Abstract
Cutaneous melanoma (CM) is a very aggressive disease, often characterized by unresponsiveness to conventional therapies and high mortality rates worldwide. The identification of the activating BRAFV600 mutations in approximately 50% of CM patients has recently fueled the development of novel small‐molecule inhibitors that specifically target BRAFV600 ‐mutant CM. In addition, a major progress in CM treatment has been made by monoclonal antibodies that regulate the immune checkpoint inhibitors. However, although target‐based therapies and immunotherapeutic strategies have yielded promising results, CM treatment remains a major challenge. In the last decade, accumulating evidence points to the aberrant expression of different types of noncoding RNAs (ncRNAs) in CM. While studies on microRNAs have grown exponentially leading to significant insights on CM biology, the role of circular RNAs (circRNAs) and long noncoding RNAs (lncRNAs) in this tumor is less understood, and much remains to be discovered. Here, we summarize and critically review the available evidence on the molecular functions of circRNAs and lncRNAs in BRAFV600 ‐mutant CM and CM immunogenicity, providing recent updates on their functional role in targeted therapy and immunotherapy resistance. In addition, we also include an evaluation of several algorithms and databases for prediction and validation of circRNA and lncRNA functional interactions.
Keywords: circular RNAs, cutaneous melanoma, immunotherapy, long noncoding RNAs, targeted therapy
In the last decade, accumulating evidence points to the aberrant expression of different types of noncoding RNAs in cutaneous melanoma (CM). Here, we summarize and critically review the available evidence on the molecular functions of circular RNAs (circRNAs) and long noncoding RNAs (lncRNAs) in BRAFV600 ‐mutant CM and CM immunogenicity, providing recent updates on their functional role in targeted therapy and immunotherapy resistance. In addition, we also include an evaluation of several algorithms and databases for prediction and validation of circRNA and lncRNA functional interactions.

Abbreviations
- ANRIL
antisense noncoding RNA in the INK4 locus
- ASO
antisense oligonucleotide
- ATB
activated by TGF‐beta
- BANCR
BRAF‐activated nonprotein‐coding RNA
- BRAFi
BRAF inhibitors
- BSJ
back‐spliced junction
- CDR1as
cerebellar degeneration‐associated protein 1 antisense transcript
- CeRNA
competitive endogenous RNA
- CircRNA
circular RNA
- CiRNA
circular intronic RNA
- CM
cutaneous melanoma
- CTL
cytotoxic T lymphocyte
- DIRC3
disrupted in renal carcinoma 3
- DLBCL
diffuse large B-cell lymphoma
- EcircRNA
exonic circular RNA
- EIciRNA
exon and intron-containing circular RNA
- EMT
epithelial-to-mesenchymal transition
- FENDRR
FOXF1 adjacent noncoding developmental regulatory RNA
- FLOT2
flotillin 2
- FOXD3-AS1
FOXD3 adjacent opposite strand RNA 1
- GAS5
growth arrest specific 5
- GAS6-AS2
GAS6 antisense RNA 2
- H3K27me3
trimethylation of lysine 27 on histone H3
- HCP5
HLA class I histocompatibility antigen protein P5
- HOTAIR
HOX transcript antisense RNA
- ITGB2-AS1
ITGB2 antisense RNA 1
- KCNQ1OT1
KCNQ1 opposite strand/antisense transcript 1
- LINC-PINT
long intergenic nonprotein-coding RNA, P53-induced transcript
- LincRNA
long intergenic noncoding RNA
- Lnc-CHOP
C/EBP homologous protein, long noncoding RNA
- LncRNA
long noncoding RNA
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- MDSCs
myeloid-derived suppressor cells
- MEG3
maternally expressed gene 3
- MEKi
MEK inhibitors
- MHENCR
melanoma highly expressed competing endogenous lncRNA for miR-425 and miR-489
- MIAT
myocardial infarction-associated transcript
- MIRAT
MAPK inhibitor resistance-associated transcript
- MiRNA
micro-RNA
- NcRNA
noncoding RNA
- OIS
oncogene-induced senescence
- Olfr29-ps1
olfactory receptor 29, pseudogene 1
- Orilnc1
oncogenic RAS-induced lncRNA 1
- OVAAL
ovarian adenocarcinoma amplified long noncoding RNA
- PcG
polycomb group
- PEG10
paternally expressed gene 10
- RBP
RNA-binding protein
- RMEL3
restricted to melanocyte 3
- RPAD
RNase R treatment, polyadenylation, and poly(A)+ RNA depletion
- SAMMSON
survival-associated mitochondrial melanoma-specific oncogenic noncoding RNA
- SiRNA
small interfering RNA
- SPRY4-IT1
Sprouty4-intronic transcript 1
- SRA
steroid receptor RNA activator
- TSS
transcription start site
- TUG1
Taurine upregulated 1
- UCA1
urothelial cancer associated 1
- ZEB1-AS1
zinc finger E-box binding homeobox 1 antisense RNA 1
1. Introduction
Cutaneous melanoma (CM) is a malignant neoplasm that arises from melanocytes, representing the leading cause of skin cancer‐related deaths worldwide, and its incidence is constantly growing in industrialized countries [1]. Although surgery remains the definitive treatment for early‐stage CM [2], it is rarely curative for advanced CM; moreover, metastatic CM is characterized by a substantial unresponsiveness to conventional therapies, including chemotherapy and radiotherapy [3]. A recent analysis of whole genome alterations in 183 CM samples indicated BRAF and NRAS as the most frequently mutated genes in CM [4]. In particular, approximately 50% of patients with CM harbor activating BRAFV600 mutations, and in 90% of those mutations, a single nucleotide alteration (nucleotide 1799T>A) results in single amino acid substitution of valine by glutamic acid (BRAFV600E ) [5]. In these patients, the constitutive activation of MAPK signaling caused by BRAFV600 appears as a major driver of CM tumorigenic potential and survival [6]. Accordingly, BRAFV600 mutation is an important factor to guide CM treatment, and BRAF and MEK inhibitors (BRAFi/MEKi) represent the best therapeutic strategy for BRAF‐mutated CM patients so far. In fact, the first‐line therapy with BRAFi, alone or in combination with MEKi, has shown remarkable response rates and a significantly improved progression‐free and overall survival in the advanced disease [7]. Despite these findings, about 15% of CM patients do not achieve tumor regression, due to primary resistance to BRAFi/MEKi, and progress more rapidly [8]. In addition, about 50% of CM patients, who initially respond to targeted therapy, ultimately develop an acquired resistance within 7 months from the start of the treatment [9].
The landscape of therapeutic strategies for CM has been revolutionized with the development of a new class of immune modulators, including checkpoint inhibitors targeting CTLA‐4 and PD‐1, which have demonstrated to provide durable responses in the metastatic disease regardless of mutation status [10]. However, primary resistance to immune checkpoint blockade occurs in approximately 40–65% of CM patients treated with PD‐1‐targeting therapy and in about 70% of those treated with anti‐CTLA‐4 therapy [11]. Furthermore, late relapses were also reported, suggesting the emergence of acquired resistance; indeed, 43% of CM responders to anti‐PD‐1 immunotherapy develop acquired resistance by 3 years [12]. Therefore, to advance in this field, novel targets and therapeutic approaches for more effective and long‐lasting treatments for CM patients must be explored. Although noncoding RNAs (ncRNAs) were for years considered as an irrelevant part of the genome, they have recently emerged as important modulators of several cancers [13, 14, 15], including CM [16, 17, 18, 19, 20, 21, 22, 23, 24, 25], and found to act as mediators of drug resistance mechanisms [26].
Based on these considerations, this review will provide novel insights on the function of selected circular RNAs (circRNAs) and long noncoding RNAs (lncRNAs) in BRAFV600 ‐mutant CM and in CM immunogenicity (Table S1). In addition, we present software algorithms currently available for the prediction and validation of the functional interactions of circRNAs and lncRNAs.
2. CircRNAs and lncRNAs
CircRNAs are circular loop structures with covalently linked ends that are mainly generated by pre‐mRNA backsplicing, which connects a downstream 5′ splice donor site to an upstream 3′ splice acceptor site [27]. Due to their circular structure, circRNAs are more resistant to exonucleases that typically degrade linear RNA and much more stable in biological fluids [28]. CircRNAs are predominantly localized in the cytoplasm, whereas a limited number of circRNAs reside in the nucleus [29]. Exonic circRNAs (ecircRNAs) represent more than 80% of total circRNAs, are mainly cytoplasmic, and in some cases are expressed higher than their corresponding linear mRNAs [30]. CircRNAs can also arise from intron lariats that escape degradation after canonical splicing (ciRNAs) or from introns that have been retained between circularized exons (EIciRNAs), and both are primarily located in the nucleus, where they regulate the expression of their parental genes [31]. So far, two models of ecircRNA and EIciRNA formation have been proposed: the lariat‐driven circularization and the intron‐pairing‐driven circularization which differ for the order in which canonical and backsplicing occur [30] (Fig. 1).
Fig. 1.
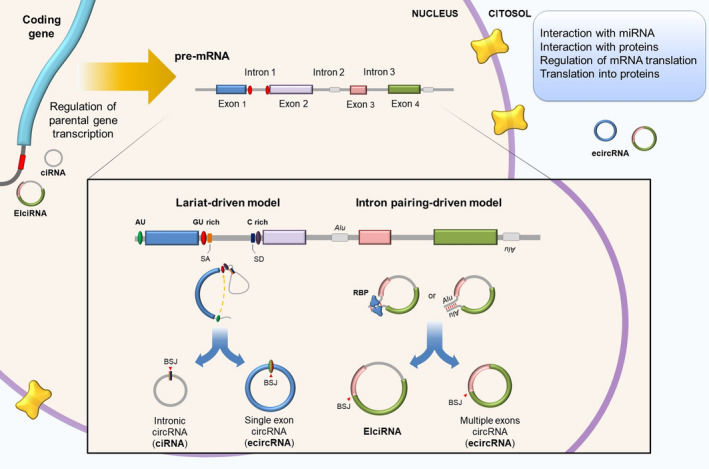
Biogenesis of circRNAs. During mRNA maturation, competition between linear splicing and backsplicing can lead to the formation of intron lariats, which can be further processed into circRNAs. Alternatively, the presence across flanking introns or within them of repeated sequences (i.e., Alu repeats with opposite directions) can produce intron‐driven circularization of RNA. In both lariat‐pairing‐driven circularization and intron‐pairing‐driven circularization, introns can be removed to originate an exonic circRNA (ecircRNA), or retained to form an intron‐containing circRNA (ciRNA or EIciRNA). CiRNA biogenesis relies on a consensus motif of a 7 nucleotide GU‐rich element near the 5′ spliced site and an 11 nucleotide C‐rich element adjacent to the branchpoint site. RNA‐binding proteins (RBPs) may actively participate in this process. EcircRNAs (exonic circRNAs) are mainly distributed in the cytoplasm, whereas ciRNAs (circular intronic RNAs) and EIciRNAs (exon‐ and intron‐containing circular RNAs) are primarily located in the nucleus.
LncRNAs are expressed at lower levels in comparison with mRNAs and display more tissue‐specific expression patterns [32]. Additionally, lncRNAs can be distributed either in the nucleus or the cytoplasm, or in both compartments simultaneously [summarized in [33]], and may or may not be subject to polyadenylation or alternative splicing [34]. Although few lncRNAs have been characterized in detail, it is clear that lncRNAs regulate various biological processes [35] in a number of different ways [summarized in [36]]. Based upon their genomic location, lncRNAs can be classified into five categories: (a) sense or (b) antisense, when the lncRNA overlaps the neighboring protein‐coding gene on the same, or opposite, strand, respectively; (c) bidirectional, when the lncRNA transcription start site (TSS) is located within 1 kb, but on the opposing strand, of the TSS of the nearest protein‐coding gene; (d) intronic, when lncRNA derives from intronic regions of protein‐coding genes; and (e) intergenic, or long intergenic noncoding RNAs (lincRNAs), when lncRNA is located within the genomic interval between two genes [37].
Besides acting as competitive endogenous RNAs (ceRNAs), both circRNAs and lncRNAs can also act through different mechanisms, as shown in Fig. 2. Interestingly, circRNAs or lncRNAs might also originate from chromosomal DNA translocations. However, the expression patterns and functions of these ncRNAs in solid tumors are still unclear [summarized in [38]].
Fig. 2.
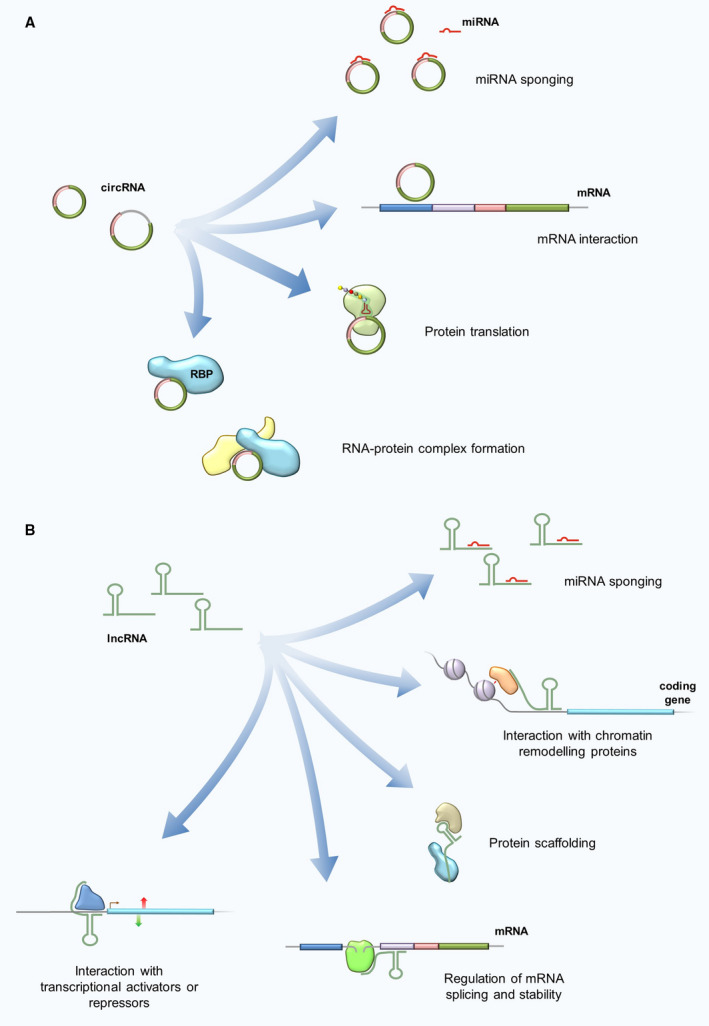
CircRNA (A) and lncRNA (B) functions. CircRNAs can modulate gene expression at different levels: by competitive miRNA sponging and sequestration, thus indirectly enabling the transcription of downstream genes, or by direct interaction with target mRNAs. In rare cases, circRNAs can be translated into proteins. Lastly, circRNAs can interact with RNA‐binding proteins (RBPs) to regulate multiple signaling pathways. LncRNAs are involved in transcriptional and post‐transcriptional regulation of gene expression. In particular, lncRNAs have been implied in different regulatory mechanisms: by competitively binding to miRNAs, by binding and redirecting chromatin remodeling proteins or transcription factors to alternatively modulate transcription of target genes, and by regulating mRNA splicing and degradation. In addition, lncRNAs can serve as scaffold for the formation of multiprotein complexes.
3. LncRNAs as regulators of the MAPK‐signaling cascades
MAPK pathways are cascades of four kinases that regulate a range of biological processes [summarized in [39, 40]]. So far, there are a number of studies aimed at elucidating lncRNA‐MAPK‐signaling interaction networks in CM harboring BRAF or RAS mutations, whereas no information on circRNAs is available (Table 1, Fig. 3).
Table 1.
