68(65) | 0.89 | 41(69) | 117(62) | 0.35 |
| No | 89 | 52(37) | 37(35) | 18(31) | 71(38) | ||
| Tumor location | |||||||
| Left lung | 122 | 74(52) | 48(46) | 0.37 | 35(59) | 87(46) | 0.10 |
| Right lung | 125 | 68(48) | 57(54) | 24(41) | 101(54) | ||
| Clinical stage | |||||||
| I | 78 | 47(33) | 31(30) | 0.02* | 21(36) | 57(30) | 0.28 |
| II | 68 | 47(33) | 21(20) | 19(32) | 49(26) | ||
| III | 101 | 48(34) | 53(50) | 19(32) | 82(44) | ||
| Vascular tumor thrombus | |||||||
| Yes | 125 | 76(54) | 49(47) | 0.31 | 35(59) | 87(46) | 0.10 |
| No | 122 | 66(46) | 56(53) | 24(41) | 101(54) | ||
| Pleural invasion | |||||||
| Yes | 170 | 91(64) | 79(75) | 0.07 | 42(71) | 128(68) | 0.75 |
| No | 77 | 51(36) | 26(25) | 17(29) | 60(32) | ||
| Lymph node metastasis | |||||||
| Yes | 143 | 75(53) | 68(65) | 0.07 | 28(47) | 115(61) | 0.07 |
| No | 104 | 67(47) | 37(35) | 31(53) | 73(39) | ||
| Bronchus invasion | |||||||
| Yes | 210 | 119(84) | 91(87) | 0.59 | 50(85) | 160(85) | 1.00 |
| No | 37 | 23(16) | 14(13) | 9(15) | 28(15) | ||
| Vascular invasion | |||||||
| Yes | 206 | 117(82) | 89(85) | 0.73 | 44(75) | 162(86) | 0.045* |
| No | 41 | 25(18) | 16(15) | 15(25) | 26(14) | ||
| Nerve invasion | |||||||
| Yes | 84 | 40(28) | 44(42) | 0.03* | 18(31) | 66(35) | 0.64 |
| No | 163 | 102(72) | 61(58) | 41(69) | 122(65) | ||
| STAS | |||||||
| Yes | 182 | 104(73) | 78(74) | 0.89 | 40(68) | 142(76) | 0.24 |
| No | 65 | 38(27) | 27(26) | 19(32) | 46(24) | ||
| Necrosis proportion | |||||||
| ≤30% | 172 | 97(68) | 75(71) | 0.68 | 37(63) | 135(72) | 0.20 |
| >30% | 75 | 45(32) | 30(29) | 22(37) | 53(28) | ||
| Fibrosis proportion | |||||||
| ≤10% | 116 | 75(53) | 41(39) | 0.04* | 30(51) | 86(46) | 0.55 |
| >10% | 131 | 67(47) | 64(61) | 29(49) | 102(54) | ||
| TILs | |||||||
| ≤30% | 212 | 124(87) | 88(84) | 0.46 | 49(83) | 163(87) | 0.52 |
| >30% | 35 | 18(13) | 17(16) | 10(17) | 25(13) | ||
p value <0.05.
Expression of ASCL1/DLL3 and correlation with clinicopathological features
ASCL1 expression was localized in the nucleus of SCLC tumor cells (Figure 1(a)–(c)), and 105 (42.5%) patients showed high expression. ASCL1 high expression was associated with clinical stage (p = 0.02) and nerve invasion (p = 0.03). DLL3 expression was localized in the cytoplasm and membrane of tumor cells (Figure 1(d)–(f)), and DLL3 high expression was observed in 188 (72.8%) patients. DLL3 high expression was correlated with vascular invasion (p = 0.04). No significant difference was found in ASCL1/DLL3 expression and the remaining clinicopathological features. Co‐expression of ASCL1/DLL3 was observed in 124 (50.2%) patients, and 87 of which showed co‐high expression of these two markers. Subsequently, we analyzed the relationship between ASCL1 and DLL3 expression in SCLC, and a significantly positive association was observed (p = 0.035, Table 2).
