Abstract
Cannabis has been used for various medicinal applications including, but not limited to, cancer: most commonly to treat chemotherapy-associated side effects. Cannabis is often used for its palliative effects in the form of purified cannabinoids, or as extracts. This study was conducted using two breast cancer cell lines and aimed to evaluate potential anti-proliferative “intra-entourage effects” between purified phytocannabinoids resembling the THC and CBD ratios of medicinal and recreational cannabis strains, as well as to investigate potential “inter-entourage effects” between the different ratios and the phytochemicals found in a Cannabis sativa extract. This study also aimed to evaluate the potential interaction between cannabinoids and chemotherapeutic agents. The data identified an intra-entourage effect present in the MCF-7 cells when treated with a recreational, but not a medicinal, cannabis formulation. This effect may be due to THC partially exerting its anti-proliferative effects through the estrogen receptor (ER), present in the MCF-7 cell line. Little to no intra-entourage effects were observed in the MDA-MB-231 cell line and no inter-entourage effects were observed in either cell line. The simultaneous treatment of the MCF-7 cell line with various cannabinoid formulations and the common breast cancer treatment, tamoxifen, resulted in the diminished anti-proliferative activity of tamoxifen, an effect that was more evident when combined with recreational cannabis formulations. Since cannabis is commonly used in palliative care to treat chemotherapy-associated side effects, further research is required to investigate the potential interference of various cannabis formulations to ensure that the efficacy of chemotherapeutic agents is not compromised.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-03102-1.
Keywords: Cannabinoids, Cannabis extract, Combination therapy, Breast cancer
Introduction
The medicinal applications of cannabis have been recorded for centuries (Mouhamed et al. 2018). Besides the well-known use of cannabinoids for their palliative effects to alleviate the side effects associated with cancer treatment regimes, cannabis has also gained interest for its potential use as a chemotherapeutic agent. Several legal and regulatory barriers have limited investigation into the widespread medicinal claims made by cannabis users in the treatment of cancer, and cancer- and chemotherapy-related symptoms. Consequently, the available data on the medicinal uses of cannabis for the management of cancer-related symptoms have mainly come from non-scientific observations collected from websites, lay press, and community interactions, as opposed to comprehensive scientific research (Pergam et al. 2017). Additionally, the frequency of cannabis use in cancer patients is not well documented (Martell et al. 2018). Saadeh and Rustem (2018) conducted a study on the use of medicinal cannabis and found that 75% of the patients who used cannabis did so of their own volition or at the advice of a friend. They also found a general lack of understanding regarding cannabis use, with most of the users having received no counseling regarding the potential harmful or beneficial effects, potential drug–drug interactions, and drug–disease interactions.
One of the major drawbacks associated with chemotherapy is the non-specific nature of the treatment, resulting in the development of several side effects that may be immediate, short, or long term. The intensity of the side effects range from mild to life-threatening. The documented immediate side effects include loss of appetite, nausea and vomiting, hair loss, fatigue, mouth sores, weight changes, fertility problems, and various toxicities including renal, neuro, cardiac, hepatic, and lung toxicity (Tao et al. 2015), which often results in non-adherence to chemotherapy protocols. The increasing demand for alleviative therapies has led to several cancer patients resorting to the use of cannabis to alleviate chemotherapy-associated side effects (Parker et al. 2011; Wasik et al. 2011). The anti-emetic and analgesic use of cannabis is well documented. Various pure and synthetic cannabinoid formulations have been approved to treat cancer-associated pain and chemotherapy-induced nausea and vomiting in patients who failed to respond to conventional anti-emetic and analgesic treatments. The most common anti-emetic treatments include corticosteroids, serotonin receptor agonists, and neurokinin receptor antagonists (May and Glode 2016). Analgesic treatment in cancer patients can range from non-steroidal anti-inflammatory drugs to weak opioids and then potent opioids, depending on pain severity (Jose et al. 2020). The types of cannabinoid formulations approved for use include THC-dominant, balanced THC/CBD, and CBD-dominant products (Steele et al. 2019). THC-dominant products include a synthetic Δ9-THC product, dronabinol (Marinol® capsules or Syndros®, the liquid formulation) and a synthetic Δ9-THC analog product, nabilone (Cesamet™). Balanced THC/CBD products include nabiximols (Sativex), which are refined extraction products that contain roughly equal amounts of THC and CBD. CBD-dominant products also include refined extraction products that contain 99% pure oil-based CBD (Epidiolex®). Various studies—many of which were controlled clinical trials—have assessed the impact of these products on cancer-related pain, as well as chemotherapy-associated nausea and vomiting. Nabiximols have frequently been studied for their effect on cancer-associated pain (Johnson et al. 2010, 2013; Portenoy et al. 2012; Lynch et al. 2014; Fallon et al. 2017; Lichtman et al. 2018), while dronabinol and nabilone have frequently been studied for their effect on a wide range of chemotherapy-induced side effects including nausea and vomiting (Zutt et al. 2006; Meiri et al. 2007; Elder and Knoderer 2015), as well as anorexia and appetite suppression (Jatoi et al. 2002; Walsh et al. 2005). These trials generally studied the efficacy of the anti-emetic agent to alleviate the chemotherapy-induced side effects, but failed to report on the potential cannabinoid–chemotherapy interactions that could potentially have affected the efficacy of the chemotherapeutic agent. Synergistic interactions between CBD and various agents have been observed in vitro, including 7-ethyl-10-hydroxycamptothecin and vinorelbine in the MCF-7 cell line (Alsherbiny et al. 2021) and paclitaxel and doxorubicin in the MCF-7 and MDA-MB-231 cell lines (Fraguas-Sánchez et al. 2020). A 2018 study by Kosgodage et al. (2018) reported a novel role for CBD as a potent inhibitor of the release of exosomes and microvesicles (EMV) from three cancer cell lines. Various studies have shown that EMV shedding from cancer cells results in increased drug efflux and contributes to their resistance to chemotherapeutic agents; therefore, an inhibition of EMV would sensitize cancer cells to chemotherapeutic agents, which various in vitro and in vivo studies have demonstrated (Federici et al. 2014; Jorfi et al. 2015; Koch et al. 2016; Muralidharan-Chari et al. 2016; Kosgodage et al. 2017). Consistent with these studies, Kosgodage et al. (2018) found that the CBD-mediated EMV inhibition sensitized HEPG2 and MDA-MB-231 cell lines to cisplatin-induced apoptosis. CBD has also been shown to sensitive glioblastoma to temozolomide, carmustine, and doxorubicin (Nabissi et al. 2012).