CircRNAs and lncRNAs that are aberrantly expressed in BRAF/RAS‐mutant CM.
| Functional pathway | NcRNA ID | Expression change | Cell lines | BRAF/RAS mutational status | Target gene(s) | Notes | References |
|---|---|---|---|---|---|---|---|
| MAPK/ERK pathway | ATB | Up |
A375 A2058 |
BRAFV600E | MiR‐590‐5p/YAP‐1 | [119] | |
| BANCR | Up |
A375 1205Lu SK‐MEL‐5 |
BRAFV600E | ERK1/2 and JNK pathway components | BANCR expression is downregulated by LINC‐PINT | [43, 44] | |
| MIR31HG | Up | Human diploid fibroblasts expressing a constitutively activated form of the mouse BRAFV600E fused to the estrogen receptor | BRAFV600E | p16INK4A | [48] | ||
| MIR4435‐2HG | Up |
A375 A2058 |
BRAFV600E | MiR‐802/FLOT2 (MAPK/ERK?) | [57] | ||
| MIRAT | Up |
DO4 MM415 |
NRASQ61L | MAPK pathway/IQGAP1 | [61] | ||
| Orilnc1 | Up |
A2058 LOX‐IMVI UACC‐257 WM9 WM983B 1205Lu 451Lu |
BRAFV600E | Cyclin E1 | [51] | ||
|
SK‐MEL‐2 WM3936 |
NRASQ61L | ||||||
| OVAAL | Up | ME4405 | NRASQ61L | p27 | [47] | ||
| RMEL3 | Up |
WM278 WM1617 |
BRAFV600E | MAPK pathway components | [49] | ||
| ZEB1‐AS1 | Up | TGCA data |
BRAFV600E NRASQ61L |
MAPK/ERK? | [52] | ||
| p38/JNK pathway | BANCR | Up |
A375 1205Lu SK‐MEL‐5 |
BRAFV600E | ERK1/2 and JNK pathway components | BANCR expression is downregulated by LINC‐PINT | [43, 44] |
| FENDRR | Down |
A375 SK‐Mel‐28 |
BRAFV600E | MMP2, MMP9, JNK pathway component | [67] | ||
| SK‐MEL‐110 | KRASE63K | ||||||
| SPRY4‐IT1 | Up |
A375 WM1552C |
BRAFV600E | MiR‐22‐3p/p38MAPK/MAPKAPK/Hsp27 | [73] | ||
| SRA | Up |
A375 SK‐MEL‐1 |
BRAFV600E | p38 | [74] | ||
| ERK5 pathway | FOXD3‐AS1 | Up |
A375 SK‐Mel‐1 |
BRAFV600E | MiR‐325/MAP3K2 (ERK5?) | [79] | |
| PI3K/AKT pathway | H19 | Up |
C32 SK‐MEL‐28 |
BRAFV600E | PI3K/AKT and NF‐kB pathway components | [200] | |
| LINC00961 | Down |
A375 SK‐MEL‐28 |
BRAFV600E | MiR‑367/PTEN | [89] | ||
| MHENCR | Up | A375 | BRAFV600E | MiR‐425/489/PI3K‐Akt pathway | [85] | ||
| SK‐MEL‐2 | NRASQ61L | ||||||
| MIAT | Up |
A375 A2058 M21 SK‐MEL‐28 |
BRAFV600E | PI3K‐Akt pathway components | [158] | ||
| PEG10 | Up | A375 | BRAFV600E | MiR‐33a/PI3K‐Akt and mTOR pathways | [83] | ||
| RMEL3 | Up |
WM278 WM1617 |
BRAFV600E | PI3K/Akt pathway components | [49] | ||
| GAS6/AXL pathway | GAS6‐AS2 | Up |
A375 SK‐MEL‐5 |
BRAFV600E | GAS6, AXL | [94] | |
| SK‐MEL‐2 | NRASQ61L | ||||||
| MITF pathway | DIRC3 | Down |
SK‐MEL‐28 A375 501mel |
BRAFV600E | IGFBP5 | [100] | |
| PRC2 complex | ANRIL | Up | A375 | BRAFV600E | CDKN2A/B | [130] | |
| CDR1as | Up | Cancer Cell Line Encyclopedia | BRAFV600E | IGF2 mRNA‐binding protein 3 | CD1R arises from the PRC2‐mediated epigenetic silencing of the lncRNA LINC00632 | [128] | |
| CircANRIL | Up | BJ | BRAFV600E | PRC proteins | [129] | ||
| GAS5 | Down | A375 | BRAFV600E | EZH2 | [135] | ||
| SK‐MEL‐110 | KRASE63K | ||||||
| PVT1 | Up |
A375 SK‐MEL‐5 |
BRAFV600E | MiR‐200c/EZH2 | [137] | ||
| EMT/invasion/metastasis | BANCR | Up |
A375 A875 M14 |
BRAFV600E | MiR‐204/Notch2 | [201] | |
| CASC2 | Down | A375 | BRAFV600E | MiR‐18a‐5p/RUNX1 | [202] | ||
|
A375 M14 |
BRAFV600E | MiR‐181a/PLXNC1 | [203] | ||||
| Circ_0016418 | Up |
SK‐MEL‐1 SK‐MEL‐5 |
BRAFV600E | MiR‐625/YY1 | [115] | ||
| Circ_0084043 | Up |
A375 A875 |
BRAFV600E | MiR‐153‐3p/Snail | [119] | ||
|
A375 SK‐MEL‐28 |
BRAFV600E | Wnt/β‐catenin pathway through miR‐429/TRIB2 axis | [120] | ||||
| CRNDE | Up |
A375 M14 |
BRAFV600E | MiR‐205/CCL18 | [204] | ||
| GAS5 | Down |
A375 M21 SK‐Mel‐28 |
BRAFV600E | MMP2, MMP9 | [205] | ||
| SK‐Mel‐110 | KRASE63K | ||||||
| HOTAIR | Up | A375 | BRAFV600E | MMP2, MMP9 | [102] | ||
|
A375 A875 SK‐MEL‐1 SK‐MEL‐5 SK‐MEL‐28 |
BRAFV600E | MiR‐152‐3p/c‐MET | [104] | ||||
| KCNQ1OT1 | Up |
A375 A875 MuM‐2C |
BRAFV600E | MiR‐153/c‐MET | [206] | ||
| LINC00173 | Up |
A375 A2058 HT144 SK‐MEL‐1 |
BRAFV600E | MiR‐493/IRS4 | [207] | ||
| LINC00518 | Up |
A375 A2058 SK‐MEL‐28 |
BRAFV600E | MiR‐204‐5p/AP1S2 | [112] | ||
| LINC00963 | Up |
A375 A2058 |
BRAFV600E | MiR‐608/NACC1 | [208] | ||
| MALAT1 | Up |
A375 SK‐MEL‐5 |
BRAFV600E | MiR‐22/MMP14/Snail | [209] | ||
| SK‐MEL‐2 | NRASQ61L | ||||||
| MIAT | Up |
A375 SK‐MEL‐28 |
BRAFV600E | MiR‐150 | [210] | ||
| NEAT1 | Up |
A375 A2058 SK‐MEL‐28 |
BRAFV600E | MiR‐495‐3p/E2F3 | [211] | ||
|
A375 A875 A2058 M14 451LU |
BRAFV600E | MiR‐23a‐5p/KLF3 | [144] | ||||
| MEG3 | Down |
A375 A875 |
BRAFV600E | MiR‐499‐5p/CYLD | [126] | ||
| MiR‐21/E‐cadherin | [127] | ||||||
| SSATX | Up |
A375 A875 |
BRAFV600E | Wnt/β‐catenin pathway | Alternative splicing variant of the SAT1 gene, it might function as a lncRNA prior to its degradation | [212] | |
| SLNCR1 | Up | A375 | BRAFV600E | MMP9 | [213] | ||
| TUG1 | Up |
A375 SK‐MEL‐5 WM35 |
BRAFV600E | MiR‐129‐5p/AEG‐1 | [123] | ||
| SK‐MEL‐2 | NRASQ61L | ||||||
| A375 | BRAFV600E | MiR‑29c‑3p/RGS1 | [214] | ||||
| SK‐MEL‐2 | NRASQ61L | ||||||
| UCA1 | Up | A375 | BRAFV600E | MiR‐507/FOXM1 | [215] | ||
| SK‐MEL‐2 | NRASQ61L | MiR‐185‐5p/Wnt/β‐catenin pathway | [122] | ||||
|
A375 A2058 HS294T WM266‐4 |
BRAFV600E | ||||||
| Metabolism | CircMYC | Up | Mel‐CV | BRAFV600E | MiR‐1236/LDHA | c‐MYC‐SRSF1 axis regulates the production of circMYC | [141] |
| Circ_ITCH | Down |
A375 M21 |
BRAFV600E | GLUT1 | Circ_ITCH is generated from several exons of ITCH | [140] | |
| Circ_0016418 | Up |
A375 A875 |
BRAFV600E | MiR‐605‐5p/GLS | [145] | ||
| Circ_0025039 | Up |
A375 A2058 SK‐MEL‐1 |
BRAFV600E | MiR‐198/CDK4 | Circ_0025039 originates from the NM_202002 fragment of chromosome 12, which is homologous to the protein‐coding gene FOXM1 | [142] | |
| Circ_0084043 | Up |
A375 A378 |
BRAFV600E | MiR‐31/KLF3 axis | [143] | ||
| H19 | Up |
A375 SK‐MEL‐1 SK‐MEL‐5 |
BRAFV600E | MiR‐106a‐5p/E2F3 | [216] | ||
| OIP5‐AS1 | Up | A375 | BRAFV600E | MiR‐217/GLS | [146] |
Fig. 3.
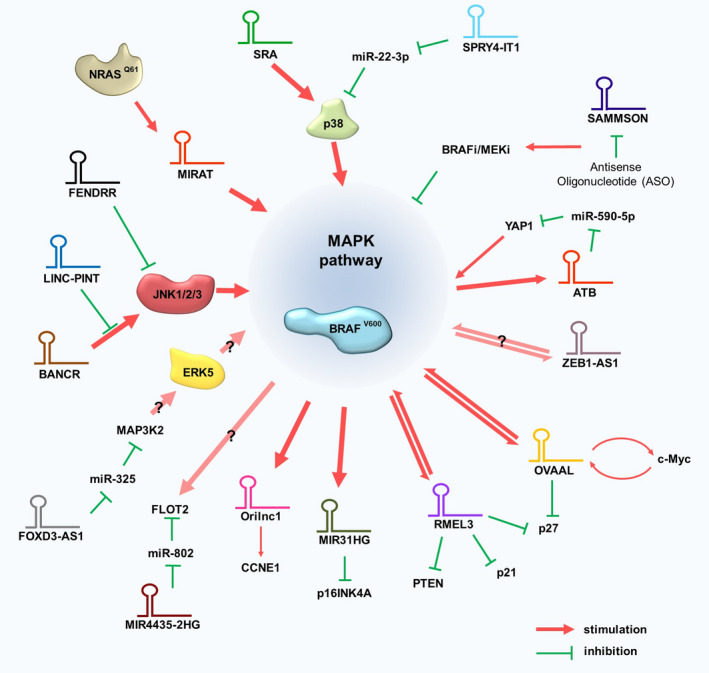
LncRNAs associated with the MAPK pathways in CM. Red arrows and green blocking bars indicate a positive or negative regulation, respectively.
3.1. LncRNAs related to MAPK/ERK signaling pathway
As stated above, the MAPK/ERK cascade plays a key role in BRAFV600 ‐mutant CM development, making it the most prominent and clinically utilized therapeutic target [summarized in [41]]. In this context, a number of lncRNAs were shown to actively interact with BRAFV600 and/or MAPK/ERK pathway in CM, including the oncogenic BRAF‐activated nonprotein‐coding RNA (BANCR). BANCR was originally correlated with BRAFV600 activation since it was found to be overexpressed in BRAFV600 ‐mutant CM in comparison with normal melanocytes. Although BANCR was initially described as a regulator of CM migration [42], subsequent studies demonstrated that BANCR regulated CM progression through activating the ERK1/2 and JNK/MAPK pathways both in vitro and in vivo [43]. In BRAF‐mutant A375 cells, BANCR expression appeared to depend on the long intergenic nonprotein‐coding RNA p53‐induced transcript (LINC‐PINT) [44], which is known to function as a tumor suppressor [45] and to interact with MAPK [46]. Despite these findings, the question of whether LINC‐PINT might regulate the BANCR/MAPK axis to inhibit BRAF‐mutant CM progression deserves further study.
In their study, Sang et al. reported a significant upregulation of the lncRNA ovarian adenocarcinoma amplified long noncoding RNA (OVAAL) in BRAF‐mutant CM compared with wild‐type CM in a TCGA dataset. Detailed mechanistic insights revealed that OVAAL was bound to STK3, enhanced the structural association of STK3 with Raf‐1, and activated the MAPK/ERK signaling pathway which, in turn, promoted c‐Myc‐driven proliferation. Following treatment with the MEKi UO126, OVAAL failed to influence c‐Myc levels, thus confirming that the OVAAL‐mediated upregulation of c‐Myc was depended on the MAPK pathway. In addition, silencing of c‐Myc reduced, whereas overexpression of c‐Myc increased, OVAAL expression levels. These results clearly suggested a positive feedback loop between c‐Myc, OVAAL, and MAPK/ERK signaling pathway in controlling tumor growth [47]. The same group reported that OVAAL competed with p27 mRNA for binding to PTBP1, thus impairing p27 mRNA translation and allowing CM cells to escape from cellular senescence [47]. However, since these studies have been performed in BRAFWT /NRASQ61R CM cells, it would be of interest to address in more detail the specific role of OVAAL in BRAF‐mutant CM.
Alike to OVAAL, the lncRNA MIR31HG was implicated in CM senescence. Intriguingly, both activity and subcellular localization of MIR31HG were strictly dependent on BRAFV600E [48]. Under normal conditions, MIR31HG was predominantly located in the nucleus of CM cells, where it recruited polycomb group (PcG) proteins to the INK4 locus to repress p16INK4A expression. Interestingly, MIR31HG knockdown reduced PcG chromatin occupancy and induced p16INK4A‐dependent senescence, which was reverted by MIR31HG overexpression. RNA‐seq analysis of CM samples holding normal diploid INK4A loci revealed a negative correlation between MIR31HG and p16INK4A expression, indicating that MIR31HG‐mediated repression of p16INK4A might drive CM progression. The same authors also observed that following BRAFV600E activation, MIR31HG translocated to the cytoplasm, whereas CM cells acquired an oncogene‐induced senescence (OIS) phenotype along with an increased expression of p16INK4A protein. Consistent with this, MIR31HG depletion reduced BRAFV600E CM cell growth and promoted OIS. However, although p16INK4A levels decreased upon MIR31HG overexpression, OIS was not reverted, thus highlighting the complexity of molecular mechanisms involved in BRAFV600E ‐induced senescence [48].
Compared with wild‐type CM, BRAFV600 ‐mutant CM exhibited a significant upregulation of the oncogenic restricted to melanocyte 3 (RMEL3) [49]. Enforced RMEL3 expression enhanced BRAFV600 ‐mutant CM cell proliferation and clonogenic ability both in vitro and in vivo [50], whereas RMEL3 abrogation decreased cell survival and proliferation along with an increase in PTEN and cell cycle inhibitors p21 and p27 protein levels [49]. Aberrant expression levels of MAPK/ERK and PI3K/AKT pathway effectors were also observed upon RMEL3 silencing [49], thus indicating that MAPK/ERK activation and RMEL3 expression might be coordinately regulated through a positive feedback loop. Similarly to RMEL3, the oncogenic RAS‐induced lncRNA 1 (Orilnc1) was found increased in BRAFV600 ‐mutant CM in respect to wild‐type CM. In line with the observation that Orilnc1 was induced by RAS‐RAF‐MEK‐ERK pathway activation, Orilnc1 acted as a mediator of RAS signaling and promoted an oncogenic CM phenotypes by regulating cyclin E1 in BRAF‐mutant CM cells [51]. In addition to RMEL3 and Orilnc1, CM samples carrying BRAF or NRAS mutations overexpressed the lncRNA ZEB1 antisense RNA 1 (ZEB1‐AS1) [52]. However, the correlation between ZEB1‐AS1 deregulation and MAPK activation in BRAF‐mutant CM has not been assessed so far.
The lncRNA activated by TGF‐beta (ATB) could enhance the expression of YAP‐1 by sponging miR‐590‐5p to promote proliferation, migration, and invasion of BRAF‐mutant CM cells. Of note, YAP‐1 activation induced the ERK/MAPK‐signaling pathway in gallbladder [53] and papillary thyroid cancers [54] which commonly harbor BRAF mutations [55, 56]. Hence, it would be interesting to study ATB regulation and to investigate whether the ATB/YAP‐1 axis triggers the ERK/MAPK pathway in BRAF‐mutant CM.
Recently, a positive correlation between MIR4435‐2HG and flotillin 2 (FLOT2) expression was identified in A375 and A2058 cells, where MIR4435‐2HG sponged miR‐802 to upregulate FLOT2 [57]. Small interfering RNA (siRNA) targeting FLOT2 restrained A375 cell proliferation, migration, and invasion, whereas MIR4435‐2HG upregulation or miR‐802 silencing abrogated the inhibitory effects of FLOT2 knockdown. FLOT2 is highly expressed in CM and was related to lymph node CM metastasis [58]. Of interest, MAPK/ERK pathway was predicted to play a key role in the signaling cascade caused by FLOT2 overexpression in CM [59]. Hence, further investigation is needed to determine whether MIR4435‐2HG/miR‐802/FLOT2 axis might affect BRAF‐mutant CM progression through modulating MAPK/ERK signaling.