FIGURE 1.
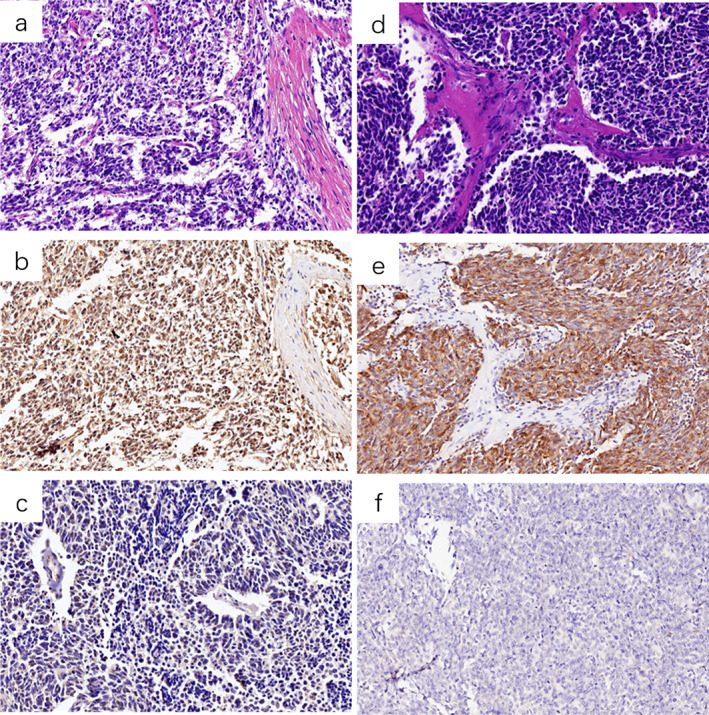
Representative slides of SCLC morphology and ASCL1/DLL3 expression level (×200). (a) and (b) SCLC H&E staining and the corresponding ASCL1 high expression, respectively, (c) ASCL1 low expression; (d) and (e) SCLC H&E staining and the corresponding DLL3 high expression, respectively, (f) DLL3 low expression
TABLE 2.
Relationship between ASCL1 and DLL3 expressions
| DLL3 expression | Patients (N = 247) | ASCL1 expression | p value | |
|---|---|---|---|---|
| Low (%) | High (%) | |||
| Low | 59 | 41(29) | 18(17) | 0.035* |
| High | 188 | 101(71) | 87(83) | |
p value <0.05.
ASCL1/DLL3 expression and correlation with conventional NE markers
Because ASCL1/DLL3 was highly expressed in SCLC tumor cells, we next examined the correlation between ASCL1/DLL3 expression and conventional NE markers (Syn, CgA, CD56, and TTF1). According to the H‐Score of these markers, their expressions were divided into low and high expression groups. Expression levels of DLL3 had a strongly positive association with those of Syn (p = 0.01), CgA (p = 0.02), CD56 (p < 0.01), and TTF1 (p < 0.01), as shown in Table 3. However, expression of ASCL1 was only positively correlated with CgA (p = 0.031), but not correlated with Syn, CD56, and TTF1 (p > 0.05).
TABLE 3.
Relationship between expressions of DLL3 and conventional NE markers
| NE marker | Cases | DLL3 expression | p value | |
|---|---|---|---|---|
| Low (%) | High (%) | |||
| CD56 expression | n = 207 | |||
| Low | 67 | 30(58) | 37(24) | <0.01* |
| High | 140 | 22(42) | 118(76) | |
| ChrA expression | n = 213 | |||
| Low | 134 | 40(77) | 94(58) | 0.02* |
| High | 79 | 12(23) | 67(42) | |
| Syn expression | n = 213 | |||
| Low | 72 | 28(55) | 44(27) | <0.01* |
| High | 141 | 23(45) | 118(73) | |
| TTF1 expression | n = 189 | |||
| Low | 46 | 31(66) | 15(11) | <0.01* |
| High | 143 | 16(34) | 127(89) | |
p value <0.05.
