Abstract
Genomes of TT virus (TTV) and TTV-like minivirus DNA were detected in 80% and 61% of cervical swabs from healthy women, respectively, regardless of concurrent human papillomavirus infection. These results show that the potential exists for sexual transmission of TTV and related viruses.
TT virus (TTV) is a recently described DNA virus of humans that shows a number of unusual properties (9, 11). From the clinical microbiology standpoint, a most remarkable feature of TTV is its widespread presence in the blood of infected individuals—often at high titers and for prolonged or indefinite periods of time—in the apparent total absence of associated pathological effects (1). For example, in recent studies of the general population of Tuscany, Italy, the prevalence of subjects who carry TTV in the blood was on the order of 90%, and viremia loads ranged between 7.7 × 102 and 6.2 × 108 DNA copies per ml of plasma, as determined with an open reading frame 1-targeted real-time PCR assay (8, 12). Similarly high prevalence rates have been reported from other countries, whenever detection methods of comparable sensitivity were used (5, 10). Thus, two aspects of TTV biology appear especially intriguing: the routes of interindividual transmission that permit such a dissemination and the type(s) of relationship with the infected organism that permits TTV to replicate extensively with no apparent damage to the host. A clear understanding of the body sites where TTV dwells and persists can help shed light on both of these aspects.
Here we examined for the presence of TTV DNA 50 consecutive cervical swabs sent to our laboratory for human papillomavirus (HPV) testing between October 1999 and May 2000. The specimens were collected from apparently healthy women using routine procedures for prophylactic screening of cervical cancer. Tests were performed without knowledge of cytology and clinical data. Each swab was soaked and vortexed gently in sterile phosphate-buffered saline, which was then stored in aliquots at −70°C until use. HPV detection and genotyping were carried out by amplifying a segment of the L1 gene with consensus primers MY09 and MY11 and subsequent restriction fragment length polymorphism analysis, as reported previously (2). Thirty-eight swabs were found to be HPV DNA positive; of these, 20 carried low-risk HPV and 12 carried high-risk HPV, while 6 HPV isolates were not typed (Table 1).
TABLE 1.
Results of TTV and TLMV detection in cervical swabs, grouped by HPV DNA status
| HPV status | Results
|
|||||
|---|---|---|---|---|---|---|
| TTV
|
TLMV
|
|||||
| No. of samples tested | No. (%) positive | Viral load (copies/μg of DNA)
|
No. of samples tested | No. (%) positive | ||
| Range | Mean (median) | |||||
| HPV negative | 12 | 10 (83) | 1.6 × 104–7.2 × 106 | 2.5 × 106 (2.5 × 106) | 7 | 5 (71) |
| HPV positive | ||||||
| Low riska | 20 | 15 (75) | 1.3 × 103–1.7 × 108 | 1.6 × 107 (1.2 × 106) | 11 | 7 (64) |
| High riskb | 12 | 9 (75) | 1.0 × 104–5.1 × 107 | 7.2 × 106 (3.4 × 105) | 11 | 6 (55) |
| Untypedc | 6 | 6 (100) | 6.6 × 105–6.8 × 106 | 2.5 × 106 (1.9 × 106) | 4 | 2 (50) |
| Total | 50 | 40 (80) | 1.3 × 103–1.7 × 108 | 8.8 × 106 (1.4 × 106) | 33 | 20 (61) |
Low-risk group includes HPV genotypes 6, 53, and 64.
High-risk group includes HPV genotypes 16, 31, 33, 39, 52, and 58.
Determination of HPV genotype was not done.
TTV detection was carried out using a TaqMan real-time PCR assay (8, 12) that, being targeted to a highly conserved segment of the nontranslated region (UTR) of the viral DNA, detects a wider range of genotypes than most TTV detection methods described to date, including the ones used by us in previous studies (6). Forward and reverse primers were 5′-GTGCCGIAGGTGAGTTTA-3′ (positions 177 to 194) and 5′-AGCCCGGCCAGTCC-3′ (positions 226 to 239), respectively. The probe was 5′-TCAAGGGGCAATTCGGGCT-3′ (positions 205 to 223), which was labeled with 6-carboxy-fluorescein and 6-carboxy-tetramethyl-rhodamine at the 5′ and 3′ ends, respectively, and had a propynilic group bound to each thymidine to increase the annealing temperature. The procedures used for quantification of copy numbers and evaluation of intra- and interassay precision and reproducibility have been previously described (12). Maximum intra- and interassay variation in the threshold cycle was about 3%, and specificity was confirmed by repeatedly sequencing the products of amplification. The lower limit of sensitivity of the assay was 1.0 × 103 copies/μg of DNA. All cervical swabs were tested in triplicate twice from independent DNA extractions. Samples positive in only one replicate or with a coefficient of variation of 50% or greater constituted less than 2% of samples tested. These samples were reextracted and tested again in triplicate.
