Abstract
Background
The characterisation of functional traits of non-indigenous and invasive species is crucial to assess their impact within invaded habitats. Successful biological invasions are often facilitated by the generalist diet of the invaders which can modify their trophic position and adapt to new ecosystems determining changes in their structure and functioning. Invasive crustaceans are an illustrative example of such mechanisms since their trophic habits can determine important ecological impacts on aquatic food webs. The Atlantic blue crab Callinectessapidus is currently established and considered invasive in the Mediterranean Sea where it has been recorded for the first time between 1947 and 1949. In the last decade, the blue crab colonised most of the eastern and central Mediterranean Sea and the Black Sea and it is currently widening its distribution towards the western region of the basin.
New information
Stable isotope analysis is increasingly used to investigate the trophic habits of invasive marine species. Here, we collated individual measures of the blue crab δ13C and δ15N values and of its potential invertebrate prey into a geo-referenced dataset. The dataset includes 360 records with 236 isotopic values of the blue crab and 224 isotopic data of potential prey collected from five countries and 12 locations between 2014 and 2019. This dataset allows the estimation of the trophic position of the blue crab within a variety of invaded ecosystems, as well as advanced quantitative comparisons of the main features of its isotopic niche.
Keywords: invasive species, Atlantic blue crab, transitional water, stable isotope, trophic position, isotopic niche
Introduction
The concern about the impacts of non-indigenous species (NIS) has grown steeply over the past half-century (David et al. 2017), and a large amount of evidence suggests that NIS can alter the structure of natural communities and the integrity of ecosystems causing substantial ecological, economic, and cultural losses, especially in case they become invasive (Invasive Alien Species, IAS hereafter; Anton et al. 2019, see also Thomsen 2020). The impact of IAS is mediated by biotic interactions with the native communities and changes in the ecosystem processes including nutrient dynamics, fluxes of energy, and material cycling (Gallardo et al. 2016, Corrales et al. 2020). The monitoring and assessment of the impact of IAS and the prioritisation of adequate management actions are crucial steps to mitigate and/or limit the potential adverse effects of those species that are more likely to represent a serious threat to native ecosystems (Dick et al. 2017, Cuthbert et al. 2019).
The Atlantic blue crab (Callinectessapidus Rathbun, 1896) is considered one of the worst invasive species in the Mediterranean Sea owing to its impact on local biodiversity, fisheries, and aquaculture (Streftaris and Zenetos 2006). The Atlantic blue crab (hereafter blue crab) was first recorded in Europe at the start of the past century (Bouvier 1901) and appeared in the Mediterranean Sea between 1947 and 1949 (Giordani Soika 1951, Serbetis 1959). Over the last decade, the blue crab has spread almost ubiquitously in the eastern and central Mediterranean Sea and in the Black Sea, and it is currently widening its distribution towards the western sectors of the basin (Mancinelli et al. 2017a, Mancinelli et al. 2021).
The blue crab is an opportunistic omnivore, feeding on a variety of food sources from plants and detritus, to molluscs, arthropods (including conspecifics), polychaetes, and fish (Belgrad and Griffen 2016). Similar to other IAS, such trophic plasticity represents a key adaptive feature explaining the establishment success of the blue crab in non-native ecosystems and its impact on invaded food webs (Mancinelli et al. 2017b). However, while the crucial role played by the blue crab in regulating the structure and functioning of native food webs is well recognised, the number of studies investigating the trophic role of this invader in non-native ecosystems is remarkably lower (Mancinelli et al. 2017b, but see Kampouris et al. 2019 for a counter-example). In native coastal ecosystems, the blue crab acts as a keystone species by regulating the carbon cycle and prey/predator abundance through both bottom-up and top-down interactions (Altieri et al. 2012, Pugesek et al. 2013). Thus, understanding how the trophic ecology of this taxon shapes benthic food webs in invaded ecosystems is crucial for an accurate assessment of its impact.
