Abstract
Background
Several outpatient coronavirus disease 2019 (COVID-19) therapies have reduced hospitalization in randomized controlled trials. The choice of therapy may depend on drug efficacy, toxicity, pricing, availability, and available infrastructure. To facilitate comparative decision-making, we evaluated the efficacy of each treatment in clinical trials and estimated the cost per hospitalization prevented.
Methods
Wherever possible, we obtained relative risk for hospitalization from published randomized controlled trials. Otherwise, we extracted data from press releases, conference abstracts, government submissions, or preprints. If there was >1 study, the results were meta-analyzed. Using relative risk, we estimated the number needed to treat (NNT), assuming a baseline hospitalization risk of 5%, and compared the cost per hospitalization prevented with the estimate for an average Medicare COVID-19 hospitalization ($21 752). Drug pricing was estimated from GoodRx, from government purchases, or manufacturer estimates. Administrative and societal costs were not included. Results will be updated online as new studies emerge and/or final numbers become available.
Results
At a 5% risk of hospitalization, the estimated NNT was 80 for fluvoxamine, 91 for colchicine, 72 for inhaled corticosteroids, 24 for nirmatrelvir/ritonavir, 50 for molnupiravir, 28 for remdesivir, 25 for sotrovimab, 29 for casirivimab/imdevimab, and 29 for bamlanivimab/etesevimab. For drug cost per hospitalization prevented, colchicine, fluvoxamine, inhaled corticosteroids, and nirmatrelvir/ritonavir were below the Medicare estimated hospitalization cost.
Conclusions
Many countries are fortunate to have access to several effective outpatient therapies to prevent COVID-19 hospitalization. Given differences in efficacy, toxicity, cost, and administration complexity, this assessment serves as one means to frame treatment selection.
Keywords: antivirals, COVID-19, monoclonal antibodies, repurposed medications, SARS-CoV-2
The coronavirus disease 2019 (COVID-19) pandemic has fueled an explosion of scientific inquiry. Since the initial reports of overwhelmed health systems and hospitals, there has been tremendous interest in finding outpatient treatments that could prevent hospitalization among those who are symptomatic and at high risk for clinical deterioration. Initial studies looked at drug repurposing: identifying widely available, inexpensive, and safe medications that could prove effective. Initially, hydroxychloroquine was considered a leading candidate [1]; however, interest waned as randomized controlled trial evidence failed to demonstrate superiority over placebo [2, 3]. Since that time, there have been a number of promising repurposed medications including colchicine [4], inhaled corticosteroids [5], and fluvoxamine [6–8], all of which have shown a relative risk reduction of 20%–30% in hospitalization. Novel therapeutics have emerged, such as customized antispike protein monoclonal antibody products, which have shown up to a 55%–85% relative risk reduction in hospitalization [9–11]. However, these therapies are not always widely available, are more challenging to administer, are comparatively expensive, and may have reduced efficacy against newer variants. Most recently, repurposed and novel antiviral therapies have attracted attention, with relative risk reductions of 30%–85% [12–16]. The US government has proactively purchased these agents based on prepublished data [17, 18].
For policy makers and/or health care professionals, especially those without ready access to novel therapeutics, the decision might be between supportive care or repurposed drugs. For well-resourced health care systems such those in North America, antispike monoclonal antibodies, remdesivir, and oral antiviral therapies cost significantly more, are available in relatively limited quantities, and can be more complex to procure and/or administer. Our objective was to systematically quantify the effect sizes of available treatments with respect to preventing hospitalization and then to contextualize those results against the expected drug costs per hospitalization prevented.
METHODS
Review of Literature and Estimations of Effect Size
To balance efficacy with potential toxicity, the outcome of interest we selected was all-cause hospitalization among outpatients. Where this was unavailable, we used COVID-19-related hospitalization (and have indicated this). Of note, use of the latter could underestimate toxicity, as hospitalizations due to drug side effects might be excluded. We used these results to calculate the relative risk for hospitalization with 95% CIs.