Nucleotide sequence accession numbers.
Sequences of a 265-bp UTR segment from the TTV-like minivirus (TLMV) isolates listed in Fig. 1 were submitted to GenBank (accession no. AF312400 to AF312420).
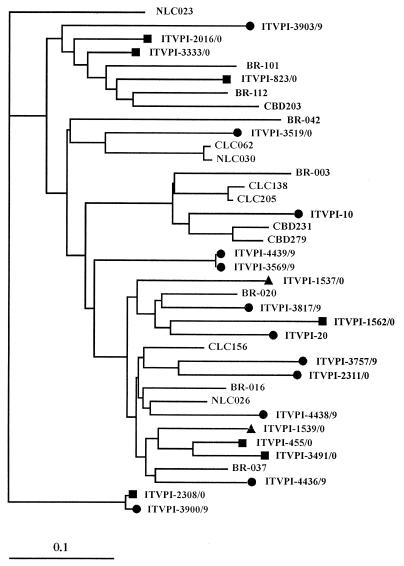
FIG. 1.
Phylogenetic analysis of the TLMV isolates based on a 265-bp UTR segment (nt 36 to 300). Isolate NLC023 (13) was used as an outgroup. The bar indicates the number of nucleotide substitutions per site. The sequences from cervical swabs are marked with ●, while reference sequences are indicated by the isolate name only. Sequences obtained in our laboratory from seven plasma samples (■) and two liver biopsy specimens (▴) are also shown.
The results of TTV testing are shown in Table 1. Overall, 80% of the cervical swabs examined were found positive, thus indicating that TTV infection of the feminine genital tract is extremely common in this select group of women. TTV loads varied widely among individual samples, ranging between 1.3 × 103 and 1.7 × 108 copies per μg of total DNA. When the swabs were grouped according to the results of HPV analysis, TTV prevalence rates and titers were similar. Thus, although the numbers of patients examined were too low to draw firm conclusions, it would appear that TTV infection is not facilitated by concurrent HPV infection (Table 1).
While this study was in progress, Takahashi et al. (13) reported on the existence of a new virus, related but clearly distinct from TTV, in the plasma of Japanese voluntary blood donors. Due to its small estimated size (less than 30 versus 30 to 50 nm) and shorter genome (approximately 2.9 versus 3.8 kb) relative to that of TTV, the virus was designated TLMV. Early data have suggested that TLMV may be as widespread as TTV, while modes of transmission and pathogenicity have yet to be investigated (13, 14). We tested 33 of the above-discussed cervical swabs (27 TTV positive and 6 TTV negative) for the presence of TLMV using a nested PCR assay targeted to a segment of the UTR of the viral DNA, which, based on the sequences available in gene banks at the time the assay was developed, was well conserved and specific for TLMV. The following oligonucleotide primers were used: external primers, 5′-ATTTGAATTGCCGACCACA-3′ (positions 30 to 48) and 5′-CGCCAGACTGATCTAGCCCGAA-3′ (positions 292 to 313); internal primers, 5′-ATTGCCGACCACAAACTGACA-3′ (positions 36 to 56) and 5′-AGCCCGAATTGCCCCTAGTC-3′ (positions 280 to 300). Bands of the expected size (265 nucleotides [nt]) were detected in 20 samples (61%), regardless of concurrent infection with HPV (Table 1) and TTV (not shown). Sequence alignment of 12 such bands with the CLUSTALW program confirmed that they belonged to TLMV, while phylogenetic relationships, estimated by using DAMBE (version 4.0.9 [15]) and the neighbor-joining method, showed a wide genetic heterogeneity (Fig. 1). TLMV isolates detected in plasma samples have also been shown to be genetically highly heterogeneous (13, 14). Interestingly, in addition to the 265-nt TLMV bands, four cervical swabs yielded bands of approximately 400 nt, which, when sequenced, were found to belong to the YONBAN strain of TTV. This strain is currently grouped together with TTV, but its high degree of divergence has led researchers to propose that it might represent a separate viral species (14). Our TLMV primers had been designed before UTR sequences of the YONBAN strain had become available, but comparison with subsequently published YONBAN sequences showed that the internal antisense primer is highly conserved between TLMV and YONBAN. This might explain why the TLMV PCR had permitted amplification of YONBAN virus from some swabs.
In conclusion, the present findings show that cervical specimens may contain a wide array of TTV and related viruses. Whether this reflects active replication of such viruses in the female genital tract, for example by lymphoid cells (7), or exudation from plasma remains to be established. Information about sexual transmission of TTV and related viruses is scarce. Recently, Inami et al. (4) detected TTV DNA in 6 of 10 human semen samples tested. Together with the latter results, the present data indicate that prerequisites exist for TTV and related viruses being commonly transmitted via the sexual route. Viral sequences obtained from sexual partners should be compared in order to shed light on the importance of this relative to other modes of transmission. Furthermore, in some studies the high prevalence of TTV infections in infants was attributed to close contact with infected mothers (3). Exposure to virus-containing vaginal fluids during delivery may contribute to perinatal transmission of these widespread viruses.
