Highlights
-
•
Development of cardiovascular disease and inflammation are heavily intertwined, and inflammasome activation is thought play an important role in this interaction.
-
•
This review provides an overview of preclinical and clinical studies supporting inflammasomes as a therapeutic target in atherosclerosis and heart failure.
-
•
Future studies exploring direct inflammasome inhibition, either NLRP3 or the lesser-studied inflammasomes, are also discussed.
Key Words: atherosclerosis, cardiovascular disease, heart failure, IL-1, inflammasome, NLRP3
Abbreviations and Acronyms: ACS, acute coronary syndrome; AIM2, absent in melanoma 2; ASC, apoptosis associated speck-like protein; ATP, adenosine triphosphate; CAD, coronary artery disease; CRP, C-reactive protein; CVD, cardiovascular disease; DAMP, damage associated molecular pattern; GSDMD, gasdermin-D; GSDMD-NT, gasdermin-D N-terminal; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; IL, interleukin; LDL, low-density lipoprotein; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NF-κB, nuclear factor κB; NLR, NOD-like receptor; NLRP3, NOD-like receptor family pyrin domain containing 3; NOD, nucleotide-binding oligomerization domain; PRR, pattern recognition receptor; STEMI, ST-elevation myocardial infarction; TLR, toll-like receptor
Summary
The pathogenesis of cardiovascular disease (CVD) is complex and multifactorial, and inflammation plays a central role. Inflammasomes are multimeric protein complexes that are activated in a 2-step manner in response to infection or tissue damage. Upon activation the proinflammatory cytokines, interleukins-1β and -18 are released. In the last decade, the evidence that inflammasome activation plays an important role in CVD development became stronger. We discuss the role of different inflammasomes in the pathogenesis of CVD, focusing on atherosclerosis and heart failure. This review also provides an overview of existing experimental studies and clinical trials on inflammasome inhibition as a therapeutic target in these disorders.
Central Illustration
Persistent inflammation is an established and major part of the pathogenesis of cardiovascular disease (CVD). In fact, the bidirectional interaction between lipids and inflammation is regarded as a hallmark of atherosclerotic disorders. The activation of inflammatory pathways and interaction with pathways that regulate myocyte function, the vasculature, and extracellular matrix are involved in the pathogenesis of heart failure (HF).
Inflammasomes are intracellular multiprotein complexes that promote the maturation and release of tightly regulated, highly inflammatory cytokines, ie, interleukin (IL)-1β and IL-18. Of these, IL-1β is particularly potent: it acts on most cell types and has the potential to initiate several downstream inflammatory pathways through the induction of other cytokines in target cells, including tumor necrosis factor, IL-6, and IL-8 (1). The function of IL-18 depends on context. In the presence of IL-12, IL-18 promotes a type 1 immune response by inducing interferon gamma synthesis in T cells (2) and natural killer cells (3). However, in the absence of IL-12, IL-18 may promote type 2 immune responses (eg, IL-4, IL-13, and immunoglobulin E synthesis) (4,5). Inflammasome-dependent cytokines are powerful molecules that have a myriad of functions and are widely and rapidly induced in both sterile and nonsterile inflammation. Whereas acute activation of inflammasomes is critical for host defense against infections, they could also promote harmful effects if the response is overwhelming or prolonged.
During the last decades, several studies have convincingly shown that the activation of inflammasomes plays an important role in CVD, with the strongest evidence in atherosclerotic disorders. The CANTOS (Canakinumab Anti-inflammatory Thrombosis Outcome Study) was the first large study to show that blocking IL-1β reduced clinical endpoints in patients with atherosclerotic disease (6).
In this review article, we discuss how to target inflammasomes in the management of cardiovascular disease (CVD), not only in atherosclerosis but also in HF. Most research has focused on the nucleotide-binding oligomerization domain (NOD)-like receptor family pyrin domain containing 3 (NLRP3) inflammasome; however, we will also discuss the potential of targeted therapy against other NOD-like receptors (NLRs) and related inflammasomes in CVD.
Inflammasomes
Inflammasomes are large protein complexes that are activated in response to infection or tissue damage. Upon activation, they assemble in the cytosol and trigger cytokine release (ie, IL-1β and IL-18) and pyroptosis—a proinflammatory form of cell death (Figure 1). Humans have a repertoire of different inflammasomes that function in a similar way. However, they may be differently expressed throughout the body and, importantly, have distinct triggers for activation.
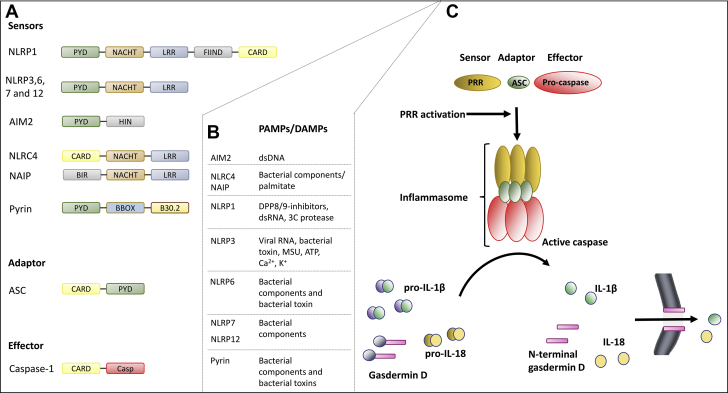
Figure 1.
Inflammasome Activation—Components and Cascade
(A) Pattern recognition receptors (PRRs). The PRRs consist of different domains: pyrin domains (PYD), NACHT, leucine-rich repeats (LRR), function-to-find domains (FIIND), caspase recruitment domains (CARD), a hematopoietic interferon-inducible nuclear (HIN) domain, and B-BOX and B30.2 domains. (B) Examples of pathogen-associated molecular patterns (PAMPs) (nonsterile) and damage-associated molecular patterns (DAMPs) (sterile). (C) Danger and/or damage are sensed by PRRs. Activated PRRs recruit the adapter protein apoptosis associated speck-like protein (ASC), which binds and activates effector caspase-1. The active inflammasome cleaves and activates pro-forms of interleukin (IL)-1β, IL-18, and gasdermin. (D) The active N-terminal gasdermin D oligomerizes in the cell membrane and forms a pore that allows secretion of the inflammatory cytokines IL-1β and IL-18, together with osmotic swelling and pyroptotic cell death, which also promote inflammation. ATP = adenosine triphosphate; BIR = baculovirus IAP-repeat; Casp = caspase; dsDNA = double-stranded DNA; dsRNA = double-stranded RNA; MSU = monosodium urate; NACHT = NAIP, CIITA, HET-E, and TP-1; NLRP = NOD-like receptor family pyrin domain.
Pattern recognition receptors detect danger
The innate immune system detects both microbes and sterile danger signals through pattern recognition receptors (PRRs). Microbial structures recognized by PRRs are named pathogen-associated molecular patterns, and endogenous danger molecules are termed damage-associated molecular patterns (DAMPs). DAMPs are typically products of tissue damage (eg, intracellular molecules released to the extracellular space) and oxidative stress. PRRs are classified into 5 major families based on protein domain homology: toll-like receptors (TLRs), C-type lectin receptors, NLRs, absent in melanoma 2 (AIM2)–like receptors, and retinoic acid inducible gene-I (RIG-I)–like receptors (7). Whereas TLRs and C-type lectin receptors are transmembrane PRRs that monitor the extracellular space, AIM2-like receptors, RIG-I–like receptors, and NLRs are cytoplasmic PRRs and survey the intracellular space. Some of the intracellular PRRs can assemble into inflammasomes, and targeted therapy against these structures will be the focus of this review.
Activated PRRs assemble into inflammasomes
Upon recognition of pathogen-associated molecular patterns or DAMPs, some PRRs can oligomerize and assemble with other proteins to construct an inflammasome (Figure 1). Pro-caspase-1 is recruited and bound to the protein complex through the adaptor protein apoptosis associated speck-like protein (ASC). Pro-caspase-1 is then activated by autolytic cleavage, and active caspase-1 cleaves and activates procytokines, pro-IL-1β, and pro-IL-18. Caspase-1 also cleaves gasdermin-D (GSDMD), generating an active N-terminal cleaving product (GSDMD-NT). GSDMD-NT translocates to the plasma membrane and oligomerizes to form a pore that allows secretion of IL-1β and IL-18, with subsequent osmotic swelling that may result in pyroptotic cell death (8, 9, 10). Pyroptosis is typically induced in monocytes/macrophages (11) but can also occur in other cell types such as endothelial cells (12) and cardiomyocytes (13).
