Abstract
Purpose
To evaluate functional clinical endpoints and their structural correlations in AMD, with a focus on subretinal drusenoid deposits (SDD).
Methods
This prospective study enroled 50 participants (11 controls, 17 intermediate AMD (iAMD) with no SDD, 11 iAMD with SDD and 11 non-foveal atrophic AMD). Participants underwent best-corrected visual acuity (BCVA), low luminance visual acuity (LLVA), low luminance questionnaire (LLQ), scotopic thresholds, rod-intercept time (RIT), photopic flicker electroretinograms and multimodal imaging. Functional and structural relationships were assessed.
Results
Compared with healthy participants, BCVA, LLVA, scotopic thresholds were depressed, and RIT prolonged in iAMD patients with SDD (p = 0.028, p = 0.045, p = 0.014 and p < 0.0001 respectively). Patients with SDD also had reduced scotopic function and delayed RIT compared to iAMD without SDD (p = 0.005 and p < 0.0001). Eyes with SDD and non-foveal atrophy did not differ functionally. Nor did healthy subjects compared with iAMD without SDD. Functional parameters were significantly associated with scotopic thresholds (r = 0.39–0.64). BCVA, LLVA and scotopic thresholds correlated well with ONL volume, ONL thickness and choroidal thickness (r = 0.34–0.61).
Conclusion
Eyes with SDD are surrogate markers of photoreceptor abnormalities comparable with non-central atrophy and should be sub-analysed in clinical trials evaluating potential prophylactic agents to decrease the progression of AMD and may even require different therapeutic interventions.
Subject terms: Prognostic markers, Macular degeneration
Introduction
Age-related macular degeneration (AMD) is clinically characterised by the presence of drusen [1]. However, subretinal drusenoid deposits (SDD) have also been recognised as a distinct morphological feature that confers increased risk of developing advanced AMD [2, 3]. Over a quarter of people with intermediate AMD (iAMD) have SDD on multimodal imaging [4]. Clinical trials that evaluated interventions to prevent progression of iAMD to advanced AMD had not stratified the eligibility criteria into eye with and without SDD [5, 6]. However, secondary analysis of the Laser Intervention in Age-Related Macular Degeneration (LEAD) trial showed that eyes with iAMD with SDD did not benefit from nanosecond laser intervention suggesting that future trials in iAMD should re-define the selection criteria.
A major challenge in conducting preventive trials in AMD is the lack of validated functional end-points. Best-corrected visual acuity (BCVA) is the only validated functional end-point that is accepted by regulatory authorities as a clinical trial end-point in retinal diseases. However, changes in BCVA do not necessarily parallel disease progression from intermediate to late AMD or the progression of non-central geographic atrophy (GA) to fovea-involving GA as BCVA is only affected when the disease involves the fovea [7, 8]. Therefore, there is an unmet need to capture other changes in retinal function that are experienced by subjects that may be used as an independent end-point or can be correlated with anatomical changes at the macula so that the structural change can be used as a surrogate marker of functional decline [9]. Developing and validating these end-points will enable the evaluation of novel therapeutic agents to prevent or delay the progression to foveal involving advanced AMD better, a disease of paramount public health importance with significant societal burden [8].
There are limited studies evaluating visual function changes in ageing and eyes that are phenotypically at risk of development or progression of advanced AMD [10–13]. With the recent evidence that photoreceptors may be affected early in some eyes with iAMD, it is also important to stratify iAMD into sub-groups based on visual function changes [14, 15].
Rod dysfunction is particularly affected in eyes with iAMD with SDD [16, 17].
This study sought to evaluate functional differences and correlation between AMD severity groups when eyes with iAMD and SDD are considered a separate phenotype.
Methods
This single centre, exploratory prospective cohort study was approved by the Camden and Kings Cross NRES Committee London REC 16/LO/1317. Written informed consent was obtained from all participants and the study followed the tenets of the Declaration of Helsinki. This paper presents the baseline results relating the outcomes of psychophysical tests of visual function to varying severity of AMD and the relationship of these tests to each other. In addition, we evaluated correlation between the structure and function.
Study participants
The participants in this study had BCVA better than 50 ETDRS letters and belonged to one of these categories: (1) Healthy aging (No AMD or presence of drupelets), (2) Intermediate AMD with no SDD (iAMD with no SDD), (3) Intermediate AMD with at least five visible SDD on OCT B-scans that interrupted the ellipsoid zone (SDD group) and (4) non-foveal atrophic AMD with background drusen with or without SDD. Intermediate AMD was defined as having at least one large drusen (>125 µm), with or without pigmentary abnormalities. Participant were classified based on at least two imaging methods; infrared reflectance, autofluorescence and spectral domain optical coherence tomography (SD-OCT) on Spectralis OCT2 (Heidelberg Engineering, Heidelberg, Germany); and colour fundus photography (CFP) of the macula (Topcon; Tokyo, Japan).
Exclusion criteria included co-existent ocular disease (neovascular AMD, glaucoma or diabetic retinopathy, substantial cataract) in the study eye, significant systemic disease or history of medication known to affect visual function, epilepsy, history of major ocular surgery in the last 3 months or anticipated within the next 6 months following enrolment in the study eye and any allergies to adhesives or any other component used. Demographic and clinical characteristics were collected.