Stable isotope analysis of δ13C and δ15N has become an increasingly popular methodology in studies focusing on food web structure and functioning. Specifically, δ13C can trace the flow of matter and nutrients from basal to higher trophic levels and δ15N can clarify trophic interactions (Fry 2006). In addition, even though species-specific physiological or metabolic factors can influence δ15N values (Tibbets et al. 2007; Doi et al. 2017), δ15N is commonly used to infer the species trophic position within food webs (Post 2002). In fact, as the trophic level increases, the nitrogen enriches predictably in its heavier isotope 15N due to the preferential excretion of the lighter isotope 14N (Minagawa and Wada 1984; Post 2002). Noticeably, the assessment of the effects of non-indigenous species on native communities is one of the first applications of stable isotope analysis in invasion ecology (Mitchell et al. 1996). Mancinelli and Vizzini (2015)reviewed the advantages and limitations of the method for identifying and quantifying the ecological impact of invasive species, clearly emphasising how the estimation of invasive species trophic position, using stable isotopes, can be successfully used for assessing direct predatory impacts, as well as community-scale effects on the whole trophic structure. For the blue crab, recent evidence indicated substantial variability in its trophic level across closely-located coastal ecosystems and size-related shifts in individual trophic position within invaded food webs (Mancinelli et al. 2016, Mancinelli et al. 2017b). Ultimately, these studies demonstrate the utility of stable isotope analysis in detecting changes in food web structure after the invasion; an advanced understanding of such trophic interactions can, in turn, help to predict and quantify the impact of biological invasions on aquatic food webs (Jackson et al. 2012).
General description
Purpose
This dataset collates available geo-referenced and individual-based isotopic values (δ13C and δ15N) of C.sapidus and its potential animal prey in Mediterranean waters. The isotopic values, included in the dataset, are expressed in delta notation (‰ deviation from atmospheric nitrogen and from Pee Dee Belemnite [PDB] limestone used as standards for N and C, respectively) and δ15N or δ13C = [(RSample/RStandard) – 1] × 1000, where R = 15N/14N or 13C/12C. The analytical precision of measurements for all δ13C and δ15N values was 0.2‰ as calculated by the standard deviation of replicates of the internal standards. This dataset can be used for a variety of comparative analyses including the calculation of the trophic position of the crab and/or metrics and descriptors of its isotopic niche, and it was conceived as one of the input files for the Functional biogeography of invaders workflow of the LifeWatch ERIC Internal Joint Initiative. Specifically, the analytical workflow aims at identifying climatic predictors of the trophic position of two invasive crustaceans, i.e. the blue crab C.sapidus and the Louisiana crayfish Procambarusclarkii. For P.clarkii, the workflow runs on an aggregated dataset resolved at population scale, whereas for C.sapidus, two datasets can be used as input files to run the analyses: (i) an aggregated dataset at the population scale similar to the one built for P.clarkii and (ii) an individual-based dataset with isotopic values of single specimens as described in the present article. All datasets include isotopic information for potential prey to allow the estimation of the trophic position of the invasive species under analysis.
Project description
Title
LifeWatch ERIC Internal Joint Initiative - Functional biogeography of invaders: the case of two widely distributed omnivorous crustaceans (https://bit.ly/iji-crustaceans).
Personnel
Cristina Di Muri, Giorgio Mancinelli, Ilaria Rosati, Lucia Vaira
Study area description
Coastal and transitional areas of the Mediterranean Sea colonised by C.sapidus. The westernmost records are located in Spain, the northernmost in Croatia, the easternmost in Turkey, and the southernmost in Greece. The majority of records lie in Italy.