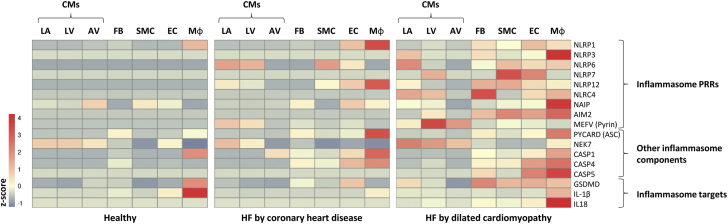
The expression levels of PRRs and inflammasome components differ with cell type and condition. As shown in Figure 2, messenger RNA expression of the inflammasome components is low in healthy hearts but may rise upon stimulation or disease, as exemplified here by HF caused by coronary heart disease or dilated cardiomyopathy.
Figure 2.
Cardiac Inflammasome mRNA Expression Varies According to Cell Type and Condition
A heatmap illustrating cardiac mRNA expression of inflammasome components in healthy hearts, HF caused by coronary heart disease, and HF caused by dilated cardiomyopathy. The Figure shows 3 CM populations: CMs originating from the LA appendage, CMs originating from LV, and a population of CMs clustering together originating from both the LA and LV (AV). The heatmap is generated based on single-cell RNA-sequencing data GSE121893, available at the Gene Expression Omnibus database (14). ASC = acute coronary syndrome; AV = atrial-ventricle; CASP = caspase; CM = cardiomyocyte; EC = endothelial cell; FB = fibroblasts; GSDMD = gasdermin-D; HF = heart failure; IL = interleukin; LA = left atrial; LV = left ventricle; Mφ = macrophage; MEFV = Mediterranean fever; mRNA = messenger RNA; NAIP = neuronal apoptosis inhibitory protein; NEK = NIMA-related kinase; NLRP = NOD-like receptor family pyrin domain; PRR = pattern recognition receptor; PYCARD = PYD And CARD domain; SMC = smooth muscle cell.
Activation of the NLRP3 inflammasome
The NLRP3 inflammasome is the most studied inflammasome. It can be activated by a wide range of chemically unrelated molecules reflecting cellular damage and metabolic stress, such as extracellular adenosine triphosphate (ATP), cholesterol crystals, angiotensin II, saturated fatty acids, and glucose. Like other inflammasomes, the NLRP3 inflammasome consists of multimers of ASC and caspase-1. The serine-threonine kinase NIMA-related kinase 7 (NEK7) interacts with NLRP3 and is essential for NLRP3-dependent caspase-1 activation.
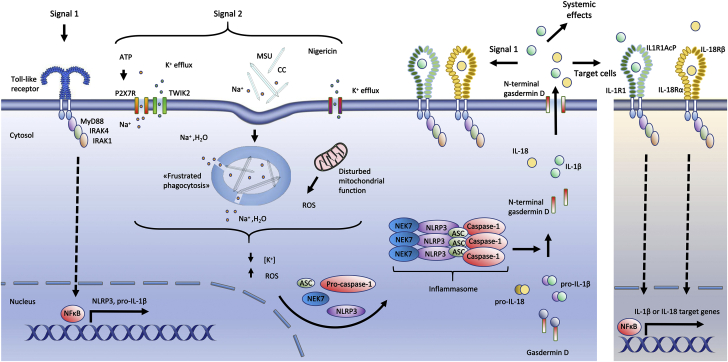
NLRP3 inflammasomes are potent inflammatory structures, and a 2-step activation process prevents excessive signaling or inappropriate activation (Figure 3). Signal 1 is a priming signal leading to the transcription of IL-1β and NLRP3 precursors. Pro-IL-18, however, is constitutively expressed in many cell types (eg, monocytes, macrophages, and epithelial cells). Priming signals are typically induced by activated PRRs, such as TLRs, which further activate the transcription factor nuclear factor κB (NF-κB). In cell experiments, lipopolysaccharide, a specific TLR4 agonist that is also relevant to CVD, is a typical signal 1. NLRP3 proteins undergo posttranslational modifications, including ubiquitination, phosphorylation, and small ubiquitin-related modifier (SUMO)ylation, to remain in an autosuppressed stable form until signal 2 is initiated. Signal 2 is an activation signal that leads to the assembly of the inflammasome, including caspase-1. The activation signals, such as extracellular ATP, urate crystals, or pore-forming toxins, do not interact directly with NLRP3. Instead, all NLRP3-activating molecules induce independent upstream events that reduce intracellular K+ concentration and increase levels of reactive oxygen species. Under these conditions, NEK7 will activate NLRP3, and the inflammasomes are formed. Caspase-1 is then activated, and IL-1β and IL-18 are released in a GSDMD-NT–dependent manner (15,16).
Figure 3.
Nlrp3 Inflammasome Activation
A priming signal (signal 1) indicative of the presence of microbes or tissue damage activates the transcription factor NF-κB, which induces the transcription of NLRP3 and pro-IL-1β. NLRP3 can then be activated by a wide range of molecules reflecting cellular stress (signal 2), resulting in assembly of the inflammasome, activation of caspase-1, and subsequent activation of IL-1β and IL-18. Caspase-1 also cleaves gasdermin D. The N-terminal gasdermin D peptides form a pore in the plasma membrane through which IL-1β and IL-18 are released. In more detail, extracellular ATP activates the ion channel P2X7R. This activation induces Ca2+ and Na+ influx and leads to efflux of K+ through TWIK2 (17). Monosodium urate and cholesterol crystals could activate NLRP3 via “frustrated phagocytosis,” where cells fail to eliminate engulfed crystals, resulting in lysosomal damage and leakage into the cytosol. Lysosomal Na+ and water then reduce the intracellular K+ concentration, which increase ROS production (18). In the extracellular space (right), IL-1β binds to IL-1 receptor 1 (IL-1R1), promoting heterodimerization with IL-1R accessory protein (IL-1RAcP). IL-18 binds to its receptor consisting of IL-18 receptor α (IL-18Rα) and IL-18 receptor β (IL-18Rβ). IL-1R/IL-18R may re-engage signal 1, stimulate target cells, and initiate systemic inflammatory functions such as neutrophil recruitment, production of acute-phase proteins, and fever. ATP = adenosine triphosphate; CC = cholesterol crystals; MSU = monosodium urate; NF-κB = nuclear factor κB; ROS = reactive oxygen species; other abbreviations as in Figure 2.
Inflammasome Activation in Atherosclerosis, MI, and Ischemic Stroke
The basis for using the NLRP3 inflammasome as a target for therapy
Inflammation and metabolic disturbances are hallmarks of atherosclerosis (19), and one of the inflammatory pathways frequently activated during atherogenesis is the NLRP3 inflammasome. Levels of IL-1β and IL-18 are elevated in coronary artery disease (CAD), and the levels are particularly high in patients with ACS (20,21). The up-regulation of these ILs also occurs at the cellular level in circulating leukocytes and in human atherosclerotic lesions. In carotid atherosclerosis, there is enhanced expression of the different components of the NLRP3 inflammasome (ie, NLRP3, ASC, caspase-1, IL-1β, and IL-18). The levels are particularly high in symptomatic lesions (22,23). Moreover, transcripts of NLRP3 and IL-1β have been found to be up-regulated in peripheral blood mononuclear cells in patients with CAD, with particularly high levels in patients with ACS (24). Furthermore, IL-1β and IL-18 give prognostic information in patients with CAD (25,26). In preclinical studies, IL-1β deficiency is shown to result in smaller aortic atherosclerotic lesions in mice lacking ApoE (27). Furthermore, administration of IL-18 is shown to increase lesion size (28), and endogenous inhibition of IL-18 in mice prevented early lesion development and promoted a more stable plaque phenotype (29). In 2010, Duewell et al (30) published a groundbreaking study showing that atherosclerotic lesions in ApoE-deficient mice on a high-fat diet contained cholesterol crystals that increased with disease progression. Notably, these crystals were colocalized with NLRP3 inflammasomes and promoted the release of IL-1β (30). Moreover, it showed that low-density lipoprotein (LDL) receptor–deficient mice that received bone marrow transplant with NLRP3-deficient, ASC-deficient, or IL-1α/β–deficient bone marrow had markedly decreased atherosclerosis (30).
Several studies have suggested a role for the NLRP3 inflammasome during acute ischemia, although results seem to depend on the experimental setup. In CD-1 mice with transient ligation of the left anterior descending coronary artery, silencing NLRP3 during reperfusion ameliorated the reperfusion damage after acute MI (31). Sandanger et al (32) showed in an ex vivo ischemia/reperfusion Langendorff model that NLRP3 deficiency induced reduced infarct size. However, in vivo ischemia reperfusion showed increased infarct size in NLRP3-deficient C57Bl6 mice (33). These contradictory results may reflect model-dependent effects of NLRP3 inhibition. Wild-type mice receiving transplants with NLRP3-/- bone marrow showed improved survival after permanent ligation, indicating that post-MI NLRP3 activation in residential cells seems to be beneficial (34). Taken together, these results could point at a beneficial effect of initial NLRP3 activation during ischemia that later should be inhibited to improve tissue healing.