Assessments
Participants underwent best-corrected visual acuity (BCVA) followed immediately by low luminance visual acuity (LLVA, by placing a 2.0-log neutral density filter over the eye) measurement at 4 m using a standard Early Treatment in Diabetic Retinopathy Study (ETDRS). Low luminance deficit (LLD) was defined as the difference between BCVA and LLVA in ETDRS letters. Following pupillary dilatation and dark adaptation for 40 min participants underwent scotopic perimetric assessment and dark adaptometry. Multimodal imaging was completed after these rod function tests. Low luminance questionnaire (LLQ) consisting of 32-item questions with six subscales related to low luminance settings were also administered.
Scotopic thresholds
Scotopic perimetry was assessed using a Medmont dark-adapted chromatic perimeter which is able to perform two-colour perimetric analysis for rod function; one at 505 nm (cyan, range 0–75 dB) and the other at 625 nm (red range 0–50 dB). Appropriate corrective lenses were placed in the lens holder to account for participant’s refraction for a viewing distance of 30 cm. Fixation was monitored using an infrared camera built in the perimeter. The light stimulus was 1.73° in size (Goldmann size V) and was presented for 170 ms in a random order across central 17 retinal locations (4°, 8°, 12° eccentricity to the fovea with one added location at 6° inferior in the vertical meridian) using 3 dB steps. We performed two scotopic perimetric examinations on each participant and calculated the mean retinal sensitivity across all locations.
Dark adaptometry
Dark adaptometry was measured using the AdaptDx (MacuLogix, Hummelstown, PA), a computer-automated dark adaptometer. Patients were light adapted in the room for 3–5 min and pupil size was measured (at least 6 mm) before being instructed to place their chin and forehead on the instrument, focusing on the red central light. Corrective lenses were inserted in the lens holder for a viewing distance of 30 cm. The test eye monocularly was exposed to the equivalent rhodopsin bleach of 82% with the delivery of a 505 nm photoflash subtending 4° and centred at 5° on the inferior vertical meridian (~0.80 ms duration). Light stimuli were presented for 200 ms using a 3-down/1-up staircase and thresholds were measured until the rod-intercept (time taken to recover 5.0 × 10−3 scotopic cd/m2 or 3.1 log units of stimulus attenuation) was reached or up to 20 min post-bleach, which ever was shorter. The participant was instructed to press on the response button when light stimuli were seen and had 15 s rest between each threshold measurement.
Photopic ERGs
Cone function was assessed by measuring full field flicker electroretinograms (ERGs) using a small handheld portable commercial device called the RETeval system (LKC Technologies, Inc., Gaithersburg, MD, USA). Self-adhesive skin electrode arrays, were placed 2 mm below the lower lid margins and eyes were tested monocularly. The device emitted a fixed flash (stimulus strength, 3.0 cd s/m2) with a background of 30 cd/m2 at 28.3 Hz frequency.
Measurement of retinal volume and choroidal thickness
The SD-OCT retinal scans were obtained on Spectralis (Heidelberg Engineering, Heidelberg, Germany). For thickness analysis of retinal layers, volumetric SD-OCT data were initially automatically segmented with the Heidelberg Eye Explorer software, then each segmentation of the multiple B-scans was reviewed carefully and manually corrected if required. The following layer volumes and thicknesses were measured: the total retinal volume (TRV), total retinal thickness (TRT), retinal pigment epithelium drusen complex (RPEDC) thickness and volume, outer nuclear layer (ONL) thickness and volume [18]. Enhanced depth imaging optical coherence tomography (EDI-OCT was performed for all patients. Choroidal thickness (CT) was measured manually with the help of built-in callipers in the OCT software. Measurements were made from the outer portion of hyperreflective line corresponding to retinal pigment epithelium to the inner portion of hyperreflective zone corresponding to the choroidoscleral junction. They were obtained at the subfoveal point (SFCT), and also at a distance of 1500 µ and 3000 µ from the locus of measurement of SFCT in the nasal and temporal quadrants. The mean of these 5 values was taken as the mean CT and used for analysis.
Statistical analysis
All statistical analysis and graphs were generated using GraphPad Prism (Version 8.2.1). The normal quantile-quantile plots (Q–Q plots) and Shapiro–Wilk test were used to assess whether the data was normally distributed.
Patient characteristics were analysed using chi squared or Fisher exact tests for categorical variables, ANOVA or Kruskal–Wallis tests for continuous data. Cross-sectional analysis between groups was acquired by applying unpaired, non-parametric Kruskal–Wallis test followed by post hoc uncorrected Dunn’s test. The relationship between functional parameters and the association between functional and structural measures was assessed using Spearman correlation coefficient. The nominal level of statistical significance was set at α = 0.05. Non-parametric bootstrap was performed (STATA R version3.6.3) on 1000 bootstrap replicates, using a pooled method to obtain adjusted p values for the ANOVA test, validating one-way ANOVA in non-normal data and overcoming limitations due to small sample size [19]. Due to the exploratory nature of the study with small sample size, the p values for pairwise comparisons were not adjusted for multiple comparisons.