Design description
The dataset contains geographical and temporal information on the sampling event including country, location, geographical coordinates, type of habitat, year, and month or season in which the sampling occurred. Biological features of the species included are also specified, such as the invasive or native nature of the species for each location and the prey-predator relationship. Such attributes, together with δ13C and δ15N values, can be used for downstream analyses including the calculation of the blue crab trophic position, which can be estimated using two different approaches. The first method estimates the trophic position using the following equation:
Trophic Position δ15N = (δ15NConsumer - δ15NBaseline)/Δ15N + λ
This equation is a generalisation of the formula presented in Jepsen and Winemiller (2002), where δ15NConsumer is the nitrogen isotopic value of the blue crab, Δ15N is the trophic level fractionation of δ15N, δ15NBaseline and λ are the nitrogen isotopic value and the trophic level of the baseline species (e.g. for Phorcusturbinatus, λ = 2). Alternatively, the blue crab trophic position can be estimated using a Bayesian approach implemented in the R package tRophicPosition (Quezada‐Romegialli et al. 2018, R Core Team 2021). In this case, the original δ13C and δ15N values can be back-estimated using mean values, standard deviations, and sample size for each location and species and assuming a normal distribution.
Sampling methods
Study extent
The literature search for compiling this dataset ended on 31st April 2021.
Sampling description
The online platforms ISI Web of Science and Scopus were searched using multiple search criteria including the terms “Callinectessapidus” and “stable isotopes” in conjunction with “non-indigenous”, “alien”, “invasive”, “Mediterranean Sea”, and “Black Sea”. The results were integrated with those obtained by querying Google Scholar using the same search criteria, together with the corresponding terms in Spanish or Portuguese (e.g. “jaiba azul”, “cangrejo azul”, “siri azul”) in order to access additional literature published in languages other than English. Google Scholar search results were saved using the freeware Publish or Perish ver. 7.27.2849 (Harzing 2007).
Quality control
Only records with defined locations whose accuracy was checked using Google Earth were included in the dataset; geographic coordinates were converted to decimal degrees when not originally specified as such. The taxonomic check was performed using the World Register of Marine Species.
Step description
The blue crab preys preferentially on bivalves (Hines 2007); accordingly, this taxonomic group was chosen as a reference for the selection of baseline species included in the dataset. M.galloprovincialis was generally used in the dataset given the almost ubiquitous distribution in Mediterranean marine coastal waters. If not available, other bivalves and herbivorous gastropods occurring at the study sites were chosen. On only one occasion, the omnivorous polychaete (Alittasuccinea) was used as the baseline species. The trophic level of prey species was assigned, based on their trophic habits as follows: bivalves’ trophic position = 2 (filter feeders); gastropod Phorcusturbinatus trophic position = 2 (herbivore; Aslan and Polito 2021); polychaete A.succinea trophic position = 2.78 (omnivore; Jumars et al. 2015, Como et al. 2018). Both M.galloprovincialis and Arcuatulasenhousia are filter feeders and their diets mainly rely on phytoplankton and suspended particulate matter (Inoue and Yamamuro 2000, Ezgeta-Balić et al. 2014); hence, in agreement with the Sea Around Us database, we assumed for both taxa a trophic position = 2. However, it has been also repeatedly indicated that zooplankton can be included in the diet of M.galloprovincialis depending on local conditions, as well as on seasonal variations in resource availability (Ezgeta-Balić et al. 2012, Peharda et al. 2012). Our dataset is freely downloadable and the trophic position of C.sapidus prey can be modified according to the user's specific needs and/or if and when more updated information becomes available. Within our dataset a considerable variation in individual δ15N values occur for M.galloprovincialis as well as for other baseline species (i.e. Phorcusturbinatus). Such variation could be due to individual-scale differences in feeding habits, ontogeny, and metabolism (Tibbets et al. 2007, Doi et al. 2017). The user is, therefore, free to calculate the trophic position of C.sapidus by adopting, for example, the grand mean of the δ15N values or any other estimation of central tendency (e.g. mode or median). Alternatively, individual δ15N values of baseline taxa can be selected by eliminating the outliers or by choosing only a subset of the specimens included (e.g. the lower quartile of the isotopic distribution).