The above reported data clearly indicate that altered lncRNA expression contributes to the abnormal MAPK signaling in BRAFV600 ‐mutant CM. In addition, considering that MAPK pathway activation represents a frequent mechanism of resistance for small molecules directed against BRAFV600, it is reasonable to believe that aberrant lncRNA expression might also influence CM resistance to BRAFi. Actually, little is known about the role of lncRNAs in the establishment of CM resistance to BRAFi [60], and only few studies have explored the impact of lncRNA silencing in restoring sensitivity to target therapies in CM. For instance, an induced expression of the lncRNA‐MAPK inhibitor resistance‐associated transcript (MIRAT) was found in NRAS‐mutant CM cells with acquired resistance to BRAFi/MEKi [61]. Gain‐ and loss‐of‐function assays, as well as RNA–protein interaction assays, indicated that MIRAT modulated the MAPK‐signaling pathway by binding to the scaffold protein IQGAP1 [61], which could promote RAS‐MAPK‐driven cancer invasion [62]. Interestingly, MIRAT depletion did not significantly affect cell viability in resistant NRAS‐mutant CM cells, thus suggesting that, despite its role in regulating MAPK activation, MIRAT silencing was not sufficient to revert resistance to targeted therapy [61]. However, since no additional studies have been performed correlating MIRAT with BRAFi/MEKi resistance in BRAF‐mutant CM, this lncRNA should be further investigated. Differently, the silencing of the lncRNA survival‐associated mitochondrial melanoma‐specific oncogenic noncoding RNA (SAMMSON) drastically impaired CM cell viability irrespective of their BRAF, NRAS, or p53 mutational status and improved sensitivity toward targeted therapy in patient‐derived xenograft models of BRAFV600 ‐mutant CM [63]. Mechanistically, SAMMSON interacted with p32 to maintain its mitochondrial localization and to enhance its function. Concordantly, SAMMSON targeting using antisense oligonucleotides (ASOs) decreased mitochondrial ribosome biogenesis, oxidative phosphorylation, and respiratory chain complex activity. Therefore, the synergistic killing of BRAFV600 ‐mutant CM cells observed upon co‐targeting of SAMMSON and MAPK pathway components was likely to arise because BRAFi elevated oxidative phosphorylation [64], whereas SAMMSON silencing led to mitochondrial dysfunction.
3.2. LncRNAs related to JNK and p38 MAPK‐signaling pathways
Besides MAPK/ERK cascade, stress‐activated MAPK pathways, such as JNK and p38, play important modulatory roles that can influence the response of CM cells to targeted therapy [65, 66]. For instance, as reported above, BANCR was demonstrated to activate JNK along with MAPK/ERK signaling pathway [43]. Another lncRNA, namely FOXF1 adjacent noncoding developmental regulatory RNA (FENDRR), mediated proliferation, migration, and invasion of BRAF‐ and KRAS‐mutated CM cells through the JNK pathway. Contrary to BANCR, FENDRR was downregulated in CM with the lowest expression in CM with metastasis [67]. In vitro and in vivo functional analyses revealed that FENDRR not only antagonized the JNK pathway, but also inhibited matrix metallopeptidases expression. So far, three different JNK isoforms have been identified, namely JNK1, JNK2, and JNK3. Interestingly, a paper of Du et al. [68] reported that JNK2 expression was significantly higher than JNK1 in CM and was specifically required for cell proliferation, invasiveness, and adaptive BRAFi resistance. However, the studies of Li et al. and Chen et al. did not indicate which JNK isoform interacted with BANCR and FENDRR, respectively.
Other studies reported that p38/MAPK might mediate cell survival [69] or cell death in BRAF‐mutant CM [70] depending on the cell context and the type of stimulus. For instance, p38/MAPK signaling was reported to be involved in biological processes associated with CM progression and mediated by the lncRNAs SPRY4 intronic transcript 1 (SPRY4‐IT1), which was initially identified to be upregulated in BRAF‐mutant WM1552C and A375 cells in comparison with melanocytes. SiRNA‐mediated SPRY4‐IT1 knockout was shown to inhibit CM cell proliferation, motility, and invasion, while increasing apoptosis [71]. SPRY4‐IT1 is transcribed from the second intron of the SPRY4 gene, a regulator of the MAPK cascade [72], indicating that SPRY4‐IT1 may also affect the MAPK‐signaling pathway. To investigate this further, A375 cells were transfected with short hairpin RNA targeting SPRY4‐IT1. Results demonstrated that SPRY4‐IT1 depletion reduced the phosphorylation levels of p38, MAPKAPK, and Hsp27. In addition, SPRY4‐IT1 knockdown enhanced miR‐22‐3p levels and inhibited CM proliferation and metastasis. Hence, Li et al. [73] proposed that SPRY4‐IT1 could act as ceRNA via sponging miR‐22‐3p to activate the p38 MAPK‐signaling pathway in CM.
Alike to SPRY4‐IT1, the lncRNA steroid receptor RNA activator (SRA) was upregulated in A375 and SK‐MEL‐1, both of which are BRAF‐mutant CM cell lines. Functional assays showed that SRA mediated cell proliferation and regulated cell invasion in the A375 cell line and in B16 murine CM cells. Of interest, a shift from p38 to MEK1/2 and BRAF phosphorylation emerged in B16 cells when SRA was inhibited with siRNAs [74]. However, since B16 cells do not harbor a BRAF mutation, future studies would be necessary to further explore whether SRA influences MAPK signals in BRAF‐mutant CM.
3.3. LncRNAs related to ERK5 signaling pathway
ERK5 was recently shown to be activated in BRAF‐mutant CM and to be involved in BRAFi/MEKi resistance [75, 76, 77, 78]. Of interest, the lncRNA FOXD3 antisense RNA 1 (FOXD3‐AS1) sponged miR‐325 to positively regulate MAP3K2, an upstream activator of ERK5, in A375 and SK‐MEL‐1 cells. In addition, MAP3K2 overexpression could rescue the effect induced by FOXD3‐AS1 silencing and improved proliferation, invasion, and migration of BRAF‐mutant CM [79]. At the moment, however, it remains to be clarified whether the FOXD3‐AS1/miR‐325/MAP3K2 axis also affects the ERK5 pathway and/or has a role in targeted therapy resistance.
4. Pleiotropic effects of circRNAs and lncRNAs in BRAF‐mutant CM
In addition to MAPK‐related lncRNAs, several other circRNAs and lncRNAs have demonstrated aberrant expression in CM. Since these studies were mainly conducted in BRAF‐ and RAS‐mutant CM cell lines, circRNA and lncRNA deregulation likely represents a mechanism for strengthening the already activated MAPK signaling. Consistent with this hypothesis, most of these ncRNAs were proven to target molecular pathways that cooperate with MAPK family members and/or are known to be involved in BRAFi/MEKi resistance of CM cells. More importantly, restoration of their expression could revert the malignant phenotype both in vitro and in vivo, thus confirming their pathogenic relevance (Table 1).
4.1. LncRNA modulation of PI3K/AKT signaling
The PI3K/AKT signaling pathway is one of the major regulators of cell survival and apoptotic cell death. PI3K/AKT and MAPK/ERK pathways strictly regulate each other; therefore, the inhibition of one of these two pathways can promote the activity of the other one [80]. PI3K/AKT aberrant activation is a common phenomenon in CM cells, where increased PI3K/AKT signaling, with or without concomitant MAPK activity, represents an alternative path to both innate and acquired BRAFi/MEKi resistance [81].
Microarray analysis in 18 melanocytic nevi with and four nevi without the BRAFV600E mutation revealed 92 upregulated genes in nevi with the BRAF mutation, including the lncRNA paternally expressed gene 10 (PEG10) [82], thus suggesting that gain of PEG10 expression might occur early during BRAF‐mutant CM development. Functional analyses demonstrated that PEG10 silencing reduced cyclin D1 and CDK4 expression, triggered apoptosis, and impaired A375 CM cell migration and invasion. More specifically, PEG10 knockdown obstructed PI3K/AKT pathway by enhancing the expression of miR‐33a [83] which functions as a tumor suppressor in CM [84]. Although these results enforced PEG10 involvement in the progression of BRAFV600 CM, the deeper correlation between PEG10 and PI3K/AKT pathway remains to be further explored.
Chen et al. [85] identified melanoma highly expressed noncoding RNA (MHENCR) as a critical regulator of PI3K/AKT. Mechanistically, MHENCR associated with miR‐425 and miR‐489 which inhibit PI3K/AKT pathway via targeting IGF1 and spindlin 1, respectively. PI3K/AKT activation through IGF1 deregulation has been shown to result in CM metastasis [86] and resistance to BRAFi‐induced apoptosis [81]. In patients with BRAFi resistance, deregulation of the PI3K/AKT pathway may be mediated by several mechanisms, including the loss of function of the tumor suppressor PTEN [87]. NcRNAs have been shown to regulate PTEN, thus contributing to the aberrant activation of the PI3K/AKT pathway. Among them, miR‐367 was reported to directly regulate PTEN protein expression to promote CM development [88]. Recently, Mu et al. provided the first evidence that the lncRNA LINC00961 acted as a micro‐RNA (miRNA) sponge for miR‐367. By sponging miR‐367, LINC00961 restored PTEN expression and suppressed migration and invasion of the BRAF‐mutant A375 and SK‐MEL‐28 cells [89]. Hence, whether MHENCR and LINC00961 are involved in CM resistance to BRAFi/MEKi requires further study.
4.2. LncRNA regulation of Gas6/AXL signaling pathway
GAS6 is a ligand for several receptor tyrosine kinases, including AXL which is usually highly expressed in BRAFi/MEKi‐resistant CM [90, 91, 92]. Furthermore, recent genomic and transcriptomic data from metastatic CM patients indicated that AXL overexpression might cause resistance to anti‐PD‐1 therapy [93]. In a recent study, Wen et al. found that the antisense RNA 2 of GAS6 (GAS6‐AS2) promoted the secretion of GAS6 in the CM cell supernatants and further increased the phosphorylation levels of AXL, AKT, and ERK in an autocrine manner. In addition, GAS6‐AS2 accelerated CM cell proliferation, and inhibited CM cell apoptosis both in vitro and in vivo. Notably, ectopic expression of GAS6‐AS2 activated the pro‐survival GAS6/AXL/AKT/ERK signals not only in BRAFV600 CM cells but also in NRAS‐mutant CM, thus supporting the rationale for further investigation on the potential implications of GAS‐AS2 in BRAFi/MEKi resistance [94].
4.3. LncRNAs in MITF signaling pathway
MITF is a master regulator transcription factor with well‐documented roles not only in melanocytes, but also in CM progression. Three major subpopulations of cells with different MITF expression levels have been detected in CM, some with high MITF levels, which were more proliferative, some exhibiting low MITF levels along with higher invasive and tumor‐forming capacities, and others expressing markers of both signatures [95]. In BRAFi/MEKi‐resistant CM cells, low MITF expression could induce high levels of tyrosine kinase receptors, such as AXL and EGFR, thus contributing to prolonged therapy resistance [92, 96]. However, MITF overexpression could also drive resistance, indicating its complex role in CM resistance to targeted therapy [97]. SOX10 activates MITF transcription in a cis‐acting fashion in melanocytes and CM [98] and cooperates with MITF in activating further downstream targets [99]. Coe et al. identified 245 CM‐associated lncRNAs whose loci were cobound by MITF and SOX10, including disrupted in renal carcinoma 3 (DIRC3). DIRC3 was described as a nuclear regulatory lncRNA that activated the expression of the neighboring IGFBP5 tumor suppressor gene. DIRC3 loss of function in three BRAF‐mutant CM cell lines led to increased anchorage‐independent growth and SOX10 occupancy at putative regulatory elements within the DIRC3 locus [100]. Furthermore, DIRC3 depletion enhanced SOX10‐mediated repression of IGFBP5 [100], which negatively regulated MAPK kinase signaling to inhibit BRAF‐mutant A375 cell proliferation and metastasis [100].
4.4. CircRNA and lncRNA involvement in epithelial‐to‐mesenchymal transition (EMT), invasion, and metastasis
It is widely recognized that oncogenic BRAF and RAS modulate the expression of cell adhesion‐associated proteins and induce an EMT switch that promotes metastasis and CM progression [101]. Consistently, a close correlation between an EMT‐like phenotype and ncRNAs deregulation was found in CM cells carrying BRAF or RAS mutations. As shown in Table 1, deregulated circRNAs and lncRNAs can impact CM epithelial plasticity by affecting different target genes, and their effects are mainly ascribed to their ability to act as ceRNAs.
HOX transcript antisense RNA (HOTAIR) has emerged as a critical factor for CM metastatic state since its expression was dramatically increased not only in metastatic respect to primary CM [102], but also in lymphocytes surrounding metastatic CM cells [103]. Luan et al. [104] suggested that HOTAIR might promote CM invasion and migration by competitively binding to miR‐152‐3p to upregulate the tyrosine kinase c‐MET, which is known to be involved in CM metastasis [105]. The activation of c‐MET by the lncRNA KCNQ1 opposite strand/antisense transcript 1 (KCNQ1OT1) was also found to increase the metastatic growth of A375 cells. Importantly, besides promoting CM metastasis, c‐MET upregulation was recognized to contribute to BRAFi resistance [106], whereas both HOTAIR and KCNQ1OT1 were supposed to play a role in chemoresistance [107, 108, 109] and radioresistance [110, 111]. Despite these findings, however, no study has demonstrated their possible involvement in c‐MET‐induced BRAFi resistance so far.
LINC00518 promoted in vitro invasion and migration of BRAF‐mutant A375 and A2058 cells and in vivo pulmonary metastasis through decoying miR‐204‐5p to upregulate AP1S2 expression [112]. Interestingly, a previous study demonstrated that BRAFV600 negatively regulated miR‐204 through the MAPK/ERK pathway, whereas treatment with BRAFi/MEKi induced its expression. Furthermore, miR‐204 overexpression potentiated anti‐migratory activity of BRAFi‐resistant CM cells by targeting mRNA [113].
Using microarray analysis, several aberrantly expressed circRNAs were identified in the BRAF‐mutant WM35 and WM451 cell lines compared with normal melanocytes. Functional tests revealed that, among these circRNAs, circ_0000082, circ_0008157, circ_0016418, circ_0023988, and circ_0030388 regulated proliferation and invasion of CM cells [114]. Further research indicated that circ_0016418 contributed to SK‐MEL‐1 and SK‐MEL‐5 cell proliferation and metastasis in skin melanoma by sponging miR‐625 to activate YY1 [115]. Of note, Du et al. [116] uncovered that YY1 suppression enhanced antitumor efficacy of BRAFi both in vitro and in vivo. Nevertheless, whether circ_0016418/miR‐625/YY1 axis takes part in regulating the response to BRAFi is still unknown.
In a study by Luan et al., circRNA_0084043 was reported to directly bind to miR‐153‐3p, a tumor suppressor capable of regulating EMT through targeting SNAIL [117, 118]. The use of siRNA targeting circRNA_0084043 and miR‐153‐3p mimics significantly repressed proliferation, migration, and invasion abilities of BRAF‐mutant A375 and A875 cells. Furthermore, circRNA_0084043 knockdown decreased both mRNA and protein levels of SNAIL, and this inhibition was attenuated by cotransfection of a miR‐153‐3p inhibitor. Therefore, circRNA_0084043 might play a pivotal role in BRAF‐mutant CM progression via sponging miR‐153‐3p to upregulate SNAIL [119]. In a subsequent study, Chen et al. further evaluated the effects of circ_0084043 knockdown through in vivo and in vitro experiments that confirmed its oncogenic role in CM. In particular, the authors unveiled that circ_0084043 positively controlled TRIB2 expression through sponging miR‐429. Notably, the downregulation of TRIB2 following circ_0084043 knockdown not only reduced proliferation, migration, and invasion of BRAF‐mutant A375 and SK‐MEL‐28 cells, but also inhibited β‐catenin, c‐Myc, and cyclin D1 expression. These results highlighted the ability of circ_0084043/miR‐429/TRIB2 axis to control the Wnt/β‐catenin signaling pathway [120], which is frequently activated in EMT and metastasis [101], and was recently found to correlate with overall immune suppression and to drive immunotherapy resistance in CM as well [121]. Hence, the potential effects of ncRNAs/Wnt/β‐catenin network on resistance to both targeted agents and immune checkpoint inhibitors should be considered for future studies. Similarly to circ_0084043, the lncRNA urothelial carcinoma associated 1 (UCA1) modulated the expression of β‐catenin and c‐Myc through a competitive ceRNA network, leading to EMT in BRAF‐mutant CM cells [122]. Taurine upregulated 1 (TUG1) sequestered miR‐129‐5p to upregulate AEG‐1, a downstream target of Ras and c‐Myc [123]. The use of shRNAs targeting TUG1 alleviated the invasive and migratory abilities of A375 cells and inhibited AEG‐1 protein expression. Furthermore, effects of TUG1 silencing were abrogated by AEG‐1 cotransfection, thus confirming that TUG1 functions were mediated by AEG‐1. Of interest, Zhang et al. [124] have previously reported that ectopic expression and/or silencing of AEG‐1 influenced the expression of several EMT regulators through the Wnt/β‐catenin pathway, suggesting that TUG1 might indirectly regulate EMT and Wnt signaling through the miR‐129‐5p/AEG‐1 axis.