Results
Demographic and clinical characteristics of the participants
A total of 50 participants were recruited: healthy ageing group (n = 11), iAMD no SDD (n = 17), iAMD with SDD (n = 11) and non-foveal atrophy due to AMD (n = 11). The mean age of the cohort was 69.3 (±7.6) years, with participants in iAMD with SDD (74.2 ± 5.6 years) and non-foveal atrophy (73.0 ± 6.0 years) groups being older than the healthy ageing (65.1 ± 6.2) and iAMD without SDD (66.3 ± 8.1) groups. There were more female participants (60%) than male (40%). There were no differences in smoking status, blood pressure, diabetes, or lipid profile between groups but body mass index was significantly higher in the group with non-foveal atrophy [20]. Functional outcome measures were not age-adjusted as SDD are associated with advancing disease and linear regression showed no significant relationship between age in the healthy aging group and study-eye BCVA (p = 0.317), LLVA (p = 0.112), LLD (p = 0.617), LLQ (p = 0.793), scotopic thresholds (p = 0.400), rod-intercept time (p = 0.822), ERG amplitude (p = 0.175). ERG timing was found to be significantly related to age (p = 0.041), although was not adjusted for analysis. Additionally, all these parameters are affected in advanced disease and less so in normal aging.
Table 1 shows the visual function differences between groups. Statistically significant difference between groups was found for BCVA ( p = 0.0005), LLVA ( p = 0.008), LLQ composite score ( p = 0.032), scotopic thresholds (cyan, p = 0.0006) and rod-intercept time ( p < 0.0001). However, LLD score and photopic ERG parameters did not differ between groups ( p = 0.424, p = 0.142 and p = 0.067 respectively).
Table 1.
Cross-sectional analysis at baseline for all functional outcome measures for all groups.
| Group, mean ± SD, median (minimum, maximum) | Pairwise comparisons post hoc uncorrected Dunn’s test (p value) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | Healthy aging (n = 11)a | iAMD no SDD (n = 17)a | iAMD with SDD (n = 11)a | Non-foveal atrophic AMD (n = 11)a | Overall p value* | Bootstrap Adjusted pvalue | Healthy aging vs. iAMD no SDD | Healthy aging vs. iAMD with SDD | Healthy aging vs. non-foveal atrophic AMD | iAMD no SDD vs. iAMD with SDD | iAMD no SDD vs. Non-foveal atrophic AMD | iAMD with SDD vs. non-foveal atrophic AMD |
| BCVA (ETDRS letters) Mean (± SD) Minimum, median, maximum | 87.6 (±4.8) 78, 89, 93 | 85.7 (±6.1) 67, 87, 93 | 83.6 (±4.3) 77, 83, 90 | 74.2 (±11.2) 50, 76.5, 87 | 0.0005 | <0.001 | 0.237 | 0.028 | <0.0001 | 0.218 | 0.001 | 0.065 |
| LLVA (ETDRS letters) Mean (± SD) Minimum, median, maximum | 74.1 (±3.89) 68, 75, 82 | 71.1 (±8.2) 47, 73, 80 | 67.3 (±8.2) 56, 67, 79 | 60.8 (±12.1) 40, 64.5, 74 | 0.008 | 0.005 | 0.385 | 0.045 | 0.002 | 0.181 | 0.009 | 0.230 |
| LLD (ETDRS letters) Mean (± SD) Minimum, median, maximum | 13.5 (±2.3) 10, 14, 17 | 14.6 (±4.3) 9, 14, 22 | 16.3 (±4.6) 11, 15, 26 | 13.4 (±3.7) 9, 12.5, 20 | 0.424 | 0.274 | – | – | – | – | – | – |
| LLQ composite score Mean (± SD) Minimum, median, maximum | 94.8 (±5.2) 83.6, 96.1, 100.0 | 87.3 (±9.1) 71.9, 90.6, 99.2 | 87.7 (± 12.9) 66.4, 93.8, 100.0 | 72.8 (± 24.6) 27.3, 71.1, 98.0 | 0.032 | 0.005 | 0.044 | 0.203 | 0.004 | 0.543 | 0.24 | 0.106 |
| Scotopic Thresholds cyan (dB) Mean (± SD) Minimum, median, maximum | 58.02 (±3.45) 53.53, 59.18, 63.06 | 58.05 (±2.29) 53.97, 58.68, 61.47 | 52.90 (±4.31) 45.41, 55.38, 57.50 | 47.68 (±9.47) 32.35, 46.56, 58.74 | 0.0006 | <0.001 | 0.913 | 0.014 | 0.003 | 0.005 | 0.0008 | 0.624 |
| Rod-intercept time (RIT) (minutes) Mean (± SD) Minimum, median, maximum | 4.77 (±1.29) 2.90, 4.65, 7.62 | 7.42 (±4.51) 3.68, 6.17, 20.00 | 20.00 (±0.00) 20.00, 20.00, 20.00 | 19.51 (±1.49) 15.26, 20.00, 20.00 | <0.0001 | <0.001 | 0.240 | <0.0001 | <0.0001 | <0.0001 | 0.0003 | 0.717 |
| Photopic ERG Amplitude (µV) Mean (± SD) Minimum, median, maximum | 16.8 (±7.2) 7.6, 15.3, 35.0 | 17.8 (±5.6) 11.1, 17.8, 31.5 | 14.0 (±5.4) 7.5, 13.55, 25.1 | 13.3 (±5.6) 5.9, 13.1, 25.0 | 0.142 | 0.258 | – | – | – | – | – | – |
| Photopic ERG Time (ms) Mean (± SD) Minimum, median, maximum | 27.9 (±2.5) 24.9, 27.5, 31.8 | 27.7 (±2.0) 25.2, 27.2, 30.9 | 28.2 (±1.0) 27.1, 28.05, 30.0 | 30.2 (±2.5) 26.2, 30.15, 34.4 | 0.067 | 0.043 | – | – | – | – | – | – |
Photopic flicker ERGs were measured in only 42 participants of the total 50 recruited due to a temporary mechanical fault with instrumentation (healthy aging group N = 10, iAMD no SDD, N = 14, iAMD with SDD N = 8, Non-foveal atrophy N = 10).