Results for colchicine were taken from COLCORONA [4] and PRINCIPLE [19] and meta-analyzed using a fixed-effects model (I2 = 0.0%). For the inhaled corticosteroids, we used the results of our fixed-effects meta-analysis [5] of all available trials [20–23], with the caveat that the fixed-effects model may overestimate efficacy (I2 = 49.2%). For fluvoxamine, we obtained the number of all-cause hospitalizations in both arms directly from the authors of the 3 completed clinical trials [6, 7, 24]. In the TOGETHER trial [7], the authors originally included ER visits of ≥6 hours as a proxy for hospitalization due to the prohibitive number of admissions in Brazil exhausting capacity. To be more conservative, we chose only to include patients who spent >24 hours in the emergency department as equivalent to being hospitalized. The trial results were combined using a fixed-effects meta-analysis (I2 = 0.2%) [8].
Results for outpatient antispike protein antibody randomized controlled trials were limited to most recent phase 3 studies as most used an integrated phase 1/2/3 design that led to multiple publications describing the same patients. We limited our analysis to the latest phase 3 studies for bamlanivimab/etesevimab [9], casirivimab/imdevimab [10], and sotrovimab [11]. Bamlanivimab monotherapy was not included as it is no longer a recommended treatment. Of important note, it appears that casirivimab/imdevimab and bamlanivimab/etesevimab may not be effective against the Omicron variant [25].
Results for outpatient antiviral therapies included 1 phase 3 trial of remdesivir [15]. For molnupiravir, we included the published phase 3 trial [14], the analogous subgroup (≤5 days of symptoms and at least 1 high-risk criterion) from the phase 2 trial [26], and a press release from an Indian trial (fixed-effects model I2 = 47%; may overestimate efficacy) [12]. For nirmatrelvir with ritonavir, we relied on the Food and Drug Administration (FDA) Emergency Use Authorization [16] and press release interim analysis for a second trial [13]. A random-effects meta-analysis was conducted for a sensitivity analysis and is presented in the Supplementary Data. Recognizing that the field moves very quickly, we have developed a webpage (https://read.idtrials.com/outptcovid) that will be updated monthly at least until the end of 2022 to contain the most up-to-date efficacy and cost data possible.
Estimation of Costs
Where available, the lowest drug pricing was taken from GoodRx (www.goodrx.com). We chose budesonide for the inhaled corticosteroid analysis because it was the first corticosteroid to demonstrate a reduction in hospitalizations [22]. For antiviral and monoclonal antibody therapies, we used any publicly available data on US government purchasing and/or the manufacturer’s quoted price. Prices for injectable agents did not include the price of administration, which varies across agents. Societal costs were also not factored in and are beyond the scope of this analysis.
Estimation of Events Prevented and Costs per Event Prevented
For each drug, we took the estimates of relative risk of hospitalization and the 95% CI to generate the estimated absolute risk reduction (and CI) assuming a moderate baseline risk of hospitalization of 5%. Sensitivity analyses were conducted for 2.5% (low) and 10% (high) risk of hospitalization. For colchicine, where the CI touched 1.00 in the meta-analysis, we used an upper bound of 0.999 for calculating the NNT. By dividing 100 by the absolute risk reduction (rounded up), we estimated the number needed to treat (NNT) to prevent 1 hospitalization with corresponding CIs. We then multiplied the NNT by the drug costs per patient treated to arrive at the estimated drug cost to prevent 1 admission. For comparison, the mean cost of a COVID-19 admission to Medicare has been estimated at $21 752 [27].
Patient Consent
This study does not include factors necessitating patient consent.
