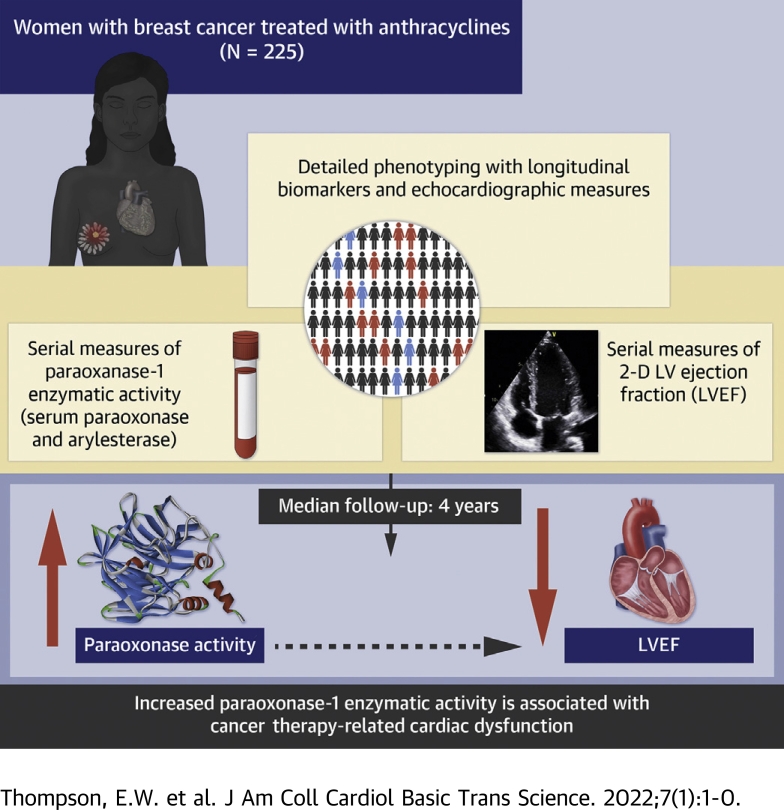
Visual Abstract
Key Words: cardiac dysfunction, cardiotoxicity, doxorubicin, heart failure, paraoxonase-1
Abbreviations and Acronyms: Aryl, arylesterase; BMI, body mass index; CTRCD, cancer therapy–related cardiac dysfunction; CVD, cardiovascular disease; HDL, high-density lipoprotein; HER2, human epidermal growth factor receptor 2; LVEF, left ventricular ejection fraction; LDL, low-density lipoprotein; PON-1, paraoxonase-1; Pon, paraoxonase
Highlights
-
•
PON-1 is an HDL-associated cardioprotective enzyme that prevents oxidized-LDL formation and has not previously been studied in cardio-oncology.
-
•
To determine the associations between PON-1 and the development of CTRCD, the Pon and Aryl serum enzymatic activity levels of PON-1 were quantified in a cohort of 225 patients with breast cancer receiving doxorubicin with or without trastuzumab.
-
•
After doxorubicin completion, the activity levels of both Pon and Aryl were significantly decreased.
-
•
Early increases in the Pon enzymatic activity of PON-1 were associated with increased risk of CTRCD.
-
•
With further study, PON-1 activity may provide insight into mechanistic risk prediction of CTRCD with doxorubicin chemotherapy.
Summary
The objective of this study was to determine associations of paraoxonase-1 (PON-1) with development of cancer therapy–related cardiac dysfunction (CTRCD). PON-1 is a cardioprotective enzyme associated with high-density lipoprotein that prevents oxidized low-density lipoprotein formation. Given the role of oxidative stress in doxorubicin-induced cardiotoxicity, PON-1 activity may have relevance for the prediction of CTRCD. In 225 patients with breast cancer receiving doxorubicin with or without trastuzumab, we quantified PON-1 activity through its paraoxonase (Pon) and arylesterase (Aryl) enzymatic activity at baseline, during, and after doxorubicin completion. Echocardiograms were performed at baseline, during therapy, and annually. CTRCD was defined as a decrease in left ventricular ejection fraction by ≥10% from baseline to <50%. Associations between baseline biomarkers and clinical variables were determined using multivariable linear regression. Associations between changes in biomarker activity and time to CTRCD were evaluated using Cox regression. Pon was directly associated with Black race and inversely associated with Stage 2 cancer. Aryl was inversely associated with body mass index. After doxorubicin completion, activity levels of Pon and Aryl were significantly decreased (median ratio compared with baseline for Pon: 0.95 [Q1-Q3: 0.81-1.07, P < 0.001]; for Aryl: 0.97 [Q1-Q3: 0.85-1.08, P = 0.010]). A total of 184 patients had an available quantitated echocardiogram at baseline and at least 1 follow-up visit. Increases from baseline in Pon at doxorubicin completion were independently associated with increased CTRCD risk (per 10% increase: hazard ratio [HR]: 1.21; 95% confidence interval [CI]: 1.05-1.39; P = 0.007). Associations between increases in Aryl and CTRCD tended in the same direction but were of borderline statistical significance (HR: 1.17; 95% CI: 0.99-1.38; P = 0.071). In patients with breast cancer treated with doxorubicin with or without trastuzumab, increases in the Pon enzymatic activity level of PON-1 were associated with increased CTRCD risk. PON-1 activity may be relevant to mechanistic risk prediction of cardiotoxicity with anthracyclines.