Atherogenesis and MI: Trials and treatments targeting the NLRP3 inflammasome
The NLRP3 inflammasome is an interesting therapeutic target because of its effect on the development of atherosclerosis. Several clinical studies with drugs suppressing the inflammasome directly, or indirectly via IL-1 activity, are either completed or ongoing. In this section, we focus on large cardiovascular outcome studies. So far, to our knowledge, no results are available from clinical trials specifically evaluating the inhibition of NLRP3 inflammasome formation to reduce atherosclerosis and MI.
Colchicine
Colchicine was the first drug to show that patients with CAD could possibly benefit from additional inflammatory suppression, as reflected by reduced levels of CRP in the treatment arm (35). Colchicine is a widely available, safe, and inexpensive drug that has broad anti-inflammatory effects, and it is a potent inhibitor of tubule polymerization. Microtubules are responsible for the subcellular transport of ASC and NLRP3 within macrophages and are necessary for proper cytosolic localization and activation of the inflammasome components. Thus, molecules that affect microtubular function can potentially alter the assembly and function of the NLRP3 inflammasome. In the first large trial with cardiovascular endpoints, the LoDoCo (Low-Dose Colchicine) study, 532 patients with stable CAD were randomized to receive colchicine or placebo and followed up for a median of 3 years (Table 1) (36). Treatment with colchicine led to a significantly lower risk of cardiovascular events. These promising results were corroborated in the randomized LoDoCo2 trial, which comprised 5,522 patients (37). There was a 31% decrease in the primary composite endpoint of cardiovascular death, spontaneous MI, ischemic stroke, or ischemia-driven coronary revascularization in the colchicine arm. COLCOT (Colchicine Cardiovascular Outcomes Trial) showed that patients with MI within the last 30 days, who were randomized to colchicine on top of standard treatment, had a reduced incidence of adverse coronary and cerebral atherothrombotic events compared to placebo at the 1.9-year follow-up (38). Importantly, a substudy suggested that the greatest benefit was observed in patients who were randomized within 3 days after the index MI (39). Recently, the relatively small COVERT-MI (Colchicine for Left Ventricular Infarct Size Treatment in Acute Myocardial Infarction) trial was published, where 192 patients with first time ST-segment elevation MI (STEMI) received either a high loading dose of colchicine followed by a low dose twice a day for 5 days or placebo treatment (40). No differences were observed between the 2 groups with respect to infarct size 5 days and 3 months post-MI, as well as left ventricular (LV) remodeling and end-diastolic function.
Table 1.
Overview of Clinical Trials Targeting Inflammation in Coronary Artery Disease
| Clinical Trial | Intervention | n | Indications | Treatment Length | Main Outcomes | Adverse Outcomes | First Author (Ref. #) |
|---|---|---|---|---|---|---|---|
| LoDoCo | Colchicine 0.5 mg daily |
532 | Stable CAD | 3 y, median | Reduced cardiovascular events | — | Nidorf et al (36) |
| LoDoCo2 | Colchicine 0.5 mg daily |
5,522 | Chronic, stable CAD | 2.4 y, median | Reduced cardiovascular events | Increased non–CVD-related deaths | Nidorf et al (37) |
| COLCOT | Colchicine 0.5 mg daily |
4,745 | Recent AMI (<30 d) | 1.9 y, median | Reduced ischemic cardiovascular events | Increased risk of pneumonia | Tardif et al (38) |
| COVERT-MI | Colchicine 2 mg loading dose, 0.5 mg twice daily |
192 | First STEMI (<12 h after onset of chest pain) | 5 d + 3 mo follow-up | No difference in infarct size, LV remodeling, LV end-diastolic volume | Increased incidence of gastrointestinal adverse events 3-fold increased incidence of LV thrombus |
Mewton et al (40) |
| CANTOS | Canakinumab 50/150/300 mg every 3 months |
10,061 | Prior AMI (>30 d), CRP >2 mg/L | 3.7 y, median | Reduced CRP, IL-6, and adverse cardiac events | Increased risk for fatal infections | Ridker et al (6) |
| MRC-ILA Heart Study | Anakinra 100 mg daily |
182 | Non-STEMI (<48 h after chest pain) | 14 d + 1 y follow-up | Reduced CRP and IL-6 | Increased major adverse cardiac events at 1 year | Morton et al (41) |
| VCUART3 | Anakinra 100 mg once or twice daily |
99 | Acute STEMI (<12 h of symptoms) | 14 d + 1 y follow-up | Reduced CRP, death/new heart failure, and heart failure hospitalization | — | Abbate et al (44) |
AMI = acute myocardial infarction; CAD = coronary artery disease; CANTOS = Canakinumab Anti-inflammatory Thrombosis Outcome Study; COLCOT = Colchicine Cardiovascular Outcomes Trial; COVERT- MI = Colchicine for Left Ventricular Infarct Size Treatment in Acute Myocardial Infarction; MRC-ILA = Medical Royal Council InterLeukin-1 Antagonist; CRP = C-reactive protein; IL = interleukin; LoDoCo = Low-Dose Colchicine; LoDoCo2 = Low-Dose Colchicine 2; LV = left ventricular; STEMI = ST-segment elevation myocardial infarction; VCUART3 = Virginia Commonwealth University Anakinra Remodeling Trial 3.
There are several ongoing trials that investigate if colchicine can prevent new cardiovascular events in patients with CVD—among others, COACS (Colchicine for Acute Coronary Syndromes; NCT01906749), CLEAR SYNERGY (Colchicine and Spironolactone in Patients with MI/SYNERGY Stent Registry; NCT03048825), and CONVINCE (Colchicine for Prevention of Vascular Inflammation in Noncardio Embolic Stroke; NCT02898610). The results of these trials may be available this or next year.
Anakinra
Anakinra blocks IL-1 receptor 1, thereby inhibiting both IL-1β and IL-1α, and is approved for other inflammatory conditions. In the MRC-ILA (Medical Royal Council InterLeukin-1 Antagonist) heart study, patients with non–ST-segment elevation ACS were randomized to daily administration of anakinra or matching placebo for 2 weeks (41). Treatment with anakinra led to a temporary reduction in CRP and IL-6; however, the number of major adverse cardiac events at 1 year was higher in the anakinra arm than in the placebo arm. After 2 successful clinical pilot studies (42,43), the VCU-ART3 (Virginia Commonwealth University Anakinra Remodeling Trial 3) investigated the effect of anakinra in 99 patients with STEMI within 12 hours after the onset of symptoms (44). Two weeks of treatment with anakinra led to a reduction in CRP levels and a lower incidence of new-onset HF and hospitalization for HF.
Canakinumab
The CANTOS trial, the largest cytokine inhibition study ever completed, included 10,061 patients and provided strong evidence that inflammation plays an important role in the pathogenesis of CVD (6). Canakinumab is a monoclonal human antibody that selectively binds to and inhibits IL-1β. It is approved by the U.S. Food and Drug Administration for the treatment of systemic juvenile idiopathic arthritis, active Still disease, and certain types of autoinflammatory periodic fever syndromes. In the CANTOS trial, patients with an MI >30 days before enrollment and CRP level of >2 mg/L were randomly allocated to placebo or to 1 of 3 doses of canakinumab (50, 150, or 300 mg), given subcutaneously once every 3 months. The patients were followed up for a median of 3.7 years. Patients who received the intermediate canakinumab dose (150 mg) had reduced occurrence of the primary endpoint, a composite of cardiovascular death, nonfatal acute MI, or nonfatal stroke, accompanied by a reduction in IL-6 and CRP (6,45). Interestingly, LDL cholesterol was not reduced by canakinumab. A small but significant increase in the incidence of fatal infections was observed in the canakinumab arm. Substudies of the CANTOS trial have suggested that canakinumab is effective in high-risk patients with chronic kidney disease and in patients with and without diabetes. However, it is unlikely that canakinumab will be a standard care therapy for patients with CAD because of its high costs (46). Nevertheless, this landmark trial was the first to establish solid evidence for the inflammatory hypothesis of CAD.