RESULTS
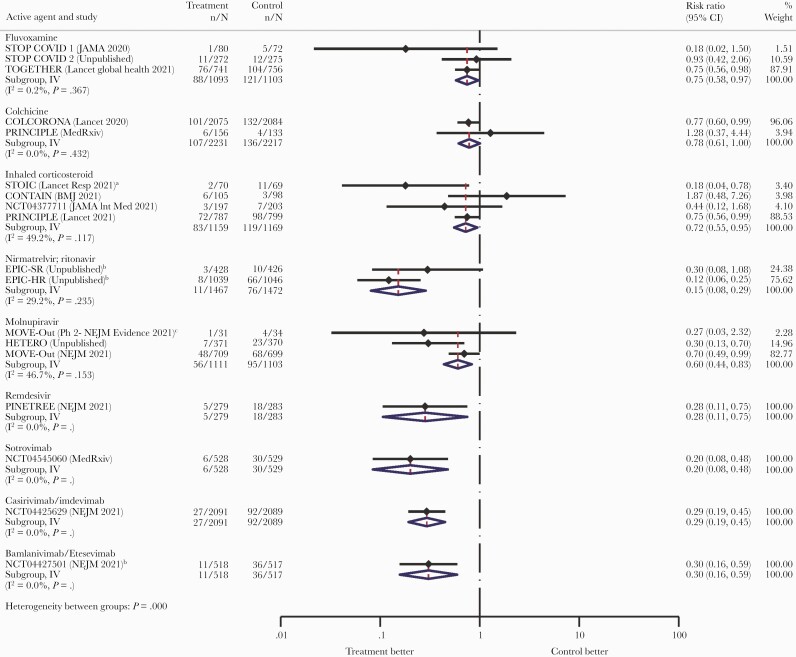
The included studies are summarized in Table 1. The results of the analysis are presented in Figure 1 and Table 2, with a random-effects meta-analysis presented in Supplementary Figure 1. The repurposed drugs fluvoxamine and colchicine and inhaled corticosteroids had smaller effect sizes and larger numbers needed to treat assuming a 5% hospitalization risk at 80 (95% CI, 48–667), 91 (95% CI, 52–20 000), and 72 (95% CI, 45–400), respectively. By contrast, the antiviral and antibody therapies had larger effect sizes and smaller numbers needed to treat at 24 (95% CI, 22–29) for nirmatrelvir/ritonavir (COVID-19 hospitalization), 28 (95% CI, 23–80) for remdesivir, 50 (95% CI, 36–118) for molnupiravir, 25 (95% CI, 22–39) for sotrovimab, 29 (95% CI, 25–37) for casirivimab/imdevimab, and 29 (95% CI, 24–50) for bamlanivimab/etesevimab (COVID-19 hospitalization). However, the latter 2 antibody therapies likely do not retain activity against Omicron [25].
Table 1.
Summary of Included Clinical Trials
| Study | Location | Original Primary Outcome | Inclusion Criteria | Demographics |
|---|---|---|---|---|
| Fluvoxamine | ||||
| Stop Covid 1 (NCT04342663) | USA | Clinical deterioration: hospitalization or new hypoxemia within 15 d | Age ≥18 unvaccinated Positive test with: ≤7 d symptoms |
Median age 46; 72% female; 70% White; 56% BMI ≥30; 20% hypertension; 11% diabetes Median 4 d of symptoms |
| Stop Covid 2 (NCT04668950) | USA and Canada | Clinical deterioration: hospitalization or new hypoxemia within 15 d | Age ≥30 unvaccinated Positive test with: ≤6 d symptoms Criterion for high risk |
Median age 47; 62% female; 73% White; 44% BMI ≥30; 21% hypertension; 9% diabetes Median 5 d of symptoms |
| Together (NCT04727424) | Brazil | ER visit ≥6 h or hospitalization within 28 d | Age ≥18 unvaccinated Positive test with: ≤7 d symptoms Criterion for high risk |
Median age 50; 55% female; 96% mixed race; 51% BMI ≥30; 13% hypertension; 16% diabetes Mean 3.8 d of symptomsb |
| Colchicine | ||||
| Colcorona (NCT04322682) | Multiple countries (majority Canada) | COVID-19-related hospitalization or death from any cause | Age ≥40 unvaccinated 93% had positive test with: Diagnosis within 24 h Criterion for high risk |
Median age 53–54; 54% female; 93% White; mean BMI 30; 36% hypertension; 20% diabetes Mean 5.3 d of symptoms |
| PRINCIPLEa (ISRCTN86534580) | UK | COVID-19-related hospitalization or death from any cause | Age ≥65 or age ≥18 with comorbidity or dyspnea 58% vaccinated ≥1 dosea Positive test with ongoing symptoms of fever, new continuous cough, or change in smell or taste within 14 d |
Median age 48; 54% female; 89% White; BMI not reported; 24% hypertension; 13% diabetes Median 6 d of symptoms |
| Inhaled corticosteroids | ||||
| STOICa (NCT04416399) | UK | COVID-19 urgent visits | Age ≥18 unvaccinated 94% had positive test with: ≤7 d of symptoms (≥1 of cough and fever or anosmia) |
Mean age 45; 56% female; 93% White; mean BMI 26–27; N/A hypertension; 4% diabetes Median 3 d of symptoms |
| CONTAIN (NCT04435795) | Canada | Resolution of cough, dyspnea, and fever day 7 | Age ≥18 unvaccinated Positive test with: ≤6 d of symptoms (≥1 of fever, cough, or dyspnea) |
Median age 35; 54% female; 61% White; BMI not reported; 6% hypertension; 3% diabetes Duration of symptoms not reported |
| Covis Pharma (NCT04377711) | USA | Time to symptom-free | Age ≥12 unvaccinated Positive test with: ≥1 of fever, cough, or dyspnea |