In the United States, approximately 1 in 3 people will be diagnosed with cancer in their lifetime, and in women, nearly 1 in 8 will develop breast cancer (1). Fortunately, breast cancer has become a more curable disease, commonly treated with surgery, radiation therapy, and systemic cancer therapy. The chemotherapeutic doxorubicin is an anthracycline that interferes with topoisomerase II activity to prevent DNA replication. Approximately 15% to 30% of breast cancers are also positive for human epidermal growth receptor 2 (HER2) upregulation and are often treated with HER2-targeted therapies such as the monoclonal antibody trastuzumab (2, 3, 4, 5).
Although doxorubicin is an effective anticancer agent, the associated dose-dependent risk of cardiotoxicity leading to cardiomyopathy and heart failure remains a significant concern. Cardiotoxicity, defined by declines in left ventricular ejection fraction (LVEF), may occur in up to 26% of patients exposed to doxorubicin, with the risk being amplified with sequential doxorubicin and trastuzumab (6). In the short term, the development of cardiotoxicity may result in treatment delays and even discontinuation, and in the long term, there is a risk of cardiovascular morbidity and mortality (7).
A fundamental mechanism of doxorubicin-induced cardiotoxicity is increased oxidative stress. DNA damage leads to the generation of superoxide radicals, associated with increases in intracellular iron and calcium. This stress not only causes direct cellular damage through mitochondrial dysfunction, decreased ATP production, and apoptosis, it also disrupts paracrine signaling between the myocardium and the endothelium through nitric oxide uncoupling (8). In addition, oxidative stress may lead to phospholipid membrane damage and subsequent lipid peroxidation (9).
Paraoxonase-1 (PON-1) is an antioxidant enzyme that associates with high-density lipoprotein (HDL) and helps to prevent the formation of oxidized low-density lipoprotein (LDL), a contributor to the development of atherosclerotic plaque (10). The enzymatic effects of PON-1 can be measured by its activity, specifically its ability to hydrolyze paraoxon (paraoxonase [Pon]) and phenylacetate (arylesterase [Aryl]) (11). PON-1 has been studied in both cancer and cardiovascular disease (CVD); in general, PON-1 activity, quantified by PON-1 concentration and Pon and Aryl enzymatic activity, appears to be lower in patients with cancer (12). In CVD, PON-1 has been studied in association with atherosclerotic disease and heart failure. Multiple studies have shown that perturbations in PON-1 enzymatic activity, measured by Aryl activity, are associated with adverse outcomes in patients with heart failure (13,14). Altogether, these findings indicate that PON-1 has a complex but incompletely defined role in both cancer and CVD.
The relevance of PON-1 activity in cardio-oncology, however, remains unknown. We hypothesized that early changes in PON-1 activity with anthracycline chemotherapy may be indicative of cancer therapy–related cardiac dysfunction (CTRCD). Thus, we first sought to determine the changes in PON-1 activity with anthracyclines, then to understand the associations between early changes in PON-1 activity and the development of CTRCD in patients with breast cancer treated with doxorubicin with or without trastuzumab. To analyze PON-1 activity in this subcohort, we quantified individual enzymatic activity levels of Pon and Aryl.
Methods
Study population
The study population is a subcohort of the CCT (Cardiotoxicity of Cancer Therapy) study, a longitudinal, prospective cohort study of women with breast cancer receiving anthracyclines and/or trastuzumab therapy recruited from the Abramson Cancer Center at the University of Pennsylvania (Philadelphia, Pennsylvania) (NCT01173341). Patients with 1 of 2 treatment regimens were included: 1) doxorubicin (240 mg/m2) and cyclophosphamide for a total of 4 cycles every 2 weeks, followed by paclitaxel for 12 weeks; or 2) doxorubicin (240 mg/m2) and cyclophosphamide for a total of 4 cycles every 2 weeks, followed by paclitaxel and trastuzumab for 12 weeks, and trastuzumab for a total duration of 1 year. The University of Pennsylvania Institutional Review Board approved the study, and all patients provided written informed consent.
Study procedures
Detailed clinical data were collected using standardized questionnaires before the initiation of doxorubicin (ie, baseline) and during prespecified follow-up visits. All data were obtained through patient interview and medical record review. Blood samples were drawn at baseline (before cancer therapy initiation), during doxorubicin therapy (∼1 month after cancer therapy initiation), and at the completion of doxorubicin (∼2 months after cancer therapy initiation). Transthoracic echocardiograms were performed by a dedicated sonographer team at prespecified standardized intervals using Vivid 7, E9, or E95 machines (GE Healthcare), and images were digitally archived for detailed analyses. In patients treated with doxorubicin without trastuzumab, echocardiograms were performed at baseline, at the completion of paclitaxel (∼4 months after cancer therapy initiation), and then annually. In patients treated with sequential doxorubicin and trastuzumab therapy, echocardiograms were performed at baseline, at the completion of doxorubicin (∼2 months after cancer therapy initiation), every 3 months during trastuzumab therapy as per clinical indications, and then annually (Supplemental Figure 1).