*Overall P value based on Kruskal–Wallis test of difference among medians.
aOutliers excluded following ROUT analysis; one participant for BCVA, LLVA, LLD (non-foveal atrophy group) and 2 for RIT (1 AMD with SDD and 1 from non-foveal atrophy group).
Scotopic thresholds were reduced in participants with iAMD with SDD ( p = 0.014) and in patients with extrafoveal atrophic AMD ( p = 0.003) when compared to healthy controls. Mean retinal sensitivity was also significantly depressed between both iAMD with SDD ( p = 0.005) and non-foveal AMD ( p = 0.0008) groups compared to iAMD without SDD participants. There was no difference in sensitivity thresholds between healthy controls and eyes with iAMD without SDD.
The rod-intercept time was longer for both iAMD with SDD and AMD patients with extrafoveal atrophy compared with controls ( p < 0.0001 for both). Dark adaptation was also delayed for both of these groups when compared to patients with iAMD no SDD (iAMD with SDD, p < 0.0001 and non-foveal atrophy group, p = 0.0003).
With the exception of LLQ composite score, no statistically significant difference was found either between healthy controls and AMD no SDD group, or between AMD with SDD and non-foveal atrophy group for any subjective functional outcome measures. Non-parametric bootstrap analysis yielded similar statistical outcome in all outcome measures compared to traditional ANOVA tests.
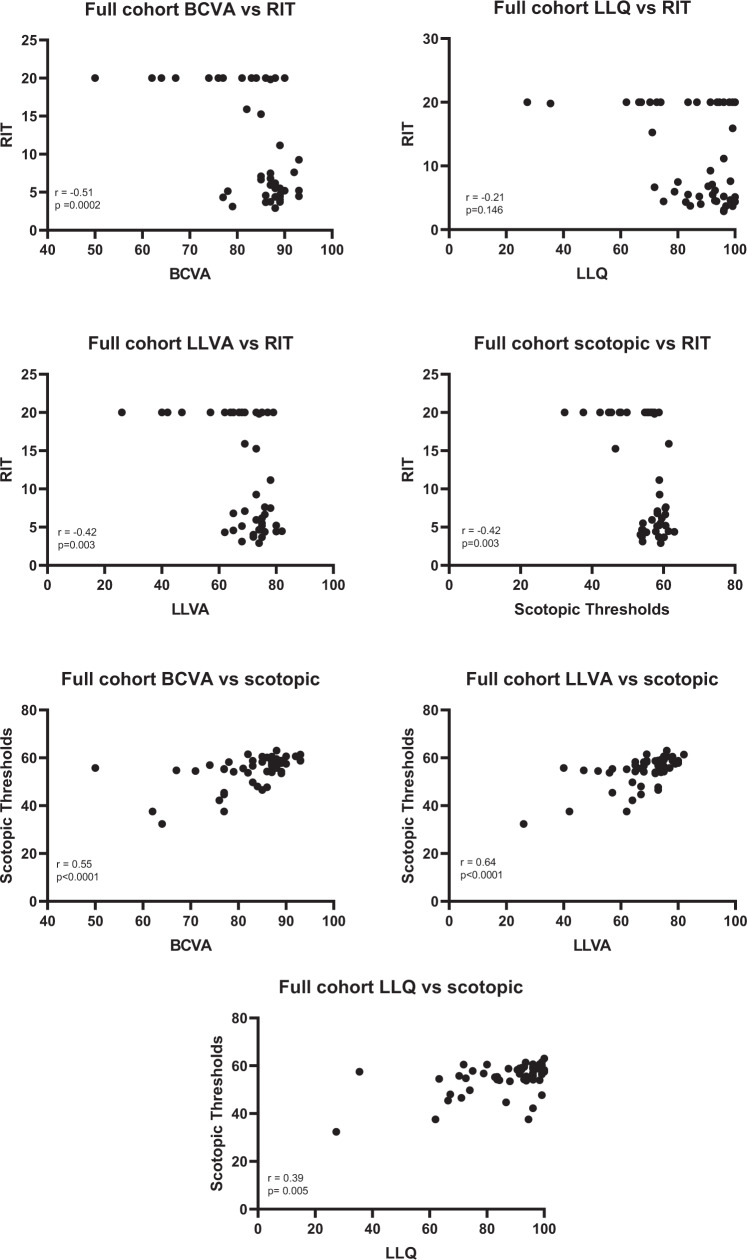
The relationships between rod-mediated functional outcome measures and other visual function tests were investigated and shown in Fig. 1. The BCVA, LLVA and scotopic thresholds were moderately correlated with RIT (r = −0.51, −0.42 and −0.42 respectively) where as LLQ was only weakly correlated with RIT (r = −0.21). By contrast, BCVA (r = 0.55), LLVA (r = 0.64) and LLQ (r = 0.39) correlated well with scotopic thresholds.