Mean age 43; 55% female; 86% White; mean BMI 29.4; 22% hypertension; 8% diabetes Duration of symptoms not reported |
| PRINCIPLEa (ISRCTN86534580) | UK | COVID-19-related hospitalization or death from any cause | Age ≥65 or ≥50 with comorbidity 14% vaccinated ≥1 dosea Positive test with ongoing symptoms of fever, new continuous cough, or change in smell or taste within 14 d |
Mean age 64–65; 51% female; 93% White; BMI not reported; 45% hypertension; 21% diabetes Mean 6 d of symptoms |
| Nirmatrelvir/ritonavir | ||||
| EPIC-HR (NCT04960202) | Multiple countries (USA 45%) | COVID-19-related hospitalization or death from any cause | Age ≥18 unvaccinated Positive test with: ≤5 d of symptoms (≥1 at randomization) Criterion for high risk |
Based on FDA Emergency Use Authorization: Mean age 46; 49% female; 72% White; 36% BMI ≥30; NA hypertension; 12% diabetes 66% had symptoms ≤3 d |
| EPIC-SR (NCT05011513) | Multiple countries | Time to sustained alleviation of all targeted COVID-19 signs/symptoms | Age ≥18 unvaccinated Positive test with: ≤5 d symptoms Vaccinated if criterion for high riska |
Not available at time of this analysis |
| Molnupiravir | ||||
| Hetero Pharmaa (CTRI2021/05/033739) | India | Hospitalization | Age ≥18 and ≤60 (vaccination unspecified) Positive test with: ≤5 d of symptoms |
Not available at time of this analysis |
| MOVe-Out Ph 2 (NCT04575597) | Multiple | All-cause hospitalization or death (included ER visit ≥24 h) | Age ≥18 unvaccinated Positive test with: ≤7 d of symptoms (at least 1 at randomization) ≥75% with criterion for high risk |
Mean age 49; 47% female; 72% White; 49% BMI ≥30; NA hypertension; 17% diabetes 67% had symptoms ≤5 d |
| MOVe-Out (NCT04575597) | Multiple countries (majority Latin America) | All-cause hospitalization or death (included ER visit ≥24 h) | Age ≥18 unvaccinated Positive test with: ≤5 d of symptoms (≥1 at randomization) Criterion for high risk |
Median age 43; 51% female; 79% White; 74% BMI ≥30; N/A hypertension; 16% diabetes 48% had symptoms ≤3 d |
| Remdesivir | ||||
| PINETREE (NCT04501952) | Multiple countries (95% USA) | COVID-19-related hospitalization or all-cause death | Age ≥18 unvaccinated: Positive test with: ≤7 d of symptoms Criterion for high risk |
Mean age 50; 48% female; 80% White; 55% BMI ≥30; 48% hypertension; 62% diabetes Median duration of symptoms 5 d |
| Antibody therapies | ||||
| Sotrovimab (NCT04545060) | Multiple countries (92% USA) | Hospitalization for ≥24 h or death | Age ≥18 unvaccinated Positive test with: ≤5 d of symptoms Criterion for high risk |
Median age 53; 54% female; 87% White; mean BMI 32; N/A hypertension; 22% diabetes 59% had duration of symptoms ≤3 d |
| Casirivimab/imdevimab (NCT04425629) | USA and Mexico | COVID-19-related hospitalization or death from any cause | Age ≥18 unvaccinated Positive test with: ≤7 d of symptoms Criterion for high risk |
Median age 48–50; 52% female; 84% White; 57% BMI ≥30; 36% hypertension; 15% diabetes Median duration of symptoms 3 d |
| Bamlanivimab/etesevimab (NCT04427501) | USA | COVID-19-related hospitalization or death from any cause | Age ≥12 unvaccinated Positive test with: Symptoms Criterion for high risk |
Mean age 54; 52% female; 87% White; mean BMI 34; 34% hypertension; 28% diabetes Median duration of symptoms 4 d |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; ER, emergency room; FDA, Food and Drug Administration.