Heart Failure
The basis for targeting the NLRP3 inflammasome in HF
HF is a clinical syndrome caused by structural and/or functional cardiac abnormality corroborated by elevated natriuretic peptide and evidence of congestion (47). In addition to the more common causes, CAD and MI, there are several other etiologies of this disorder, including hypertension, aortic stenosis, infections, and genetics. About one-half of the patients with HF exhibit a preserved ejection fraction (HFpEF) (LV ejection fraction [LVEF] ≥50%) in contrast to the more traditional HF with reduced ejection fraction (HFrEF) (LVEF ≤40%), which is the most prevalent form of HF in elderly patients (48). Regardless of classification, the overall survival rate is poor, with mortality rates close to 50% in 5 years, despite therapeutic improvement the last decades (49).
In HF, independent of LVEF, persistent immune activation and inflammation play a central role. In ischemic HF, there is substantial evidence to suggest that inflammation contributes to disease progression in its early stages. Proinflammatory markers such as CRP, IL-6, and IL-1 are increased, and their levels are associated with the severity and prognosis of HF (50).
In healthy mice, injections of IL-1β or IL-18 led to a transient and reversible systolic dysfunction and reduced LV contractility (51,52). Furthermore, plasma from patients with HF and elevated levels of CRP induced a similar pattern of systolic dysfunction and impaired contractile reserves when injected in mice (53). Treatment with either an IL-1β antibody or anakinra fully prevented the observed cardiac dysfunction. These studies corroborate the role of IL-1β in the progression of HF and highlight the cardiodepressive effects of IL-1β, which also have been observed in patients with sepsis (54).
Cardiac remodeling and fibrosis are both dominant mechanisms in HF development. Some studies suggest that NLRP3 effectuates maladaptive remodeling after experimental MI and point to a key role for (proinflammatory) macrophages (34,55). Furthermore, genetic deletion of NLRP3 leads to a favorable outcome with improved survival, attenuated LV remodeling, and reduced fibrosis (34). Similarly, myocardial remodeling in diet-induced obese mice was modulated by the NLRP3 inflammasome, at least partly through its effects on systemic inflammation (56). In experimental pressure overload, the expression and function of NLRP3 inflammasomes were elevated (57,58). Interestingly, in mice subjected to transverse aortic constriction, where Ca2+/calmodulin-dependent protein kinase II δ (CaMKIIδ) was selectively deleted from cardiomyocytes, NLRP3 inflammasome activation was reduced and associated with a diminished accumulation of macrophages, attenuated fibrosis, and reduced contractile dysfunction as well as ventricular dilation. This suggests that CaMKIIδ plays a role in the activation of NLRP3 (59).
Trials and treatments targeting inflammation in HF
Based on the potential role of inflammation, particularly the NLRP3 inflammasome, in the pathogenesis of HF, several clinical trials have targeted IL-1 (Table 2).
Table 2.
Overview of Inflammasome Targeted Studies in Patients with HF
| Clinical Trial | Intervention | n | Indications | Treatment Length | Main Outcomes | First Author (Ref. #) |
|---|---|---|---|---|---|---|
| Anti-inflammatory treatment with colchicine in stable HF | Colchicine 0.5 mg twice daily |
279 | Stable HFrEF | 6 mo | Reduced CRP and IL-6 | Deftereos et al (60) |
| DHART-2 | Anakinra 100 mg daily |
31 | Stable HFpEF CRP >2 mg/L |
12 wk, 12 wk follow-up | Reduced CRP and NT-proBNP Increased exercise time and QoL |
Van Tassel et al (62) |
| ADHF | Anakinra 100 mg twice per day for 3 d; thereafter, 100 mg daily for 11 d |
30 | Acute decompensated HFrEF CRP >5 mg/L |
2 wk | Reduced CRP and IL-6 Increased LVEF |
Van Tassel et al (63) |
| REDHART | Anakinra 100 mg daily |
60 | Postdischarge HFrEF CRP >2 mg/L |
12 wk | Reduced CRP and NT-proBNP Increased peak Vo2 exercise time and QoL |
Van Tassel et al (64) |
| Study of Dapansutrile Capsules in Heart Failure | Dapansutrile 500 mg, either 1, 2, or 4 times daily |
30 | Stable HFrEF (NYHA class II or III) hCRP >2 mg/L | 2 wk, 2 wk follow-up | Increased LVEF and exercise time at 2 wk with 2,000 mg | Wohlford et al (65) |
| CANTOS | Canakinumab 50/150/300 mg every 3 months |
15 | HFrEF CRP >2 mg/L |
1 y | Reduced CRP Increased LVEF and peak Vo2 |
Trankle et al (66) |
ADHF = Acute Decompensated Heart Failure; CANTOS = Canakinumab Anti-inflammatory Thrombosis Outcome Study; CRP = C-reactive protein; DHART2 = Diastolic Heart Failure Anakinra Response Trial; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; IL = interleukin; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart Association; peak Vo2 = maximal oxygen consumption during cardiopulmonary exercise testing; QoL = quality of life; REDHEART = Recently Decompensated Heart Failure Anakinra Response Trial.
Colchicine
In a single-center trial, 279 patients with stable HFrEF were randomly assigned to receive colchicine 0.5 mg daily or placebo for 6 months. Levels of CRP and IL-6 were lower in patients allocated to colchicine than in patients allocated to placebo; however, this did not translate to an effect on clinical outcomes, exercise capacity, or improvement in mortality (60).
Anakinra
Most of the trials targeting the NLRP3 inflammasome in patients with HF have used anakinra. In the Diastolic Heart Failure Anakinra Response Trial (D-HART) and D-HART2 trials, patients with stable HFpEF were randomized to anakinra for 2 or 12 weeks, respectively (61,62). In both studies, a reduction in CRP was observed, as were increased exercise time and quality of life. Two weeks of treatment resulted in a modest, but significant, increase in peak Vo2, whereas 12 weeks of treatment led to a reduction in N-terminal pro–B-type natriuretic peptide levels compared to placebo.
In the ADHF (Acute Decompensated Heart Failure) trial, patients with acute decompensated HF were randomized to treatment with anakinra or placebo for 14 days. CRP and IL-6 levels were markedly reduced in patients receiving anakinra compared to placebo (63), and improved LVEF was observed. In another clinical trial designed for patients with HFrEF, the REDHART (Recently Decompensated Heart Failure Anakinra Response Trial) study, anakinra was supplied at hospital discharge for 12 weeks (64). A daily dosage of 100 mg anakinra resulted in improved exercise time, quality of life, and Vo2, together with a reduction in CRP and N-terminal pro–B-type natriuretic peptide. The largest clinical trial with anakinra to date, the REDHART2 trial (NCT03797001), investigates the effect of 100 mg anakinra per day versus placebo for 24 weeks in 102 patients after hospital discharge for HFrEF and is ongoing.
Dapansutrile
Dapansutrile, also known as OLT1177, is a specific NLRP3 inflammasome inhibitor. It was recently tested in a phase 1B trial in 30 patients with stable HFrEF and was well tolerated and safe (65). At the highest tested dose of 2,000 mg, an increased LVEF and exercise time after 2 weeks of treatment was observed. These results are very promising for a first clinical trial with a selective oral NLRP3 inhibitor in HF.
Canakinumab
In a small substudy of the CANTOS trial, the effect of canakinumab on 15 patients with HFrEF was investigated (66). Despite the small group size caused by early enrollment halting of the CANTOS trial, in the 150 mg treatment arm, peak Vo2 was improved at 3 months, and at 12 months, an increased LVEF was observed. At both timepoints, a decreased CRP level was found. Although the sample size was small, these results underline the potential of canakinumab in HF, and the outcomes are in line with the positive results in the CANTOS trial.
HF and NLRP3: The need for larger clinical trials including focus on HFpEF
Overall, the results in these clinical trials with patients with HF support the hypothesis that the NLRP3 inflammasome and its product IL-1 can modulate cardiac function by acting as a soluble cardiodepressant factor. The observed results of the different drugs are consistent in patients with HF. It is striking to observe the limited number of studies that have investigated the effect of the inflammasome on HFpEF. Only the D-HART pilot study and DHART-2 trial, both on treatment with anakinra and with a small number of subjects, looked into HFpEF (62,67), and both HFpEF and HFrEF patient groups have not been investigated simultaneously. Because the exact etiology of HFpEF is unresolved, it is difficult to pinpoint the mechanism for how HFpEF is activating the inflammasome. However, because treatment options for patients with HFpEF are fairly limited, intervention in the inflammasome could be a potential therapeutic option. Moreover, whereas its seems that the NLRP3 inflammasome may be more activated in dilated cardiomyopathy than in ischemic cardiomyopathy (Figure 2), data on NLRP3 activation in different HF subgroups are scarce.