In addition to its palliative effects, there is evidence that suggests that cannabis may be used as a potential chemotherapeutic agent. Munson et al. (1975) showed that mice with lung adenocarcinoma exhibited decreased tumor growth when given oral THC, prompting further investigations into the anti-tumor mechanisms of THC. The in vitro anti-proliferative action of CBD was first investigated by Massi et al. (2004) in human glioma cell lines. They showed that CBD inhibited the growth of implanted human glioma cells, which highlighted the potential application of CBD as a chemotherapeutic agent. CBD and THC have since been extensively studied for their anti-tumor effects. Specific cannabis strains are characterized by the content of these two phytocannabinoids (Namdar et al. 2019). The first systematic chemotaxonomy of the various cannabis strains was proposed by Small and Beckstead (1973), who classified strains into three chemical types according to the THC and CBD content of the strain. The three chemotypes are chemotype I (high THC; > 85% of total extract), chemotype II (dominant CBD content, THC at varying concentrations), and chemotype III (high CBD; > 85% of total extract). However, this THC/CBD-based classification does not consider the role of secondary metabolites in cannabis strains. Cannabis can produce more than 600 different secondary metabolites, many of which are biologically active (Russo 2011), and have been found to enhance the bioactivity of single, purified phytocannabinoids (Russo 2011, 2019; Andre et al. 2016). This phenomenon was coined as the “entourage effect” (Blasco-Benito et al. 2018). These entourage effects are further classified as the “intra-entourage effect” which refers to enhanced bioactivity due to interaction between different phytocannabinoids, and the “inter-entourage effect”, which refers to enhanced bioactivity associated with the presence of secondary plant metabolites, primarily terpenoids. It has also been suggested that synthetic and/or pure cannabinoid formulations may induce increased health risks compared to cannabis plant extracts (Kisková et al. 2019). An example of this includes the documented increase in patients admitted to emergency rooms in the USA and Europe presenting toxic side effects attributed to the use of illicit synthetic cannabinoid products, called “Spice” (Von Bueren et al. 2008).
Cannabis is used in various forms ranging from crude extracts to purified ingredients with different THC/cannabinoid ratios, and the drug interactions that are potentially caused by cannabis not only depend on the drugs involved, but also on the chemical profile of the cannabis preparations used (Alsherbiny and Li 2019). Breast cancer is the second most commonly diagnosed cancer worldwide, second only to lung cancer and the most commonly diagnosed cancer in women. It is also ranked as the leading cause of cancer-related death in women in less developed regions; as the second leading cause of death in women in more developed regions; and as the fifth leading cause of cancer-related death overall (Ferlay et al. 2015). This indicates that breast cancer is one of the most commonly treated cancers worldwide. With the exponential increase in the use of cannabis for both its palliative and potential anti-tumor effects, more understanding regarding the effects of different cannabinoid ratios and potential interaction between cannabis and chemotherapeutic agents is required. The estrogen receptor (ER) positive (MCF-7) and estrogen receptor negative (MDA-MB-231) cell lines represent two common types of cancer, and the dose-dependent effects of THC and CBD on these cell lines is well established, making them good models for further investigation into the effect of cannabis on breast cancer. This study aimed to evaluate the potential anti-proliferative “intra-entourage effects” between purified phytocannabinoids resembling the THC:CBD content of general medicinal and recreational cannabis strains in the MCF-7 and MDA-MB-231 cell lines. This study also aimed to evaluate the potential “inter-entourage effect” between the phytocannabinoids and the phytochemicals extracted from C. Sativa in these cell lines. Additionally, both the purified cannabinoid formulations and the cannabis extract were investigated for their “entourage effect” in combination with the conventional breast cancer treatment, tamoxifen, in the MCF-7 cell line.
Materials and methods
Cell maintenance
The cell lines used in this study were the human breast cancer cell lines, MDA-MB-231 and MCF-7. The MDA-MB-231 cell line was cultured in Leibovitz’s L-15 medium (Sigma-Aldrich) supplemented with 10% (v/v) fetal bovine serum (FBS, Biowest), while the MCF-7 cell line was cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM, Biowest) supplemented with 10% (v/v) FBS. Cultures were maintained at 37 °C in a humidified atmosphere. The MCF-7 cell line was incubated in 5% CO2, while the MDA-MB-231 cell line was incubated in a CO2-free environment. All cell lines were obtained from the American Type Culture Collection (ATCC). At approximately 80% confluence, cells were either sub-cultured or seeded at a density of 10 000 cells per well in 96-well plates and allowed to attach for 24 h before treatment.
Phytochemical extraction
An aged cannabis extract (10 g) was dried and ground using a mortar and pestle. The phytochemicals were extracted by the addition of 10 mL analytical grade chloroform (Merck). After 1 h at room temperature, the chloroform was removed, and the procedure was repeated twice more. The three chloroform fractions were pooled, filtered, and evaporated under nitrogen gas to yield a resin. The resin was resuspended in 100% analytical-grade methanol (Merck), flushed with nitrogen, and stored in the dark under a vacuum at 4 °C (Ben-Shabat et al. 1998).
Phytocannabinoid quantification using RP-HPLC
Reverse-phase high-performance liquid chromatography (RP-HPLC—Agilent Technologies Infinity 1260) was used to construct calibration curves of commercial cannabinoid standards (0–100 ng/µL), including Δ9-THC (Leco) and CBD (LGC Standards). The standards were resuspended in the relevant mobile phase (0.1% (v/v) formic acid acidified acetonitrile: 0.1% (v/v) formic acid acidified water, 75:25). The elution profiles were obtained using a C18 column (Restek RaptorTM ARC-18 column; 4.6 mm × 150 mm) with isocratic elution. The injection volume was 1 µL with a flow rate of 1.5 mL/min (Ramlugon et al. 2018). The absorbance was recorded at 214 nm and chromatograms were analyzed using the DioArray detection Agilent software. The retention times and peak areas of commercial cannabinoid standards were used to putatively identify and quantify the respective cannabinoids in the cannabis extract.
Formulation of the recreational and medicinal cannabinoid combinations
To mimic a chemotype I cannabis strain (commonly referred to as a recreational cannabis strain), a high THC:CBD (9:1) ratio was prepared. Hereafter, this ratio will be referred to as the recreational cannabinoid-only formulation (RCOF) and was based on the THC and CBD content of a recreational cannabis strain, previously quantified by our research group. To mimic a chemotype II/chemotype III cannabis strain (commonly referred to as a medicinal cannabis strain), a moderate THC and high CBD ratio of THC:CBD (1:3) was prepared. Hereafter, this ratio will be referred to as the medicinal cannabinoid-only formulation (MCOF) and was based on a medicinal cannabis ratio sold in cannabis dispensaries.