Geographic coverage
Description
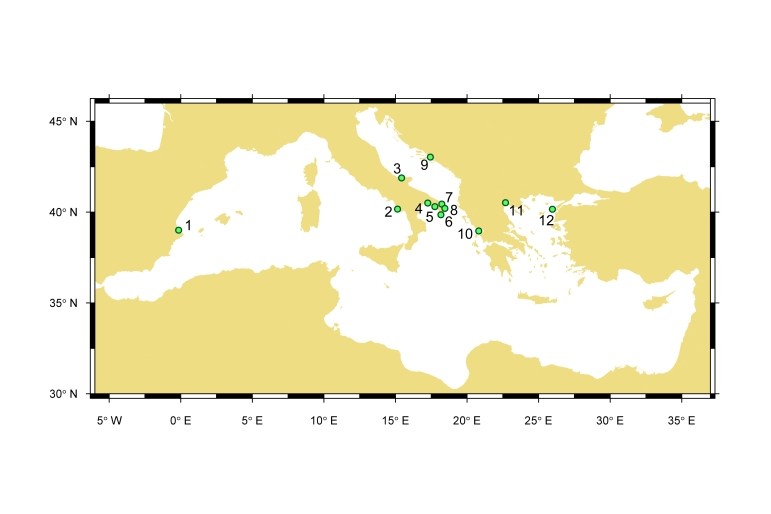
The dataset gathers isotopic values of different Mediterranean areas colonised by C.sapidus including seven study sites in Italy, two study sites in Greece, and one study site in Croatia, Spain, and Turkey (Fig. 1, Table 1). The westernmost records are located in Spain (39.00494; -0.148602), the northernmost in Croatia (43.027423; 17.436056), the easternmost in Turkey (40.157275; 25.96473), and the southernmost in Greece (38.961748; 20.815487).
Figure 1.
Map of the Mediterranean sites included in the dataset. The study sites represent coastal and transitional systems where established populations of Callinectessapidus were investigated.
Table 1.
List of locations included in the dataset with names, countries, ecosystem types, and years in which the sampling events occurred. The last column states the source from which the information was extracted.
| Location ID | Location name | Country | Habitat | Sampling year | Reference |
| 1 | Gandia | Spain | Estuary | 2016 | De Giorgi et al. (2022) |
| 2 | Alento | Italy | Estuary | 2019 | Li Veli et al. (2022) |
| 3 | Lesina | Italy | Lagoon | 2016 | De Giorgi et al. (2022) |
| 4 | Mar Piccolo | Italy | Lagoon | 2014 | Mancinelli et al. (2017b) |
| 5 | Torre Colimena | Italy | Lagoon | 2014 | Mancinelli et al. (2017b) |
| 6 | Spunderati | Italy | Lagoon | 2014 | Mancinelli et al. (2017b) |
| 7 | Acquatina | Italy | Lagoon | 2016 | Mancinelli et al. (2017b) |
| 8 | Alimini | Italy | Lagoon | 2014 | Mancinelli et al. (2017b) |
| 9 | Parila | Croatia | Lagoon | 2015 | Mancinelli et al. (2016) |
| 10 | Pogonitsa | Greece | Lagoon | 2016 | De Giorgi et al. (2022) |
| 11 | Loudias | Greece | Coastal | 2016 | De Giorgi et al. (2022) |
| 12 | Gökçeada | Turkey | Lagoon | 2017 | Aslan and Polito (2021) |
Coordinates
38.96175 and 43.02742 Latitude; -0.1486 and 25.96473 Longitude.
Taxonomic coverage
Description
The dataset is a collection of individual-based isotopic values belonging to C.sapidus and its potential prey including: P.turbinatus, A.succinea, Arcuatulasenhousia, and M.galloprovicialis.
Taxa included
| Rank | Scientific Name | Common Name |
|---|---|---|
| species | Alittasuccinea | Pile worm |
| species | Arcuatulasenhousia | Asian date mussel |
| species | Callinectessapidus | Blue crab |
| species | Mytilusgalloprovincialis | Mediterranean mussel |
| species | Phorcusturbinatus | Turbinate monodont |
Usage licence
Usage licence
Creative Commons Public Domain Waiver (CC-Zero)
IP rights notes
This work is licensed under a Creative Commons Attribution (CC-BY) 4.0 Licence.