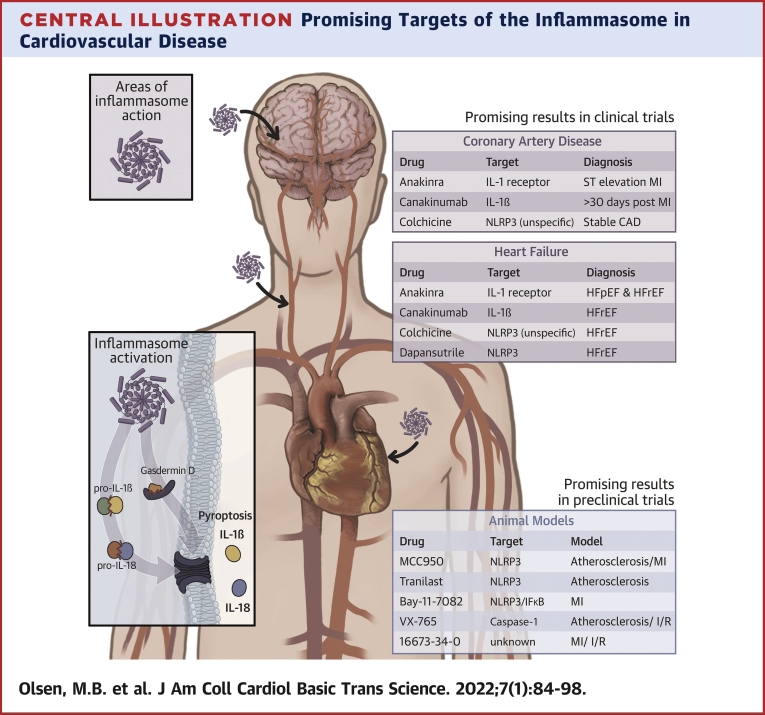
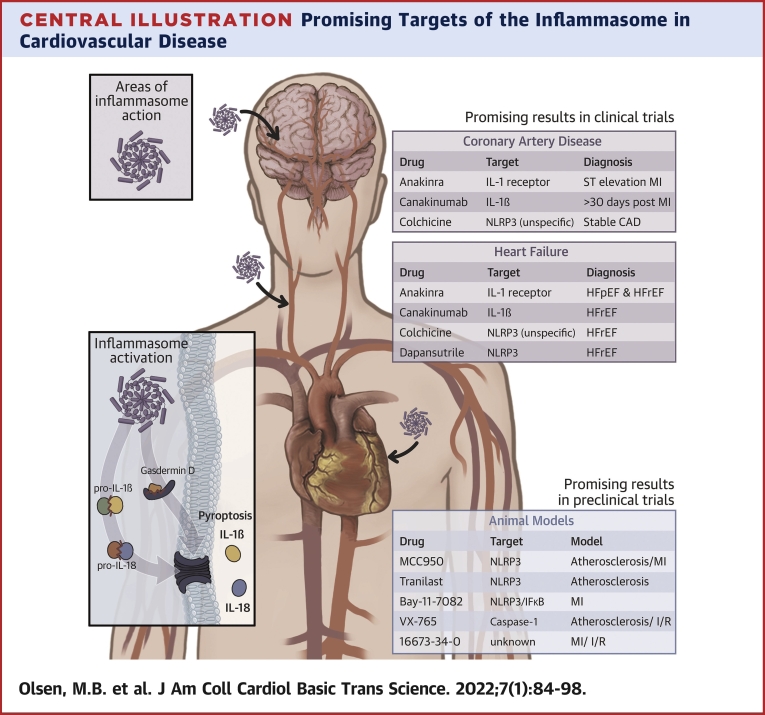
Additionally, in contrast to trials in patients with CAD, described in the “Atherogenesis and MI—Trials and Treatments Targeting the NLRP3 Inflammasome” section, the trials in patients with HF are small with fewer clinical endpoints. Therefore, further and larger trials are needed before conclusions can be drawn. The most interesting and promising study for patients with HF is, however, the dapansutrile study, showing a potential for direct inhibition of NLRP3 activation and not only IL-1β. The main current preclinical and clinical trials targeting the inflammasome in CVD are summarized in the Central Illustration.
Central Illustration.
Promising Targets of the Inflammasome in Cardiovascular Disease
MI = myocardial infarction; NLRP3 = NOD-like receptor family pyrin domain containing 3; STEMI = ST-segment elevation myocardial infarction.
Targeting Inflammasomes in Cardiovascular Disease: The Road Ahead
So far, mainly therapeutic strategies targeting the products of inflammasome activation, in particular IL-1β, have made it to large clinical trials for CVD. However, targeting the inflammasomes directly could be more beneficial, because this will also inhibit GSDMD pore formation and pyroptosis. Also, specific inhibition reduces the risk of off-target effects.
Significant potential of NLRP3 inhibitors
Although many NLRP3 inhibitors have been tested in vitro and in animal models of inflammatory disease, only a few have shown promising results in preclinical studies of CVD (Table 3). One of these is the small-molecule inhibitor MCC950, which directly targets the NACHT domain of NLRP3 (68). MCC950 has been shown to inhibit macrophage plaque infiltration and the development of atherosclerosis in ApoE-deficient mice (69). Furthermore, it reduced infiltration of neutrophils to the heart and infarct size as well as preserved cardiac function in a pig model of MI (70). Molecule 16673-34-0 is an oral active compound modified with permission from the NLRP3 inhibitor glyburide. Its exact mode of action is not known, but it is shown in several mouse models to have therapeutic potential by limiting inflammation and myocardial injury after ischemia-reperfusion injury (31,71) and also after nonischemic cardiac injury (72), suggesting potential for clinical testing. Another compound inhibiting NLRP3 is Bay-11-7082, shown to have positive effect in a rat MI model (73). Bay-11-7082 is, however, also an NF-κB inhibitor, and in an in vivo setting, these effects might be difficult to distinguish and may preclude its clinical utility. Tranilast was recently shown to inhibit NLRP3 oligomerization and activation by facilitating NLRP3 ubiquitination (74). Tranilast is shown to reduce plaque development in LDLr- and ApoE-deficient mice (75). These are only some examples of specific NLRP3 inhibitors under testing in preclinical models of CVD, and more comprehensive overviews can be found elsewhere (74). Nevertheless, it will be intriguing to follow this development further to fully elucidate the potential of NLRP3 as a therapeutic target for CVD, fitted for clinical practice.
Table 3.
Overview of Discussed Inflammasome Targeted Preclinical Studies
| Compound | Target | Species and Model | Main Outcomes | First Author (Ref. #) |
|---|---|---|---|---|
| MCC950 (CRID3, CP-456,773) |
Inhibition of ASC oligomerization | ApoE-/- mice, atherosclerosis | Inhibition of MΦ infiltration in plaque, expression of adhesion molecules, reduced atherosclerosis development | van der Heijden et al (69) |
| Pig, MI | Reduced neutrophil infiltration and IL-1β, smaller infarct size, preserved cardiac function | van Hout et al (70) | ||
| 16673-34-0 | Unknown | ICR mice, I/R | Reduced caspase-1 activity in the heart, smaller infarct size | Marchetti et al (71) |
| ICR mice: either I/R, permanent LAD occlusion, or doxorubicin-induced toxicity | Smaller infarct size, improved LVFS reduced cardiac troponin; less LV dilatation, improved LVFS; and reduced fibrosis, improved LVFS, respectively | Marchetti et al (72) | ||
| Bay-11-7082 | Alkylation of cysteine in NLRP3 ATPase region, inhibition of kappa B kinase | Rat, I/R | Smaller infarct size preserved systolic function, reduced fibrosis and apoptosis | Kim et al (73) |
| Tranilast | NACHT domain of NLRP3, inhibits oligomerization | LDLr-/- and ApoE-/- mice, atherosclerosis | Reduced lesion size and MΦ content; increased collagen; reduced cardiac expression of NLRP3, IL-1β, and caspase-1 | Chen et al (75) |
| VX-765 | Caspase-1 inhibition | ApoE-/-mice, atherosclerosis | Inhibits vascular muscle cell pyroptosis and atherogenesis | Li et al (80) |
| Sprague Dawley rat, Langendorff I/R | Smaller infarct size | Do Carmo et al (81) |
ASC = apoptosis associated speck-like protein; ATPase = adenosine triphosphatase; ICR = Institute for Cancer Research; I/R = ischemia/reperfusion; IL = interleukin; LAD = left anterior descending aorta; LV = left ventricular; LVFS = left ventricular fractional shortening; MΦ = macrophages; MI = myocardial infarction; NLRP3 = NOD-like receptor family pyrin domain containing 3; NACHT = NAIP, CIITA, HET-E, and TP-1.