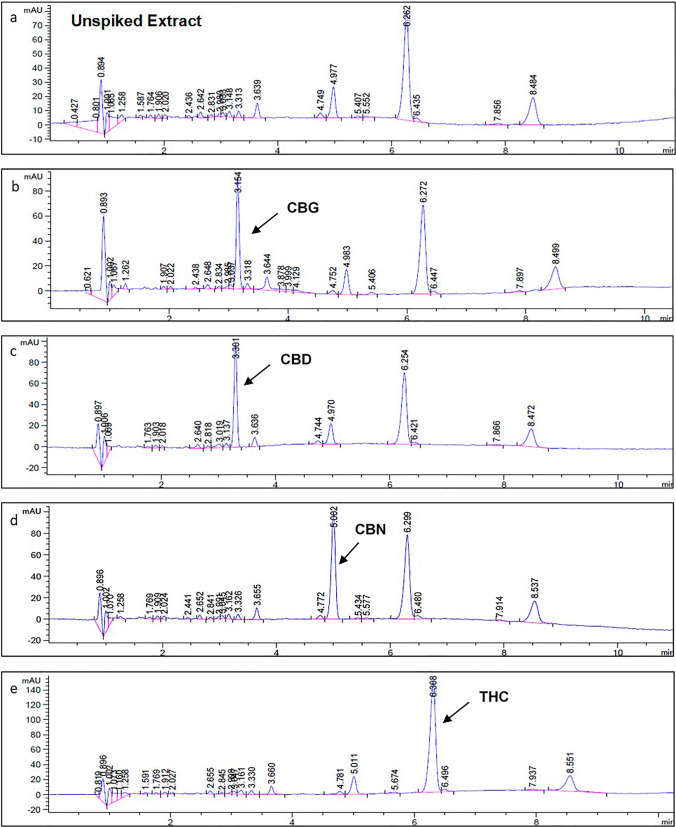
To evaluate the inter-entourage effects between the cannabinoids and phytochemicals found in cannabis, the THC and CBD content in 5 mg/ml phytochemicals (extracted as described in Section "Phytochemical extraction") was quantified using RP-HPLC (as described in Section "Phytocannabinoid quantification using RP-HPLC"). Calibration curves of the cannabinoid standards were constructed using RP-HPLC (supplementary Fig. S1). The retention times (in minutes) for CBG, CBD, CBN, and THC were 3.15 min, 3.30 min, 5.00 min, and 6.26 min, respectively. After the retention times were confirmed, the THC and CBD content in a recreational cannabis strain was quantified (Fig. 1). The cannabinoid content was adjusted to 1) the same final THC and CBD concentration (9:1) present in the RCOF, with a total THC content of 74% of the extract, and 2) the same THC and CBD concentration (1:3) present in the MCOF where the total CBD content was 83% of the extract. These ratios will hereafter be referred to as the 1) recreational cannabis extract formulation (RCEF) and 2) medicinal cannabis extract formulation (MCEF), respectively (Table 1). The interactions between cannabinoid combinations were evaluated using the median-effect method described by Chou (2010), using the CompuSyn software version 1.0 (available at: www.combosyn.com).
Fig. 1.
Representative chromatograms of recreational C. sativa extract. a Unspiked recreational cannabis extract (1 mg/ml). The extract was spiked with 20 µl (1 mg/ml) of commercial cannabinoid standards to identify and quantify the presence of the major and minor cannabinoids of interest. Cannabinoid standards included: b CBG, c CBD, d CBN and e THC. Retention times (in minutes) for CBG, CBD, CBN and THC were 3.15, 3.30, 5.00, and 6.26 min, respectively
Table 1.
The various cannabinoid formulations evaluated in this study and their respective codes
| Ratio type | Code | Formulation |
|---|---|---|
| Recreational | RCOF | THC:CBD – 9:1 |
| RCEF | THC:CBD + Phytochemicals* – 9:1 + 5 μg/mL | |
| Medicinal | MCOF | THC:CBD – 1:3 |
| MCEF | THC:CBD + Phytochemicals* – 1:3 + 5 μg/mL |
*Phytochemicals extracted from C. sativa
MTT cell viability assay
To evaluate the effects of the various treatments on the breast cancer cell lines, spent media were removed and replaced with MTT solution (0.5 mg/mL final concentration) in the respective media. The cells were incubated for 2 h at 37 °C (Mosmann 1983). The MTT reagent was removed, and dimethyl sulfoxide (DMSO, Merck) was added to solubilize the formazan crystals. The absorbance was measured at 550 nm using an EPOCH 2 microplate reader™ (BioTek® Instruments Inc., USA). Cell density standard curves were used to normalize the data to cell number.
Results and discussion
Medicinal vs. recreational cannabis formulations
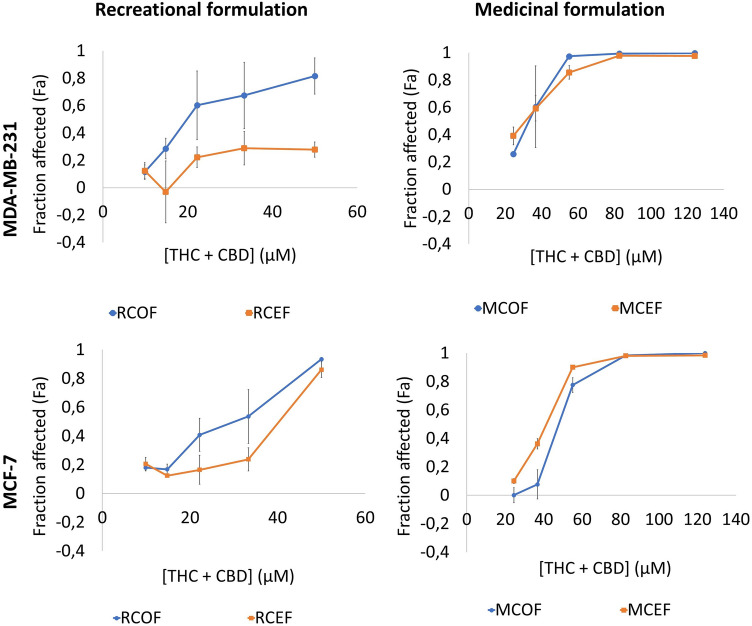
The MDA-MB-231 and MCF-7 cell lines were treated with the RCOF and MCOF (Table 1) for 48 h. These ratios were chosen to represent commonly used recreational (high THC) and medicinal (high CBD) cannabis strains. The highest concentration of the ratios tested was THC:CBD—45 µM:5 µM and 34 µM:88 µM for the recreational and medicinal formulations, respectively. The ratios were diluted to create the various concentrations used to construct the dose–response curves (Fig. 2), maintaining the respective ratios.