Biomarker measurements
Serum PON-1 activity was quantitated based on its Pon and Aryl enzymatic activities (not concentrations). Serum Pon activity was measured by spectrophotometry in an open channel on a Roche Cobas ce6000 platform (Roche Diagnostics). The rate of generation of para-nitrophenol was determined at 405 nm in 40-fold diluted serum (final) in reaction mixtures composed of 1.5 mM paraoxon (Sigma-Aldrich), 10 mM Tris hydrocholoride, pH 8, 1M sodium chloride, and 2 mM calcium chloride at 24°C. An extinction coefficient (at 405 nm) of 17,000 M−1·cm−1 was used for calculating units of Pon activity, which is expressed as nanomoles of para-nitrophenol produced per minute per milliliter of serum. Serum Aryl activity was measured in a 96-well plate format (Spectramax 384 Plus; Molecular Devices). Initial hydrolysis rates were determined at 270 nm in 50-fold diluted serum (final) in reaction mixtures composed of 3.4 mM phenylacetate (Sigma-Aldrich), 9 mM Tris hydrocholoride, pH 8, and 0.9 mM calcium chloride at 24°C. An extinction coefficient (at 270 nm) of 1310 M−1·cm−1 was used for calculating units of Aryl activity, which are expressed as micromoles of phenyl acetate hydrolyzed per minute per milliliter of serum.
Quantitative echocardiography and outcome definitions
Quantitative echocardiography was performed in a blinded fashion at the University of Pennsylvania Center for Quantitative Echocardiography (Philadelphia, Pennsylvania) using the TomTec Imaging Systems platform (Unterschleissheim, Germany). Left ventricular end-diastolic and end-systolic volumes were calculated using the Simpson’s method of discs, and these were used to derive LVEF, with an intraobserver coefficient of variation of 4.9%. The primary outcome measure was CTRCD, defined as an absolute reduction in LVEF by ≥10% from baseline to a value <50% (15).
Statistical analysis
Baseline characteristics are presented using frequency (percentage) for categorical variables, and mean ± SD, or median with 25th and 75th percentiles (Q1-Q3) for normally and non-normally distributed variables, respectively. To examine the determinants of PON-1 enzymatic activity, the associations between clinical variables and baseline biomarker levels were evaluated. Univariable, followed by multivariable linear regression models, were developed individually for Pon and Aryl, with the biomarker level as the dependent variable and age, race, disease stage, hypertension, hyperlipidemia, body mass index (BMI), systolic blood pressure, tobacco use, and cardiac medications (statins, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and beta blockers) as the independent variables. In all models, biomarkers were transformed by dividing the baseline value by the median baseline value and then taking the natural logarithm of this ratio. The exponentiated coefficients from the regression models can thus be interpreted as the estimated multiple of the median change according to the relevant clinical variable.
Associations between baseline clinical variables and changes in Pon and Aryl activity levels were modeled using repeated measures linear regression estimated via generalized estimating equations. The exponentiated coefficients can be interpreted as the estimated multiple of change in biomarker activity level relative to independent variable under consideration.
To characterize the early changes in Pon and Aryl, the ratios of enzymatic activity levels at 1 month after cancer therapy initiation (mid-point through doxorubicin) and 2 months after cancer therapy initiation (completion of doxorubicin) relative to baseline were calculated. Differences in enzyme activity level relative to baseline were assessed using the Wilcoxon signed-rank test.
Next, the associations between baseline levels of Pon and Aryl and time to CTRCD were evaluated using Cox proportional hazards models. The associations between early changes in Pon and Aryl and the time to CTRCD were similarly examined. Two sets of models were developed for each of the biomarkers; one including change from baseline to the 1-month visit and another including change from baseline to the 2-month visit. Biomarker levels were transformed in these models such that the resulting hazard ratios (HRs) with 95% confidence intervals (CIs) can be interpreted as the expected change in the hazard of CTRCD for each 10% increase in activity level. All models were adjusted for baseline LVEF, cancer therapy regimen, age, race, hypertension, smoking, diabetes, disease stage, BMI, and statin use. These variables were chosen a priori based on clinical judgment, as they were hypothesized to be potentially relevant as confounders. We further evaluated CTRCD-free survival rates according to the tertiles of biomarker ratio at the 2-month visit relative to baseline. Here, adjusted survival curves were generated for Pon and Aryl based on multivariable Cox proportional hazards models that included the aforementioned covariates and categorical biomarker variables.
Two-sided alpha levels <0.05 were considered statistically significant. Analyses were performed on complete cases and no imputations were performed for missing data. All analyses were performed using R 3.4.0 (R Foundation for Statistical Computing).
Results
Study population
Baseline characteristics of the study population are summarized in Table 1. The study population consisted of 225 women with a median age of 49, of whom 29.8% were Black. Most patients (60%) had stage 2 breast cancer, and 66.5% of patients received radiation therapy. Most (82.2%) of the patients received doxorubicin, and the remaining 17.8% received doxorubicin followed by trastuzumab. The median baseline LVEF was 54% (Q1-Q3: 51%-58%). Cardiovascular risk factors included diabetes mellitus (9.4%), hypertension (26.8%), and hyperlipidemia (24.2%). The numbers of available biomarker measurements at each timepoint according to cancer therapy group are provided in Supplemental Table 1. The median duration of study follow-up was 4.0 (Q1-Q3: 2.5–6.9) years. The median Pon activity level before anthracycline initiation was 1,006 (Q1-Q3: 449–1,391) nmol/min/mL; the median Aryl activity level was 99 (Q1-Q3: 79–120) μmol/min/L.
Table 1.