Caspase-1 inhibition
In addition to cleavage and activation of inflammatory cytokines, caspase-1 activation stimulates pyroptosis through activation of GSDMD, causing pore formation. Pyroptosis is associated with pathologic processes of atherosclerosis and MI and is suggested as a therapeutic target for CVD (76). Caspase-1 deficiency is shown to reduce the development of atherosclerosis in mice (77) and to reduce monocyte recruitment to the plaque and endothelial cell activation (78). Pyroptosis is also thought to be involved in ischemia-reperfusion injury (79). The caspase-1 inhibitor VX-765 is subsequently shown to inhibit vascular muscle cell pyroptosis and atherogenesis in ApoE-deficient mice (80). Furthermore, VX-765 is shown to reduce MI size in a rat ischemia-reperfusion injury model (81); thus, caspase-1 is an interesting target for several CVDs. Of note, caspase-1 is not uniquely expressed by the NLRP3 inflammasome, and inhibiting its effect would therefore also stop the effects of other inflammasomes.
Targeting novel inflammasomes: An exciting future
Whereas NLRP3 is the most described inflammasome, there are many other inflammasomes with potential importance for CVD, who also can serve as possible therapeutic targets. The first inflammasome to be described was NLRP1, in 2002 (82). Some activators and inhibitors of this inflammasome are identified (83, 84, 85, 86), but the role of NLRP1 and its inhibition in CVD is not known. Expression levels of NLRP1, as well as NLRP6, -7, and -12, are up-regulated in failing hearts (Figure 2); thus, it will be exciting to unravel their role in the pathogenesis of CVD. The NAIP/NLRC4 inflammasome has been described in more detail. Interestingly, the nonbacterial components lysophosphatidylcholine and palmitate have been found to activate this inflammasome in brain microglia (87,88), and NLRC4 is activated in post–ischemic brain injury and is proposed as a contributing factor to microglial cell death (89,90). As shown in Figure 2, the expression levels of NLRC4 and NAIP are increased in dilated cardiomyopathy HF, especially in fibroblasts and macrophages, respectively. As for the NLRC4/NAIP inflammasome, AIM2 expression also seems to be increased during dilated cardiomyopathy HF (Figure 2). This inflammasome responds to endogenous DNA and double-stranded DNA in the cytosol originating from virus or bacteria (91,92). Factors that trigger DNA damage, including release of mitochondrial DNA, could therefore be indirect triggers of the AIM2 inflammasome, and notably, cholesterol overload could also activate the AIM2 inflammasome (93). Indeed, the AIM2 inflammasome is shown to contribute to CVD in several animal models (89,94,95) and has been detected in human atherosclerotic lesions (96). Very recently, AIM2 activation was shown to exacerbate atherosclerosis in a mouse model of clonal hematopoiesis (97); thus, AIM2 is an intriguing future therapeutic target for CVD. Of note, AIM2 also has anti-inflammatory effects by restraining neuroinflammation (98) and preserving regulatory T-cell function (99). Thus, cell- and tissue-specific effects are also important factors to consider when evaluating the clinical potential of inflammasome-targeted therapy, as summarized in Figure 4.
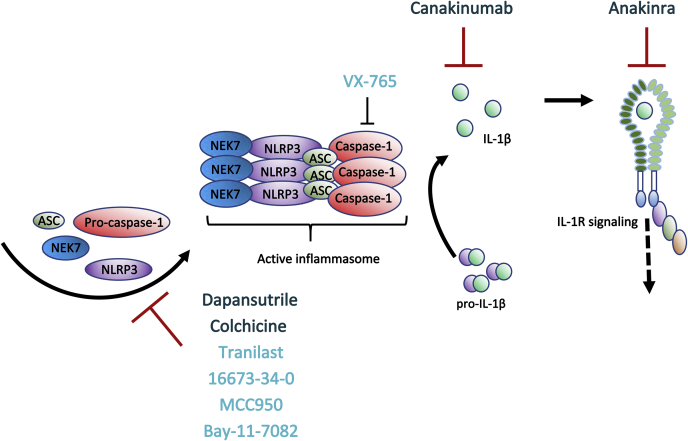
Figure 4.
Overview of Main Inflammasome Treatment Options and Their Modes of Action
Currently, drugs targeting IL-1β signaling, ie, anakinra and canakinumab, and tubuli formation (colchicine) are the only treatment options in clinical trials for CVD (dark blue). There are, however, promising results from smaller preclinical studies using compounds more directly targeting the NLRP3 inflammasome (ie, 16673-34-0, MCC950, Bay-11-7082, tranilast) or caspase-1 (VX-765) (light blue). For more details on drugs targeting NLRP3, see the review by Mezzaroma et al (74).
Concluding Remarks
We believe that therapeutic options targeting inflammasomes may play an important role in the future therapy of CVD. However, even in the short term, there are some challenges. Although the focus has been directed against IL-1β, recent studies suggest that IL-1α may play an important role in CVD. For example, the ability of neutrophil extracellular traps to elicit inflammatory responses from human endothelial cells depends on the α-isoform rather than on the β-isoform (100). The granulocyte serine proteinase cathepsin G can process IL-1α (100), augmenting its proinflammatory action, relating IL-1α to neutrophils rather than monocyte/macrophages. From a therapeutic point of view, anakinra, but not canakinumab, will block IL-1α, and antibodies against IL-1α have undergone clinical testing.
Furthermore, in addition to IL-1β, activated NLRP3 inflammasomes release large amounts of IL-18. The effect of IL-1β and IL-18 on cardiac function seems to be synergistic. Mice lacking IL-18 did not suffer reduced cardiac contractility when treated with recombinant IL-1β, whereas the control group did (51). This suggests that, whereas IL-18 is not necessary for IL-1β–mediated inflammation, it seems to be vital for an IL-1β–mediated reduction in cardiac contractility. The reversibility of cardiac dysfunction in mice in vivo provides a rationale for blocking these cytokines, either directly or upstream via NLRP3 inhibition, to improve cardiac function. There are some clinical studies targeting IL-18 inhibition in autoimmune disorders, but firm evidence for its effects are lacking. In the CANTOS study, the blockade of IL-1β did not reduce IL-18 concentrations, indicating a selective effect of canakinumab on one but not the other major inflammatory cytokines that are released from activated NLRP3 inflammasomes (101).
An important factor that too seldom is addressed in both preclinical and clinical studies is sex. A study published last year showed that NLRP3 has a higher impact on lesion development in female LDL-/- mice, an effect that is sex hormone dependent (102). Furthermore, it is acknowledged that immune regulation differs depending on sex, and sex-related inflammatory responses in CAD was recently reviewed (103). To develop better treatment options, future preclinical and clinical trials should strive to achieve better determination of sex-dependent effects, with a sex subgroup analysis at a minimum, like that performed in the LoDoCo2 trial (37).
In addition to treatment, Zuurbier (104) recently reviewed and concluded on a role of the NLRP3 inflammasome in cardioprotective pathways (104); thus, the timepoint of the disease course in which the inflammasome is targeted will matter. Supporting this, treatment with a high dose of colchicine from the time of reperfusion in first-time patients with STEMI did not reduced myocardial damage or inflammation (40). Additionally, we have shown that targeting IL-6 (a downstream mediator of IL-1β) in STEMI seems to be less beneficial in patients with symptom duration of <3 hours (105). This illustrates that inflammation, in principle, is protective, even though too much and too long may be harmful, and it is important that the treatment of chronic inflammation in CVD does not move the patient from one ditch to another. However, the optimal timepoint for the administration of drugs targeting the inflammasome is still not known. This is an important topic that depends on the specific drug, type of cardiovascular disease, and severity degree as well as the need for biomarkers or other aims to identify the patients who will benefit the most in the clinical setting. Future studies should also aim at mapping the oscillation of the drug targets (ie, IL-1β) throughout the day to be able to administer the drugs at the most efficient time. It is important to note that not all patients should receive such therapy. Dosages and the duration of treatment will be important aspects in future research to achieve a beneficial balance between favorable and harmful effects on individual patients, pointing toward the era of personalized and precision medicine.