Data resources
Data package title
An individual-based dataset of carbon and nitrogen isotopic data of Callinectessapidus in invaded Mediterranean waters.
Resource link
https://doi.org/10.48372/9CD2907F-8F44-4DF6-9070-6B21E1991B94
Number of data sets
1
Data set 1.
Data set name
An individual-based dataset of carbon and nitrogen isotopic data of Callinectessapidus in invaded Mediterranean waters.
Data format
csv
Number of columns
13
Download URL
https://dataportal.lifewatchitaly.eu/view/urn%3Auuid%3Ae869c2cc-2011-461d-99cc-8d6d642b1624
Description
A description of the dataset format is provided below. The dataset attributes were labelled using standard glossaries harvested from Darwin Core, LifeWatch ERIC Ecoportal, and NERC Vocabulary Server.
Data set 1.
| Column label | Column description |
|---|---|
| catalogNumber | An identifier (preferably unique) for the record within the dataset or collection. |
| country | The name of the country or major administrative unit in which the Location occurs. |
| locality | The specific description of the place. |
| habitat | A category or description of the habitat in which the Event occurred. |
| eventDate | The date-time or interval during which an Event occurred. |
| decimalLatitude | The geographic latitude (in decimal degrees, using the spatial reference system given in geodeticDatum) of the geographic centre of a Location. Positive values are north of the Equator, negative values are south of it. |
| decimalLongitude | The geographic longitude (in decimal degrees, using the spatial reference system given in geodeticDatum) of the geographic centre of a Location. Positive values are east of the Greenwich Meridian, negative values are west of it. |
| establishmentMeans | Statement about whether an organism or organisms have been introduced to a given place and time through the direct or indirect activity of modern humans (https://dwc.tdwg.org/em/#dwcem_e). |
| scientificName | The full scientific name, with authorship and date information, if known. When forming part of an Identification, this should be the name in lowest level taxonomic rank that can be determined. This term should not contain identification qualifications, which should instead be supplied in the IdentificationQualifier term. |
| trophicRole | Statement specifying whether the species is a predator or a prey. |
| carbon-13 | A value of the isotope of the chemical element carbon, expressed in permil (‰). |
| nitrogen-15 | A value of the isotope of the chemical element nitrogen, expressed in permil (‰). |
| trophicLevel | Any of the feeding levels through which the passage of energy through an ecosystem proceeds; examples are photosynthetic plants, herbivorous animals, and microorganisms of decay. |
Acknowledgements
The authors thank all the Italian, Croatian, Greek, and Spanish colleagues who have contributed to the data collection and Lucia Vaira (LifeWatch ERIC) for her assistance with the data and metadata publications.
Contributor Information
Cristina Di Muri, Email: cristina.dimuri@iret.cnr.it.
Giorgio Mancinelli, Email: giorgio.mancinelli@unisalento.it.
Author contributions
Cristina Di Muri: conceptualisation, data analysis, writing - final review and editing.
Ilaria Rosati: dataset formatting and quality check - final review and editing.
Roberta Bardelli and Salvatrice Vizzini: dataset creation, stable isotope analysis - final review and editing.
Lucrezia Cilenti, Daniel Li Veli, Silvia Falco, George Katselis, Kosmas Kevrekidis, and Luka Glamuzina: sample collection - final review and editing.
Giorgio Mancinelli: conceptualisation, formal analysis, methodology, writing - original draft, final review and editing.