So far, a limited number of lncRNAs with metastatic suppressor function has been reported in CM, including maternally expressed gene 3 (MEG3) [125, 126, 127]. MEG3 restoration could limit EMT‐like phenotype in BRAF‐mutant CM cells through regulating E‐cadherin expression by targeting miR‐21 [127] and miR‐499‐5p, which negatively regulated CYLD [126]. Importantly, high levels of plasma MEG3 were linked with longer survival in BRAFi‐treated CM patients, whereas CYLD downregulation might protect CM cells from BRAFi/MEKi‐induced apoptosis. Hence, the role of MEG3/miR‐499‐5p/CYLD in CM resistance to BRAFi would require further evaluation.
4.5. CircRNA and lncRNA interaction with epigenetic complexes
Some ncRNAs have shown to affect the chromatin landscape of CM cells by interacting with epigenetic enzymes, and, in turn, they can be themselves targets of these epigenetic mediators. For instance, the circRNA cerebellar degeneration‐associated protein 1 antisense transcript (CDR1as) has been proven to directly arise from the PRC2‐mediated epigenetic silencing of the lncRNA LINC00632 [128], whose function in CM has yet to be defined. Downregulation of CDR1as positively correlated with CM progression since CDR1as reduction resulted in CM invasion and metastasis by enhancing IGF2BP3. Interestingly, 18/21 cell lines with low CDR1as levels (CDR1aslow) harbored BRAF mutation, suggesting that CDR1as loss might be required for pro‐metastatic functions of IGF2BP3 in BRAF‐mutant CM. Furthermore, CDR1aslow was more sensitive to several MAPK pathway inhibitors, suggesting that CDR1as expression levels might be a useful marker to predict the response to targeted therapy [128].
Antisense noncoding RNA in the INK4 locus (ANRIL) is a well‐established example of lncRNA that interacts with PRC2 to mediate epigenetic silencing of p15INK4b and p16INK4a genes [129]. ANRIL was highly expressed in BRAF‐mutant A375 and OM431 cell lines, and its silencing activated p15INK4b and p16INK4a expression, thus significantly reducing CM growth both in vitro and in vivo [130]. Recently, Sakar et al. described several circular isoforms of the ANRIL, called circANRIL, which were all expressed in the cytoplasm of CM cell lines, thus suggesting their involvement in post‐transcriptional regulatory mechanisms. Importantly, since the expression of the linear ANRIL was specifically enriched in the nucleus, these results also indicated divergent activities for linear and circular isoforms of ANRIL [131]. Consistent with this hypothesis, a study of Muniz et al. speculated that, in proliferative cells, ANRIL would prevent senescence by repressing INK4 locus through PRC2 recruitment. On the contrary, during BRAF‐ and MEK‐induced senescence, circular ANRIL species would sequester PRC2 proteins in the cytoplasm to prevent them from being recruited to the INK4 locus [129].
PRC2 contains different catalytic components, including the histone methyltransferase EZH2 that catalyzes the trimethylation of histone H3 lysine 27 (H3K27me3) [132]. EZH2 has been evidenced to have a crucial role in CM progression [133], especially in BRAF‐mutant CM where BRAFV600 mutation and EZH2 gain often coexist [134]. Mechanistic investigations revealed that the silencing of the lncRNA GAS5 accelerated EZH2 expression to suppress the transcription of CDKN1C in A375 BRAF‐mutant CM cells [135]. On the other hand, when overexpressed, GAS5 inhibited EZH2, prevented H3K27me3, and upregulated CDKN1C expression, thus suppressing CM cells viability, and inducing apoptosis and oxidative stress [135]. Oxidative stress is a cellular characteristic of CM that has acquired BRAFi resistance and that likely renders them more sensitive to pro‐oxidative agents [136]. Hence, further studies are warranted to clarify whether the GAS5/EZH2 axis is implicated in the oxidative state of CM resistant to BRAFi.
By using BRAF‐mutant SK‐MEL‐5, Chen et al. [137] discovered that the oncogenic plasmacytoma variant translocation 1 (PVT1) directly bound to EZH2 in order to epigenetically inhibit the expression of miR‐200c, which has been described as a potential therapeutic target for overcoming BRAFi resistance [138]. In fact, loss of miR‐200c expression was found to promote a BRAFi‐resistant phenotype in CM cells and tissues with a mechanism that involved both MAPK and PI3K/AKT signaling pathways [138]. Therefore, PVT1 might be a key molecule in the development of BRAFi resistance in CM.
4.6. CircRNAs and lncRNAs as metabolism regulators
BRAF mutation dramatically affects CM metabolism, depending mainly on glycolytic metabolism [summarized in [139]]. In this context, ncRNAs were found to regulate glucose metabolism and lactate production in BRAF‐mutant CM cells. For example, the overexpression of a circRNA namely circ_ITCH restrained glucose uptake in BRAF‐mutant A375 and M21 cell lines, thereby preventing CM cell proliferation. Notably, circ_ITCH did not act as a miRNA sponge since it directly downregulated glucose transporter 1 expression [140]. On the other hand, circMYC was shown to promote Mel‐CV proliferation and to accelerate glycolysis by binding to miR‐1236, a negative regulator of lactate dehydrogenase A. CircMYC silencing significantly decreased lactate production, whereas its overexpression generated opposite effects [141]. Evidence from both in vitro and in vivo studies revealed that circ_0025039 also facilitated glucose metabolism in BRAF‐mutant CM cells by negatively regulating miR‐198 to promote CDK4 activity. Circ_0025039 depletion significantly reduced glucose consumption rate and inhibited CM cell proliferation and invasion [142]. Circ_0084043 expression was abnormally enhanced in BRAF‐mutant CM cells, as above reported. Of interest, circ_0084043 could also contribute to glycolysis in A375 and A378 cells via the modulation of the miR‐31/KLF3 axis [143]. In a similar way, the lncRNA H19 sponged miR‐106a‐5p to upregulate E2F3 expression and consequently enhanced glucose metabolism in A375 cells. Notably, both KLF3 and E2F3 participated with the lncRNA NEAT1 to form a regulatory axis that promoted BRAF‐mutant CM cell proliferation, migration, and invasion [144]. These data clearly confirm that circRNAs and lncRNAs closely cooperate to regulate BRAF‐mutant CM through different pathways.
Besides regulating the miR‐625/YY1 axis, circ_0016418 acted as a decoy for miR‐605‐5p which directly bound to glutaminase, the rate‐limiting enzyme in glutamine metabolism [145]. Consequently, circ_0016418 depletion impeded glutamine catabolism in A375 and A875 cells and impeded tumor progression. Similarly, the lncRNA OIP5 antisense RNA (OIP5‐AS1) sponged miR‐217 to upregulate glutaminase expression, thus promoting glutamine catabolism in SK‐MEL‐1 and SK‐MEL‐5 [146]. A switch from glucose to glutamine metabolism and an enhanced dependence on glutamine over glucose for cell proliferation is usually observed in BRAFi‐resistant CM [147]. Hence, these data provide valuable insights for future research, which may be directed to evaluate relationship between ncRNAs, glutamine metabolism, and response to targeted therapy in BRAF‐mutant CM.
5. CircRNAs and lncRNAs in CM immune regulation
At present, little is known about the effects of circRNAs on immune regulation in CM. However, it has become recently clear that their targeting may have therapeutic potential for overcoming immunotherapy resistance. This is supported by the study of Wei CY et al., who focused on circ_0020710, that derives from the CD151 gene. Besides promoting CM cell proliferation, migration, and invasion both in vitro and in vivo, elevated circ_0020710 levels could favor tumor immune escape. Mechanistically, circ_0020710 sponged miR‐370‐3p to protect CXCL12 from downregulation, thus creating an immunosuppression microenvironment that finally led to the exhaustion of cytotoxic T lymphocytes (CTL). Interestingly, the use of a CXCL12‐specific siRNA or the CXCL12 inhibitor AMD3100 reduced the circ_0020710‐induced malignant phenotype of CM cells. More importantly, treatment with AMD3100 and anti‐PD‐1 significantly attenuated in vivo tumor growth, indicating that the inhibition of circ_0020710/CXCL12 increased CTL infiltration and restored the efficacy of anti‐PD‐1 immunotherapy [148] (Fig. 4).
Fig. 4.
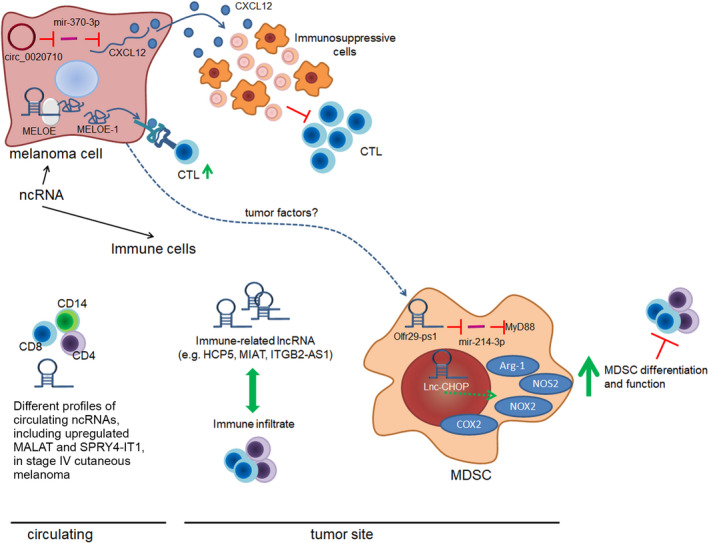
Roles of ncRNAs in CM‐immune system interaction. NcRNAs can impact on immune cell differentiation, function, and interaction with CM by acting either in cancer cells or in immune cells. In CM cells, the expression of ncRNAs could be both immunosuppressive and immunostimulating. Indeed, an impaired CTL (cytotoxic lymphocyte) infiltration can be observed in tumors expressing circ_020710, whereas the translation of lncRNA MELOE into the MELOE‐1 protein can improve CM immunogenicity. Immune cells, as well, express plenty of lncRNA. The mechanistic activity of lncRNA was studied more in detail in myeloid‐derived suppressor cells (MDSCs), where Olfr29‐ps1 and Lnc‐CHOP, with the possible contribution of tumor factors, are involved in MDSC differentiation and function. In line with the role of lncRNA in immune cell functions and with notion that the immune system is altered in cancer, CD4, CD8, and CD14 circulating cells from patients with stage IV CM were demonstrated to have different lncRNA profiles than those in healthy people. Green arrows and blocking bars indicate, respectively, the positive or negative regulation.
On the other hand, no data regarding lncRNAs and immune checkpoint inhibitors relationship are available in the literature. However, lncRNAs might play vital roles in immunotherapy resistance, since they are likely to control the homeostasis and functions of immune cells in CM (Fig. 4). In fact, RNA sequencing (RNA‐seq) analysis in diverse immune cell types (i.e., CD4+, CD8+, and CD14+ cells) identified a differential lncRNA expression profile between healthy subjects and stage IV CM patients, which usually develop resistance upon immunotherapy treatment. Functional enrichment analysis revealed that these lncRNAs were associated with several immune‐related and the PD‐1 checkpoint pathways. Metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1) and SPRY4‐IT1 expression was also detected in stage IV CM patients and showed differential expression patterns between healthy subjects and patients with stage IV melanoma and in each of the three cell types [149]. Interestingly, MALAT1 was recently found to positively regulate PD‐L1 in non‐small‐cell lung cancer [150] and diffuse large B‐cell lymphoma (DLBCL) [151]; furthermore, MALAT1 expression promoted DLBCL immune escape by regulating the proliferation and apoptosis of CD8+ T cells. This evidence would support future research aimed at exploring the role of MALAT1 in regulating immune cell function and immune response in CM. A more recent analysis found that among lncRNAs whose expression was correlated with immunology in CM, 56% were significantly associated with CD8+ T‐cell infiltration in CM [152], which has been demonstrated to be a useful biomarker to predict prognosis and response to therapy in CM patients [153]. Intriguingly, some of these lncRNAs have already been demonstrated to participate in immune regulation. In particular, the integrin subunit beta 2 antisense RNA 1 (ITGB2‐AS1) was found to be involved in the regulation of T‐cell and B‐cell activation [154], whereas the HLA class I histocompatibility antigen protein P5 (HCP5) is known for its functional roles in adaptive and innate immune responses [155]. Hence, besides controlling the miR‐1286/RARRP3 axis [156], HCP5 might also regulate CM immunogenicity. The myocardial infarction‐associated transcript (MIAT) was another lncRNAs which expression was significantly associated with the infiltration of immune cells in CM [152, 157]. Notably, although MIAT expression promoted CM cell proliferation, invasion, and migration [158], in a study of Liu et al. [157] CM patients with high expression of MIAT carried out a better prognosis, raising questions about its function in the control of immune response in CM.
Interestingly, lncRNAs were also shown to improve antigen presentation in CM (Fig. 4). For example, MELOE RNA represents a polycistronic lncRNA which is translated into MELOE‐1, MELOE‐2, and MELOE‐3 by different translational approaches: MELOE‐1 and MELOE‐2 are translated by an alternative internal ribosome entry sequence‐dependent mechanism exclusively in CM cell lines while MELOE‐3 is translated in a cap‐dependent manner, both in melanocytes and in CM cell lines [159, 160, 161]. In vitro experiments revealed a very scarce MELOE‐3‐specific T‐cell repertoire as compared to MELOE‐1 which could be recognized by tumor‐infiltrating lymphocytes and displayed the highest immunogenicity [159]. Based on these data, MELOE‐1 antigen is currently exploited as an immunotherapeutic target in a T‐cell immunotherapy clinical trial to treat metastatic CM patients (NCT02424916). LncRNAs have also proven to be associated with immune evasion since they may regulate the recruitment and activity of immunosuppressive cells, such as myeloid‐derived suppressor cells (MDSCs) (Fig. 4). As published by Shang et al. [162], the lncRNA olfactory receptor 29, pseudogene 1 (Olfr29‐ps1) could sponge miR‐214‐3p to promote MDSC differentiation into monocytic MDSCs with higher suppressive activities. By using a murine B16 melanoma model, in vivo experiments further demonstrated that Olfr29‐ps1 knockdown on MDSC decreased their immunosuppressive function. Moreover, smaller tumor volume and lighter tumor weight were detected in mice injected with Olfr29‐ps1‐knockdown MDSCs, and an increased number of CD4+ and CD8+ T cells was found in the tumor tissues compared with the control group. On the other hand, the mice injected with Olfr29‐ps1‐overexpressing MDSCs exhibited faster tumor development, larger tumor volume, heavier tumor weight, and fewer CD4+ and CD8+ T cells respect to control mice [162]. Likewise, the intronic C/EBP homologous protein long noncoding RNA (lnc‐CHOP) positively regulated MDSC generation and promoted tumor growth in murine B16 melanoma model [163]. Mechanistically, as observed by Gao et al. [163], lnc‐CHOP bound to CHOP and liver‐enriched inhibitory protein to regulate a large set of target transcripts in MDSCs, thus promoting their differentiation and immunosuppressive function in inflammatory and tumor environments. Altogether, these data indicate that the targeting of these immune‐related lncRNAs might negatively regulate the immunosuppressive abilities of MDSCs, and possibly improve CM patient’s response to immunotherapy.
In summary, there is still a lack of research on how lncRNAs regulate the function of tumor immune cells; therefore, further investigation in this field will be crucial to better elucidate the immune pathway regulation in CM in order to improve immunotherapy effectiveness.
6. Databases for the prediction and validation of circRNAs and lncRNAs
The last years have seen a rapid expansion in the number of bioinformatic resources for circRNA study, including circRNA identification algorithms, circRNA annotation databases and other tools implemented to create networks, or for visualization and computing their expression.