ASCL1/DLL3 expression and patients’ outcome
All the 247 SCLC patients were followed up, with a median follow‐up time of 48 months ranging from 0 to 167 months. Univariate analysis revealed that patients with ASCL1 high expression had a worse OS (p = 0.047), whereas ASCL1 expression was not associated with DFS (p > 0.05). No significant difference was found in DLL3 expression level and patients OS/DFS (p > 0.05). Further multivariate cox regression analysis showed neither ASCL1 nor DLL3 expression was an independent prognostic factor (p > 0.05).
DISCUSSION
SCLC is a highly aggressive cancer with poor prognosis. Although most patients are sensitive to the initial chemotherapy and radiotherapy, frequent relapse and resistance to traditional drugs will eventually result in treatment failure. Therapeutic options for recurrent patients were limited, and only a few survived more than 5 years from diagnosis. Current advances in molecular biology established by using xenografts and cell lines proposed the concept of RNA‐based molecular subtyping, which had great significance in exploration of therapeutic targets for distinct subgroups. Our study observed protein expressions of ASCL1 (an essential biomarker for SCLC‐A subgroup) and DLL3 (a ROVA‐T drug target), and analyzed their correlations with clinicopathological features, in an attempt to identify potential patients in SCLC‐A subgroup who would benefit from ROVA‐T treatment. In the current study, ASCL1 and DLL3 were found to be highly expressed in a large cohort of surgically resected SCLC samples (n = 247), and a significantly positive correlation between these two markers were identified.
ASCL1 is one of the master transcription factors in SCLC and plays an important role in cell fate decisions during neurogenesis. Our previous study revealed that ASCL1 protein expression was strongly associated with ASCL1 mRNA expression and NE differentiation score, and ASCL1‐high patients had a lower OS, 15 suggesting that examination of ASCL1 expression by IHC staining could be an alternative option for SCLC subgrouping. In this study, ASCL1 expression was scored according to H‐Score criteria, and then subdivided into ASCL1 low and high expression groups. ASCL1 was highly expressed in 42.5% (105/247) of SCLC. Compared to previous studies, it was consistent with one study as 64% of SCLC expressing ASCL1 in at least 5% tumor cells, 12 whereas a bit lower than the other study as 80% of cases showing ASCL1 expression. 16 As shown in the study by Baine et al., 16 60% cases were small biopsies and 15% were cytology samples, which was different from ours, as all the cases in our study were surgically resected samples and representative tissue cores were selected for tissue array construction. Therefore, regarding specimen sources, our data were more consistent, and it would be better in result interpretation and observation.