References
- Altieri A. H., Bertness M. D., Coverdale T. C., Herrmann N. C., Angelini C. A trophic cascade triggers collapse of a salt‐marsh ecosystem with intensive recreational fishing. Ecology. 2012;93(6):1402–1410. doi: 10.1890/11-1314.1. [DOI] [PubMed] [Google Scholar]
- Anton A., Geraldi N. R., Lovelock C. E., Apostolaki E. T., Bennett S., Cebrian J., Krause-Jensen D., Marbà N., Martinetto P., Pandolfi J. M., Santana-Garcon J., Duarte C. M. Global ecological impacts of marine exotic species. Nature Ecology & Evolution. 2019;3(5):787–800. doi: 10.1038/s41559-019-0851-0. [DOI] [PubMed] [Google Scholar]
- Aslan H., Polito M. J. Trophic ecology of the Atlantic blue crab Callinectessapidus as an invasive non-native species in the Aegean Sea. Biological Invasions. 2021;23:2289–2304. doi: 10.1007/s10530-021-02506-7. [DOI] [Google Scholar]
- Belgrad B. A., Griffen B. D. The influence of diet composition on fitness of the blue crab, Callinectessapidus. PLOS One. 2016;11:0145481. doi: 10.1371/journal.pone.0145481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier E. L. Sur un Callinectessapidus M. Rathbun Trouvé à Rochefort. Bulletin du Muséum d’Histoire Naturelle Paris. 1901;7:16. [Google Scholar]
- Como S., Velde G., Magni P. Temporal variation in the trophic levels of secondary consumers in a Mediterranean coastal lagoon (Cabras lagoon, Italy) Estuaries and Coasts. 2018;41(1):218–232. doi: 10.1007/s12237-017-0265-7. [DOI] [Google Scholar]
- Corrales X., Katsanevakis S., Coll M., Heymans J. J., Piroddi C., Ofir E., Gal G. Advances and challenges in modelling the impacts of invasive alien species on aquatic ecosystems. Biological Invasions. 2020;22(3):907–934. doi: 10.1007/s10530-019-02160-0. [DOI] [Google Scholar]
- Cuthbert R. N., Dickey J. W., Coughlan N. E., Joyce P. W., Dick J. T. The Functional Response Ratio (FRR): advancing comparative metrics for predicting the ecological impacts of invasive alien species. Biological Invasions. 2019;21(8):2543–2547. doi: 10.1007/s10530-019-02002-z. [DOI] [Google Scholar]
- David P., Thebault E., Anneville O., Duyck P. F., Chapuis E., Loeuille N. Impacts of invasive species on food webs: a review of empirical data. Advances in Ecological Research. 2017;56:1–60. doi: 10.1016/bs.aecr.2016.10.001. [DOI] [Google Scholar]
- De Giorgi R., Cilenti L., Falco S., Katselis G., Kevrekidis K., Papadia P., Fanizzi F. P., Mancini F., Bardelli R., Vizzini S., Mancinelli G. Trace metals in five Mediterranean populations of the invasive Atlantic blue crab Callinectessapidus: do variations in trophic position matter? Submitted to Journal of Applied Ecology 2022
- Dick J. T., Laverty C., Lennon J. J., Barrios‐O'Neill D., Mensink P. J., Robert Britton J., Médoc V., Boets P., Alexander M. E., Taylor N. G., Dunn A. M., Hatcher M. J., Rosewarne P. J., Crookes S., MacIsaac H. J., Xu M., Ricciardi A., Wasserman R. J., Ellender B. R., Weyl O. L.F., Lucy F. E., Banks P. B., Dodd J. A., MacNeil C., Penk M. R., Aldridge D. C., Caffrey J. M. Invader relative impact potential: a new metric to understand and predict the ecological impacts of existing, emerging and future invasive alien species. Journal of Applied Ecology. 2017;54(4):1259–1267. doi: 10.1111/1365-2664.12849. [DOI] [Google Scholar]
- Doi Hideyuki, Akamatsu Fumikazu, González Angélica L. Starvation effects on nitrogen and carbon stable isotopes of animals: an insight from meta-analysis of fasting experiments. Royal Society Open Science. 2017;4(8) doi: 10.1098/rsos.170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezgeta-Balić Daria, Najdek Mirjana, Peharda Melita, Blažina Maria. Seasonal fatty acid profile analysis to trace origin of food sources of four commercially important bivalves. Aquaculture. 2012:89–100. doi: 10.1016/j.aquaculture.2011.12.041. [DOI]
- Ezgeta-Balić Daria, Lojen Sonja, Dolenec Tadej, Žvab Rožič Petra, Dolenec Matej, Najdek Mirjana, Peharda Melita. Seasonal differences of stable isotope composition and lipid content in four bivalve species from the Adriatic Sea. Marine Biology Research. 2014;10(6):625–634. doi: 10.1080/17451000.2013.833338. [DOI] [Google Scholar]
- Fry Brian. Stable Isotope Ecology. Vol. 521. Springer; New York: 2006. [DOI] [Google Scholar]
- Gallardo B., Clavero M., Sánchez M. I, Vilà M. Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology. 2016;22(1):151–163. doi: 10.1111/gcb.13004. [DOI] [PubMed] [Google Scholar]
- Giordani Soika A. Il Neptunuspelagicus (L.) nell’alto Adriatico. Natura. 1951;42(1-2):18–20. [Google Scholar]
- Harzing A. W. Publish or Perish. https://harzing.com/resources/publish-or-perish. 2007 ver. 7.27.2849.