The two fundamental steps that allow circRNA identification are represented by the RNA library construction and sequencing. The RNase R treatment, polyadenylation, and poly(A)+ RNA depletion (RPAD) method enable the isolation of highly pure circRNA [164], whereas the RNA‐seq of RPAD‐isolated RNA analysis can be used to uncover new circRNAs. However, other library preparation strategies can be applied for circRNA identification [165], with a variable specificity in their detection. In addition, paired‐end sequencing method is also preferred to single end, improving the discovery of back‐spliced junction (BSJ) reads, that represent a molecular signature to detect circRNAs [166]. Most of the tools implemented for the identification of circRNA are stand‐alone and perform a remapping of the sequenced reads. A list of representative circRNAs identification tools is shown in Table 2. Among them, Find_circ [29], CIRI [167], and CIRCexplorer [168] use raw RNA‐seq reads, while DCC [169] employs the output of STAR aligner to detect BJS reads. Other tools largely used for circRNA identification, and based on BJS reads, are KNIFE [170], segemehl [171], Ularcirc [172], and UROBORUS [173]. Recently, machine learning approaches have also been applied to predict circRNAs, using several models classified on their known features (i.e., the conservation of transposable element, tandem repeats, open reading frame length, and single nucleotide polymorphism density). These tools mainly include DeepCirCode [174], PredcircRNA [175], WebCircRNA [176], and PredicircRNATool [177]. It is noteworthy that integration of different circRNA identification tools can reduce the false‐positive rate [178, 179, 180]. Users can combine or compare the results of different circRNA prediction tools to improve sensitivity and specificity of circRNA identification. Most of these pipelines are implemented in Python, Perl, or R and run in Linux or Unix‐like system. Although these tools are well‐documented with tutorials to help users, some computer science skills may be needed to perform an analysis. Therefore, a stand‐alone tool with a user‐friendly interface or a web‐tool could help users without advanced computational training. Of note, a comprehensive overview and evaluation of the circRNA detection tools have recently been described by Zeng and colleagues [181] and Chen and colleagues [182]. The quantification of circRNA expression is another important step in studying this class of ncRNAs. Generally, it is performed from the tools designed to identify them, and it is determined computing the ratio between back‐spliced junction reads and normal splicing junction reads, named circular‐to‐linear ratio. It represents the ratio of circRNA and linear RNA to obtain an overall expression value [183]. However, other strategies have been implemented, such as the one applied in Sailfish‐circ tool (https://github.com/zerodel/sailfish‐cir) [184] that quantifies circRNA abundance by transforming circRNA to pseudolinear transcript.
Table 2.
Selected circRNA identification tools. The column “Category” describes the type of the tool. “Annotation” label indicates tool using a gene annotation file; otherwise, it is labeled with “De novo.”
| Name | Last update | Category | Link | Reference |
|---|---|---|---|---|
| CIRCexplorer | 2019 | De novo; annotation | https://github.com/YangLab/CIRCexplorer2 | [168] |
| CIRI | 2017 | De novo | https://sourceforge.net/projects/ciri/ | [167] |
| DCC | 2019 | Annotation | https://github.com/dieterich‐lab/ | [169] |
| DeepCirCode | 2019 | De novo; annotation | https://github.com/BioDataLearning/DeepCirCode | [174] |
| Find_circ | 2015 | De novo | https://github.com/marvin‐jens/find_circ | [29] |
| KNIFE | 2016 | Annotation | https://github.com/lindaszabo/KNIFE | [170] |
| PredcircRNA | 2017 | De novo; annotation | https://github.com/xypan1232/PredcircRNA | [175] |
| PredicircRNA Tool | 2016 | Annotation | https://sourceforge.net/projects/predicircrnatool/files/ | [177] |
| Segemehl | 2018 | Annotation | https://www.bioinf.uni‐leipzig.de/Software/segemehl/ | [171] |
| Ularcirc | 2019 | Annotation | https://github.com/VCCRI/Ularcirc | [172] |
| UROBORUS | 2018 | Annotation | https://github.com/WGLab/UROBORUS | [173] |
| WebCircRNA | 2018 | De novo; annotation | https://rth.dk/resources/webcircrna/ | [176] |
At present, several circRNA databases have been established, all containing a large number of circRNAs (Table 3) [summarized in [185]]. For instance, circBase [186] annotates circRNAs based on data from nine published papers, and for each circRNA reports several types of information, such as the sequence and the genomic coordinates. CircFunBase [187] and CIRCpedia [188] also represent useful tools that resume circRNA expression profiles and annotation from six species with data from different cell types or tissues. Other databases of note are CircRNADb [189], which contains information on circRNA with protein‐coding potential, CircInteractome [190, 191], that includes interaction of circRNAs with other ncRNAs as well as expression data, and CircNet [192], that integrates miRNA‐target networks, genomic annotation, expression profiles, and circRNA sequences. Due to clinical implication of circRNAs, some databases link circRNAs and diseases. For example, circ2Traits [193] lists 1951 human circRNAs potentially associated with 105 different diseases and details miRNA–circRNA–mRNA–lncRNA interaction network for each of these diseases. The main problem in circRNA databases is given by the nomenclature. To date, there is no unified nomenclature for circRNAs, and IDs used in the different databases are not universal. A standard unified nomenclature would facilitate data integration from different databases.
Table 3.
Selected circRNA databases.
| Database | Year | Annotation tool | Link | Reference |
|---|---|---|---|---|
| Circ2Disease | 2018 | Manually curated | http://bioinformatics.zju.edu.cn/Circ2Disease/index.html | [217] |
| Circ2Traits | 2019 | NA | https://github.com/shaoli86/circ2Traits | [193] |
| Circbase | 2017 | Manually curated | http://www.circbase.org/ | [186] |
| CircFunBase | 2019 | Manually curated | http://bis.zju.edu.cn/CircFunBase/index.php | [187] |
| Circinteractome | 2018 | circBase | https://circinteractome.nia.nih.gov/ | [190, 191] |
| CircNet | 2016 | Manually curated | http://circnet.mbc.nctu.edu.tw/ | [192] |
| Circpedia | 2018 | CIRCexplorer2 | http://www.picb.ac.cn/rnomics/circpedia | [188] |
| CircR2Disease | 2018 | Manually curated | http://bioinfo.snnu.edu.cn/CircR2Disease/ | [218] |
| CircRNADb | 2016 | Manually curated | http://202.195.183.4:8000/circrnadb/circRNADb.php | [189] |
| CircRNADisease | 2018 | Manually curated | http://cgga.org.cn:9091/circRNADisease/ | [219] |
LncRNA association with other regulatory RNAs and proteins can be computationally determined using several approaches, previously used to predict miRNA or transcription factor targets. These strategies are generally based on the identification of functional similarity patterns extracted from sequences, of gene co‐expression, and of evolutionary conservation relationships [194]. Machine learning approaches have also been applied to predict RNA–RNA or RNA–protein interaction, starting from a large collection of known lncRNA–RNA interactions [195]. In view of the increasing interest in lncRNAs, several databases comprising experimentally validated and computationally predicted lncRNA interactions have recently been developed. For instance, STARBase deciphers protein–RNA and miRNA‐target interactions, thus allowing to decode lncRNA/miRNA/mRNA interaction networks [196]. Other databases of interest are listed in Table 4.
Table 4.
Selected lncRNA databases.
| Database | Year | Link | Reference |
|---|---|---|---|
| ChIPBase | 2016 | http://rna.sysu.edu.cn/chipbase/ | [220] |
| LncBase | 2016 | https://carolina.imis.athena‐innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex‐experimental | [221] |
| LNCipedia | 2019 | https://lncipedia.org/ | [222] |
| LncRNAdb | 2010 | https://rnacentral.org/expert‐database/lncrnadb | [223] |
| LncRNADisease | 2019 | http://www.cuilab.cn/lncrnadisease | [224] |
| LncRNome | 2012 | http://genome.igib.res.in/lncRNome/ | [225] |
| miRNet | 2020 | https://www.mirnet.ca/miRNet/home.xhtml | [226] |
| Noncode v6.0 | 2017 | http://www.noncode.org/ | [227] |
| STARBase | 2013 | http://starbase.sysu.edu.cn/starbase2/index.php | [196] |
7. Conclusions
Recently, circRNAs and lncRNAs have attracted intensive interest due to their potential functions in CM biology. These ncRNAs have often pleiotropic effects by which they can affect different pathways rather than acting predominantly through a specific target gene. Therefore, by functioning as regulators of gene expression, they contribute to increase the growth and spread of CM cancer cells, making them valuable biomarkers and ideal therapeutic targets. Classical circRNA and lncRNA targeting involves the use of RNA interference approaches, whereas ASO technology can be employed to ablate lncRNA expression. Considering that circRNAs and lncRNAs could be located in the nucleus [197], genome editing using CRISPR/Cas‐9 system could also serve as an intriguing method to trigger their silencing [198] [summarized in [199]]; however, additional research is needed for its eventual application in the clinic. An alternative approach to target circRNA and lncRNA interactions would be the use of small‐molecule inhibitors that can disrupt lncRNA secondary structure or inhibit their association with miRNAs. Despite these findings, at present, circRNA and lncRNA therapeutic targeting remains mainly at the laboratory stage.
Although a large number of studies have indicated ncRNA deregulation in BRAFV600 ‐mutant CM, only a number of papers are about the role of lncRNAs in response to targeted therapies, whereas no information on circRNA involvement in BRAFi/MEKi resistance is available. Similarly, research on the role of circRNAs and lncRNAs in the resistance of CM to immunotherapy is still at the nascent stage. Therefore, there are many unknown questions about circRNAs and lncRNAs that need to be further explored in CM.
Conflict of interest
MM has served as a consultant and/or advisor to Roche, Bristol‐Myers Squibb, Merck Sharp Dohme, Incyte, AstraZeneca, Amgen, Pierre Fabre, Eli Lilly, Glaxo Smith Kline, SciClone, Sanofi, Alfasigma, and Merck Serono; MM and AC own shares in Epigen Therapeutics, SRL. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
BM and EF wrote the initial manuscript and prepared the tables. GG, GP, LS, and EF designed the figures. All authors contributed to writing and finalized the manuscript. All authors read and approved the final manuscript.
Supporting information
Table S1. Summary of circRNAs and lncRNAs discussed in the text.
Acknowledgments
This work was supported by grants from 5x1000 Ministero della Salute Ricerca Corrente, Alleanza contro il Cancro, 5 × 1000 CRO Intramural Young Investigator and Ministero della Salute (GR‐2018‐12366312) to EF, Italian Association for Cancer Research (grant number IG‐23068), University of Salerno (Fondi FARB 2017), Regione Campania, GENOMAeSALUTE (POR Campania FESR 2014/2020, azione 1.5; CUP: B41C17000080007), and RarePlatNet (CUP: B63D18000380007) to AW. GP is a PhD Student of the Doctorate in Biomedical Sciences and Technologies of the University Roma 3.
Contributor Information
Giorgio Giurato, Email: ggiurato@unisa.it.
Elisabetta Fratta, Email: efratta@cro.it.
References
- 1. Karimkhani C, Green AC, Nijsten T, Weinstock MA, Dellavalle RP, Naghavi M & Fitzmaurice C (2017) The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol 177, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prado G, Svoboda RM & Rigel DS (2019) What's new in melanoma. Dermatol Clin 37, 159–168. [DOI] [PubMed] [Google Scholar]
- 3. Singh BP & Salama AKS (2016) Updates in therapy for advanced melanoma. Cancers (Basel) 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, Patch A‐M, Kakavand H, Alexandrov LB, Burke H et al. (2017) Whole‐genome landscapes of major melanoma subtypes. Nature 545, 175. [DOI] [PubMed] [Google Scholar]
- 5. Ascierto PA, Kirkwood JM, Grob J‐J, Simeone E, Grimaldi AM, Maio M, Palmieri G, Testori A, Marincola FM & Mozzillo N (2012) The role of BRAF V600 mutation in melanoma. J Transl Med 10, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT et al. (2012) Survival in BRAF V600‐mutant advanced melanoma treated with vemurafenib. N Engl J Med 366, 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364, 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muñoz‐Couselo E, García JS, Pérez‐García JM, Cebrián VO & Castán JC (2015) Recent advances in the treatment of melanoma with BRAF and MEK inhibitors. Ann Transl Med 3, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kudchadkar R, Paraiso KHT & Smalley KSM (2012) Targeting mutant BRAF in melanoma: current status and future development of combination therapy strategies. Cancer J 18, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolchok JD, Chiarion‐Sileni V, Gonzalez R, Rutkowski P, Grob J‐J, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF et al. (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377, 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dika E, Ravaioli GM, Fanti PA, Piraccini BM, Lambertini M, Chessa MA, Baraldi C, Ribero S, Andrea A, Melotti B et al. (2017) Cutaneous adverse effects during ipilimumab treatment for metastatic melanoma: a prospective study. Eur J Dermatol 27, 266–270. [DOI] [PubMed] [Google Scholar]
- 12. Gide TN, Wilmott JS, Scolyer RA & Long GV (2018) Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res 24, 1260–1270. [DOI] [PubMed] [Google Scholar]
- 13. Diamantopoulos MA, Tsiakanikas P & Scorilas A (2018) Non‐coding RNAs: the riddle of the transcriptome and their perspectives in cancer. Ann Transl Med 6, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shang Q, Yang Z, Jia R & Ge S (2019) The novel roles of circRNAs in human cancer. Mol Cancer 18, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su M, Xiao Y, Ma J, Tang Y, Tian B, Zhang Y, Li X, Wu Z, Yang D, Zhou Y et al. (2019) Circular RNAs in Cancer: emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol Cancer 18, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abi A, Farahani N, Molavi G & Gheibi Hayat SM (2020) Circular RNAs: epigenetic regulators in cancerous and noncancerous skin diseases. Cancer Gene Ther 27, 280–293. [DOI] [PubMed] [Google Scholar]
- 17. Dika E, Riefolo M, Porcellini E, Broseghini E, Ribero S, Senetta R, Osella‐Abate S, Scarfì F, Lambertini M, Veronesi G et al. (2020) Defining the prognostic role of microRNAs in cutaneous melanoma. J Invest Dermatol 140, 2260–2267. [DOI] [PubMed] [Google Scholar]
- 18. Fattore L, Costantini S, Malpicci D, Ruggiero CF, Ascierto PA, Croce CM, Mancini R & Ciliberto G (2017) MicroRNAs in melanoma development and resistance to target therapy. Oncotarget 8, 22262–22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gajos‐Michniewicz A & Czyz M (2019) Role of miRNAs in melanoma metastasis. Cancers (Basel) 11, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lazăr AD, Dinescu S & Costache M (2020) The non‐coding landscape of cutaneous malignant melanoma: a possible route to efficient targeted therapy. Cancers (Basel) 12, 3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richtig G, Ehall B, Richtig E, Aigelsreiter A, Gutschner T & Pichler M (2017) Function and clinical implications of long non‐coding RNAs in melanoma. Int J Mol Sci 18, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riefolo M, Porcellini E, Dika E, Broseghini E & Ferracin M (2019) Interplay between small and long non‐coding RNAs in cutaneous melanoma: a complex jigsaw puzzle with missing pieces. Mol Oncol 13, 74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Safa A, Gholipour M, Dinger ME, Taheri M & Ghafouri‐Fard S (2020) The critical roles of lncRNAs in the pathogenesis of melanoma. Exp Mol Pathol 117, 104558. [DOI] [PubMed] [Google Scholar]