Funding Support and Author Disclosures
This work was supported by the South-Eastern Norway Regional Health Authority (grant 2019058 to Dr Louwe) and Throne-Holst fund (grant 511322 to Dr Belland Olsen). Dr Sokolova is currently an employee of GlaxoSmithKline. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Chauhan D., Vande Walle L., Lamkanfi M. Therapeutic modulation of inflammasome pathways. Immunol Rev. 2020;297:123–138. doi: 10.1111/imr.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamura H., Tsutsi H., Komatsu T., et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 3.Chaix J., Tessmer M.S., Hoebe K., et al. Cutting edge: priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimoto T., Mizutani H., Tsutsui H., et al. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000;1:132–137. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimoto T., Tsutsui H., Tominaga K., et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci U S A. 1999;96:13962–13966. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 7.Brubaker S.W., Bonham K.S., Zanoni I., Kagan J.C. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding J., Wang K., Liu W., et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 9.Evavold C.L., Ruan J., Tan Y., Xia S., Wu H., Kagan J.C. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48:35–44.e6. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monteleone M., Stanley A.C., Chen K.W., et al. Interleukin-1β maturation triggers its relocation to the plasma membrane for gasdermin-D-dependent and -independent secretion. Cell Rep. 2018;24:1425–1433. doi: 10.1016/j.celrep.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Miao E.A., Leaf I.A., Treuting P.M., et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H., Lu Y., Cao Z., et al. Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol Lett. 2016;246:7–16. doi: 10.1016/j.toxlet.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Du N., Zhang Q., et al. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5:e1479. doi: 10.1038/cddis.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., Yu P., Zhou B., et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat Cell Biol. 2020;22:108–119. doi: 10.1038/s41556-019-0446-7. [DOI] [PubMed] [Google Scholar]
- 15.Shi J., Zhao Y., Wang K., et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 16.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di A., Xiong S., Ye Z., et al. The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. Immunity. 2018;49:56–65. doi: 10.1016/j.immuni.2018.04.032. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins G.R., Wen H., Ting J.P. Inflammasomes and metabolic disorders: old genes in modern diseases. Mol Cell. 2014;54:297–308. doi: 10.1016/j.molcel.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libby P., Buring J.E., Badimon L., et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 20.Galea J., Armstrong J., Gadsdon P., Holden H., Francis S.E., Holt C.M. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 21.Seta Y., Kanda T., Tanaka T., et al. Interleukin 18 in acute myocardial infarction. Heart. 2000;84:243–249. doi: 10.1136/heart.84.6.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paramel Varghese G., Folkersen L., Strawbridge R.J., et al. NLRP3 Inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi X., Xie W.L., Kong W.W., Chen D., Qu P. Expression of the NLRP3 inflammasome in carotid atherosclerosis. J Stroke Cerebrovasc Dis. 2015;24:2455–2466. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Niyonzima N., Bakke S.S., Gregersen I., et al. Cholesterol crystals use complement to increase NLRP3 signaling pathways in coronary and carotid atherosclerosis. EBioMedicine. 2020;60:102985. doi: 10.1016/j.ebiom.2020.102985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartford M., Wiklund O., Hulten L.M., et al. Interleukin-18 as a predictor of future events in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol. 2010;30:2039–2046. doi: 10.1161/ATVBAHA.109.202697. [DOI] [PubMed] [Google Scholar]
- 26.Åkerblom A., James S.K., Lakic T.G., et al. Interleukin-18 in patients with acute coronary syndromes. Clin Cardiol. 2019;42:1202–1209. doi: 10.1002/clc.23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirii H., Niwa T., Yamada Y., et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 28.Whitman S.C., Ravisankar P., Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(-/-) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 29.Elhage R., Jawien J., Rudling M., et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 30.Duewell P., Kono H., Rayner K.J., et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toldo S., Marchetti C., Mauro A.G., et al. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int J Cardiol. 2016;209:215–220. doi: 10.1016/j.ijcard.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 32.Sandanger Ø, Ranheim T., Vinge L.E., et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99:164–174. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 33.Sandanger Ø, Gao E., Ranheim T., et al. NLRP3 inflammasome activation during myocardial ischemia reperfusion is cardioprotective. Biochem Biophys Res Commun. 2016;469:1012–1020. doi: 10.1016/j.bbrc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 34.Louwe M.C., Olsen M.B., Kaasbøll O.J., et al. Absence of NLRP3 inflammasome in hematopoietic cells reduces adverse remodeling after experimental myocardial infarction. J Am Coll Cardiol Basic Trans Sci. 2020;5:1210–1224. doi: 10.1016/j.jacbts.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nidorf M., Thompson P.L. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. Am J Cardiol. 2007;99:805–807. doi: 10.1016/j.amjcard.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 36.Nidorf S.M., Eikelboom J.W., Budgeon C.A., Thompson P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Nidorf S.M., Fiolet A.T.L., Mosterd A., et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 38.Tardif J.C., Kouz S., Waters D.D., et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 39.Bouabdallaoui N., Tardif J.C., Waters D.D., et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT) Eur Heart J. 2020;41:4092–4099. doi: 10.1093/eurheartj/ehaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mewton N., Roubille F., Bresson D., et al. Effect of colchicine on myocardial injury in acute myocardial infarction. Circulation. 2021;144:859–869. doi: 10.1161/CIRCULATIONAHA.121.056177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton A.C., Rothman A.M., Greenwood J.P., et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36:377–384. doi: 10.1093/eurheartj/ehu272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbate A., Kontos M.C., Grizzard J.D., et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study) Am J Cardiol. 2010;105:1371–1377.e1. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 43.Abbate A., Van Tassell B.W., Biondi-Zoccai G., et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study] Am J Cardiol. 2013;111:1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbate A., Trankle C.R., Buckley L.F., et al. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridker P.M., Libby P., MacFadyen J.G., et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) Eur Heart J. 2018;39:3499–3507. doi: 10.1093/eurheartj/ehy310. [DOI] [PubMed] [Google Scholar]
- 46.Sehested T.S.G., Bjerre J., Ku S., et al. Cost-effectiveness of canakinumab for prevention of recurrent cardiovascular events. JAMA Cardiol. 2019;4:128–135. doi: 10.1001/jamacardio.2018.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bozkurt B., Coats A.J., Tsutsui H., et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27(4):387–413. doi: 10.1016/j.cardfail.2021.01.022. [DOI] [Google Scholar]
- 48.Zile M.R., Brutsaert D.L. New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 49.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Bauersachs J., Langer H.F. Immune mechanisms in heart failure. Eur J Heart Fail. 2017;19:1379–1389. doi: 10.1002/ejhf.942. [DOI] [PubMed] [Google Scholar]
- 51.Toldo S., Mezzaroma E., O’Brien L., et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1025–H1031. doi: 10.1152/ajpheart.00795.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Tassell B.W., Seropian I.M., Toldo S., Mezzaroma E., Abbate A. Interleukin-1β induces a reversible cardiomyopathy in the mouse. Inflamm Res. 2013;62:637–640. doi: 10.1007/s00011-013-0625-0. [DOI] [PubMed] [Google Scholar]
- 53.Van Tassell B.W., Arena R.A., Toldo S., et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar A., Thota V., Dee L., Olson J., Uretz E., Parrillo J.E. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu W., Zhang X., Zhao M., et al. Activation in M1 but not M2 macrophages contributes to cardiac remodeling after myocardial infarction in rats: a critical role of the calcium sensing receptor/NRLP3 inflammasome. Cell Physiol Biochem. 2015;35:2483–2500. doi: 10.1159/000374048. [DOI] [PubMed] [Google Scholar]
- 56.Sokolova M., Sjaastad I., Louwe M.C., et al. NLRP3 inflammasome promotes myocardial remodeling during diet-induced obesity. Front Immunol. 2019;10:1621. doi: 10.3389/fimmu.2019.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gan W., Ren J., Li T., et al. The SGK1 inhibitor EMD638683, prevents angiotensin II-induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1–10. doi: 10.1016/j.bbadis.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y., Wu Y., Chen J., Zhao S., Li H. Pirfenidone attenuates cardiac fibrosis in a mouse model of TAC-induced left ventricular remodeling by suppressing NLRP3 inflammasome formation. Cardiology. 2013;126:1–11. doi: 10.1159/000351179. [DOI] [PubMed] [Google Scholar]