Open label.
Missing data on ~23%.
Figure 1.
Effect sizes of the various drugs on hospitalization. aUrgent care, emergency room, or hospitalization. bBased on COVID-19 hospitalization because all-cause not available. cSubgroups and doses matched the phase 3 trial. Abbreviation: COVID-10, coronavirus disease 2019.
Table 2.
Number Needed to Treat and Costs per Hospitalization Prevented
| Number Needed to Treat | Cost per Hospitalization Prevented | ||||||
|---|---|---|---|---|---|---|---|
| Drug | Cost/Patient | 2.5% Risk | 5% Risk | 10% Risk | 2.5% Risk | 5% Risk | 10% Risk |
| Fluvoxamine (meta-analysis) | 14 | 160 (96–1334) | 80 (48–667) | 40 (24–334) | 2244 (1346–18 709) | 1122 (673–9355) | 561 (337–4684) |
| Colchicine (meta-analysis) | 37 | 182 (103–40 000) | 91 (52–20 000) | 46 (26–10 000) | 6667 (3773–1 465 200) | 3333 (1905–732 600) | 1685 (952–366 300) |
| Inhaled corticosteroids (meta-analysis)a | 132 | 143 (89–800) | 72 (45–400) | 36 (23–200) | 18 819 (11 712–105 280) | 9475 (5922–52 640) | 4738 (3027–26 320) |
| Nirmatrelvir/ritonavir (meta-analysis)b | 530 | 48 (44–57) | 24 (22–29) | 12 (11–15) | 25 440 (23 320–30 210) | 12 720 (11 660–15 370) | 6360 (5830–7950) |
| Molnupiravir (meta-analysis)a | 700 | 100 (72–236) | 50 (36–118) | 25 (18–59) | 70 000 (50 400–165 200) | 35 000 (25 200–82 600) | 17 500 (12 600–41 300) |
| Remdesivir (phase 3) | 1872 | 56 (45–160) | 28 (23–80) | 14 (12–40) | 104 832 (84 240–299 520) | 52 416 (43 056–149 760) | 26 208 (22 464–74 880) |
| Sotrovimab (phase 3) | 2100 | 50 (44–77) | 25 (22–39) | 13 (11–20) | 105 000 (92 400–161 700) | 52 500 (46 200–81 900) | 27 300 (23 100–42 000) |
| Casirivimab/imdevimab (phase 3) | 2100 | 57 (50–73) | 29 (25–37) | 15 (13–19) | 119 700 (105 000–153 300) | 60 900 (52 500–77 700) | 31 500 (27 300–39 900) |
| Bamlanivimab/etesevimab (phase 3)b | 2100 | 58 (48–99) | 29 (24–50) | 15 (12–25) | 121 800 (100 800–207 900) | 60 900 (50 400–105 000) | 31 500 (25 200–52 500) |
Lowest drug prices for repurposed therapy (eg, GoodRx) may underestimate the costs of acquisition for patients/drug plans. Monoclonal antibody and remdesivir prices do not include price of administration. Shaded monoclonal antibodies likely have significantly reduced efficacy against the Omicron variant.
Abbreviation: COVID-19, coronavirus disease 2019.
Fixed-effects models had moderate heterogeneity.
All-cause hospitalization not provided; COVID-19-related hospitalizations used, which may inflate efficacy.
At a 5% risk of hospitalization, the corresponding drug costs per hospitalization prevented were $1122 (95% CI, $673–$9355) for fluvoxamine, $3333 (95% CI, $1905–$732 600) for colchicine, $9475 (95% CI, $5922–$52 640) for inhaled corticosteroids, $12 720 (95% CI, $11 660–$15 370) for nirmatrelvir/ritonavir, $35 000 (95% CI, $25 200–$82 600) for molnupiravir, $52 416 (95% CI, $43 056–$149 760) for remdesivir, $52 500 (95% CI, $46 200–$81 900) for sotrovimab, $60 900 (95% CI, $52 500–$77 700) for casirivimab/imdevimab, and $60 900 (95% CI, $50 400–$10 500) for bamlanivimab/etesevimab.