DLL3 is an inhibitory ligand of the Notch receptors and inhibits Notch pathway by binding to Notch within the Golgi apparatus and retaining it to endosomal compartments. The Notch pathway plays a tumor suppressive role in SCLC, and inactivation of Notch pathway results in NE transformation of SCLC. 8 As a downstream target of ASCL1, DLL3 links ASCL1‐DLL3‐Notch axis. Activation of ASCL1 upregulates DLL3 expression, which subsequently motivates Notch pathway inactivation and contributes to carcinogenesis of SCLC. DLL3 could hardly be detected in normal adult tissues, but is highly expressed in SCLC tumor cells as well as other types of NE origin tumors including melanoma, glioblastoma, small cell bladder cancer, large cell neuroendocrine carcinoma, and carcinoid of lung. 17 , 18 , 19 , 20 , 21 This finding enables the development of DLL3 targeted therapeutics. DLL3‐targeted agents have being evaluated in several clinical studies. For well‐established DLL3‐specific drugs, Rova‐T, both preclinical and clinical study revealed convincing objective response in recurrent SCLC patients, and a significantly higher efficiency was observed in DLL3‐high SCLC tumors compared to DLL3‐low tumors. 22 In the phase I clinical trial, DLL3‐high expression was defined as expression in 50% or more tumor cells by IHC staining, and 10 patients (35%) with DLL3‐high had a confirmed objective response, but none of the patients with DLL3‐low had a response. 22 The impact of DLL3 expression on response to Rova‐T was concordant in the following phase II study, 23 whereas it was not supported by the phase III trial, in which DLL3‐high patients were defined as expression in more than 75% tumor cells. 24 Although responses to Rova‐T were determined by various factors, the threshold of expression to define DLL3‐high patients that benefit from this targeted drug is still deserved to be clarified in the future. In our study, DLL3 was highly expressed in SCLC tumor cells, accounting for 188 (72.8%) patients. This is consistent with previous reports (68%–83%). 12 , 16 , 25 , 26 Considering researchers used different scoring criteria, thresholds, and IHC methodologies of DLL3 detection, direct comparison might be inappropriate. However, both our study and recent multi‐center retrospective investigation demonstrated abundant DLL3 expression in SCLC. In addition, DLL3 expression is extremely stable in SCLC samples irrespective of ethnicity, gender, age, clinical stage, biopsies or resected samples, pre‐ or post‐treatment. 25 Despite unfavorable results in some clinical studies, DLL3 remains a remarkable target in SCLC because of its high and homogeneous expression on the cell surface. DLL3 targeted therapy is expected to provide great advances in treatment of this aggressive tumor. Further studies are urgently needed to explore the molecular mechanism, to assess expression threshold for DLL3‐high patients that benefit from targeted therapy, and to focus on selection of potential molecular markers for predicting treatment response.
Our previous work already showed ASCL1 was a NE related marker. 15 As an inhibitory ligand of Notching pathway, DLL3 also plays an essential role in NE transformation and maintenance. We analyzed the relationship between DLL3 expression and several conventional NE markers, such as SYN, CgA, CD56, and TTF1 in this study. These markers were normally used for differential diagnosis of SCLC in routine clinical practices. We found a strong positive association between DLL3 and SYN, CgA, CD56, and TTF1 expressions, suggesting the NE function of DLL3. Our observation was consistent with other investigations. 27 , 28 SYN, CgA, and CD56 have been classic markers for SCLC diagnosis, whereas DLL3 showed much wider coverage of expression in SCLC than SYN and CgA. This suggests DLL3 could be a candidate NE marker for SCLC diagnosis to remedy the deficiency of present markers. Moreover, considering the strong correction between DLL3 and TTF1 expression, several recent studies suggested that TTF1 expression could be a potential surrogate of DLL3 expression to identify patients who may respond to DLL3 targeted therapy.
Finally, we analyzed the impact of ASCL1 and DLL3 expression on prognosis of SCLC patients. Although univariate analysis revealed that patients with ASCL1 high expression had a worse OS, multivariate cox regression analysis showed neither ASCL1 nor DLL3 expression was an independent prognostic factor. These results were also supported by several other findings. 12 , 26 , 29 ASCL1 and DLL3 expressions have limited impact on patient outcomes and may not be valuable indicators for prognosis prediction. Because the molecular mechanisms of SCLC are complicated, multiple signaling pathways are involved in the tumorigenicity and progression, including Hippo/Notch/EMT pathways. 30 Therefore, signal indicator cannot predict prognosis, and analysis of ASCL1/DLL3 expression could be more significant in selection of patients suitable for target therapy. As mentioned above, ASCL1 positive phenotype was the most conventional and predominant subgroup of SCLC tumor. To some extent, DLL3 is elevated as a consequence of ASCL1 overexpression, and activities of DLL3 targeted therapy can be the highest in ASCL1‐high and DLL3‐high group tumors based on preferential expression of these markers in this subtype. 22 , 31