Fig. 2.
Screening of recreational and medicinal cannabinoid formulations both in the presence and absence of phytochemicals in the MDA-MB-231 and MCF-7 cell lines. The recreational (RCOF and RCEF) and medicinal (MCOF and MCEF) formulations were evaluated against the MDA-MB-231 and MCF-7 cell lines. The cells were treated for 48 h, and cell viability was determined using the MTT cell viability assay. Error bars represent SEM (n = 3)
The dose–response curves were linearized to create median-effect plots (Supplementary Fig. S2) to obtain the median-effect parameters at inhibitory concentrations of 50, 75 and 90%, which were used to determine the CI values. The dose–response curves were linearized by plotting the logarithm of the drug concentration (log dose) against the logarithm of the fraction of the cell population affected (Fa) against the fraction unaffected (Fu).
According to Chou (2006), an R value of 0.95–1 is required for in vitro studies. All the R values were higher than 0.95, adhering to this prerequisite. The linear equations obtained from median-effect plots were then used to determine the median-effect parameters (Table 2).
Table 2.
Median-effect plot parameters obtained for the recreational and medicinal cannabinoid-only formulations
| Cell line | Formulation | Parameters# | ||||
|---|---|---|---|---|---|---|
| m | r | Dm (μM) ± SEM | ||||
| IC50 | IC75 | IC90 | ||||
| MDA-MB-231 | RCOF | 2.135 | 0.959 | 22.9 ± 4.8 | 38.3 ± 13.4 | 64.0 ± 33.9 |
| MCOF | 4.536 | 0.957 | 29.9 ± 9.1 | 38.1 ± 11.1 | 48.6 ± 13.4 | |
| MCF-7 | RCOF | 2.793 | 0.962 | 20.8 ± 3.5 | 30.9 ± 7.1 | 45.8 ± 13.7 |
| MCOF | 9.584 | 0.991 | 49.2 ± 4.0 | 55.1 ± 3.8 | 61.8 ± 4.9 | |
#Parameters consist of the slope (m), potency (Dm) and the linear correlation coefficient of the median-effect plot (r)
When comparing the efficacy of the MCOF and RCOF formulations within each cell line, the RCOF combination had greater efficacy than the MCOF in the MDA-MB-231 cell line at the IC50 value, but the MCOF combination showed greater efficacy at the IC75 and IC90 values when compared to the RCOF. In the MCF-7 cell line, the RCOF had greater efficacy than the MCOF at all tested concentrations. When comparing the efficacy of the formulations between the two cell lines, the RCOF had a greater efficacy in the MCF-7 cell line, and the MCOF in the MDA-MB-231 cell line.
Most studies have shown that THC exerts its cytotoxic effects via the cannabinoid G-protein coupled receptors (GPCR), CB1 and CB2, while non-THC cannabinoids such as CBD exert their effect through orphans GPCRs, de-orphan GPCRs, and non-GPCRs (Afrin et al. 2020; Mangal et al. 2021). Therefore, the difference observed between the recreational and medicinal formulations across the cell lines may be explained by differences in the expression of various receptors. One of these receptors is the estrogen receptor (ER). The proliferation of the MCF-7 cell line is stimulated by 17β-estradiol (E2), resulting from the activation of ERα signal transduction pathways, and ERβ is recognized as a repressor of ERα activity (Takeda et al. 2013). Takeda et al. (2013) reported that THC increases the expression of ERβ, disrupting the proliferative E2/ERα signaling pathway (Repka et al. 2006; Takeda et al. 2013). Therefore, THC may have exerted dual anti-proliferative action via both the cannabinoid receptors (CB1 and CB2), as well as upregulation of ERβ expression in the MCF-7 ER+ cell line. This effect on the estrogen receptor would not be observed in the MDA-MB-231 cell line, as it expresses very low basal levels of ERβ and lacks ERα expression. This was confirmed by Takeda et al. (2013), who showed that, in the presence of E2, THC did not show anti-proliferative effects in the MDA-MB-231 cell line, suggesting that ERα expression is required to exhibit the anti-proliferative effects of THC. This may partially explain the increased efficacy of the RCOF combination in the MCF-7 cell line.
In contrast to THC, CBD shows decreased affinity for CB1 and CB2, but has been shown to exhibit high potency at non-CB receptors, such as the G-protein coupled receptor 55 (GPR55), transient receptor potential vanilloid type 1 (TRPV1) and transient receptor potential cation channel subfamily M member 8 (TRPM8) (De Petrocellis et al. 2011). TRPM8 has been shown to play an important role in the initiation and progression of tumors (Liu et al. 2016) where the antagonization of this receptor by the phytocannabinoid, cannabigerol, led to a concentration-dependent decrease in the viability of Caco-2 cells (Borrelli et al. 2014). CBD has also been shown to be a potent TRPM8 antagonist (De Petrocellis et al. 2011) and Liu et al. (2014) showed that the MDA-MB-231 cell line had higher expression of the TRPM8 receptor compared to the MCF-7 cell line. CBD has also been shown to be a potent antagonist of GPR55 (Mangal et al. 2021), which has also been found to be more abundantly expressed in the MDA-MB-231 cell line when compared to the MCF-7 cell line (Ford et al. 2010). GPR55 has been proposed as a potential target for cancer treatment. Ferro et al. (2018) showed that antagonization of this receptor by CBD decreased proliferation of pancreatic cancer cells and also increased responsiveness to Gemcitabine treatment in mice. These differences in expression between the cell lines, combined with the varying affinities of CBD and THC for these receptors, potentially explains the increased efficacy of the high THC formulation in the MCF-7 cell line, and the high CBD formulation in the MDA-MB-231 cell line.
The combination index (CI) values were determined for the cannabinoid combinations (Table 3) to elucidate the type of interaction between THC and CBD. The CI is a practical model used to analyze the synergy of a combination in a fixed ratio. The dose–response curves of the individual components, as well as the combined components were determined, and the dose that resulted in a specified effect (e.g., 50%, 75% and 90% inhibition in this case) are determined. The doses for the individual components required in the combination to produce the same effect could be calculated, and the synergism/addition/antagonism determined (Zhou et al. 2016). The CI was calculated using Eq. 1.
| 1 |
(D)1 and (D)2 refer to the dose of each drug in the combination required to cell death by a certain level. (Dx)1 and (Dx)2 refer to the dose of the individual drugs required to induce the same level of cell death calculated using Eq. 2.
| 2 |
Dm refers to the inhibitory concentration (potency), fa refers to the fraction of the cell population affected by the drug, and m refers to the slope of the median-effect equation. The dose–response curves and corresponding values for the parameters needed to calculate the dose of the individual drugs (THC and CBD) were obtained from results that were published in a previous paper by our research group (Schoeman et al. 2020).