Baseline Characteristics of the Study Population (N = 225a)
| Age (y) | 49 [41-57] |
| Race | |
| Black | 67 (29.8) |
| White | 145 (64.4) |
| Other | 13 (5.8) |
| Breast cancer side | |
| Left | 113 (50.4) |
| Right | 98 (43.8) |
| Bilateral | 13 (5.8) |
| Breast cancer stage | |
| Stage 1 | 34 (15.1) |
| Stage 2 | 135 (60) |
| Stage 3 | 54 (24) |
| Stage 4 | 2 (0.9) |
| Radiation therapy | |
| None | 75 (33.5) |
| Left-sided | 76 (33.9) |
| Right-sided | 65 (29.0) |
| Bilateral | 8 (3.6) |
| Cancer therapy regimen | |
| Doxorubicin | 185 (82.2) |
| Doxorubicin+Trastuzumab | 40 (17.8) |
| Left ventricular ejection fraction (%) | 54 [51-58] |
| Body mass index (kg/m2) | 27 [23-32] |
| Systolic blood pressure (mm Hg) | 124 [115-134] |
| Diastolic blood pressure (mm Hg) | 76 [69-82] |
| Current or past smoking | 93 (41.5) |
| Diabetes mellitus | 21 (9.4) |
| Hypertension | 60 (26.8) |
| Hyperlipidemia | 54 (24.2) |
| Statin use | 28 (12.4) |
| ACEI/ARB use | 32 (14.2) |
| Beta blocker use | 20 (8.9) |
| ACEI/ARB or beta blocker use | 45 (20) |
| Pon (nmol/min/mL) | 1006 [449-1391] |
| Aryl (μmol/min/L) | 99 [79-120] |
Values are median [Q1, Q3] or n (%).
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; Aryl = arylesterase; Pon = paraoxonase.
The analytic patient population includes those with available Pon or Aryl measurements at baseline.
Clinical determinants of baseline and changes in PON and ARYL activity
To assess whether PON-1 baseline activity was associated with baseline clinical characteristics, multivariable linear regression analysis was performed between clinical variables and Pon (Table 2) and Aryl (Table 3) activity levels. Black race was independently associated with a 1.77-fold (95% CI: 1.41-2.22, P < 0.001) higher level of baseline Pon activity. Stage 2 disease was associated with a 0.76-fold (95% CI: 0.58-0.98, P = 0.036) lower baseline Pon activity level as compared with stage 1. We also observed a significant association between BMI and Aryl activity level. Baseline Aryl activity level was 0.96-fold (95% CI: 0.92-0.99, P = 0.028) lower with each 5 kg/m2 increase in BMI.
Table 2.
Associations Between Baseline Clinical Variables and Baseline Paraoxonase Activity
| Effect Sizea (95% CI) | P Value | |
|---|---|---|
| Age, per 10 y | 0.95 (0.87-1.05) | 0.314 |
| Black race | 1.77 (1.41-2.22) | <0.001 |
| Disease stage | ||
| Stage 1 | Ref | Ref |
| Stage 2 | 0.76 (0.58-0.98) | 0.036 |
| Stage 3 or 4 | 0.87 (0.65-1.18) | 0.376 |
| Body mass index, per 5 kg/m2 | 0.97 (0.89-1.04) | 0.374 |
| Systolic blood pressure, per 10 mm Hg | 1.05 (0.89-1.12) | 0.132 |
| Current or past smoking | 0.95 (0.79-1.14) | 0.553 |
| Diabetes mellitus | 0.99 (0.69-1.41) | 0.939 |
| Hypertension | 0.81 (0.60-1.09) | 0.158 |
| Hyperlipidemia | 1.16 (0.89-1.51) | 0.284 |
| Statin use | 0.88 (0.61-1.29) | 0.516 |
| ACEI/ARB or beta blocker use | 1.15 (0.86-1.55) | 0.346 |
The log-ratio of Pon at baseline relative to the cohort median was the dependent variable in this multivariable linear regression model.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; CI = confidence interval; Pon = paraoxonase; Ref = reference.
Exponentiated coefficients are presented and should be interpreted as the expected multiple of the median change in Pon levels per unit change in the independent variable under consideration.
Table 3.
Associations Between Baseline Clinical Variables and Baseline Arylesterase Activity
| Effect Sizea (95% CI) | P Value | |
|---|---|---|
| Age, per 10 y | 0.99 (0.95-1.04) | 0.749 |
| Black race | 1.05 (0.94-1.18) | 0.366 |
| Disease stage | ||
| Stage 1 | Ref | Ref |
| Stage 2 | 1.04 (0.92-1.19) | 0.512 |
| Stage 3 or 4 | 0.99 (0.86-1.15) | 0.950 |
| Body mass index, per 5 kg/m2 | 0.96 (0.92 – 0.99) | 0.028 |
| Systolic blood pressure, per 10 mm Hg | 1.02 (0.99-1.06) | 0.126 |
| Current or past smoking | 0.91 (0.83-1.00) | 0.052 |
| Diabetes mellitus | 0.92 (0.75-1.17) | 0.325 |
| Hypertension | 0.96 (0.83-1.11) | 0.592 |
| Hyperlipidemia | 1.06 (0.93-1.21) | 0.359 |
| Statin use | 0.94 (0.78-1.13) | 0.525 |
| ACEI/ARB or beta blocker use | 1.08 (0.93-1.25) | 0.301 |
The log-ratio of Aryl at baseline relative to the cohort median was the dependent variable in this multivariable linear regression model.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker, Aryl = arylesterase; CI = confidence interval; Ref = reference.