- 24. Wu X, Xiao Y, Ma J & Wang A (2020) Circular RNA: a novel potential biomarker for skin diseases. Pharmacol Res 158, 104841. [DOI] [PubMed] [Google Scholar]
- 25. Yu X, Zheng H, Tse G, Chan MT & Wu WK (2018) Long non‐coding RNAs in melanoma. Cell Prolif 51, e12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X, Xie K, Zhou H, Wu Y, Li C, Liu Y, Liu Z, Xu Q, Liu S, Xiao D et al. (2020) Role of non‐coding RNAs and RNA modifiers in cancer therapy resistance. Mol Cancer 19, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quan G & Li J (2018) Circular RNAs: biogenesis, expression and their potential roles in reproduction. J Ovarian Res 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, Wong DT & Xiao X (2015) The landscape of microRNA, Piwi‐interacting RNA, and circular RNA in human saliva. Clin Chem 61, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M et al. (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. [DOI] [PubMed] [Google Scholar]
- 30. Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF & Sharpless NE (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA (New York, NY) 19, 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang G, Li S, Yang N, Zou Y, Zheng D & Xiao T (2017) Recent progress in circular RNAs in human cancers. Cancer Lett 404, 8–18. [DOI] [PubMed] [Google Scholar]
- 32. Chen L, Zhang Y‐H, Pan X, Liu M, Wang S, Huang T & Cai Y‐D (2018) Tissue expression difference between mRNAs and lncRNAs. Int J Mol Sci 19, 3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aillaud M & Schulte LN (2020) Emerging roles of long noncoding RNAs in the cytoplasmic milieu. Noncoding RNA 6, 10.3390/ncrna6040044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Amaral PP, Clark MB, Gascoigne DK, Dinger ME & Mattick JS (2011) lncRNAdb: a reference database for long noncoding RNAs. Nucleic Acids Res 39, D146–D151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark MB & Mattick JS (2011) Long noncoding RNAs in cell biology. Semin Cell Dev Biol 22, 366–376. [DOI] [PubMed] [Google Scholar]
- 36. Marchese FP, Raimondi I & Huarte M (2017) The multidimensional mechanisms of long noncoding RNA function. Genome Biol 18, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ponting CP, Oliver PL & Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136, 629–641. [DOI] [PubMed] [Google Scholar]
- 38. Han C, Sun L‐Y, Wang W‐T, Sun Y‐M & Chen Y‐Q (2019) Non‐coding RNAs in cancers with chromosomal rearrangements: the signatures, causes, functions and implications. J Mol Cell Biol 11, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee S, Rauch J & Kolch W (2020) Targeting MAPK signaling in cancer: mechanisms of drug resistance and sensitivity. Int J Mol Sci 21, 10.3390/ijms21031102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Plotnikov A, Zehorai E, Procaccia S & Seger R (2011) The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta Mol Cell Res 1813, 1619–1633. [DOI] [PubMed] [Google Scholar]
- 41. Savoia P, Fava P, Casoni F & Cremona O (2019) Targeting the ERK signaling pathway in melanoma. Int J Mol Sci 20, 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flockhart RJ, Webster DE, Qu K, Mascarenhas N, Kovalski J, Kretz M & Khavari PA (2012) BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res 22, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L & Sha N (2014) Long non‐coding RNA BANCR promotes proliferation in malignant melanoma by regulating MAPK pathway activation. PLoS One 9, e100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang Q, Zhang D, Diao Q & Lin M (2019) lncRNA LINC‐PINT is downregulated in melanoma and regulates cell proliferation by downregulating lncRNA BANCR. Oncol Lett 18, 2917–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu Y, Wang H, Li F, Heindl LM, He X, Yu J, Yang J, Ge S, Ruan J, Jia R et al. (2019) Long Non‐coding RNA LINC‐PINT Suppresses Cell Proliferation and Migration of Melanoma via Recruiting EZH2. Front Cell Dev Biol 7, 10.3389/fcell.2019.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu J, Gu H, Lv X, Yuan C, Ni P & Liu F (2018) LINC‐PINT activates the mitogen‐activated protein kinase pathway to promote acute myocardial infarction by regulating miR‐208a‐3p. Circ J 82, 2783–2792. [DOI] [PubMed] [Google Scholar]
- 47. Sang B, Zhang YY, Guo ST, Kong LF, Cheng Q, Liu GZ, Thorne RF, Zhang XD, Jin L & Wu M (2018) Dual functions for OVAAL in initiation of RAF/MEK/ERK prosurvival signals and evasion of p27‐mediated cellular senescence. Proc Natl Acad Sci USA 115, E11661–E11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Montes M, Nielsen MM, Maglieri G, Jacobsen A, Højfeldt J, Agrawal‐Singh S, Hansen K, Helin K, van de Werken HJG , Pedersen JS et al. (2015) The lncRNA MIR31HG regulates p16INK4A expression to modulate senescence. Nat Commun 6, 6967. [DOI] [PubMed] [Google Scholar]
- 49. Goedert L, Pereira CG, Roszik J, Plaça JR, Cardoso C, Chen G, Deng W, Yennu‐Nanda VG, Silva WA Jr, Davies MA et al. (2016) RMEL3, a novel BRAFV600E‐associated long noncoding RNA, is required for MAPK and PI3K signaling in melanoma. Oncotarget 7, 36711–36718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cardoso C, Serafim RB, Kawakami A, Gonçalves Pereira C, Roszik J, Valente V, Vazquez VL, Fisher DE & Espreafico EM (2019) The lncRNA RMEL3 protects immortalized cells from serum withdrawal‐induced growth arrest and promotes melanoma cell proliferation and tumor growth. Pigment Cell Melanoma Res 32, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang D, Zhang G, Hu X, Wu L, Feng Y, He S, Zhang Y, Hu Z, Yang L, Tian T et al. (2017) Oncogenic RAS regulates long noncoding RNA Orilnc1 in human cancer. Cancer Res 77, 3745–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Siena ÁDD, Plaça JR, Araújo LF, de Barros II, Peronni K, Molfetta G, de Biagi CAO, Espreafico EM, Sousa JF & Silva WA (2019) Whole transcriptome analysis reveals correlation of long noncoding RNA ZEB1‐AS1 with invasive profile in melanoma. Sci Rep 9, 11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li M, Lu J, Zhang F, Li H, Zhang B, Wu X, Tan Z, Zhang L, Gao G, Mu J et al. (2014) Yes‐associated protein 1 (YAP1) promotes human gallbladder tumor growth via activation of the AXL/MAPK pathway. Cancer Lett 355, 201–209. [DOI] [PubMed] [Google Scholar]
- 54. Liao T, Wen D, Ma B, Hu J‐Q, Qu N, Shi R‐L, Liu L, Guan Q, Li D‐S & Ji Q‐H (2017) Yes‐associated protein 1 promotes papillary thyroid cancer cell proliferation by activating the ERK/MAPK signaling pathway. Oncotarget 8, 11719–11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, Duh Q‐Y, Shibru D, Bastian B & Griffin A (2007) The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 246, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saetta AA, Papanastasiou P, Michalopoulos NV, Gigelou F, Korkolopoulou P, Bei T & Patsouris E (2004) Mutational analysis of BRAF in gallbladder carcinomas in association with K‐ras and p53 mutations and microsatellite instability. Virchows Arch 445, 179–182. [DOI] [PubMed] [Google Scholar]
- 57. Ma DM, Sun D, Wang J, Jin DH, Li Y & Han YE (2020) Long non‐coding RNA MIR4435‐2HG recruits miR‐802 from FLOT2 to promote melanoma progression. Eur Rev Med Pharmacol Sci 24, 2616–2624. [DOI] [PubMed] [Google Scholar]
- 58. Doherty SD, Prieto VG, George S, Hazarika P & Duvic M (2006) High flotillin‐2 expression is associated with lymph node metastasis and Breslow depth in melanoma. Melanoma Res 16, 461–463. [DOI] [PubMed] [Google Scholar]
- 59. Hazarika P, McCarty MF, Prieto VG, George S, Babu D, Koul D, Bar‐Eli M & Duvic M (2004) Up‐regulation of Flotillin‐2 is associated with melanoma progression and modulates expression of the thrombin receptor protease activated receptor 1. Cancer Res 64, 7361–7369. [DOI] [PubMed] [Google Scholar]
- 60. Joung J, Engreitz JM, Konermann S, Abudayyeh OO, Verdine VK, Aguet F, Gootenberg JS, Sanjana NE, Wright JB, Fulco CP et al. (2017) Genome‐scale activation screen identifies a LncRNA locus that regulates a gene neighborhood. Nature 548, 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sanlorenzo M, Vujic I, Esteve‐Puig R, Lai K, Vujic M, Lin K, Posch C, Dimon M, Moy A, Zekhtser M et al. (2018) The lincRNA MIRAT binds to IQGAP1 and modulates the MAPK pathway in NRAS mutant melanoma. Sci Rep 8, 10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jameson KL, Mazur PK, Zehnder AM, Zhang J, Zarnegar B, Sage J & Khavari PA (2013) IQGAP1 scaffold‐kinase interaction blockade selectively targets RAS‐MAP kinase–driven tumors. Nat Med 19, 626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leucci E, Vendramin R, Spinazzi M, Laurette P, Fiers M, Wouters J, Radaelli E, Eyckerman S, Leonelli C, Vanderheyden K et al. (2016) Melanoma addiction to the long non‐coding RNA SAMMSON. Nature 531, 518–522. [DOI] [PubMed] [Google Scholar]
- 64. Haq R, Shoag J, Andreu‐Perez P, Yokoyama S, Edelman H, Rowe GC, Frederick DT, Hurley AD, Nellore A, Kung AL et al. (2013) Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell 23, 302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lidsky M, Antoun G, Speicher P, Adams B, Turley R, Augustine C, Tyler D & Ali‐Osman F (2014) Mitogen‐activated protein kinase (MAPK) hyperactivation and enhanced NRAS expression drive acquired vemurafenib resistance in V600E BRAF melanoma cells. J Biol Chem 289, 27714–27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lopez‐Bergami P (2011) The role of mitogen‐ and stress‐activated protein kinase pathways in melanoma. Pigment Cell Melanoma Res 24, 902–921. [DOI] [PubMed] [Google Scholar]
- 67. Chen X‐E, Chen P, Chen S, Lu J, Ma T, Shi G & Sheng L (2020) Long non‐coding RNA FENDRR inhibits migration and invasion of cutaneous malignant melanoma cells. Biosci Rep 40, BSR20191194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68. Du L, Anderson A, Nguyen K, Ojeda SS, Ortiz‐Rivera I, Nguyen TN, Zhang T, Kaoud TS, Gray NS, Dalby KN et al. (2019) JNK2 is required for the tumorigenic properties of melanoma cells. ACS Chem Biol 14, 1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Naffa R, Vogel L, Hegedűs L, Pászty K, Tóth S, Kelemen K, Singh N, Reményi A, Kállay E, Cserepes M et al. (2020) P38 MAPK promotes migration and metastatic activity of BRAF mutant melanoma cells by inducing degradation of PMCA4b. Cells 9, 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li Z, Liu X, Li M, Chai J, He S, Wu J & Xu J (2020) Juglone potentiates BRAF inhibitor‐induced apoptosis in melanoma through reactive oxygen species and the p38–p53 pathway. Mol Med Rep 22, 566–574. [DOI] [PubMed] [Google Scholar]
- 71. Khaitan D, Dinger ME, Mazar J, Crawford J, Smith MA, Mattick JS & Perera RJ (2011) The melanoma‐upregulated long noncoding RNA <em>SPRY4‐IT1</em> modulates apoptosis and invasion. Cancer Res 71, 3852–3862. [DOI] [PubMed] [Google Scholar]
- 72. Masoumi‐Moghaddam S, Amini A & Morris DL (2014) The developing story of Sprouty and cancer. Cancer Metastasis Rev 33, 695–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li Z, Tang X & Duan S (2019) Interference from LncRNA SPRY4‐IT1 restrains the proliferation, migration, and invasion of melanoma cells through inactivating MAPK pathway by up‐regulating miR‐22‐3p. Int J Clin Exp Pathol 12, 477–487. [PMC free article] [PubMed] [Google Scholar]
- 74. Hong C‐H, Ho J‐C & Lee C‐H (2020) Steroid receptor RNA activator, a long noncoding RNA, activates p38, facilitates epithelial‐mesenchymal transformation, and mediates experimental melanoma metastasis. J Investig Dermatol 140, 1355–1363.e1351. [DOI] [PubMed] [Google Scholar]
- 75. Benito‐Jardón L, Díaz‐Martínez M, Arellano‐Sánchez N, Vaquero‐Morales P, Esparís‐Ogando A & Teixidó J (2019) Resistance to MAPK inhibitors in melanoma involves activation of the IGF1R–MEK5–Erk5 pathway. Cancer Res 79, 2244–2256. [DOI] [PubMed] [Google Scholar]
- 76. Cook SJ, Tucker JA & Lochhead PA (2020) Small molecule ERK5 kinase inhibitors paradoxically activate ERK5 signalling: be careful what you wish for…. Biochem Soc Trans 48, 1859–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee B, Sahoo A, Sawada J, Marchica J, Sahoo S, Layng FIAL, Finlay D, Mazar J, Joshi P, Komatsu M et al. (2021) MicroRNA‐211 modulates the DUSP6‐ERK5 signaling axis to promote BRAFV600E‐driven melanoma growth in vivo and BRAF/MEK inhibitor resistance. J Invest Dermatol 141, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Song C, Wang L, Xu Q, Wang K, Xie D, Yu Z, Jiang K, Liao L, Yates JR, Lee JD et al. (2017) Targeting BMK1 impairs the drug resistance to combined inhibition of BRAF and MEK1/2 in melanoma. Sci Rep 7, 46244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen X, Gao J, Yu Y, Zhao Z & Pan Y (2019) LncRNA FOXD3‐AS1 promotes proliferation, invasion and migration of cutaneous malignant melanoma via regulating miR‐325/MAP3K2. Biomed Pharmacother 120, 109438. [DOI] [PubMed] [Google Scholar]
- 80. Mendoza MC, Er EE & Blenis J (2011) The Ras‐ERK and PI3K‐mTOR pathways: cross‐talk and compensation. Trends Biochem Sci 36, 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Perna D, Karreth FA, Rust AG, Perez‐Mancera PA, Rashid M, Iorio F, Alifrangis C, Arends MJ, Bosenberg MW, Bollag G et al. (2015) BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. Proc Natl Acad Sci USA 112, E536–E545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bloethner S, Snellman E, Bermejo JL, Hiripi E, Gast A, Thirumaran RK, Wellenreuther R, Hemminki K & Kumar R (2007) Differential gene expression in melanocytic nevi with the V600E BRAF mutation. Genes Chromosomes Cancer 46, 1019–1027. [DOI] [PubMed] [Google Scholar]
- 83. Fu Y, Bi Y, Wang F, Chen X & Liu H (2019) Declination of long noncoding RNA paternally expressed gene 10 inhibits A375 cells proliferation, migration, and invasion via mediating microRNA‐33a. J Cell Biochem 120, 19868–19877. [DOI] [PubMed] [Google Scholar]
- 84. Zhou J, Xu D, Xie H, Tang J, Liu R, Li J, Wang S, Chen X, Su J, Zhou X et al. (2015) miR‐33a functions as a tumor suppressor in melanoma by targeting HIF‐1α. Cancer Biol Ther 16, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen X, Dong H, Liu S, Yu L, Yan D, Yao X, Sun W, Han D & Gao G (2017) Long noncoding RNA MHENCR promotes melanoma progression via regulating miR‐425/489‐mediated PI3K‐Akt pathway. Am J Transl Res 9, 90–102. [PMC free article] [PubMed] [Google Scholar]
- 86. Liu P, Hu Y, Ma L, Du M, Xia L & Hu Z (2015) miR‐425 inhibits melanoma metastasis through repression of PI3K‐Akt pathway by targeting IGF‐1. Biomed Pharmacother 75, 51–57. [DOI] [PubMed] [Google Scholar]
- 87. Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, Wood E, Fedorenko IV, Sondak VK, Anderson AR et al. (2011) PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 71, 2750–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Long J, Luo J & Yin X (2018) miR‐367 enhances the proliferation and invasion of cutaneous malignant melanoma by regulating phosphatase and tensin homolog expression. Mol Med Report 17, 6526–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mu X, Mou KH, Ge R, Han D, Zhou Y & Wang LJ (2019) Linc00961 inhibits the proliferation and invasion of skin melanoma by targeting the miR‐367/PTEN axis. Int J Oncol 55, 708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Boshuizen J, Koopman LA, Krijgsman O, Shahrabi A, van den Heuvel EG , Ligtenberg MA, Vredevoogd DW, Kemper K, Kuilman T, Song J‐Y et al. (2018) Cooperative targeting of melanoma heterogeneity with an AXL antibody‐drug conjugate and BRAF/MEK inhibitors. Nat Med 24, 203–212. [DOI] [PubMed] [Google Scholar]