DISCUSSION
Scientific advancement has been exponential during the pandemic. We have gone from the discovery of a new disease with no treatment in December 2019 to numerous effective vaccines coupled with a suite of proven therapies that prevent hospitalization and, by extension, presumably impact progression to death. Within these treatments, several are inexpensive and widely available worldwide, while others are expensive, limited in availability, and/or limited in accessibility. The purpose of this review was to contrast the efficacy of these therapies against a measure of cost. Evidence suggests that the antiviral therapies and monoclonal antibodies are more effective than repurposed drugs. While we show that this efficacy comes at a price that often exceeds that of COVID-19 hospitalization, this was not a formal cost-effectiveness analysis and we did not factor in death, long-term outcomes, societal costs, patient preferences, or costs of administration. The higher an individual’s baseline risk of deterioration, the greater the absolute benefit and less costly these options become. If one conservatively estimates a risk of death of 5%–10% for those who are hospitalized, the NNTs for death (and costs) would be ~10–20 times higher. Furthermore, some of these therapies have already been purchased, which necessarily alters the dialogue. Essentially, at the right price or if prescribed to a high enough risk individual, most drugs on this list have the potential to be cost-saving to the system as a whole. Accurate country-specific models for prediction of hospitalization risk will be essential in contextualizing and maximizing the benefits of any therapy.
This analysis has several limitations. First, most of the agents studied have only had a single positive randomized controlled trial, and several are prepublication. Replication in science is important, and while the pandemic necessitated speed, which led to single trials, with many vaccinations and outpatient therapy options now available, the safeguards of confirmatory trials are likely needed for reproducibility and generalizability. Second, some of the data are currently limited to government documents and press releases. In normal times, peer-reviewed results would be required; however, major decisions are being made based on industry public relations material, confidential submissions, and limited data [17, 18], and our analysis can serve to inform those conversations elsewhere. Third, our sensitivity analysis for inhaled corticosteroids found that the benefits were not statistically significant in the random-effects model, and thus the results of this current analysis are predicated on additional trials confirming that these drugs have benefit. Fourth, the efficacy of antispike protein antibodies requires ongoing confirmation for emerging variants, as there are suggestions that some therapies may no longer be as effective against Omicron [25]. Fifth, 2 molnupiravir trials [14, 26] and 1 fluvoxamine trial [7] included emergency room visits of >24 hours in their outcomes, which may not necessarily be completely exchangeable with hospitalizations. Sixth, drug prices from GoodRx likely represent the lowest drug pricing, and costs for some patients could be substantially higher. Finally, very few patients in these clinical trials were vaccinated, and the risk for hospitalization may not be the same in vaccinated patients. Nonetheless, hospitalization in vaccinated patients can be estimated [28], and this is one of the reasons we present sensitivity analyses for different baseline risks of hospitalization. Prospective studies in vaccinated patients are urgently needed.
There is an ongoing need to identify effective treatments that can be administered early in the disease course to prevent COVID-19 hospitalization and death and to make them available and accessible in all regions. While many countries worldwide are fortunate to have access to novel treatments, the number and location of available doses may wax and wane over time, and access may be challenging in remote regions or in congregate care settings. Some degree of decision-making will be required at the level of the individual clinician, hospitals, and regional and federal governments to prioritize deployment of therapies and capacity-building. This analysis provides one means of contextualizing those discussions.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This project was completed without specific funding.
Potential conflicts of interest. T.C.L. and E.G.M. receive research salary support from the Fonds de Recherche du Québec – Santé. T.C.L. and E.G.M. were co-investigators on several outpatient drug repurposing randomized controlled trials for COVID-19 (hydroxychloroquine, inhaled ciclesonide, and fluvoxamine). S.M. holds the Health Research Foundation of Innovative Medicines Canada Chair in Pandemic Preparedness Research. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Conceptualization—T.C.L., E.G.M.; methodology—T.C.L., S.A.G., E.G.M.; validation—T.C.L.; formal analysis—T.C.L.; investigation—all authors; resources—T.C.L.; data curation—T.C.L., E.G.M.; writing—original draft—T.C.L., E.G.M.; writing—review and editing—all authors; visualization—T.C.L., E.G.M.