Exponentiated coefficients are presented and should be interpreted as the expected multiple of the median change in Aryl levels per unit change in the independent variable under consideration.
Pon and Aryl activity decreased over time with doxorubicin treatment (Table 4). One month after doxorubicin initiation, Pon ratio relative to baseline was significantly decreased. The median ratio was 0.97 (Q1-Q3: 0.88-1.09, P = 0.037) compared with baseline. Aryl activity at 1 month was not significantly different from baseline. However, at 2 months, the activity levels of both Pon and Aryl were significantly decreased. The median ratio compared with baseline was 0.95 for Pon (Q1-Q3: 0.81-1.07, P < 0.001) and 0.97 for Aryl (Q1-Q3: 0.85-1.08, P = 0.010).
Table 4.
Changes in Pon and Aryl Activity Levels at 1 and 2 Months
| Biomarker Activity Level | Ratio of Activity Level at 1 Month Relative to Baseline |
Ratio of Activity Level at 2 Months Relative to Baseline |
||
|---|---|---|---|---|
| Median (Q1-Q3) | P Value | Median (Q1-Q3) | P Value | |
| Pon | 0.97 (0.88-1.09) | 0.037 | 0.95 (0.81-1.07) | <0.001 |
| Aryl | 0.97 (0.87-1.10) | 0.160 | 0.97 (0.85-1.08) | 0.010 |
P value indicates the significance of the Wilcoxon signed rank test comparing the distribution of the biomarkers at baseline and at 1 or 2 months after doxorubicin initiation.
Aryl = arylesterase; Pon = paraoxonase.
When we evaluated changes in Pon (Supplemental Table 2A) and Aryl (Supplemental Table 2B) activity over time and their associations with baseline clinical variables, only BMI was associated with Pon. Each 5 kg/m2 increase in BMI was associated with a 1.03-fold (95% CI: 1.01-1.05, P = 0.004) increase in Pon activity level. In addition, current or past smoking was associated with a 1.04-fold (95% CI: 1.01-1.08, P = 0.009) increase in Aryl activity level.
Associations between CTRCD and baseline and changes in PON and ARYL activity over time
A total of 184 patients had available and quantifiable echocardiograms at baseline and during at least 1 follow-up visit. The number of patients with available LVEF at different follow-up study timepoints is provided in Supplemental Tables 3A and 3B. In this subcohort, CTRCD developed in 22 (12.0%) and 12 (33.3%) patients in the doxorubicin and sequential doxorubicin and trastuzumab groups, respectively. The median time to the development of CTRCD was (Q1-Q3: 4-27) 20 months from cancer therapy initiation in the doxorubicin group, and (Q1-Q3: 6-13) 9 months in patients treated with sequential doxorubicin and trastuzumab.
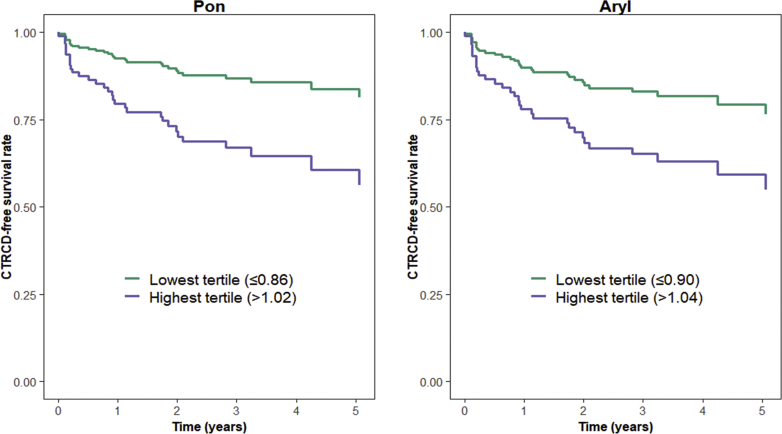
Baseline activity levels were not associated with development of CTRCD (Table 5), nor were changes from baseline to the mid-doxorubicin timepoint (1 month). However, in multivariable models, changes in Pon from baseline to doxorubicin completion (2 months post cancer therapy initiation), were significantly associated with CTRCD development. Here, each 10% increase in Pon level from baseline to doxorubicin completion was associated with an increased risk of CTRCD (HR: 1.21; 95% CI: 1.05-1.39; P = 0.007) (Table 5). Changes in Aryl from baseline to doxorubicin completion also tended to be associated with increased CTRCD risk, although this was of borderline statistical significance (HR: 1.17; 95% CI: 0.99-1.38; P = 0.071). Moreover, patients in the highest tertile of Pon activity ratio at 2 months relative to baseline (≥1.02) had greater CTRCD risk (HR: 3.08; 95% CI: 1.14-8.32; P = 0.027) compared with the lowest tertile. (Figure 1). With Aryl activity, again, the association was of borderline significance (HR: 2.47; 95% CI: 0.98-6.23; P = 0.056 comparing the highest to lowest tertile).
Table 5.
Associations Between Baseline and Changes in Pon and Aryl Activity Levels and Time to CTRCD
| Biomarker Activity Level | Baseline |
Change From Baseline at 1 Month |
Change From Baseline at 2 Months |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P value | HR (95% CI) | P Value | |
| Pon | 0.97 (0.93-1.03) | 0.337 | 1.08 (0.92-1.27) | 0.352 | 1.21 (1.05-1.39) | 0.007 |
| Aryl | 0.95 (0.87-1.05) | 0.313 | 1.17 (0.98-1.39) | 0.086 | 1.17 (0.99-1.38) | 0.071 |
HRs should be interpreted per 10% increase in biomarker value from baseline to 1 or 2 months after doxorubicin initiation; associations were modeled using Cox proportional hazards models; associations were adjusted for baseline left ventricular ejection fraction, cancer therapy regimen, age, race, hypertension, smoking, body mass index, disease stage, diabetes, and statin use at baseline.