Table 3.
Combination index values of the recreational and medicinal cannabinoid-only formulations determined using the median-effect plot parameters
| Cell line | Formulation | Combination index* | ||
|---|---|---|---|---|
| 50% | 75% | 90% | ||
| MDA-MB-231 | RCOF | 0.843 | 1.044 | 1.310 |
| MCOF | 1.740 | 1.316 | 1.015 | |
| MCF-7 | RCOF | 0.715 | 0.762 | 0.817 |
| MCOF | 2.674 | 1.867 | 1.313 | |
*CI < 1 indicates synergism, CI = 1 indicates an additive effect and CI > 1 indicates antagonism
The CI values for the RCOF were generally lower than those for the MCOF in both cell lines. The RCOF combination displayed a moderately synergistic interaction in the MDA-MB-231 cell line (CI < 1) at 50% growth inhibition. The RCOF combination displayed moderately synergistic interactions at 50%, 75% and 90% growth inhibition in the MCF-7 cell line. This synergism measured between THC and CBD suggested that an “intra-entourage effect” was evident. In contrast, the MCOF resulted in antagonistic interactions against both of the cell lines tested, with the CI values being lower in the MDA-MB-231.
Phytochemicals and cannabis efficacy
To determine the effect of the phytochemicals in C. sativa on cannabinoid efficacy, a recreational cannabis extract was supplemented with THC and CBD to the same concentrations present in the RCOF and MCOF, thereby creating the RCEF and MCEF combinations, respectively. The MDA-MB-231 and MCF-7 cell lines were treated with the RCEF and MCEF, and their efficacy was compared to the respective cannabinoid-only formulations (Fig. 2). The highest concentration of the ratios tested were THC:CBD:phytochemicals—45 µM:5 µM:5 μg/mL and 34 µM:88 µM:5 μg/mL for the recreational and medicinal formulations, respectively. The ratios were then diluted to create the concentrations used to construct the dose–response curves, maintaining the various ratios.
The anti-proliferative effects of the medicinal cannabinoid formulations remained relatively unchanged in the presence (MCEF) and absence (MCOF) of the phytochemicals. The presence of the phytochemicals (RCEF) decreased the efficacy of the recreational (RCOF) formulation in both cell lines. Although several studies have reported the enhanced bioactivity of cannabinoids in the presence of the phytochemicals in C. sativa (Russo 2011, 2019; Stuyt 2018), an “inter-entourage effect” was not evident between the phytocannabinoids and phytochemicals in this study. The difference in the effect observed between the medicinal and recreational formulations, in terms of cell death, is most likely due to the differences in the THC and CBD content of the recreational and medicinal formulations. A decrease in efficacy of the recreational strain in the presence of the phytochemicals was seen in both the ER+ and ER− cell lines, suggesting that this decrease in efficacy was most likely not a result of ER signaling. As mentioned previously, the effects of THC are predominantly mediated by CB1 and CB2 activation and since the phytochemical-induced decrease in efficacy was only observed in the recreational, but not the medicinal formulation, it suggests that the phytochemicals present in the C. sativa extract may oppose the effects of the RCOF combination in a cannabinoid receptor-dependent mechanism, due to the THC-rich content of the RCEF combination. Alternatively, one particular study found that the anti-proliferative activity of THC was only enhanced when combined with its co-related terpenoids, while the combination with other terpenoids (i.e., CBDA-related terpenoids) inhibited the anti-proliferative activity of THC. They also highlighted that the specificity of the terpenoid content was less stringent when combined with CBD (Santiago et al. 2019), supporting the results obtained in this study, where the addition of phytochemicals had little effect on the high CBD (MCEF) formulation (Fig. 2). This suggests that an “inter-entourage effect” is dependent on both the type of cannabinoid formulation (recreational or medicinal) and the phytochemical profile of the specific strain.
Cannabis and tamoxifen efficacy
Cancer patients use both pure cannabinoids and cannabis extracts in the treatment of chemotherapy-associated side effects (Ware et al. 2008). Therefore, the cannabinoid-only (RCOF and MCOF) and cannabinoid-extract (RCEF and MCEF) combinations were evaluated in combination with the common endocrine treatment, tamoxifen (Tam).
To determine the IC50 value of Tam against the MCF-7 cell line, a dose–response curve was constructed in the range of 0–88 μM. Tam was combined at a concentration of 2 × the IC50 value (18 μM × 2) with the various cannabinoid ratios. The highest concentrations for the ratios tested were THC:CBD:Tam—45 µM:5 µM:36 μM and 34 µM:88 µM:36 μM for the recreational and medicinal formulations, respectively. Tam alone, as well in combination with the recreational and medicinal cannabinoid formulations, was diluted to construct the dose–response curves, maintaining the various ratios. This was only completed against the ER+ MCF-7 cell line, since tamoxifen targets the estrogen signaling pathway.
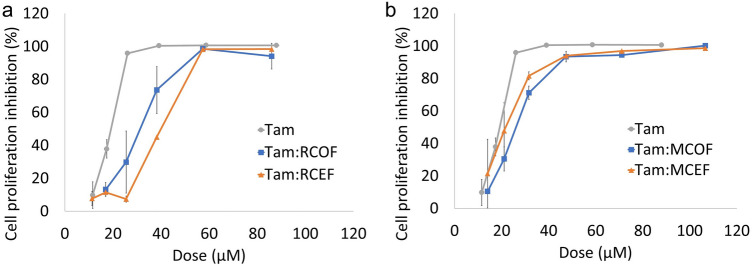
The combination of tamoxifen with both the recreational and medicinal cannabinoid formulations resulted in a decrease in tamoxifen efficacy (Fig. 3). This was highlighted by the calculated IC50 values (Table 4), which showed that the lowest IC50 was obtained for tamoxifen treatment alone and that combination with any of the cannabinoid formulations decreased the toxicity of tamoxifen. This decrease in toxicity was observed to a lesser degree when tamoxifen was combined with the medicinal cannabinoid formulations (Fig. 3b) as opposed to the recreational formulations (Fig. 3a), which is shown by the greater increase in the IC50 when combined with the recreational formulations. As mentioned previously, various studies have found synergistic interactions between CBD and various chemotherapeutic agents, including -ethyl-10-hydroxycamptothecin, vinorelbine, (Alsherbiny et al. 2021), paclitaxel and doxorubicin (Fraguas-Sánchez et al. 2020), as well as increased sensitivity to cisplatin, temozolomide, carmustine, and doxorubicin (Nabissi et al. 2012). No synergistic interactions or increase in sensitivity was observed in this study; however, this may be due to several reasons including the type of chemotherapeutic agent used or the use of a THC:CBD ratio, as opposed to CBD alone, which was used in the above-mentioned studies. With regard to the effect of the phytochemicals on tamoxifen efficacy, the high THC formulation had greater efficacy in the absence of the phytochemicals. In contrast, the high CBD formulation had greater efficacy in the presence of the phytochemicals (MCEF). This further supports the theory that the phytochemicals may oppose cannabinoid efficacy in a CB receptor-dependent mechanism, due to the decreased efficacy of the high THC formulation when combined with the phytochemicals.