Fig. 1. Scatterplots examining the relationship between rod-mediated function and other visual function outcome measures amongst all subjects.
Significant correlations (Spearman correlation: r) were found between all functional parameters with the exception of LLQ vs RIT (p = 0.146).
The means of TRV, retinal thickness, outer nuclear volume (ONL) volume, ONL thickness and CT for each group are displayed in Table 2.
Table 2.
Cross-sectional quantitative OCT parameter analysis between all groups.
| Layer | Healthy aging (N = 11) Mean (±SD) |
iAMD no SDD (N = 17) Mean (±SD) |
iAMD with SDD (N = 11) Mean (±SD) |
Non-foveal GA (N = 11) Mean (±SD) |
|---|---|---|---|---|
| Total retinal volume | 8.57 (±0.31) | 8.71 (±0.49) | 8.69 (±0.28) | 8.14 (±0.78) |
| Total retinal thickness | 311.10 (±9.84) | 316.85 (±18.87) | 317.99 (±12.74) | 291.67 (±32.26) |
| ONL volume | 1.73 (±0.27) | 1.77 (±0.14) | 1.73 (±0.13) | 1.41 (±0.30) |
| ONL thickness | 67.76 (±9.65) | 67.54 (±6.28) | 66.06 (±4.61) | 50.17 (±11.02) |
| RPEDC volume | 2.24 (±0.05) | 2.33 (±0.24) | 2.44 (±0.38) | 2.35 (±0.48) |
| RPEDC thickness | 80.75 (1.98) | 86.00 (±11.62) | 90.89 (17.57) | 87.63 (±30.59) |
| Choroidal thickness | 250.3 (±101.3) | 168.9 (±58.9) | 209.6 (±104.2) | 123.0 (±50.5) |
Table 3 shows the structure-function correlation. BCVA, LLVA, scotopic thresholds and photopic ERG showed moderate but undoubted correlation with ONL volume and thickness and CT. No significant association was found between LLD, LLQ, RIT and photopic ERG amplitude and quantitative OCT parameters. There was also no association between RPEDC parameters and visual function outcome measures.
Table 3.
Correlation between functional and structural outcome measures (Spearman’s correlation r).
| Parameters | Mean total retinal volume | Mean total retinal thickness | Mean ONL volume | Mean ONL thickness | Mean RPEDC volume | Mean RPEDC thickness | Choroidal thickness |
|---|---|---|---|---|---|---|---|
| BCVA (n = 49) | r = 0.21 p = 0.148 | r = 0.16 p = 0.266 | r = 0.42 p = 0.003 | r = 0.55 p = <0.0001 | r = −0.02 p = 0.919 | r = 0.04 p = 0.805 | r = 0.43 p = 0.002 |
| LLVA (n = 49) | r = 0.25 p = 0.083 | r = 0.22 p = 0.126 | r = 0.48 p = 0.0004 | r = 0.61 p < 0.0001 | r = −0.06 p = 0.666 | r = −0.01 p = 0.970 | r = 0.42 p = 0.003 |
| LLD (n = 49) | r = −0.08 p = 0.602 | r = −0.10 p = 0.502 | r = −0.16 p = 0.259 | r = −0.24 p = 0.099 | r = 0.09 p = 0.530 | r = 0.09 p = 0.555 | r = −0.11 p = 0.441 |
| LLQ (n = 50) | r = 0.02 p = 0.892 | r = 0.11 p = 0.431 | r = 0.10 p = 0.490 | r = 0.25 p = 0.082 | r = 0.07 p = 0.608 | r = 0.07 p = 0.606 | r = 0.25 p = 0.078 |
| Scotopic thresholds (n = 50) | r = 0.24 p = 0.094 | r = 0.18 p = 0.215 | r = 0.46 p = 0.0009 | r = 0.52 p = 0.0001 | r = 0.10 p = 0.501 | r = 0.08 p = 0.569 | r = 0.34 p = 0.015 |
| RIT (n = 48) | r = −0.03 p = 0.830 | r = −0.08 p = 0.603 | r = −0.11 p = 0.453 | r = −0.25 p = 0.082 | r = 0.10 p = 0.507 | r = 0.06 p = 0.685 | r = −0.24 p = 0.103 |
| Photopic ERG amplitude (n = 42) | r = 0.29 p = 0.066 | r = 0.31 p = 0.048 | r = 0.23 p = 0.146 | r = 0.30 p = 0.054 | r = 0.08 p = 0.618 | r = 0.12 p = 0.454 | r = 0.23 p = 0.149 |
| Photopic ERG time (n = 42) | r = −0.21 p = 0.174 | r = −0.20 p = 0.204 | r = −0.33 p = 0.033 | r = −0.37 p = 0.016 | r = −0.12 p = 0.445 | r = −0.15 p = 0.335 | r = −0.49 p = 0.001 |
Discussion
Our study showed that BCVA, LLVA, scotopic threshold and RIT were significantly reduced in iAMD with SDD and the values were comparable to eyes with parafoveal atrophy, suggesting that SDD is a marker of advanced disease despite no noticeable atrophic changes on multimodal imaging. Parafoveal atrophy indicates localised tissue loss. However, those with SDD have generalised and equal loss of function whether or not there is manifest atrophy as shown on reflection images. Sunness et al. have previously established delayed dark adaptation as a predictor of progression of GA [21]. Our findings are compatible with the concept that both SDD and parafoveal atrophy show similar magnitude of impairment of dark adaptation suggesting that SDD is as strong a predictor of disease progression as parafoveal atrophy.