Our study has some advantages. First, we observed high expression levels of ASCL1/DLL3 and their positive correlations in the largest surgically resected samples. Second, co‐analysis of ASCL1/DLL3 expression using routine IHC staining provides evidence for selection of potential subgroups, which is more applicable for advanced patients (accounting for 80% SCLC patients in clinical practice) because of insufficient sample amount for further examination. Third, unlike the study by Furuta et al., 12 we enrolled pure SCLC samples for study (that is more representative of authentic SCLC features compared to combined SCLC) because both of our previous works based on clinicopathological analysis 13 and YAP1 expression 14 revealed the distinction between pure SCLC and combined SCLC, and the latter presented much more heterogenicity. Although the cell origin of combined SCLC remains unclear, SCLC components and non‐SCLC components in combined SCLC have been reported to share almost 75% common mutations and show similar genetic background, suggesting that the SCLC components and non‐SCLC components are derived from common precursors. Moreover, this also implies that one component of combined SCLC arises from the other with subsequent acquisition of oncogenic change and microenvironment in combined SCLC. 32 In Furuta et al.'s study, 12 29 combined SCLC and 66 pure SCLC were included. Although DLL3 and ASCL1 scoring was only assessed in components of SCLC cells, but not in the components of histologically characterized as squamous cell carcinoma, adenocarcinoma, or large cell carcinoma, the SCLC components in combined SCLC is relatively different from that in pure SCLC. Combined with our previous studies, we believe that the combined SCLC as a distinct subtype should be considered as an important histological factor significantly affecting the efficacy and prognosis. Our research, with a much larger sample size, further confirmed the conclusion of the study by Furuta et al. 12 that the expression of ASCL1/DLL3 has a significant positive correlation, and DLL3 was not an independent prognostic factor and may only benefit from targeted therapy.
However, this study also has some limitations. First, we only analyzed the correlation between ASCL1/DLL3 expressions and their clinical implications and no further study was carried out on exploration of the underlying molecular mechanisms. Second, all the clinical specimen used were retrospectively surgically resected samples, DLL3 targeted therapeutic effect cannot be straightly evaluated based on subgroups. Further studies are still needed to clarify the molecular mechanisms and to predict response of target therapy.
CONCLUSION
In this study, we analyzed ASCL1 and DLL3 expression in a large scale of surgically resected pure SCLC samples. ASCL1 and DLL3 were highly expressed in SCLC tumor cells, and their expression level was positively correlated. The patients with ASCL1 high expression level represent the dominant subgroup of SCLC with NE characteristics, and DLL3 high patients indicate a subgroup suitable for DLL3 targeted therapy. Therefore, we suppose that co‐analysis of ASCL1 and DLL3 expression may contribute to selection of distinct SCLC subgroup that benefit most from DLL3 targeted therapy.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the Cancer Foundation of China, Beijing Hope Marathon Foundation (grant numbers LC2017A20), and the National Key Research and Development Program of China (grant numbers 2017YFC1308704, 2017YFC1311000, and 2017YFC1308700), and National Key R and D Program of China (grant number 2018YFC0116905), and Innovation Project‐Clinical and Translational Research Fund Project (grant number 2021‐I2M‐C&T‐B‐062).
Hu C, Dong J, Liu L, Liu J, Sun X, Teng F, et al. ASCL1 and DLL3 expressions and their clinicopathological implications in surgically resected pure small cell lung cancer: A study of 247 cases from the National Cancer Center of China . Thorac Cancer. 2022;13:338–345. 10.1111/1759-7714.14249
Chunfang Hu and Jiyan Dong contributed equally to this research.
Parts of this study have been presented at 2021 World Conference on Lung Cancer.
Funding information the National Key Research and Development Program of China, Grant/Award Numbers: 2017YFC1308704, 2017YFC1311000, 2017YFC1308700; Innovation Project‐Clinical and Translational Research Fund Project, Grant/Award Number: 2021‐I2M‐C&T‐B‐062; National Key R&D Program of China, Grant/Award Number: 2018YFC0116905; the Cancer Foundation of China, Beijing Hope Marathon Foundation, Grant/Award Number: LC2017A20
Contributor Information
Puyuan Xing, Email: xingpuyuan@cicams.ac.cn.