Fig. 3.
Dose–response curves of tamoxifen alone and in combination with (a) recreational and (b) medicinal cannabinoid-only and cannabinoid-extract formulations. The MCF-7 cell line was treated with a various concentrations of Tamoxifen alone, as well as tamoxifen in combination with a recreational cannabinoid-only and cannabinoid-extract formulation and b various concentrations of tamoxifen alone, as well as tamoxifen in combination with a medicinal cannabinoid-only and cannabinoid-extract formulation. The dose (µM) refers to the combined concentration of Tam + CBD + THC. Error bars represent SEM (n = 3)
Table 4.
IC50 values of tamoxifen with the various recreational and medicinal cannabinoid formulations, determined using GraphPad Prism® 5 (version 5.01) software
| Treatment | IC50 (μM) ± SEM | |
|---|---|---|
| Tam only | 18.2 ± 0.8 | |
| Recreational | Tam:RCOF | 33.9 ± 4.5 |
| Tam:RCEF | 39.1 ± 0.6 | |
| Medicinal | Tam:MCOF | 26.9 ± 1.9 |
| Tam:MCEF | 23.2 ± 0.9 |
Interestingly, tamoxifen has been shown to act as an inverse agonist of the cannabinoid receptors 1 and 2, thus inducing a pharmacological response opposite to that of an agonist (Chang 2012). This suggests that the reduced tamoxifen efficacy observed when combined with the cannabinoid formulations may have been mediated via the CB1 and CB2 receptors. Since the THC-mediated effects are cannabinoid receptor dependent, the inverse agonistic binding of tamoxifen may counteract the anti-proliferative effects of THC. This potentially explains the greater decrease in efficacy with the recreational cannabinoid formulations when compared to the medicinal cannabinoid formulations, which contained a lower THC content. The anti-proliferative effects of CBD are cannabinoid receptor-independent, explaining the enhanced anti-proliferative effect of the combination between tamoxifen and the medicinal cannabinoid formulations when compared to combination with the recreational cannabinoid formulations. Overall, these results suggested that the efficacy of tamoxifen is greatest when used as a monotherapy, as the efficacy decreases when used in combination with a cannabis extract.
Conclusions
With regard to the comparative effect of the RCOF and MCOF on the two cell lines tested, the RCOF was more effective against the MCF-7 cell line, displaying moderately synergistic interactions and provided evidence that an “intra-entourage effect” may be present. The RCOF contained a high THC content, and it was hypothesized that its anti-proliferative effect was more prominent in the ER+ MCF-7 cell line due to THC exerting its anti-proliferative effects via the ER (Blasco-Benito et al. 2018). The MCOF, with its high CBD content that exerts its effects independent of the CB receptors and ER (Takeda et al. 2013), was more effective against the ER− MDA-MB-231 cell line. CBD has been documented to exert its anti-proliferative effects via various receptors including—but not limited to—the GPR55 and TRM8 receptors, both of which are more highly expressed in the MDA-MB-231 cell line when compared to the MCF-7 cell line. This may explain the enhanced effect of the MCOF in the MDA-MB-231 cell line. Overall, the data highlights the need for the development of specialized cannabinoid formulations to cater for specific cancer cell types, supporting the notion for patient- and cancer-specific treatments as opposed to a “one-size-fits-all” therapy regime.
Concerning the effect of treatment with the RCEF and MCEF combinations, no “inter-entourage effects” were present in either of the cell lines, contradicting previous reports that showed evidence of an inter-entourage effect between phytocannabinoids and terpenes (Blasco-Benito et al. 2018; Russo 2019). No difference in the anti-proliferative effects of the medicinal formulations in the presence (MCEF) and absence (MCOF) of phytochemicals was observed, while the presence of the phytochemicals showed a decreased anti-proliferative effect between the RCEF and RCOF combinations, suggesting that the phytochemicals present in the C. sativa extract may antagonize the CB receptor-mediated effects of THC. With regard to the effect of the combination treatment of tamoxifen with the various cannabinoid formulations, the results indicated that the cannabinoid formulations generally reduced the efficacy of tamoxifen, with a more pronounced reduction with the recreational cannabis formulations.
The anti-emetic and analgesic prescription of cannabinoids is well-documented to alleviate cancer-associated pain and chemotherapy-induced side effects. With several cancer patients using cannabis-based products in the form of extracts, as opposed to pure cannabinoid formulations, the results of this study suggest that medicinal cannabis formulations should be recommended for two main reasons: (1) a high CBD content is crucial to counteract the potential psychosis induced by THC and (2) the potential for reduced chemotherapeutic treatment efficacy is minimized. Various studies have shown the beneficial effects of combined CBD and chemotherapeutic drug treatment; however, this same beneficial effect was not observed in this study when tamoxifen was combined with a THC:CBD ratio, or with a cannabis extract. This suggests that the synergistic effect observed by previous studies may be limited to combination with CBD alone. This means that patients should maintain caution when combining their chemotherapy regime with cannabis extracts with an unknown THC:CBD ratio and phytochemical profile, due to the potential for undesired interactions. This study highlights the need for research to holistically investigate the safety and efficacy of cannabinoids, and more specifically cannabis extracts, as anti-emetic agents as well as their effect on the efficacy on the chemotherapeutic agent it is combined with.