- Hines A. H. In: The blue crab: Callinectessapidus. Kennedy V. S., Cronin L. E., editors. Maryland Sea Grant College, College Park, Maryland: 2007. Ecology of juvenile and adult blue crabs.565-654 [Google Scholar]
- Inoue Tetsunori, Yamamuro Masumi. Respiration and ingestion rates of the filter-feeding bivalve Musculistasenhousia: implications for water-quality control. Journal of Marine Systems. 2000;26(2):183–192. doi: 10.1016/s0924-7963(00)00053-1. [DOI] [Google Scholar]
- Jackson M. C., Donohue I., Jackson A. L., Britton J. R., Harper D. M., Grey J. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PlOS One. 2012;7(2):31757. doi: 10.1371/journal.pone.0031757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen D. B., Winemiller K. O. Structure of tropical river food webs revealed by stable isotope ratios. Oikos. 2002;96(1):46–55. doi: 10.1034/j.1600-0706.2002.960105.x. [DOI] [Google Scholar]
- Jumars P. A., Dorgan K. M., Lindsay S. M. Diet of worms emended: an update of polychaete feeding guilds. Annual Review of Marine Science. 2015;7:497–520. doi: 10.1146/annurev-marine-010814-020007. [DOI] [PubMed] [Google Scholar]
- Kampouris T. E., Porter J. S., Sanderson W. G. Callinectessapidus Rathbun, 1896 (Brachyura: Portunidae): an assessment on its diet and foraging behaviour, Thermaikos Gulf, NW Aegean Sea, Greece: evidence for ecological and economic impacts. Crustacean Research. 2019;48:23–37. doi: 10.18353/crustacea.48.0_23. [DOI] [Google Scholar]
- Li Veli D., Lillo A. O, Lago N., Specchiulli A., Scirocco T., Cilenti L., Bardelli R., Vizzini S., Mancinelli G. The invasive Callinectessapidus in the Tyrrhenian Sea: a baseline assessment of the trophic position of a newly-recorded population from the Campania region (Italy) Submitted to Marine Pollution Bulletin 2022
- Mancinelli G., Vizzini S. Assessing anthropogenic pressures on coastal marine ecosystems using stable CNS isotopes: state of the art, knowledge gaps, and community-scale perspectives. Estuarine Coastal and Shelf Science. 2015;156:195–204. doi: 10.1016/j.ecss.2014.11.030. [DOI] [Google Scholar]
- Mancinelli G., Glamuzina B., Petrić M., Carrozzo L., Glamuzina L., Zotti M., Raho D., Vizzini S. The trophic position of the Atlantic blue crab Callinectessapidus Rathbun, 1896 in the food web of Parila Lagoon (South Eastern Adriatic, Croatia): a first assessment using stable isotopes. Mediterranean Marine Science. 2016;17:634–643. doi: 10.12681/mms.1724. [DOI] [Google Scholar]
- Mancinelli G., Raho D., Zotti M., Alujević K., Guerra M. T., Vizzini S. Trophic flexibility of the Atlantic blue crab Callinectessapidus in invaded coastal systems of the Apulia region (SE Italy): a stable isotope analysis. Estuarine Coastal and Shelf Science. 2017;198:421–431. doi: 10.1016/j.ecss.2017.03.013. [DOI] [Google Scholar]