Of note, all eyes with SDD in our study had delayed RIT of 20 min (the maximum limit placed in this study), indicating that RIT is a good test that can accurately discriminate eyes with iAMD with and without SDD. Studies that have not previously stratified iAMD by the presence of SDD reported delayed rod function tests in iAMD and these results may have been driven by those with SDD [22, 23]. Our results support more recent studies showing significant impaired rod function in SDD eyes [11, 13, 16, 24].
When we consider the mean scotopic threshold in the different groups, the SDD group also behaved like the group with eyes with parafoveal atrophy rather than those with iAMD without SDD. However, some eyes with SDD and parafoveal atrophy achieved scotopic thresholds comparable with iAMD without SDD and healthy eyes respectively albeit slowly. Therefore, scotopic threshold is not as accurate as RIT in terms of differentiating eyes with SDD from the other groups.
Our study results also suggest that eyes with SDD may be already on track to irreversible disease progression, and so clinical trials on prevention or treatment of progression of GA should exclude them or undertake a sub analysis of this group. The subthreshold nanosecond LEAD randomised controlled trial is an example where this was done and the SDD group did less well than those eyes without SDD [5], reinforcing the need for their segregation.
We also observed increasing thickness and volume of the ONL was associated with better photopic and scotopic function indicating that SDD is an indicator of outer retinal cell loss. However, larger sample size in each group is required to evaluate whether there are group-wise differences. Histopathological and clinical studies have also suggested a possible role for choroid in the pathogenesis of SDD [25] but we could not identify a relation between CT and RIT in this study. SDD has been linked to choroidal vascular insufficiency, further research is required to evaluate whether RIT correlates with changes in choroidal vasculature [26] or whether there is a threshold of choroidal thinning below which RIT may be affected. The Beijing Eye Study showed that visual acuity impairment correlated with subfoveal CT of 30 μm or less [27].
We also found that RIT negatively and moderately related to BCVA and LLVA and only weakly associated with LLQ. McGuiness et al. have also shown similar moderate relationships between RIT and visual acuity as did Flamendorf et al. between BCVA and RIT [24, 28]. Nevertheless, other studies have found stronger correlation between LLQ and RIT [29, 30]. An important observation is that BCVA, LLVA and LLQ could not differentiate eyes with iAMD with and without SDD. Indeed, eyes with iAMD with no SDD behaved very similar to healthy eyes in terms of all visual function tests. No difference was found between groups for photopic ERGs assessment for either the amplitude or implicit time. This is a reasonable finding in that this test evaluates pan-retinal cone function.
The strengths of this study include detailed retinal grading and disease classification with two graders based on multimodal imaging and AMD patients with varying disease severity including stratification of those with and without SDD. In addition to deep phenotyping, all assessments were carried out systematically and consistently by a single observer with full dark adaptation of the subjects for 40 min prior to scotopic testing. Despite the small sample size, we observed significant and consistent differences between AMD groups, substantiated with non-parametric bootstrap analysis, and significant associations between clinical measures, validating the findings of our cohort.
Some limitations of this pilot study include the small sample size in each group. The length of RIT test was automatically terminated at 20 min in our study and this may be a factor in not capturing any difference between patients with SDD and those with atrophy. We also classified the SDD group based on a small number of SDDs (at least five SDD that show interruption of the EZ zone as against recent studies that have used area of SDD as classification criteria [31]. For the purpose of correlating function with structure in our study, we evaluated 6 mm region which may have resulted in poor association with RPEDC. It is also important to note that visual functional parameters reflect uniocular function where as LLQ represents binocular function and therefore the results could be affected depending on the status of the non-study eye.