- 91. Konieczkowski DJ, Johannessen CM, Abudayyeh O, Kim JW, Cooper ZA, Piris A, Frederick DT, Barzily‐Rokni M, Straussman R, Haq R et al. (2014) A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov 4, 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Müller J, Krijgsman O, Tsoi J, Robert L, Hugo W, Song C, Kong X, Possik PA, Cornelissen‐Steijger PD, Geukes Foppen MH et al. (2014) Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat Commun 5, 5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu‐Lieskovan S, Berent‐Maoz B, Pang J, Chmielowski B, Cherry G et al. (2016) Genomic and transcriptomic features of response to Anti‐PD‐1 therapy in metastatic melanoma. Cell 165, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wen L, Zheng Y, Wen X, Zhang Y & Zeng W (2019) Increased expression of long noncoding RNA GAS6‐AS2 promotes proliferation and inhibits apoptosis of melanoma cells via upregulating GAS6 expression. IUBMB Life 71, 1503–1514. [DOI] [PubMed] [Google Scholar]
- 95. Ennen M, Keime C, Gambi G, Kieny A, Coassolo S, Thibault‐Carpentier C, Margerin‐Schaller F, Davidson G, Vagne C, Lipsker D et al. (2017) <em>MITF</em>‐high and <em>MITF</em>‐low cells and a novel subpopulation expressing genes of both cell states contribute to intra‐ and intertumoral heterogeneity of primary melanoma. Clin Cancer Res 23, 7097–7107. [DOI] [PubMed] [Google Scholar]
- 96. Ji Z, Erin Chen Y, Kumar R, Taylor M, Jenny Njauw CN, Miao B, Frederick DT, Wargo JA, Flaherty KT, Jönsson G et al. (2015) MITF modulates therapeutic resistance through EGFR signaling. J Invest Dermatol 135, 1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Johannessen CM, Johnson LA, Piccioni F, Townes A, Frederick DT, Donahue MK, Narayan R, Flaherty KT, Wargo JA, Root DE et al. (2013) A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature 504, 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Verastegui C, Bille K, Ortonne JP & Ballotti R (2000) Regulation of the microphthalmia‐associated transcription factor gene by the Waardenburg syndrome type 4 gene, SOX10. J Biol Chem 275, 30757–30760. [DOI] [PubMed] [Google Scholar]
- 99. Murisier F, Guichard S & Beermann F (2007) The tyrosinase enhancer is activated by Sox10 and Mitf in mouse melanocytes. Pigment Cell Res 20, 173–184. [DOI] [PubMed] [Google Scholar]
- 100. Coe EA, Tan JY, Shapiro M, Louphrasitthiphol P, Bassett AR, Marques AC, Goding CR & Vance KW (2019) The MITF‐SOX10 regulated long non‐coding RNA DIRC3 is a melanoma tumour suppressor. PLoS Genet 15, e1008501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Paluncic J, Kovacevic Z, Jansson PJ, Kalinowski D, Merlot AM, Huang MLH, Lok HC, Sahni S, Lane DJR & Richardson DR (2016) Roads to melanoma: key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim Biophys Acta Mol Cell Res 1863, 770–784. [DOI] [PubMed] [Google Scholar]
- 102. Tang L, Zhang W, Su B & Yu B (2013) Long noncoding RNA HOTAIR is associated with motility, invasion, and metastatic potential of metastatic melanoma. BioMed Res Int 2013, 251098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cantile M, Scognamiglio G, Marra L, Aquino G, Botti C, Falcone MR, Malzone MG, Liguori G, Di Bonito M, Franco R et al. (2017) HOTAIR role in melanoma progression and its identification in the blood of patients with advanced disease. J Cell Physiol 232, 3422–3432. [DOI] [PubMed] [Google Scholar]
- 104. Luan W, Li R, Liu L, Ni X, Shi Y, Xia Y, Wang J, Lu F & Xu B (2017) Long non‐coding RNA HOTAIR acts as a competing endogenous RNA to promote malignant melanoma progression by sponging miR‐152‐3p. Oncotarget 8, 85401–85414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cao H‐H, Cheng C‐Y, Su T, Fu X‐Q, Guo H, Li T, Tse AK‐W, Kwan H‐Y, Yu H & Yu Z‐L (2015) Quercetin inhibits HGF/c‐Met signaling and HGF‐stimulated melanoma cell migration and invasion. Mol Cancer 14, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhou Y, Song KY & Giubellino A (2019) The role of MET in melanoma and melanocytic lesions. Am J Pathol 189, 2138–2148. [DOI] [PubMed] [Google Scholar]
- 107. Ren K, Xu R, Huang J, Zhao J & Shi W (2017) Knockdown of long non‐coding RNA KCNQ1OT1 depressed chemoresistance to paclitaxel in lung adenocarcinoma. Cancer Chemother Pharmacol 80, 243–250. [DOI] [PubMed] [Google Scholar]
- 108. Tang X, Zhang W, Ye Y, Li H, Cheng L, Zhang M, Zheng S & Yu J (2020) LncRNA HOTAIR contributes to sorafenib resistance through suppressing miR‐217 in hepatic carcinoma. BioMed Res Int 2020, 9515071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang Y, Ai H, Fan X, Chen S, Wang Y & Liu L (2020) Knockdown of long non‐coding RNA HOTAIR reverses cisplatin resistance of ovarian cancer cells through inhibiting miR‐138‐5p‐regulated EZH2 and SIRT1. Biol Res 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. He H, Song X, Yang Z, Mao Y, Zhang K, Wang Y, Su B, Li Q, Chen H & Li Y (2020) Upregulation of KCNQ1OT1 promotes resistance to stereotactic body radiotherapy in lung adenocarcinoma by inducing ATG5/ATG12‐mediated autophagy via miR‐372‐3p. Cell Death Dis 11, 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jing L, Yuan W, Ruofan D, Jinjin Y & Haifeng Q (2014) HOTAIR enhanced aggressive biological behaviors and induced radio‐resistance via inhibiting p21 in cervical cancer. Tumour Biol 36, 3611–3619. [DOI] [PubMed] [Google Scholar]
- 112. Luan W, Ding Y, Ma S, Ruan H, Wang J & Lu F (2019) Long noncoding RNA LINC00518 acts as a competing endogenous RNA to promote the metastasis of malignant melanoma via miR‐204‐5p/AP1S2 axis. Cell Death Dis 10, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vitiello M, Tuccoli A, D’Aurizio R, Sarti S, Giannecchini L, Lubrano S, Marranci A, Evangelista M, Peppicelli S, Ippolito C et al. (2017) Context‐dependent miR‐204 and miR‐211 affect the biological properties of amelanotic and melanotic melanoma cells. Oncotarget 8, 25395–25417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang Q, Chen J, Wang A, Sun L, Qian L, Zhou X, Liu Y, Tang S, Chen X, Cheng Y et al. (2018) Differentially expressed circRNAs in melanocytes and melanoma cells and their effect on cell proliferation and invasion. Oncol Rep 39, 1813–1824. [DOI] [PubMed] [Google Scholar]
- 115. Zou Y, Wang SS, Wang J, Su HL & Xu JH (2019) CircRNA_0016418 expedites the progression of human skin melanoma via miR‐625/YY1 axis. Eur Rev Med Pharmacol Sci 23, 10918–10930. [DOI] [PubMed] [Google Scholar]
- 116. Du J, Ren W, Yao F, Wang H, Zhang K, Luo M, Shang Y, O'Connell D, Bei Z, Xiong R et al. (2019) YY1 cooperates with TFEB to regulate autophagy and lysosomal biogenesis in melanoma. Mol Carcinog 58, 2149–2160. [DOI] [PubMed] [Google Scholar]
- 117. Xia W, Ma X, Li X, Dong H, Yi J, Zeng W & Yang Z (2015) miR‐153 inhibits epithelial‐to‐mesenchymal transition in hepatocellular carcinoma by targeting Snail. Oncol Rep 34, 655–662. [DOI] [PubMed] [Google Scholar]
- 118. Xu Q, Sun Q, Zhang J, Yu J, Chen W & Zhang Z (2013) Downregulation of miR‐153 contributes to epithelial‐mesenchymal transition and tumor metastasis in human epithelial cancer. Carcinogenesis 34, 539–549. [DOI] [PubMed] [Google Scholar]
- 119. Luan W, Shi Y, Zhou Z, Xia Y & Wang J (2018) circRNA_0084043 promote malignant melanoma progression via miR‐153‐3p/Snail axis. Biochem Biophys Res Commun 502, 22–29. [DOI] [PubMed] [Google Scholar]
- 120. Chen Z, Chen J, Wa Q, He M, Wang X, Zhou J & Cen Y (2020) Knockdown of circ_0084043 suppresses the development of human melanoma cells through miR‐429/tribbles homolog 2 axis and Wnt/β‐catenin pathway. Life Sci 243, 117323. [DOI] [PubMed] [Google Scholar]
- 121. Goldsberry WN, Londoño A, Randall TD, Norian LA & Arend RC (2019) A review of the role of Wnt in cancer immunomodulation. Cancers (Basel) 11, 10.3390/cancers11060771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chen X, Gao J, Yu Y, Zhao Z & Pan Y (2018) Long non‐coding RNA UCA1 targets miR‐185‐5p and regulates cell mobility by affecting epithelial‐mesenchymal transition in melanoma via Wnt/β‐catenin signaling pathway. Gene 676, 298–305. [DOI] [PubMed] [Google Scholar]
- 123. Long J, Menggen Q, Wuren Q, Shi Q & Pi X (2018) Long noncoding RNA Taurine‐Upregulated Gene1 (TUG1) promotes tumor growth and metastasis through TUG1/Mir‐129‐5p/Astrocyte‐Elevated Gene‐1 (AEG‐1) axis in malignant melanoma. Med Sci Monit 24, 1547–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Zhang Y, Peng G, Wang Y, Cui L, Wu W, Wang L, Liu C & Han X (2017) Silencing of astrocyte elevated gene‐1 inhibits proliferation and migration of melanoma cells and induces apoptosis. Clin Exp Pharmacol Physiol 44, 815–826. [DOI] [PubMed] [Google Scholar]
- 125. Li P, Gao Y, Li J, Zhou Y, Yuan J, Guan H & Yao P (2018) LncRNA MEG3 repressed malignant melanoma progression via inactivating Wnt signaling pathway. J Cell Biochem 119, 7498–7505. [DOI] [PubMed] [Google Scholar]
- 126. Long J & Pi X (2018) lncRNA‐MEG3 suppresses the proliferation and invasion of melanoma by regulating CYLD expression mediated by sponging miR‐499‐5p. Biomed Res Int 2018, 2086564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wu L, Zhu L, Li Y, Zheng Z, Lin X & Yang C (2020) Correction to: LncRNA MEG3 promotes melanoma growth, metastasis and formation through modulating miR‐21/E‐cadherin axis. Cancer Cell Int 20, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hanniford D, Ulloa‐Morales A, Karz A, Berzoti‐Coelho MG, Moubarak RS, Sánchez‐Sendra B, Kloetgen A, Davalos V, Imig J, Wu P et al. (2020) Epigenetic silencing of CDR1as drives IGF2BP3‐mediated melanoma invasion and metastasis. Cancer Cell 37, 55–70.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Muniz L, Lazorthes S, Delmas M, Ouvrard J, Aguirrebengoa M, Trouche D & Nicolas E (2020) Circular ANRIL isoforms switch from repressors to activators of p15/CDKN2B expression during RAF1 oncogene‐induced senescence. RNA Biol 18, 404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Xu S, Wang H, Pan H, Shi Y, Li T, Ge S, Jia R, Zhang H & Fan X (2016) ANRIL lncRNA triggers efficient therapeutic efficacy by reprogramming the aberrant INK4‐hub in melanoma. Cancer Lett 381, 41–48. [DOI] [PubMed] [Google Scholar]
- 131. Sarkar D, Oghabian A, Bodiyabadu PK, Joseph WR, Leung EY, Finlay GJ, Baguley BC & Askarian‐Amiri ME (2017) Multiple isoforms of ANRIL in melanoma cells: structural complexity suggests variations in processing. Int J Mol Sci 18, 1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Margueron R & Reinberg D (2011) The Polycomb complex PRC2 and its mark in life. Nature 469, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zingg D, Debbache J, Schaefer SM, Tuncer E, Frommel SC, Cheng P, Arenas‐Ramirez N, Haeusel J, Zhang Y, Bonalli M et al. (2015) The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat Commun 6, 6051. [DOI] [PubMed] [Google Scholar]
- 134. Yu H, Ma M, Yan J, Xu L, Yu J, Dai J, Xu T, Tang H, Wu X, Li S et al. (2017) Identification of coexistence of BRAF V600E mutation and EZH2 gain specifically in melanoma as a promising target for combination therapy. J Transl Med 15, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Xu W, Yan Z, Hu F, Wei W, Yang C & Sun Z (2020) Long non‐coding RNA GAS5 accelerates oxidative stress in melanoma cells by rescuing EZH2‐mediated CDKN1C downregulation. Cancer Cell Int 20, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Corazao‐Rozas P, Guerreschi P, Jendoubi M, André F, Jonneaux A, Scalbert C, Garçon G, Malet‐Martino M, Balayssac S, Rocchi S et al. (2013) Mitochondrial oxidative stress is the Achille's heel of melanoma cells resistant to Braf‐mutant inhibitor. Oncotarget 4, 1986–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chen L, Ma D, Li Y, Li X, Zhao L, Zhang J & Song Y (2018) Effect of long non‐coding RNA PVT1 on cell proliferation and migration in melanoma. Int J Mol Med 41, 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Liu S, Tetzlaff MT, Wang T, Yang R, Xie L, Zhang G, Krepler C, Xiao M, Beqiri M, Xu W et al. (2015) miR‐200c/Bmi1 axis and epithelial‐mesenchymal transition contribute to acquired resistance to BRAF inhibitor treatment. Pigment Cell Melanoma Res 28, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Avagliano A, Fiume G, Pelagalli A, Sanità G, Ruocco MR, Montagnani S & Arcucci A (2020) Metabolic plasticity of melanoma cells and their crosstalk with tumor microenvironment. Front Oncol 10, 10.3389/fonc.2020.00722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lin Q, Jiang H & Lin D (2019) Circular RNA ITCH downregulates GLUT1 and suppresses glucose uptake in melanoma to inhibit cancer cell proliferation. J Dermatolog Treat 32, 231–235. [DOI] [PubMed] [Google Scholar]
- 141. Jin C, Dong D, Yang Z, Xia R, Tao S & Piao M (2020) CircMYC regulates glycolysis and cell proliferation in melanoma. Cell Biochem Biophys 78, 77–88. [DOI] [PubMed] [Google Scholar]
- 142. Bian D, Wu Y & Song G (2018) Novel circular RNA, hsa_circ_0025039 promotes cell growth, invasion and glucose metabolism in malignant melanoma via the miR‐198/CDK4 axis. Biomed Pharmacother 108, 165–176. [DOI] [PubMed] [Google Scholar]
- 143. Wu S, Tang Y & Liu W (2020) Circ_0084043 promotes cell proliferation and glycolysis but blocks cell apoptosis in melanoma via circ_0084043‐miR‐31‐KLF3 axis. Open Life Sci 15, 774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ding F, Lai J, Gao Y, Wang G, Shang J, Zhang D & Zheng S (2019) NEAT1/miR‐23a‐3p/KLF3: a novel regulatory axis in melanoma cancer progression. Cancer Cell Int 19, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lu R, Zhang X, Li X & Wan X (2020) Circ_0016418 promotes melanoma development and glutamine catabolism by regulating the miR‐605‐5p/GLS axis. Int J Clin Exp Pathol 13, 1791–1801. [PMC free article] [PubMed] [Google Scholar]
- 146. Luan W, Zhang X, Ruan H, Wang J & Bu X (2019) Long noncoding RNA OIP5‐AS1 acts as a competing endogenous RNA to promote glutamine catabolism and malignant melanoma growth by sponging miR‐217. J Cell Physiol 234, 16609–16618. [DOI] [PubMed] [Google Scholar]
- 147. Baenke F, Chaneton B, Smith M, Van Den Broek N, Hogan K, Tang H, Viros A, Martin M, Galbraith L, Girotti MR et al. (2016) Resistance to BRAF inhibitors induces glutamine dependency in melanoma cells. Mol Oncol 10, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wei C‐Y, Zhu M‐X, Lu N‐H, Liu J‐Q, Yang Y‐W, Zhang Y, Shi Y‐D, Feng Z‐H, Li J‐X, Qi F‐Z et al. (2020) Circular RNA circ_0020710 drives tumor progression and immune evasion by regulating the miR‐370‐3p/CXCL12 axis in melanoma. Mol Cancer 19, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang L, Felts SJ, Van Keulen VP, Scheid AD, Block MS, Markovic SN, Pease LR & Zhang Y (2018) Integrative genome‐wide analysis of long noncoding RNAs in diverse immune cell types of melanoma patients. Cancer Res 78, 4411–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wei S, Wang K, Huang X & Zhao Z (2019) LncRNA MALAT1 contributes to non‐small cell lung cancer progression via modulating miR‐200a‐3p/programmed death‐ligand 1 axis. Int J Immunopathol Pharmacol 33, 2058738419859699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wang QM, Lian GY, Song Y, Huang YF & Gong Y (2019) LncRNA MALAT1 promotes tumorigenesis and immune escape of diffuse large B cell lymphoma by sponging miR‐195. Life Sci 231, 116335. [DOI] [PubMed] [Google Scholar]
- 152. Li Y, Jiang T, Zhou W, Li J, Li X, Wang Q, Jin X, Yin J, Chen L, Zhang Y et al. (2020) Pan‐cancer characterization of immune‐related lncRNAs identifies potential oncogenic biomarkers. Nat Commun 11, 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Obeid JM, Hu Y, Erdag G, Leick KM & Slingluff CLJ (2017) The heterogeneity of tumor‐infiltrating CD8+ T cells in metastatic melanoma distorts their quantification: how to manage heterogeneity? Melanoma Res 27, 211–217. [DOI] [PubMed] [Google Scholar]
- 154. Mirsafian H, Manda SS, Mitchell CJ, Sreenivasamurthy S, Ripen AM, Mohamad SB, Merican AF & Pandey A (2016) Long non‐coding RNA expression in primary human monocytes. Genomics 108, 37–45. [DOI] [PubMed] [Google Scholar]