- Mancinelli G., Chainho P., Cilenti L., Falco S., Kapiris K., Katselis G., Ribeiro F. The Atlantic blue crab Callinectessapidus in southern European coastal waters: distribution, impact and prospective invasion management strategies. Marine Pollution Bulletin. 2017;119:5–11. doi: 10.1016/j.marpolbul.2017.02.050. [DOI] [PubMed] [Google Scholar]
- Mancinelli G., Bardelli R., Zenetos A. A global occurrence database of the Atlantic blue crab Callinectessapidus. Scientific Data. 2021;8(1):1–10. doi: 10.1038/s41597-021-00888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa Masao, Wada Eitaro. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta. 1984;48(5):1135–1140. doi: 10.1016/0016-7037(84)90204-7. [DOI] [Google Scholar]
- Mitchell M J, Mills E L, Idrisi N, Michener R. Stable isotopes of nitrogen and carbon in an aquatic food web recently invaded by Dreissenapolymorpha (Pallas) Canadian Journal of Fisheries and Aquatic Sciences. 1996;53(6):1445–1450. doi: 10.1139/f96-053. [DOI] [Google Scholar]
- Peharda Melita, Ezgeta-Balić Daria, Davenport John, Bojanić Natalia, Vidjak Olja, Ninčević-Gladan Živana. Differential ingestion of zooplankton by four species of bivalves (Mollusca) in the Mali Ston Bay, Croatia. Marine Biology. 2012;159(4):881–895. doi: 10.1007/s00227-011-1866-5. [DOI] [Google Scholar]
- Post D. M. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002;83(3):703–718. doi: 10.1890/0012-9658(2002)083. [DOI] [Google Scholar]
- Pugesek B. H., Baldwin M. J., Stehn T. The relationship of blue crab abundance to winter mortality of Whooping Cranes. The Wilson Journal of Ornithology. 2013;125(3):658–661. doi: 10.1676/12-159.1. [DOI] [Google Scholar]
- Quezada‐Romegialli C., Jackson A. L., Hayden B., Kahilainen K. K., Lopes C., Harrod C. tRophicPosition, an R package for the Bayesian estimation of trophic position from consumer stable isotope ratios. Methods in Ecology and Evolution. 2018;9(6):1592–1599. doi: 10.1111/2041-210X.13009. [DOI] [Google Scholar]
- Team R Core. R Foundation for Statistical Computing, Vienna, Austria; 2021. R: a language and environment for statistical computing. [Google Scholar]
- Serbetis C. Un nouveau crustacé commestible en mer Egeé Callinectessapidus Rath. (Decapod. Brach.). General Fisheries Council for the Mediterranean (GFMC), Proceedings and Technical papers. 1959;5:505–507. [Google Scholar]
- Streftaris N., Zenetos A. Alien marine species in the Mediterranean-the 100 ‘worst invasives’ and their impact. Mediterranean Marine Science. 2006;7(1):87–118. doi: 10.12681/mms.180. [DOI] [Google Scholar]
- Thomsen M. S. Indiscriminate data aggregation in ecological meta-analysis underestimates impacts of invasive species. Nature Ecology & Evolution. 2020;4(3):312–314. doi: 10.1038/s41559-019-0851-0. [DOI] [PubMed] [Google Scholar]
- Tibbets Teresa M., Wheeless Leslie A., del Rio Carlos Martínez. Isotopic enrichment without change in diet: an ontogenetic shift in δ15N during insect metamorphosis. Functional Ecology. 2007 doi: 10.1111/j.1365-2435.2007.01342.x. [DOI]