- 59.Suetomi T., Willeford A., Brand C.S., et al. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca(2+)/calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation. 2018;138:2530–2544. doi: 10.1161/CIRCULATIONAHA.118.034621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deftereos S., Giannopoulos G., Panagopoulou V., et al. Anti-inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. J Am Coll Cardiol HF. 2014;2:131–137. doi: 10.1016/j.jchf.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Van Tassell B.W., Arena R., Biondi-Zoccai G., et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study) Am J Cardiol. 2014;113:321–327. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Tassell B.W., Trankle C.R., Canada J.M., et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.118.005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Tassell B.W., Abouzaki N.A., Oddi Erdle C., et al. Interleukin-1 blockade in acute decompensated heart failure: a randomized, double-blinded, placebo-controlled pilot study. J Cardiovasc Pharmacol. 2016;67:544–551. doi: 10.1097/FJC.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Tassell B.W., Canada J., Carbone S., et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial) Circ Heart Fail. 2017;10:e004373. doi: 10.1161/CIRCHEARTFAILURE.117.004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wohlford G.F., Van Tassell B.W., Billingsley H.E., et al. Phase 1B, randomized, double-blinded, dose escalation, single-center, repeat dose safety and pharmacodynamics study of the oral NLRP3 inhibitor dapansutrile in subjects with NYHA II-III systolic heart failure. J Cardiovasc Pharmacol. 2020;77:49–60. doi: 10.1097/FJC.0000000000000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trankle C.R., Canada J.M., Cei L., et al. Usefulness of canakinumab to improve exercise capacity in patients with long-term systolic heart failure and elevated C-reactive protein. Am J Cardiol. 2018;122:1366–1370. doi: 10.1016/j.amjcard.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang J., Teehan D., Farioli A., Baur D.M., Smith D., Kales S.N. Sudden cardiac death among firefighters ≤45 years of age in the United States. Am J Cardiol. 2013;112:1962–1967. doi: 10.1016/j.amjcard.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coll R.C., Hill J.R., Day C.J., et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15:556–559. doi: 10.1038/s41589-019-0277-7. [DOI] [PubMed] [Google Scholar]
- 69.van der Heijden T., Kritikou E., Venema W., et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice-brief report. Arterioscler Thromb Vasc Biol. 2017;37:1457–1461. doi: 10.1161/ATVBAHA.117.309575. [DOI] [PubMed] [Google Scholar]
- 70.van Hout G.P., Bosch L., Ellenbroek G.H., et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J. 2017;38:828–836. doi: 10.1093/eurheartj/ehw247. [DOI] [PubMed] [Google Scholar]
- 71.Marchetti C., Chojnacki J., Toldo S., et al. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol. 2014;63:316–322. doi: 10.1097/FJC.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marchetti C., Toldo S., Chojnacki J., et al. Pharmacologic inhibition of the NLRP3 inflammasome preserves cardiac function after ischemic and nonischemic injury in the mouse. J Cardiovasc Pharmacol. 2015;66:1–8. doi: 10.1097/FJC.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim Y.S., Kim J.S., Kwon J.S., et al. BAY 11-7082, a nuclear factor-κB inhibitor, reduces inflammation and apoptosis in a rat cardiac ischemia-reperfusion injury model. Int Heart J. 2010;51:348–353. doi: 10.1536/ihj.51.348. [DOI] [PubMed] [Google Scholar]
- 74.Mezzaroma E., Abbate A., Toldo S. NLRP3 inflammasome inhibitors in cardiovascular diseases. Molecules. 2021;26:976. doi: 10.3390/molecules26040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen S., Wang Y., Pan Y., et al. Novel role for tranilast in regulating NLRP3 ubiquitination, vascular inflammation, and atherosclerosis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng C., Wang R., Tan H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int J Biol Sci. 2019;15:1345–1357. doi: 10.7150/ijbs.33568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gage J., Hasu M., Thabet M., Whitman S.C. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can J Cardiol. 2012;28:222–229. doi: 10.1016/j.cjca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 78.Yin Y., Li X., Sha X., et al. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler Thromb Vasc Biol. 2015;35:804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rauf A., Shah M., Yellon D.M., Davidson S.M. Role of caspase 1 in ischemia/reperfusion injury of the myocardium. J Cardiovasc Pharmacol. 2019;74:194–200. doi: 10.1097/FJC.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 80.Li Y., Niu X., Xu H., et al. VX-765 attenuates atherosclerosis in ApoE deficient mice by modulating VSMCs pyroptosis. Exp Cell Res. 2020;389:111847. doi: 10.1016/j.yexcr.2020.111847. [DOI] [PubMed] [Google Scholar]
- 81.Do Carmo H., Arjun S., Petrucci O., Yellon D.M., Davidson S.M. The caspase 1 inhibitor VX-765 protects the isolated rat heart via the RISK pathway. Cardiovasc Drugs Ther. 2018;32:165–168. doi: 10.1007/s10557-018-6781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 83.Bauernfried S., Scherr M.J., Pichlmair A., Duderstadt K.E., Hornung V. Human NLRP1 is a sensor for double-stranded RNA. Science. 2021;371(6528):eabd0811. doi: 10.1126/science.abd0811. [DOI] [PubMed] [Google Scholar]
- 84.Hollingsworth L.R., Sharif H., Griswold A.R., et al. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature. 2021;592:778–783. doi: 10.1038/s41586-021-03350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang M., Zhang X., Toh G.A., et al. Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature. 2021;592:773–777. doi: 10.1038/s41586-021-03320-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robinson K.S., Teo D.E.T., Tan K.S., et al. Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia. Science. 2020;370(6521):eaay2002. doi: 10.1126/science.aay2002. [DOI] [PubMed] [Google Scholar]
- 87.Freeman L., Guo H., David C.N., Brickey W.J., Jha S., Ting J.P. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med. 2017;214:1351–1370. doi: 10.1084/jem.20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu L., Chan C. IPAF inflammasome is involved in interleukin-1β production from astrocytes, induced by palmitate; implications for Alzheimer’s disease. Neurobiol Aging. 2014;35:309–321. doi: 10.1016/j.neurobiolaging.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Denes A., Coutts G., Lénárt N., et al. AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci U S A. 2015;112:4050–4055. doi: 10.1073/pnas.1419090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poh L., Kang S.W., Baik S.H., et al. Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behav Immun. 2019;75:34–47. doi: 10.1016/j.bbi.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 91.Di Micco A., Frera G., Lugrin J., et al. AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc Natl Acad Sci U S A. 2016;113:E4671–E4680. doi: 10.1073/pnas.1602419113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hornung V., Ablasser A., Charrel-Dennis M., et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dang E.V., McDonald J.G., Russell D.W., Cyster J.G. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell. 2017;171:1057–1071.e11. doi: 10.1016/j.cell.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paulin N., Viola J.R., Maas S.L., et al. Double-strand DNA sensing Aim2 inflammasome regulates atherosclerotic plaque vulnerability. Circulation. 2018;138:321–323. doi: 10.1161/CIRCULATIONAHA.117.033098. [DOI] [PubMed] [Google Scholar]
- 95.Wang X., Pan J., Liu H., et al. AIM2 gene silencing attenuates diabetic cardiomyopathy in type 2 diabetic rat model. Life Sci. 2019;221:249–258. doi: 10.1016/j.lfs.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 96.Hakimi M., Peters A., Becker A., Böckler D., Dihlmann S. Inflammation-related induction of absent in melanoma 2 (AIM2) in vascular cells and atherosclerotic lesions suggests a role in vascular pathogenesis. J Vasc Surg. 2014;59:794–803. doi: 10.1016/j.jvs.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 97.Fidler T.P., Xue C., Yalcinkaya M., et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 2021;592:296–301. doi: 10.1038/s41586-021-03341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma C., Li S., Hu Y., et al. AIM2 controls microglial inflammation to prevent experimental autoimmune encephalomyelitis. J Exp Med. 2021;218:e20201796. doi: 10.1084/jem.20201796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chou W.C., Guo Z., Guo H., et al. AIM2 in regulatory T cells restrains autoimmune diseases. Nature. 2021;591:300–305. doi: 10.1038/s41586-021-03231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Libby P. Targeting inflammatory pathways in cardiovascular disease: the inflammasome, interleukin-1, interleukin-6 and beyond. Cells. 2021;10(4):951. doi: 10.3390/cells10040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ridker P.M., MacFadyen J.G., Thuren T., Libby P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur Heart J. 2020;41:2153–2163. doi: 10.1093/eurheartj/ehz542. [DOI] [PubMed] [Google Scholar]
- 102.Chen S., Markman J.L., Shimada K., et al. Sex-specific effects of the Nlrp3 inflammasome on atherogenesis in LDL receptor-deficient mice. J Am Coll Cardiol Basic Trans Sci. 2020;5:582–598. doi: 10.1016/j.jacbts.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shabbir A., Rathod K.S., Khambata R.S., Ahluwalia A. Sex differences in the inflammatory response: pharmacological opportunities for therapeutics for coronary artery disease. Annu Rev Pharmacol Toxicol. 2021;61:333–359. doi: 10.1146/annurev-pharmtox-010919-023229. [DOI] [PubMed] [Google Scholar]
- 104.Zuurbier C.J. NLRP3 inflammasome in cardioprotective signaling. J Cardiovasc Pharmacol. 2019;74:271–275. doi: 10.1097/FJC.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 105.Broch K., Anstensrud A.K., Woxholt S., et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77:1845–1855. doi: 10.1016/j.jacc.2021.02.049. [DOI] [PubMed] [Google Scholar]