Limitations of the study
This paper served as an initial screening to observe the overall effect of general recreational and medicinal THC:CBD ratios, as well as a Cannabis extract on breast cancer cell lines and their effect on tamoxifen efficacy. Many of the conclusions drawn from the data are based on previous studies, as no quantitative markers were completed to confirm the findings. Another limitation of this study was the combination of tamoxifen with the THC:CBD ratios only, and not with the individual cannabinoids. THC and CBD alone should be tested in combination with tamoxifen to determine the effect of the individual cannabinoids on tamoxifen efficacy, especially due to the synergistic interactions between CBD and various chemotherapeutic drugs that have been observed in previous studies. Additionally, only one chemotherapeutic drug was tested. Ideally, multiple drugs should be tested to determine if the trends are observed across multiple agents, or if the interactions are drug specific. All of these limitations are opportunities for further investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the South African community for contacting our research group and inspiring this investigation when they discovered our research interests. We hope our limited findings will assist them in making informed health decisions. R. Schoeman and A. de la Harpe would like to acknowledge Nelson Mandela University and the National Research Foundation (NRF) for the provision of research scholarships. Running costs for this study were covered by the Nelson Mandela University (NMU).
Author contributions
RS: conceptualization; methodology; formal analysis; investigation. ADLH: writing—original draft, visualization. NB: methodology; supervision; writing—review and editing. CLF: conceptualization; methodology; supervision; writing—review and editing; resources; project administration; funding acquisition.
Funding
The funding has been received from National Research Foundation.
Declarations
Conflict of interest
All co-authors declare that they have no conflict of interest.
References
- Afrin F, Chi M, Eamens AL, Duchatel RJ, Douglas AM, Schneider J, Gedye C, Woldu AS, Dun MD. Can hemp help? Low-THC cannabis and non-THC cannabinoids for the treatment of cancer. Cancers. 2020;12:1033. doi: 10.3390/cancers12041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines. 2019;6:3. doi: 10.3390/medicines6010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsherbiny MA, Bhuyan DJ, Low MN, Chang D, Li CG. Synergistic interactions of cannabidiol with chemotherapeutic drugs in MCF7 cells: mode of interaction and proteomics analysis of mechanisms. Int J Mol Sci. 2021;22:10103. doi: 10.3390/ijms221810103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre CM, Hausman J-F, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee M-H, Vogel Z, Bisogno T, de Petrocellis L, di Marzo V, Mechoulam R. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353:23–31. doi: 10.1016/S0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- Blasco-Benito S, Seijo-Vila M, Caro-Villalobos M, Tundidor I, Andradas C, García-Taboada E, Wade J, Smith S, Guzmán M, Pérez-Gómez E. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem Pharmacol. 2018;157:285–293. doi: 10.1016/j.bcp.2018.06.025. [DOI] [PubMed] [Google Scholar]
- Borrelli F, Pagano E, Romano B, Panzera S, Maiello F, Coppola D, de Petrocellis L, Buono L, Orlando P, Izzo AA. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis. 2014;35:2787–2797. doi: 10.1093/carcin/bgu205. [DOI] [PubMed] [Google Scholar]
- Chang M. Tamoxifen resistance in breast cancer. Biomol Therap. 2012;20:256. doi: 10.4062/biomolther.2012.20.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Chou T-C. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- de Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JJ, Knoderer HM. Characterization of dronabinol usage in a pediatric oncology population. J Pediat Pharmacol Therap. 2015;20:462–467. doi: 10.5863/1551-6776-20.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon MT, Albert Lux E, Mcquade R, Rossetti S, Sanchez R, Sun W, Wright S, Lichtman AH, Kornyeyeva E. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. Br J Pain. 2017;11:119–133. doi: 10.1177/2049463717710042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, D'Ilio S, Lugini L, Violante N, Azzarito T, Majorani C, Brambilla D, Fais S. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE. 2014;9:e88193. doi: 10.1371/journal.pone.0088193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- Ferro R, Adamska A, Lattanzio R, Mavrommati I, Edling CE, Arifin SA, Fyffe CA, Sala G, Sacchetto L, Chiorino G, de Laurenzi V, Piantelli M, Sansom OJ, Maffucci T, Falasca M. GPR55 signalling promotes proliferation of pancreatic cancer cells and tumour growth in mice, and its inhibition increases effects of gemcitabine. Oncogene. 2018;37:6368–6382. doi: 10.1038/s41388-018-0390-1. [DOI] [PubMed] [Google Scholar]
- Ford LA, Roelofs AJ, Anavi-Goffer S, Mowat L, Simpson DG, Irving AJ, Rogers MJ, Rajnicek AM, Ross RA. A role for L-alpha-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br J Pharmacol. 2010;160:762–771. doi: 10.1111/j.1476-5381.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraguas-Sánchez AI, Fernández-Carballido A, Simancas-Herbada R, Martin-Sabroso C, Torres-Suárez AI. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int J Pharm. 2020;574:118916. doi: 10.1016/j.ijpharm.2019.118916. [DOI] [PubMed] [Google Scholar]
- Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, Pundaleeka S, Kardinal CG, Fitch TR, Krook JE, Novotny PJ, Christensen B. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567–573. doi: 10.1200/jco.2002.20.2.567. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symp Manag. 2010;39:167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. 2013;46:207–218. doi: 10.1016/j.jpainsymman.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Jorfi S, Ansa-Addo EA, Kholia S, Stratton D, Valley S, Lange S, Inal J. Inhibition of microvesiculation sensitizes prostate cancer cells to chemotherapy and reduces docetaxel dose required to limit tumor growth in vivo. Sci Rep. 2015;5:13006. doi: 10.1038/srep13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose A, Thomas L, Baburaj G, Munisamy M, Rao M. Cannabinoids as an alternative option for conventional analgesics in cancer pain management: a pharmacogenomics perspective. Indian J Palliat Care. 2020;26:129–133. doi: 10.4103/ijpc.ijpc_155_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisková T, Mungenast F, Suváková M, Jäger W, Thalhammer T. Future aspects for cannabinoids in breast cancer therapy. Int J Mol Sci. 2019;20:1673. doi: 10.3390/ijms20071673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R, Aung T, Vogel D, Chapuy B, Wenzel D, Becker S, Sinzig U, Venkataramani V, von Mach T, Jacob R, Truemper L, Wulf GG. Nuclear trapping through inhibition of exosomal export by indomethacin increases cytostatic efficacy of doxorubicin and pixantrone. Clin Cancer Res. 2016;22:395–404. doi: 10.1158/1078-0432.ccr-15-0577. [DOI] [PubMed] [Google Scholar]