References
- 1. Pastick KA, Okafor EC, Wang F, et al. Review: hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19). Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med 2020; 173:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitjà O, Corbacho-Monné M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis 2021; 73:e4073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tardif J-C, Bouabdallaoui N, L’Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med 2021; 9:924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee TC, Bortolussi-Courval E, Belga S, et al. Inhaled corticosteroids for outpatients with Covid-19: a meta-analysis. medRxiv 2021.11.04.21265945 [Preprint]. 5 November 2021. Available at: 10.1101/2021.11.04.21265945. Accessed 25 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA 2020; 324:2292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022; 10:e42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee TC, Vigod S, Bortolussi-Courval E, et al. Fluvoxamine for outpatient COVID-19 to prevent hospitalization: a systematic review and meta-analysis. medRxiv 2021.12.17.21268008 [Preprint]. 21 December 2021. Available at: 10.1101/2021.12.17.21268008. Accessed 25 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med 2021; 385:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med 2021; 385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of the neutralizing SARS-CoV-2 antibody sotrovimab in preventing progression of COVID-19: a randomized clinical trial. N Engl J Med 2021; 385:1941–50. [DOI] [PubMed] [Google Scholar]
- 12. Hetero Pharmaceuticals. Molnupiravir interim clinical results. 2021. Available at: https://www.heteroworld.com/images/Press_Release_Molnupiravir_Interim_Clinical_Results_Final_090721.pdf. Accessed 25 December 2021.
- 13. Pfizer Inc. Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk of hospitalization or death. 2021. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results. Accessed 25 December 2021.
- 14. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients [manuscript published online ahead of print 16 December 2021]. N Engl J Med 2021. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. 2021. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19. Accessed 25 December 2021.
- 17. US Department of Health and Human Services. Biden Administration announces U.S. government procurement of Merck’s investigational antiviral medicine for COVID-19 treatment. 2021. Available at: https://www.hhs.gov/about/news/2021/06/09/biden-administration-announces-us-government-procurement-mercks-investigational-antiviral-medicine-covid-19-treatment.html. Accessed 25 December 2021.
- 18. US Department of Health and Human Services. Biden Administration secures 10 million courses of Pfizer’s COVID-19 oral antiviral medicine as additional tool to reduce hospitalizations and save lives. 2021. Available at: https://www.hhs.gov/about/news/2021/11/18/biden-administration-secures-10-million-courses-pfizers-covid-19-oral-antiviral-medicine-as-additional-tool-reduce-hospitalizations-save-lives.html. Accessed 25 December 2021.
- 19. Dorward J, Yu L-M, Hayward G, et al. ; PRINCIPLE Trial Collaborative Group. Colchicine for COVID-19 in adults in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial. medRxiv 2021.09.20.21263828 [Preprint]. 23 September 2021. Available at: 10.1101/2021.09.20.21263828. Accessed 16 January 2022. [DOI] [Google Scholar]
- 20. Ramakrishnan S, Nicolau DV, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Res Med 2021; 9:763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ezer N, Belga S, Daneman N, et al. Inhaled and intranasal ciclesonide for the treatment of COVID-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ 2021; 375:e068060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. YL, -M, Bafadhel M, et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 2021; 398:843–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clemency BM, Varughese R, Gonzalez-Rojas Y, et al. Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial. JAMA Intern Med 2022; 182:42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lenze E. Fluvoxamine for early treatment of Covid-19: a fully-remote, randomized placebo controlled trial. Report No. NCT04668950. 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT04668950. Accessed 15 November 2021.
- 25. Gruell H, Vanshylla K, Tober-Lau P, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med 2022:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caraco Y, Crofoot GE, Moncada PA, et al. Phase 2/3 trial of molnupiravir for treatment of COVID-19 in nonhospitalized adults. N Engl J Med2021; 1. [DOI] [PubMed] [Google Scholar]
- 27. Tsai Y, Vogt TM, Zhou F.. Patient characteristics and costs associated with COVID-19–related medical care among Medicare fee-for-service beneficiaries. Ann Intern Med 2021; 174:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bierle DM, Ganesh R, Tulledge-Scheitel S, et al. Monoclonal antibody treatment of breakthrough COVID-19 in fully vaccinated individuals with high-risk comorbidities [manuscript published online ahead of print 16 November 2021]. J Infect Dis 2021. doi: 10.1093/infdis/jiab570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.