In conclusion, our study reiterates that eyes with SDD are surrogate markers of photoreceptor abnormalities, although not necessarily cell death that are as significant as eyes with non-central atrophy and so are unlikely to show an improvement in visual functions with potential novel prophylactic agents that are evaluated to decrease the rate of progression of AMD.
Summary
What was known before
SDD have been recognised as a distinct morphological feature that confer increased risk of developing advanced AMD.
Clinical trials that evaluated interventions to prevent progression of iAMD to advanced AMD have not stratified the eligibility criteria into eyes with and without SDD.
A major challenge in conducting preventive trials in AMD is the lack of validated functional end-points.
With the recent evidence that photoreceptors may be affected early in some eyes with iAMD, it is also important to stratify iAMD into sub-groups based on visual function changes.
What this study adds
SDD is as strong a predictor of disease progression as parafoveal atrophy.
Eyes with SDD may be already on track to irreversible disease progression, and so clinical trials on prevention or treatment of progression of GA should exclude them or undertake a sub analysis of this group.
SDD is an indicator of outer retinal cell loss.
SDD are surrogate markers of photoreceptor abnormalities, although not necessarily cell death that are as significant as eyes with non-central atrophy and so are unlikely to show an improvement in visual functions with potential novel prophylactic agents.
Acknowledgements
The research was funded by Fight for Sight (Ref 1905) and supported by the NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology and the NIHR Moorfields Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding
The project is funded by Fight For Sight grant number—1905. SC and RR are supported by ORNATE India Project (GCRF UKRI MR/P207881/1).
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigl B. Age-related maculopathy—linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog Retin Eye Res. 2009;28:63–86. doi: 10.1016/j.preteyeres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Finger RP, Wu Z, Luu CD, Kearney F, Ayton LN, Lucci LM, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014;121:1252–6. doi: 10.1016/j.ophtha.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg JS, Göbel AP, Fleckenstein M, Holz FG, Schmitz-Valckenberg S. Reticular drusen in eyes with high-risk characteristics for progression to late-stage age-related macular degeneration. Br J Ophthalmol. 2015;99:1289–94. doi: 10.1136/bjophthalmol-2014-306535. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Ayton LN, Luu CD, Baird PN, Guymer RH. Reticular pseudodrusen in intermediate age-related macular degeneration: prevalence, detection, clinical, environmental, and genetic associations. Investig Ophthalmol Vis Sci. 2016;57:1310–6. doi: 10.1167/iovs.15-18682. [DOI] [PubMed] [Google Scholar]
- 5.Guymer RH, Wu Z, Hodgson LAB, Caruso E, Brassington KH, Tindill N, et al. Subthreshold nanosecond laser intervention in age-related macular degeneration: the LEAD randomized controlled clinical trial. Ophthalmology. 2019;126:829–38. doi: 10.1016/j.ophtha.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, Gregori G, Penha FM, Moshfeghi AA, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121:693–701. doi: 10.1016/j.ophtha.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogg RE, Chakravarthy U. Visual function and dysfunction in early and late age-related maculopathy. Prog Retin Eye Res. 2006;25:249–76. doi: 10.1016/j.preteyeres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Beirne RO, Hogg RE, Stevenson MR, Zlatkova MB, Chakravarthy U, Anderson RS. Severity staging by early features of age-related maculopathy exhibits weak relationships with functional deficits on SWS grating acuity. Investig Ophthalmol Vis Sci. 2006;47:4624–31. doi: 10.1167/iovs.05-1227. [DOI] [PubMed] [Google Scholar]
- 9.Bird A. Role of retinal pigment epithelium in age-related macular disease: a systematic review. Br J Ophthalmol. 2020. 10.1136/bjophthalmol-2020-317447. [DOI] [PubMed]
- 10.Dimitrov PN, Robman LD, Varsamidis M, Aung KZ, Makeyeva GA, Guymer RH, et al. Visual function tests as potential biomarkers in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2011;52:9457–69. doi: 10.1167/iovs.10-7043. [DOI] [PubMed] [Google Scholar]
- 11.Tan RS, Guymer RH, Aung KZ, Caruso E, Luu CD. Longitudinal assessment of rod function in intermediate age-related macular degeneration with and without reticular pseudodrusen. Investig Ophthalmol Vis Sci. 2019;60:1511–8. doi: 10.1167/iovs.18-26385. [DOI] [PubMed] [Google Scholar]
- 12.Finger RP, Fenwick E, Hirneiss CW, Hsueh A, Guymer RH, Lamoureux EL, et al. Visual impairment as a function of visual acuity in both eyes and its impact on patient reported preferences. PLoS ONE. 2013;8:e81042. doi: 10.1371/journal.pone.0081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen CT, Fraser RG, Tan R, Caruso E, Lek JJ, Guymer RH, et al. Longitudinal changes in retinotopic rod function in intermediate age-related macular degeneration. Investig Ophthalmol Vis Sci. 2018;59:Amd19–24. doi: 10.1167/iovs.17-23084. [DOI] [PubMed] [Google Scholar]