Aryl = arylesterase; CI = confidence interval; CTRCD = cancer therapy–related cardiac dysfunction; HR = hazard ratio; Pon = paraoxonase.
Figure 1.
Risk of CTRCD According to Pon and Aryl Activity Ratios at Doxorubicin Completion
CTRCD-free survival curves comparing highest versus lowest tertiles of Pon and Aryl ratios at 2 months relative to baseline; models adjusted for baseline LVEF, cancer therapy regimen, age, race, hypertension, smoking, BMI, disease stage, diabetes, and statin use at baseline. Aryl = arylesterase; BMI = body mass index; CTRCD = cancer therapy–related cardiac dysfunction; LVEF = left ventricular ejection fraction; Pon = paraoxonase.
In exploratory subgroup analyses of biomarker/CTRCD associations according to race, with limited confounder adjustment (age, cancer therapy regimen, hypertension) given sample size considerations, there were very modest differences in the effect sizes between Black and White/Other participants with HR of 1.24 (95% CI: 0.89-1.74) versus 1.15 (95% CI: 0.99-1.35) for Pon, although the confidence intervals were largely overlapping (Supplemental Table 4). Likewise, no notable differences were observed in effect sizes between participants based on age (Supplemental Table 5).
Discussion
Our study sought to define the potential relevance of Pon and Aryl activities of the antioxidant enzyme PON-1 as novel biomarkers for the development of CTRCD in patients with breast cancer treated with doxorubicin. The main findings of our study are as follows: 1) baseline Pon is associated with Black race and breast cancer stage, and baseline Aryl is associated with BMI; 2) Pon and Aryl decreased over time with doxorubicin; 3) increases in Pon are associated with a higher risk of CTRCD; and 4) increases in Aryl and associations with a higher risk of CTRCD are of borderline statistical significance.
Our current understanding is that doxorubicin can both damage DNA and generate free radicals, resulting in increased oxidative and nitrosative stress, increased intracellular iron and calcium, and damage to both cardiomyocytes and endothelial cells (8). Although various studies have shown decreased antioxidant activity to be associated with cancer and CVD separately, there are limited human translational data on the antioxidant response to chemotherapy.
We determined associations between baseline Pon and Aryl enzymatic activities of PON-1 and Black race, cancer stage, and BMI. Prior studies have also shown that Black patients have higher levels of Pon activity compared with White patients (16,17), which may be related to specific genetic polymorphisms that are more common in Black patients (18). We also observed modest differences in the association between Pon activity and CTRCD in Black patients as compared with White patients, although given the exploratory nature of this finding it should be viewed as hypothesis generating.
Cancer is generally associated with lower PON-1 activity (12), and our results showed that Pon activity was inversely associated with cancer stage, although Aryl was not. It is important to note that Pon and Aryl each reflect distinct enzymatic activities of PON-1 in response to different substrates (paraoxon and phenylacetate, respectively); thus, activity of each enzyme may be associated with a unique pathophysiologic state. The inverse relationship between the Aryl activity of PON-1 and BMI is hypothesized to be a reflection of elevated oxidative stress in patients with obesity (19). Altogether, our findings indicate that certain patient-specific modifiable and nonmodifiable clinical factors are associated with baseline PON-1 activity and may be important in determining risk of CTRCD. This is an area in need of further study.
Longitudinal measurements of PON-1 activity have not previously been reported with chemotherapy, nor have associations with CTRCD. It is widely accepted that there is a global worsening in oxidative and nitrosative stress with doxorubicin chemotherapy. We observed decreases in Pon and Aryl activity over time, consistent with the overall hypothesis that PON-1 activity is affected by doxorubicin exposure. Our results also suggest that increases in Pon activity over time are associated with an increased risk of CTRCD. Although this initially may be counterintuitive given some of the existent albeit limited data in chronic heart failure (13,14), most of the other published data have focused on single levels at one timepoint, and not the change over time. Moreover, others have reported results with a similar directionality. In a case-cohort study of 211 women with coronary heart disease and 71 women with acute myocardial infarction, higher Pon activity was associated with an increased risk of myocardial infarction (20). Interestingly, similar to our cohort, these were all women, and all activity levels were assayed on nonfasting samples.
However, given the observational nature of our data, we are limited in our mechanistic understanding of the directionality of this change. We offer a number of potential explanations. It is possible that decreased activity may reflect increased utilization and consumption of enzyme binding sites due to increased toxin accumulation. In line with this hypothesis, a recent study of the Pon and Aryl activities of PON-1 during coronary artery bypass grafting surgery demonstrated that Pon and Aryl activities both decreased following aortic cross-clamping as compared with preoperative levels, likely representing an inflammatory reaction in response to surgery and inflicted myocardial damage (21). Alternatively, declines in PON-1 activity may represent decreased hepatic production of PON-1 due to the effects of doxorubicin on hepatic synthetic function (22). Moreover, the complexities of PON-1 activity cannot be elucidated via observation alone, although our work generates important hypotheses that motivate additional mechanistic studies and suggests potential clinical relevance of this pathway to anthracycline cardiotoxicity.