Lin Yang, Email: linyang0616@126.com.
REFERENCES
- 1. Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA. Current diagnosis and management of small‐cell lung cancer. Mayo Clin Proc. 2019;94:1599–622. [DOI] [PubMed] [Google Scholar]
- 2. Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang XD, Hu R, Ding Q, Savage TK, Huffman KE, Williams N, et al. Subtype‐specific secretomic characterization of pulmonary neuroendocrine tumor cells. Nat Commun. 2019;10:3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borromeo MD, Savage TK, Kollipara RK, He M, Augustyn A, Osborne JK, et al. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep. 2016;16:1259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ireland AS, Micinski AM, Kastner DW, Guo B, Wait SJ, Spainhower KB, et al. MYC drives temporal evolution of small cell lung cancer subtypes by reprogramming neuroendocrine fate. Cancer Cell. 2020;38:60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang W, Girard L, Zhang YA, Haruki T, Papari‐Zareei M, Stastny V, et al. Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Transl Lung Cancer Res. 2018;7:32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leonetti A, Facchinetti F, Minari R, Cortellini A, Rolfo CD, Giovannetti E, et al. Notch pathway in small‐cell lung cancer: from preclinical evidence to therapeutic challenges. Cell Oncol (Dordr). 2019;42:261–73. [DOI] [PubMed] [Google Scholar]
- 9. Owen DH, Giffin MJ, Bailis JM, Smit MA, Carbone DP, He K. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol. 2019;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsoukalas N, Aravantinou‐Fatorou E, Baxevanos P, Tolia M, Tsapakidis K, Galanopoulos M. Advanced small cell lung cancer (SCLC): new challenges and new expectations. Ann Transl Med. 2018;6:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Amar N, Zhu Y, Wang C, Xia C, Yang X, et al. Combined DLL3‐targeted bispecific antibody with PD‐1 inhibition is efficient to suppress small cell lung cancer growth. J Immunother Cancer. 2020;8 (1):e000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furuta M, Sakakibara‐Konishi J, Kikuchi H, Kikuchi H, Yokouchi H, Nishihara H, et al. Analysis of DLL3 and ASCL1 in surgically resected small cell lung cancer (HOT1702). Oncologist. 2019;24:e1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo Y, Yang L, Liu L, Wei J, Teng F, Zhang J, et al. Comparative study of clinicopathological characteristics and prognosis between combined and pure small cell lung cancer (SCLC) after surgical resection. Thorac Cancer. 2020;11:2782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X, Guo Y, Liu L, Wei J, Zhang J, Xie T, et al. YAP1 protein expression has variant prognostic significance in small cell lung cancer (SCLC) stratified by histological subtypes. Lung Cancer. 2021;160:166–174. [DOI] [PubMed] [Google Scholar]
- 15. Wei J, Liu L, Guo Y, Zhang J, Wang X, Dong J, et al. Clinicopathological features and prognostic implications of ASCL1 expression in surgically resected small cell lung cancer. Thorac Cancer. 2021;12:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15:1823–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hermans BCM, Derks JL, Thunnissen E, van Suylen RJ, den Bakker MA, Groen HJ, et al. DLL3 expression in large cell neuroendocrine carcinoma (LCNEC) and association with molecular subtypes and neuroendocrine profile. Lung Cancer. 2019;138:102–8. [DOI] [PubMed] [Google Scholar]
- 18. Xie H, Boland JM, Maleszewski JJ, Aubry MC, Eunhee SY, Jenkins SM, et al. Expression of delta‐like protein 3 is reproducibly present in a subset of small cell lung carcinomas and pulmonary carcinoid tumors. Lung Cancer. 2019;135:73–9. [DOI] [PubMed] [Google Scholar]