- 155. Kulski JK (2019) Long noncoding RNA HCP5, a hybrid HLA class I endogenous retroviral gene: structure, expression, and disease associations. Cells 8, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wei X, Gu X, Ma M & Lou C (2019) Long noncoding RNA HCP5 suppresses skin cutaneous melanoma development by regulating RARRES3 gene expression via sponging miR‐12. Onco Targets Ther 12, 6323–6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Liu N, Liu Z, Liu X & Chen H (2019) Comprehensive analysis of a competing endogenous RNA network identifies seven‐lncRNA signature as a prognostic biomarker for melanoma. Front Oncol 9, 10.3389/fonc.2019.00935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Yang Y, Zhang Z, Wu Z, Lin W & Yu M (2019) Downregulation of the expression of the lncRNA MIAT inhibits melanoma migration and invasion through the PI3K/AKT signaling pathway. Cancer Biomark 24, 203–211. [DOI] [PubMed] [Google Scholar]
- 159. Charpentier M, Croyal M, Carbonnelle D, Fortun A, Florenceau L, Rabu C, Krempf M, Labarrière N & Lang F (2016) IRES‐dependent translation of the long non coding RNA meloe in melanoma cells produces the most immunogenic MELOE antigens. Oncotarget 7, 59704–59713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Godet Y, Moreau‐Aubry A, Guilloux Y, Vignard V, Khammari A, Dreno B, Jotereau F & Labarriere N (2008) MELOE‐1 is a new antigen overexpressed in melanomas and involved in adoptive T cell transfer efficiency. J Exp Med 205, 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Godet Y, Moreau‐Aubry A, Mompelat D, Vignard V, Khammari A, Dreno B, Lang F, Jotereau F & Labarriere N (2010) An additional ORF on meloe cDNA encodes a new melanoma antigen, MELOE‐2, recognized by melanoma‐specific T cells in the HLA‐A2 context. Cancer Immunol Immunother 59, 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shang W, Gao Y, Tang Z, Zhang Y & Yang R (2019) The pseudogene <em>Olfr29‐ps1</em> promotes the suppressive function and differentiation of monocytic MDSCs. Cancer Immunol Res 7, 813–827. [DOI] [PubMed] [Google Scholar]
- 163. Gao Y, Wang T, Li Y, Zhang Y & Yang R (2018) Lnc‐chop promotes immunosuppressive function of myeloid‐derived suppressor cells in tumor and inflammatory environments. J Immunol 200, 2603–2614. [DOI] [PubMed] [Google Scholar]
- 164. Pandey PR, Munk R, Kundu G, De S, Abdelmohsen K & Gorospe M (2020) Methods for analysis of circular RNAs. Wiley Interdiscip Rev RNA 11, e1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Szabo L & Salzman J (2016) Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet 17, 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Salzman J, Gawad C, Wang PL, Lacayo N & Brown PO (2012) Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 7, e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gao Y, Wang J & Zhao F (2015) CIRI: an efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol 16, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zhang X‐O, Wang H‐B, Zhang Y, Lu X, Chen L‐L & Yang L (2014) Complementary sequence‐mediated exon circularization. Cell 159, 134–147. [DOI] [PubMed] [Google Scholar]
- 169. Cheng J, Metge F & Dieterich C (2016) Specific identification and quantification of circular RNAs from sequencing data. Bioinformatics 32, 1094–1096. [DOI] [PubMed] [Google Scholar]
- 170. Szabo L, Morey R, Palpant NJ, Wang PL, Afari N, Jiang C, Parast MM, Murry CE, Laurent LC & Salzman J (2015) Statistically based splicing detection reveals neural enrichment and tissue‐specific induction of circular RNA during human fetal development. Genome Biol 16, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hoffmann S, Otto C, Doose G, Tanzer A, Langenberger D, Christ S, Kunz M, Holdt LM, Teupser D, Hackermüller J et al. (2014) A multi‐split mapping algorithm for circular RNA, splicing, trans‐splicing and fusion detection. Genome Biol 15, R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Humphreys DT, Fossat N, Tam PPL & Ho JWK (2018) Ularcirc: visualisation and enhanced analysis of circular RNAs via back and canonical forward splicing. bioRxiv 318436. 10.1101/318436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Song X, Zhang N, Han P, Moon B‐S, Lai RK, Wang K & Lu W (2016) Circular RNA profile in gliomas revealed by identification tool UROBORUS. Nucleic Acids Res 44, e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wang J & Wang L (2019) Deep learning of the back‐splicing code for circular RNA formation. Bioinformatics 35, 5235–5242. [DOI] [PubMed] [Google Scholar]
- 175. Pan X & Xiong K (2015) PredcircRNA: computational classification of circular RNA from other long non‐coding RNA using hybrid features. Mol Biosyst 11, 2219–2226. [DOI] [PubMed] [Google Scholar]
- 176. Pan X, Xiong K, Anthon C, Hyttel P, Freude KK, Jensen LJ & Gorodkin J (2018) WebCircRNA: classifying the circular RNA potential of coding and noncoding RNA. Genes (Basel) 9, 10.3390/genes9110536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Liu Z, Han J, Lv H, Liu J & Liu R (2016) Computational identification of circular RNAs based on conformational and thermodynamic properties in the flanking introns. Comput Biol Chem 61, 221–225. [DOI] [PubMed] [Google Scholar]
- 178. Hansen TB (2018) Improved circRNA identification by combining prediction algorithms. Front Cell Dev Biol 6, 10.3389/fcell.2018.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Hansen TB, Venø MT, Damgaard CK & Kjems J (2016) Comparison of circular RNA prediction tools. Nucleic Acids Res 44, e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB & Kjems J (2019) The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 20, 675–691. [DOI] [PubMed] [Google Scholar]
- 181. Zeng X, Lin W, Guo M & Zou Q (2017) A comprehensive overview and evaluation of circular RNA detection tools. PLoS Comput Biol 13, e1005420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Chen L, Wang C, Sun H, Wang J, Liang Y, Wang Y & Wong G (2020) The bioinformatics toolbox for circRNA discovery and analysis. Brief Bioinform 22, 1706–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Rybak‐Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal‐Fluss R et al. (2015) Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 58, 870–885. [DOI] [PubMed] [Google Scholar]
- 184. Li M, Xie X, Zhou J, Sheng M, Yin X, Ko EA, Zhou T & Gu W (2017) Quantifying circular RNA expression from RNA‐seq data using model‐based framework. Bioinformatics 33, 2131–2139. [DOI] [PubMed] [Google Scholar]
- 185. Xu Y (2017) An overview of the main circRNA databases. Noncoding RNA Investig 1. [Google Scholar]
- 186. Glažar P, Papavasileiou P & Rajewsky N (2014) circBase: a database for circular RNAs. RNA 20, 1666–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Meng X, Hu D, Zhang P, Chen Q & Chen M (2019) CircFunBase: a database for functional circular RNAs. Database 2019, 10.1093/database/baz003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Dong R, Ma X‐K, Li G‐W & Yang L (2018) CIRCpedia v2: an updated database for comprehensive circular RNA annotation and expression comparison. Genomics Proteomics Bioinformatics 16, 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Chen X, Han P, Zhou T, Guo X, Song X & Li Y (2016) circRNADb: a comprehensive database for human circular RNAs with protein‐coding annotations. Sci Rep 6, 34985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K & Gorospe M (2016) CircInteractome: a web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol 13, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Panda AC, Dudekula DB, Abdelmohsen K & Gorospe M (2018) Analysis of circular RNAs using the web tool circinteractome. Methods Mol Biol 1724, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Liu YC, Li JR, Sun CH, Andrews E, Chao RF, Lin FM, Weng SL, Hsu SD, Huang CC, Cheng C et al. (2016) CircNet: a database of circular RNAs derived from transcriptome sequencing data. Nucleic Acids Res 44, D209–D215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ghosal S, Das S, Sen R, Basak P & Chakrabarti J (2013) Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Front Genet 4, 10.3389/fgene.2013.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Muniategui A, Pey J, Planes FJ & Rubio A (2012) Joint analysis of miRNA and mRNA expression data. Brief Bioinform 14, 263–278. [DOI] [PubMed] [Google Scholar]
- 195. Glazko GV, Zybailov BL & Rogozin IB (2012) Computational prediction of polycomb‐associated long non‐coding RNAs. PLoS One 7, e44878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Li J‐H, Liu S, Zhou H, Qu L‐H & Yang J‐H (2014) starBase v2.0: decoding miRNA‐ceRNA, miRNA‐ncRNA and protein‐RNA interaction networks from large‐scale CLIP‐Seq data. Nucleic Acids Res 42, D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG et al. (2012) The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 22, 1775–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA & Zhang F (2013) Genome engineering using the CRISPR‐Cas9 system. Nat Protoc 8, 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Behlke M (2016) Mini‐review on current strategies to knockdown long non‐coding RNAs. J Rare Dis Res Treat 1, 66–70. [Google Scholar]
- 200. Liao Z, Zhao J & Yang Y (2018) Downregulation of lncRNA H19 inhibits the migration and invasion of melanoma cells by inactivating the NF‐κB and PI3K/Akt signaling pathways. Mol Med Rep 17, 7313–7318. [DOI] [PubMed] [Google Scholar]
- 201. Cai B, Zheng Y, Ma S, Xing Q, Wang X, Yang B, Yin G & Guan F (2017) BANCR contributes to the growth and invasion of melanoma by functioning as a competing endogenous RNA to upregulate Notch2 expression by sponging miR‐204. Int J Oncol 51, 1941–1951. [DOI] [PubMed] [Google Scholar]
- 202. Zhang Y, Qian W, Feng F, Cao Q, Li Y, Hou Y, Zhang L & Fan J (2019) Upregulated lncRNA CASC2 may inhibit malignant melanoma development through regulating miR‐18a‐5p/RUNX1. Oncol Res 27, 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Wang Z, Wang X, Zhou H, Dan X, Jiang L & Wu Y (2018) Long non‐coding RNA CASC2 inhibits tumorigenesis via the miR‐181a/PLXNC1 axis in melanoma. Acta Biochim Biophys Sin 50, 263–272. [DOI] [PubMed] [Google Scholar]
- 204. Xu L, Zhang Y, Zhao Z, Chen Z, Wang Z, Xu S, Zhang X, Liu T & Yu S (2018) The long non‐coding RNA CRNDE competed endogenously with miR‐205 to promote proliferation and metastasis of melanoma cells by targeting CCL18. Cell Cycle 17, 2296–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Chen L, Yang H, Xiao Y, Tang X, Li Y, Han Q, Fu J, Yang Y & Zhu Y (2016) LncRNA GAS5 is a critical regulator of metastasis phenotype of melanoma cells and inhibits tumor growth in vivo. Onco Targets Ther 9, 4075–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Guo B, Zhang Q, Wang H, Chang P & Tao K (2018) KCNQ1OT1 promotes melanoma growth and metastasis. Aging 10, 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 207. Yang F, Lei P, Zeng W, Gao J & Wu N (2020) Long noncoding RNA LINC00173 promotes the malignancy of melanoma by promoting the expression of IRS4 through competitive binding to microRNA‐493. Cancer Manag Res 12, 3131–3144. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 208. Jiao H, Jiang S, Wang H, Li Y & Zhang W (2018) Upregulation of LINC00963 facilitates melanoma progression through miR‐608/NACC1 pathway and predicts poor prognosis. Biochem Biophys Res Commun 504, 34–39. [DOI] [PubMed] [Google Scholar]
- 209. Luan W, Li L, Shi Y, Bu X, Xia Y, Wang J, Djangmah HS, Liu X, You Y & Xu B (2016) Long non‐coding RNA MALAT1 acts as a competing endogenous RNA to promote malignant melanoma growth and metastasis by sponging miR‐22. Oncotarget 7, 63901–63912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zhu L, Wang Y, Yang C, Li Y, Zheng Z, Wu L & Zhou H (2020) Long non‐coding RNA MIAT promotes the growth of melanoma via targeting miR‐150. Hum Cell 33, 819–829. [DOI] [PubMed] [Google Scholar]
- 211. Xia Y, Zhou Y, Han H, Li P, Wei W & Lin N (2019) lncRNA NEAT1 facilitates melanoma cell proliferation, migration, and invasion via regulating miR‐495‐3p and E2F3. J Cell Physiol 234, 19592–19601. [DOI] [PubMed] [Google Scholar]
- 212. Yang Q, Deng Y, Xu Y, Ding N, Wang C, Zhao X, Lou X, Li Y, Zhao H & Fang X (2019) Knockdown of SSATX, an alternative splicing variant of the SAT1 gene, promotes melanoma progression. Gene 716, 144010. [DOI] [PubMed] [Google Scholar]
- 213. Schmidt K, Joyce CE, Buquicchio F, Brown A, Ritz J, Distel RJ, Yoon CH & Novina CD (2016) The lncRNA SLNCR1 mediates melanoma invasion through a conserved SRA1‐like region. Cell Rep 15, 2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Wang Y, Liu G, Ren L, Wang K & Liu A (2019) Long non‐coding RNA TUG1 recruits miR‐29c‐3p from its target gene RGS1 to promote proliferation and metastasis of melanoma cells. Int J Oncol 54, 1317–1326. [DOI] [PubMed] [Google Scholar]
- 215. Wei Y, Sun Q, Zhao L, Wu J, Chen X, Wang Y, Zang W & Zhao G (2016) LncRNA UCA1‐miR‐507‐FOXM1 axis is involved in cell proliferation, invasion and G0/G1 cell cycle arrest in melanoma. Med Oncol 33, 88. [DOI] [PubMed] [Google Scholar]
- 216. Luan W, Zhou Z, Ni X, Xia Y, Wang J, Yan Y & Xu B (2018) Long non‐coding RNA H19 promotes glucose metabolism and cell growth in malignant melanoma via miR‐106a‐5p/E2F3 axis. J Cancer Res Clin Oncol 144, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yao D, Zhang L, Zheng M, Sun X, Lu Y & Liu P (2018) Circ2Disease: a manually curated database of experimentally validated circRNAs in human disease. Sci Rep 8, 11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Fan C, Lei X, Fang Z, Jiang Q & Wu F‐X (2018) CircR2Disease: a manually curated database for experimentally supported circular RNAs associated with various diseases. Database 2018, 10.1093/database/bay044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Zhao Z, Wang K, Wu F, Wang W, Zhang K, Hu H, Liu Y & Jiang T (2018) circRNA disease: a manually curated database of experimentally supported circRNA‐disease associations. Cell Death Dis 9, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Zhou K‐R, Liu S, Sun W‐J, Zheng L‐L, Zhou H, Yang J‐H & Qu L‐H (2016) ChIPBase v2.0: decoding transcriptional regulatory networks of non‐coding RNAs and protein‐coding genes from ChIP‐seq data. Nucleic Acids Res 45, D43–D50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Paraskevopoulou MD, Vlachos IS, Karagkouni D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P, Floros E, Dalamagas T et al. (2016) DIANA‐LncBase v2: indexing microRNA targets on non‐coding transcripts. Nucleic Acids Res 44, D231–D238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Volders P‐J, Anckaert J, Verheggen K, Nuytens J, Martens L, Mestdagh P & Vandesompele J (2018) LNCipedia 5: towards a reference set of human long non‐coding RNAs. Nucleic Acids Res 47, D135–D139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Amaral PP, Clark MB, Gascoigne DK, Dinger ME & Mattick JS (2010) lncRNAdb: a reference database for long noncoding RNAs. Nucleic Acids Res 39, D146–D151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Chen G, Wang Z, Wang D, Qiu C, Liu M, Chen X, Zhang Q, Yan G & Cui Q (2013) LncRNADisease: a database for long‐non‐coding RNA‐associated diseases. Nucleic Acids Res 41, D983–D986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Bhartiya D, Pal K, Ghosh S, Kapoor S, Jalali S, Panwar B, Jain S, Sati S, Sengupta S, Sachidanandan C et al. (2013) lncRNome: a comprehensive knowledgebase of human long noncoding RNAs. Database 2013, 10.1093/database/bat034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Chang L, Zhou G, Soufan O & Xia J (2020) miRNet 2.0: network‐based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res 48, W244–W251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Fang S, Zhang L, Guo J, Niu Y, Wu Y, Li H, Zhao L, Li X, Teng X, Sun X et al. (2018) NONCODEV5: a comprehensive annotation database for long non‐coding RNAs. Nucleic Acids Res 46, D308–D314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of circRNAs and lncRNAs discussed in the text.