The complex interaction of a patient’s baseline antioxidant status, genetic and environmental contributions, high free radical production in patients with breast cancer (23), and the potential for chemotherapy and radiation to increase PON-1 activity, taken together with our results, suggest that PON-1 activity quantified through Pon enzymatic activity may act as an early indicator of doxorubicin-associated cardiac damage. There is basic evidence to support that HDL protects against cardiomyocyte apoptosis and myocardial atrophy with exposure to doxorubicin, with protection against various forms of cell death (24). HDL composition may also be relevant to these cardioprotective effects, including the abundance and function of ApoA1. In animal models, high PON-1 activity has been observed with an increase in the catabolic rate of ApoA1 (24). As such, it may be that increases in PON-1 activity are associated with an increase in the catabolic rate of ApoA1, and a shift in HDL subclass to less favorable, less protective distribution. Although this is very speculative, and we are still in the early stages of understanding the role of PON-1 in cardio-oncology, mechanistic translational research is needed to elucidate the precise actions of PON-1 in response to various physiological compounds and stressors. Additional clinical studies are also needed to externally validate our findings; these efforts are ongoing (R21HL141802). This work includes ascertaining the effect of drugs such as statins, which increase HDL levels, on PON-1 activity and their role in cardioprotection (PREVENT [Preventing Anthracycline Cardiovascular Toxicity With Statins]; NCT01988571).
Study limitations
Limitations to our study must be acknowledged. We did not quantify serum PON-1 concentrations, which would have served as a complement to the enzymatic activity we measured. In addition, we did not have access to HDL concentrations, which may also affect PON-1 activity. Methodologically, length of storage and number of freeze-thaw cycles may also affect PON-1 enzymatic activity, but these changes are not yet well-understood (25). However, all of our samples were consistently handled, and activity levels were measured on previously unthawed samples. The exact timing of echocardiograms, although standardized, differed slightly between the doxorubicin and doxorubicin + trastuzumab subgroups. We also did not collect genotype data, which would have provided more insight into the baseline risk of our cohort as well as associations with CTRCD. In addition, PON-1 data from a race- and age-matched cohort without cancer would have provided a helpful control dataset. Although our study is one of the larger in cardio-oncology with comprehensive biomarker and echocardiography data, we are still limited by sample size. Each of these limitations should be addressed in future studies.
Conclusions
We determined that in patients with breast cancer undergoing treatment with doxorubicin with or without trastuzumab, early increases in the Pon enzymatic activity of PON-1 were associated with increased risk of CTRCD. These results suggest a complex homeostatic interplay between oxidative stress and antioxidant status, which may result in cardiac dysfunction in patients treated with anthracyclines. Further clinical and basic research is warranted to advance our understanding of this relationship and determine the clinical utility of PON-1 enzymatic activities as biomarkers for CTRCD.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: PON-1 is an HDL-associated cardioprotective enzyme that prevents oxidized-LDL formation. In a longitudinal prospective cohort of 225 patients with breast cancer receiving doxorubicin with or without trastuzumab, Pon was directly associated with Black race and inversely associated with Stage 2 cancer. Aryl was inversely associated with BMI. After doxorubicin completion, activity levels of both Pon and Aryl were significantly decreased. Early increases in the Pon enzymatic activity of PON-1 were significantly associated with increased risk of CTRCD in patients with breast cancer treated with doxorubicin with or without trastuzumab.
TRANSLATIONAL OUTLOOK: Future studies of PON-1 activity in larger cohorts of patients with cancer treated with anthracyclines and potentially other cardiotoxic cancer therapies are needed to further clarify the role of PON-1 in cardio-oncology. Moreover, basic science research is needed to understand precise mechanisms of PON-1 in cardio-oncology, and specifically further our insight into race-specific differences. The interaction between PON-1 activity and HDL and statin therapy also deserves further study. With future detailed mechanistic and outcome data, PON-1 activity may be used to understand and mitigate CTRCD risk.
Funding Support and Author Disclosures
Dr Fradley has received a research grant from Medtronic and has served as a consultant for Abbott and AstraZeneca, all unrelated to the contents of this paper. Dr Tang is a consultant for Sequana Medical A.G., Owkin Inc, Relypsa Inc, PreCardia Inc, and CardiolRx; and has received honoraria from Springer Nature for authorship/editorship and American Board of Internal Medicine for exam writing committee participation, all unrelated to the contents of this paper. Dr Ky has served as a consultant for Cytokinetics; and has received honoraria from Roche, unrelated to the contents of this paper. Ms Thompson is supported by a National Institutes of Health Medical Scientist Training Program Predoctoral T32 grant. For this work, she received additional support from the American Heart Association Student Scholarship in Cardiovascular Disease, the Perelman School of Medicine Center for Clinical Epidemiology and Biostatistics Summer Fellowship, and the Okun Family Cardiovascular Scholarship. This work is supported by R01HL118018 and R21HL141802, to Dr Ky. Dr Tang is partially supported by grants from the National Institutes of Health (R01HL126827). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and a figure, please see the online version of this paper.