- 19. Konstantakou EG, Velentzas AD, Anagnostopoulos AK, Litou ZI, Konstandi OA, Giannopoulou AF, et al. Deep‐proteome mapping of WM‐266‐4 human metastatic melanoma cells: from oncogenic addiction to druggable targets. PLoS One. 2017;12:e0171512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spino M, Kurz SC, Chiriboga L, Serrano J, Zeck B, Sen N, et al. Cell surface notch ligand DLL3 is a therapeutic target in isocitrate dehydrogenase‐mutant glioma. Clin Cancer Res. 2019;25:1261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koshkin VS, Garcia JA, Reynolds J, Elson P, Magi‐Galluzzi C, McKenney JK, et al. Transcriptomic and protein analysis of small‐cell bladder cancer (SCBC) identifies prognostic biomarkers and DLL3 as a relevant therapeutic target. Clin Cancer Res. 2019;25:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab tesirine, a DLL3‐targeted antibody‐drug conjugate, in recurrent small‐cell lung cancer: a first‐in‐human, first‐in‐class, open‐label, phase 1 study. Lancet Oncol. 2017;18:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morgensztern D, Besse B, Greillier L, Santana‐Davila R, Ready N, Hann CL, et al. Efficacy and safety of rovalpituzumab tesi rine in third‐line and beyond patients with DLL3‐expressing, relapsed/refractory small‐cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res. 2019;25:6958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phase 3 Trial of Rova‐T as Second‐line Therapy for Advanced Small‐Cell Lung Cancer (TAHOE Study) Halted. AbbVie. Published December 5, 2018. https://bit.ly/2KZcyVl. Accessed December 6, 2018. https://news.abbvie.com/news/phase-3-trial-rova-t-as-second-line-therapy-foradvanced-small-cell-lung-cancer-tahoe-study-halted
- 25. Rojo F, Corassa M, Mavroudis D, Öz AB, Biesma B, Brcic L, et al. International real‐world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer. 2020;147:237–43. [DOI] [PubMed] [Google Scholar]
- 26. Tendler S, Kanter L, Lewensohn R, Ortiz‐Villalón C, Viktorsson K, De Petris L. The prognostic implications of Notch1, Hes1, Ascl1, and DLL3 protein expression in SCLC patients receiving platinum‐based chemotherapy. PLoS One. 2020;15:e0240973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cardnell RJ, Li L, Sen T, Bara R, Tong P, Fujimoto J, et al. Protein expression of TTF1 and cMYC define distinct molecular subgroups of small cell lung cancer with unique vulnerabilities to aurora kinase inhibition, DLL3 targeting, and other targeted therapies. Oncotarget. 2017;8:73419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan LX, Liu YH, Li Z, Luo DL, Li YF, Yan JH, et al. Prognostic value of delta‐like protein 3 combined with thyroid transcription factor‐1 in small‐cell lung cancer. Oncol Lett. 2019;18:2254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanaka K, Isse K, Fujihira T, Takenoyama M, Saunders L, Bheddah S, et al. Prevalence of delta‐like protein 3 expression in patients with small cell lung cancer. Lung Cancer. 2018;115:116–20. [DOI] [PubMed] [Google Scholar]
- 30. Tlemsani C, Pongor L, Elloumi F, Girard L, Huffman KE, Roper N, et al. SCLC‐CellMiner: a resource for small cell lung cancer cell line genomics and pharmacology based on genomic signatures. Cell Rep. 2020;33:108296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3‐targeted antibody‐drug conjugate eradicates high‐grade pulmonary neuroendocrine tumor‐initiating cells in vivo. Sci Transl Med. 2015;7:302ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao X, McCutcheon JN, Kallakury B, Chahine JJ, Pratt D, Raffeld M, et al. Combined small cell carcinoma of the lung: is it a single entity? J Thorac Oncol. 2018;13:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