Acknowledgments
This work was supported in part by grants from the Ministero della Università e Ricerca Scientifica.
REFERENCES
- 1.Bendinelli M, Pistello M, Maggi F, Fornai C, Freer G, Vatteroni M L. Molecular properties, biology and clinical implications of TT virus, a recently identified widespread infectious agent of man. Clin Microbiol Rev. 2001;14:98–113. doi: 10.1128/CMR.14.1.98-113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard H U, Chan S Y, Manos M M, Ong C K, Villa L L, Delius H, Peyton C L, Bauer H M, Wheeler C M. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J Infect Dis. 1994;170:1077–1085. doi: 10.1093/infdis/170.5.1077. [DOI] [PubMed] [Google Scholar]
- 3.Davidson F, MacDonald D, Mokili J L K, Prescott L E, Graham S, Simmonds P. Early acquisition of TT virus (TTV) in an area endemic for TTV infection. J Infect Dis. 1999;179:1070–1076. doi: 10.1086/314730. [DOI] [PubMed] [Google Scholar]
- 4.Inami T, Konomi N, Arakawa Y, Abe K. High prevalence of TT virus DNA in human saliva and semen. J Clin Microbiol. 2000;38:2407–2408. doi: 10.1128/jcm.38.6.2407-2408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irving W L, Ball J K, Berridge S, Curran R, Grabowska A M, Jameson C L, Neal K R, Ryder S D, Thomson B J the Trent HCV Study Group. TT virus infection in patients with hepatitis C: frequency, persistence, and sequence heterogeneity. J Infect Dis. 1999;180:27–34. doi: 10.1086/314825. [DOI] [PubMed] [Google Scholar]
- 6.Maggi F, Fornai C, Morrica A, Casula F, Vatteroni M L, Marchi S, Ciccorossi P, Riente L, Pistello M, Bendinelli M. High prevalence of TT virus viremia in Italian patients, regardless of age, clinical diagnosis, and previous interferon treatment. J Infect Dis. 1999;180:838–842. doi: 10.1086/314961. [DOI] [PubMed] [Google Scholar]
- 7.Maggi F, Fornai C, Zaccaro L, Morrica A, Vatteroni M L, Isola P, Marchi S, Ricchiuti A, Pistello M, Bendinelli M. TT virus (TTV) loads associated with different peripheral blood cell types and evidence for TTV replication in activated mononuclear cells. J Med Virol. 2001;64:1–6. doi: 10.1002/jmv.1035. [DOI] [PubMed] [Google Scholar]
- 8.Morrica A, Maggi F, Vatteroni M L, Fornai C, Pistello M, Ciccorossi P, Grassi E, Gennazzani A, Bendinelli M. TT virus: evidence for transplacental transmission. J Infect Dis. 2000;181:803–804. doi: 10.1086/315296. [DOI] [PubMed] [Google Scholar]
- 9.Mushahwar I K, Erker J C, Muerhoff A S, Leary T P, Simons J N, Birkenmeyer L G, Chalmers M L, Pilot-Matias T J, Dexai S M. Molecular and biophysical characterization of TT virus: evidence for a new virus family infecting humans. Proc Natl Acad Sci USA. 1999;96:3177–3182. doi: 10.1073/pnas.96.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa N, Ikoma J, Ishihara T, Yasui-Kawamura N, Fujita N, Iwasa M, Kaito M, Watanabe S, Adachi Y. Biliary excretion of TT virus (TTV) J Med Virol. 2000;61:462–467. doi: 10.1002/1096-9071(200008)61:4<462::aid-jmv8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241:92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- 12.Pistello M, Morrica A, Maggi F, Vatteroni M L, Freer G, Fornai C, Casula F, Marchi S, Ciccorossi P, Rovero P, Bendinelli M. TT virus levels in the plasma of infected individuals with different hepatic and extrahepatic pathologies. J Med Virol. 2001;63:189–195. [PubMed] [Google Scholar]
- 13.Takahashi K, Iwasa Y, Hijikata M, Mishiro S. Identification of a new human DNA virus (TTV-like mini virus, TLMV) intermediately related to TT virus and chicken anemia virus. Arch Virol. 2000;145:979–993. doi: 10.1007/s007050050689. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Hijikata M, Samokhvalov E I, Mishiro S. Full or near length nucleotide sequences of TT virus variants (types SANBAN and YONBAN) and the TT virus-like mini virus. Intervirology. 2000;43:119–123. doi: 10.1159/000025034. [DOI] [PubMed] [Google Scholar]
- 15.Xia X. Data analysis in molecular biology and evolution. Boston, Mass: Kluwer Academic Publishers; 2000. [Google Scholar]