Appendix
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N. Advances in targeting HER2-positive breast cancer. Curr Opin Obstet Gynecol. 2018;30:55–59. doi: 10.1097/GCO.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed S., Sami A., Xiang J. HER2-directed therapy: current treatment options for HER2-positive breast cancer. Breast Cancer. 2015;22:101–116. doi: 10.1007/s12282-015-0587-x. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D.J., Godolphin W., Jones L.A., et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 5.Madden R., Kosari S., Peterson G.M., Bagheri N., Thomas J. Lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer: a systematic review. Int J Clin Pharmacol Ther. 2018;56:72–80. doi: 10.5414/CP203123. [DOI] [PubMed] [Google Scholar]
- 6.Narayan H.K., Finkelman B., French B., et al. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation. 2017;135:1397–1412. doi: 10.1161/CIRCULATIONAHA.116.023463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Qadir H., Austin P.C., Lee D.S., et al. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017;2:88–93. doi: 10.1001/jamacardio.2016.3841. [DOI] [PubMed] [Google Scholar]
- 8.Luu A.Z., Chowdhury B., Al-Omran M., Teoh H., Hess D.A., Verma S. Role of endothelium in doxorubicin-induced cardiomyopathy. J Am Coll Cardiol Basic Trans Science. 2018;3:861–870. doi: 10.1016/j.jacbts.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrelia S., Fiorentini D., Maraldi T., et al. Doxorubicin induces early lipid peroxidation associated with changes in glucose transport in cultured cardiomyocytes. Biochim Biophys Acta. 2002;1567:150–156. doi: 10.1016/s0005-2736(02)00612-0. [DOI] [PubMed] [Google Scholar]
- 10.Chistiakov D.A., Melnichenko A.A., Orekhov A.N., Bobryshev Y.V. Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie. 2017;132:19–27. doi: 10.1016/j.biochi.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Arenas M., Rodriguez E., Sahebkar A., et al. Paraoxonase-1 activity in patients with cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2018;127:6–14. doi: 10.1016/j.critrevonc.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Bacchetti T., Ferretti G., Sahebkar A. The role of paraoxonase in cancer. Semin Cancer Biol. 2019;56:72–86. doi: 10.1016/j.semcancer.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Tang W.H., Wu Y., Mann S., et al. Diminished antioxidant activity of high-density lipoprotein-associated proteins in systolic heart failure. Circ Heart Fail. 2011;4:59–64. doi: 10.1161/CIRCHEARTFAILURE.110.958348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammadah M., Kalogeropoulos A.P., Georgiopoulou V.V., et al. High-density lipoprotein-associated paraoxonase-1 activity for prediction of adverse outcomes in outpatients with chronic heart failure. Eur J Heart Fail. 2017;19:748–755. doi: 10.1002/ejhf.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardinale D., Colombo A., Bacchiani G., et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 16.Tripi L.M., Manzi S., Chen Q., et al. Relationship of serum paraoxonase 1 activity and paraoxonase 1 genotype to risk of systemic lupus erythematosus. Arthritis Rheum. 2006;54:1928–1939. doi: 10.1002/art.21889. [DOI] [PubMed] [Google Scholar]
- 17.Coombes R.H., Crow J.A., Dail M., et al. Relationship of human paraoxonase-1 serum activity and genotype with atherosclerosis in individuals from the Deep South. Pharmacogenet Genomics. 2011;21:867–875. doi: 10.1097/FPC.0b013e32834cebc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDaniel C.Y., Dail M.B., Wills R.W., Chambers H.W., Chambers J.E. Paraoxonase 1 polymorphisms within a Mississippi USA population as possible biomarkers of enzyme activities associated with disease susceptibility. Biochem Genet. 2014;52:509–523. doi: 10.1007/s10528-014-9663-8. [DOI] [PubMed] [Google Scholar]
- 19.Cervellati C., Bonaccorsi G., Trentini A., et al. Paraoxonase, arylesterase and lactonase activities of paraoxonase-1 (PON1) in obese and severely obese women. Scand J Clin Lab Invest. 2018;78:18–24. doi: 10.1080/00365513.2017.1405274. [DOI] [PubMed] [Google Scholar]
- 20.van Himbergen T.M., van der Schouw Y.T., Voorbij H.A., et al. Paraoxonase (PON1) and the risk for coronary heart disease and myocardial infarction in a general population of Dutch women. Atherosclerosis. 2008;199:408–414. doi: 10.1016/j.atherosclerosis.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Wysocka A., Cybulski M., Berbec H., et al. Dynamic changes of paraoxonase 1 activity towards paroxon and phenyl acetate during coronary artery surgery. BMC Cardiovasc Disord. 2017;17:92. doi: 10.1186/s12872-017-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damodar G., Smitha T., Gopinath S., Vijayakumar S., Rao Y. An evaluation of hepatotoxicity in breast cancer patients receiving injection Doxorubicin. Ann Med Health Sci Res. 2014;4:74–79. doi: 10.4103/2141-9248.126619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray G., Batra S., Shukla N.K., et al. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat. 2000;59:163–170. doi: 10.1023/a:1006357330486. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Olmos V., Carreon-Torres E., Luna-Luna M., et al. Increased HDL size and enhanced Apo A-I catabolic rates are associated with doxorubicin-induced proteinuria in New Zealand White rabbits. Lipids. 2016;51:311–320. doi: 10.1007/s11745-016-4120-6. [DOI] [PubMed] [Google Scholar]
- 25.Rossi G., Richardson A., Jamaludin H., Secombe C. Preanalytical variables affecting the measurement of serum paraoxonase-1 activity in horses. J Vet Diagn Invest. 2021;33:59–66. doi: 10.1177/1040638720974745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.