- 14.Rogala J, Zangerl B, Assaad N, Fletcher EL, Kalloniatis M, Nivison-Smith L. In vivo quantification of retinal changes associated with drusen in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2015;56:1689–700. doi: 10.1167/iovs.14-16221. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz-Valckenberg S, Steinberg JS, Fleckenstein M, Visvalingam S, Brinkmann CK, Holz FG. Combined confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography imaging of reticular drusen associated with age-related macular degeneration. Ophthalmology. 2010;117:1169–76. doi: 10.1016/j.ophtha.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 16.Tan R, Guymer RH, Luu CD. Subretinal drusenoid deposits and the loss of rod function in intermediate age-related macular degeneration. Investig Ophthalmol Vis Sci. 2018;59:4154–61. doi: 10.1167/iovs.18-23970. [DOI] [PubMed] [Google Scholar]
- 17.Flynn OJ, Cukras CA, Jeffrey BG. Characterization of rod function phenotypes across a range of age-related macular degeneration severities and subretinal drusenoid deposits. Investig Ophthalmol Vis Sci. 2018;59:2411–21. doi: 10.1167/iovs.17-22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saßmannshausen M, Zhou J, Pfau M, Thiele S, Steinberg J, Fleckenstein M. Longitudinal analysis of retinal thickness and retinal function in eyes with large drusen secondary to intermediate age-related macular degeneration. Ophthalmol Retina. 2020;S2468-6530:30304–3. doi: 10.1016/j.oret.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Dwivedi AK, Mallawaarachchi I, Alvarado LA. Analysis of small sample size studies using nonparametric bootstrap test with pooled resampling method. Stat Med. 2017;36:2187–205. doi: 10.1002/sim.7263. [DOI] [PubMed] [Google Scholar]
- 20.Grewal MK, Chandra S, Gurudas S, Bird A, Jeffery G, Sivaprasad S, et al. Exploratory study on visual acuity and patient-perceived visual function in patients with subretinal drusenoid deposits. J Clin Med. 2020;9:2832. doi: 10.3390/jcm9092832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunness JS, Rubin GS, Applegate CA, Bressler NM, Marsh MJ, Hawkins BS, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677–91. doi: 10.1016/S0161-6420(97)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu ST, Thompson AC, Stinnett SS, Luhmann UFO, Vajzovic L, Horne A, et al. Longitudinal study of visual function in dry age-related macular degeneration at 12 months. Ophthalmol Retina. 2019;3:637–48. doi: 10.1016/j.oret.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cocce KJ, Stinnett SS, Luhmann UFO, Vajzovic L, Horne A, Schuman SG, et al. Visual function metrics in early and intermediate dry age-related macular degeneration for use as clinical trial endpoints. Am J Ophthalmol. 2018;189:127–38. doi: 10.1016/j.ajo.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuinness MB, Fraser RG, Tan R, Luu CD, Guymer RH. Relationship between rod-mediated sensitivity, low-luminance visual acuity, and night vision questionnaire in age-related macular degeneration. Transl Vis Sci Technol. 2020;9:30. doi: 10.1167/tvst.9.6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudtson MD, Klein R, Klein BE, Lee KE, Meuer SM, Tomany SC. Location of lesions associated with age-related maculopathy over a 10-year period: the Beaver Dam Eye Study. Investig Ophthalmol Vis Sci. 2004;45:2135–42. doi: 10.1167/iovs.03-1085. [DOI] [PubMed] [Google Scholar]
- 26.Grewal DS, Chou J, Rollins SD, Fawzi AA. A pilot quantitative study of topographic correlation between reticular pseudodrusen and the choroidal vasculature using en face optical coherence tomography. PLoS ONE. 2014;9:e92841. doi: 10.1371/journal.pone.0092841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao L, Xu L, Wei WB, Chen CX, Du KF, Li XP, et al. Visual acuity and subfoveal choroidal thickness: the Beijing Eye Study. Am J Ophthalmol. 2014;158:702–9. doi: 10.1016/j.ajo.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Flamendorf J, Agrón E, Wong WT, Thompson D, Wiley HE, Doss EL, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122:2053–62. doi: 10.1016/j.ophtha.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson AC, Luhmann UFO, Stinnett SS, Vajzovic L, Horne A, Toth CA, et al. Association of low luminance questionnaire with objective functional measures in early and intermediate age-related macular degeneration. Investig Ophthalmol Vis Sci. 2018;59:289–97. doi: 10.1167/iovs.17-22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yazdanie M, Alvarez J, Agrón E, Wong WT, Wiley HE, Ferris FL, 3rd, et al. Decreased visual function scores on a low luminance questionnaire is associated with impaired dark adaptation. Ophthalmology. 2017;124:1332–9. doi: 10.1016/j.ophtha.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sassmannshausen M, Pfau M, Thiele S, Fimmers R, Steinberg JS, Fleckenstein M, et al. Longitudinal analysis of structural and functional changes in presence of reticular pseudodrusen associated with age-related macular degeneration. Investig Ophthalmol Vis Sci. 2020;61:19. doi: 10.1167/iovs.61.10.19. [DOI] [PMC free article] [PubMed] [Google Scholar]