- Kosgodage US, Trindade RP, Thompson PR, Inal JM, Lange S. Chloramidine/bisindolylmaleimide-I-mediated inhibition of exosome and microvesicle release and enhanced efficacy of cancer chemotherapy. Int J Mol Sci. 2017 doi: 10.3390/ijms18051007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosgodage US, Mould R, Henley AB, Nunn AV, Guy GW, Thomas EL, Inal JM, Bell JD, Lange S. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front Pharmacol. 2018;9:889–889. doi: 10.3389/fphar.2018.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W, Wright S, Kornyeyeva E, Fallon MT. Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J Pain Symp Manage. 2018;55:179–188.e1. doi: 10.1016/j.jpainsymman.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen Y, Shuai S, Ding D, Li R, Luo R. TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3β pathway. Tumor Biol. 2014;35:8969–8977. doi: 10.1007/s13277-014-2077-8. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wu H, Wei Z, Wang X, Shen P, Wang S, Wang A, Chen W, Lu Y. TRPM8: a potential target for cancer treatment. J Cancer Res Clin Oncol. 2016;142:1871–1881. doi: 10.1007/s00432-015-2112-1. [DOI] [PubMed] [Google Scholar]
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47:166–173. doi: 10.1016/j.jpainsymman.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Mangal N, Erridge S, Habib N, Sadanandam A, Reebye V, Sodergren MH. Cannabinoids in the landscape of cancer. J Cancer Res Clin Oncol. 2021;147:2507–2534. doi: 10.1007/s00432-021-03710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell K, Fairchild A, Legerrier B, Sinha R, Baker S, Liu H, Ghose A, Olivotto I, Kerba M. Rates of cannabis use in patients with cancer. Curr Oncol. 2018;25:219–225. doi: 10.3747/co.25.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Ceruti S, Colombo A, Abbracchio MP, Parolaro D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther. 2004;308:838–845. doi: 10.1124/jpet.103.061002. [DOI] [PubMed] [Google Scholar]
- May MB, Glode AE. Dronabinol for chemotherapy-induced nausea and vomiting unresponsive to antiemetics. Cancer Manag Res. 2016;8:49. doi: 10.2147/CMAR.S81425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang HM, Baranowski V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23:533–543. doi: 10.1185/030079907x167525. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mouhamed Y, Vishnyakov A, Qorri B, Sambi M, Frank SS, Nowierski C, Lamba A, Bhatti U, Szewczuk MR. Therapeutic potential of medicinal marijuana: an educational primer for health care professionals. Drug Healthc Patient Saf. 2018;10:45. doi: 10.2147/DHPS.S158592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson A, Harris L, Friedman M, Dewey W, Carchman R. Antineoplastic activity of cannabinoids. J Natl Cancer Inst. 1975;55:597–602. doi: 10.1093/jnci/55.3.597. [DOI] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Kohan HG, Asimakopoulos AG, Sudha T, Sell S, Kannan K, Boroujerdi M, Davis PJ, Mousa SA. Microvesicle removal of anticancer drugs contributes to drug resistance in human pancreatic cancer cells. Oncotarget. 2016;7:50365–50379. doi: 10.18632/oncotarget.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabissi M, Morelli MB, Santoni M, Santoni G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2012;34:48–57. doi: 10.1093/carcin/bgs328. [DOI] [PubMed] [Google Scholar]
- Namdar D, Voet H, Ajjampura V, Nadarajan S, Mayzlish-Gati E, Mazuz M, Shalev N, Koltai H. Terpenoids and phytocannabinoids co-produced in Cannabis sativa strains show specific interaction for cell cytotoxic activity. Molecules. 2019;24:3031. doi: 10.3390/molecules24173031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Rock EM, Limebeer CL. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol. 2011;163:1411–1422. doi: 10.1111/j.1476-5381.2010.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergam SA, Woodfield MC, Lee CM, Cheng G-S, Baker KK, Marquis SR, Fann JR. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer. 2017;123:4488–4497. doi: 10.1002/cncr.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, McQuade R, Wright S, Fallon MT. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13:438–449. doi: 10.1016/j.jpain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Ramlugon S, Levendal RA, Frost C. Time-dependent effect of phytocannabinoid treatments in fat cells. Phytother Res. 2018;32:1080–1089. doi: 10.1002/ptr.6047. [DOI] [PubMed] [Google Scholar]
- Repka MA, Munjal M, Elsohly MA, Ross SA. Temperature stability and bioadhesive properties of Δ9-tetrahydrocannabinol incorporated hydroxypropylcellulose polymer matrix systems. Drug Dev Ind Pharm. 2006;32:21–32. doi: 10.1080/03639040500387914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: no “strain”, no gain. Front Plant Sci. 2019;9:1969. doi: 10.3389/fpls.2018.01969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadeh CE, Rustem DR. Medical marijuana use in a community cancer center. J Oncol Pract. 2018;14:e566–e578. doi: 10.1200/JOP.18.00057. [DOI] [PubMed] [Google Scholar]
- Santiago M, Sachdev S, Arnold JC, McGregor IS, Connor M. Absence of entourage: terpenoids commonly found in cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannab Cannabin Res. 2019;4:165–176. doi: 10.1089/can.2019.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeman R, Beukes N, Frost C. Cannabinoid combination induces cytoplasmic vacuolation in mcf-7 breast cancer cells. Molecules. 2020;25:4682. doi: 10.3390/molecules25204682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E, Beckstead H. Cannabinoid phenotypes in Cannabis sativa. Nature. 1973;245:147–148. doi: 10.1038/245147a0. [DOI] [PubMed] [Google Scholar]
- Steele G, Arneson T, Zylla D. A comprehensive review of cannabis in patients with cancer: availability in the USA, general efficacy, and safety. Curr Oncol Rep. 2019;21:10. doi: 10.1007/s11912-019-0757-7. [DOI] [PubMed] [Google Scholar]
- Stuyt E. The problem with the current high potency THC marijuana from the perspective of an addiction psychiatrist. Missour Med. 2018;115:482. [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Yoshida K, Nishimura H, Harada M, Okajima S, Miyoshi H, Okamoto Y, Amamoto T, Watanabe K, Omiecinski CJ. Δ9-Tetrahydrocannabinol disrupts estrogen-signaling through up-regulation of estrogen receptor β (ERβ) Chem Res Toxicol. 2013;26:1073–1079. doi: 10.1021/tx4000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. The Breast. 2015;24:S149–S153. doi: 10.1016/j.breast.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bueren A, Schlumpf M, Lichtensteiger W. Delta (9)-tetrahydrocannabinol inhibits 17β-estradiol-induced proliferation and fails to activate androgen and estrogen receptors in MCF7 human breast cancer cells. Anticancer Res. 2008;28:85–89. [PubMed] [Google Scholar]
- Walsh D, Kirkova J, Davis MP. The efficacy and tolerability of long-term use of dronabinol in cancer-related anorexia: a case series. J Pain Symp Manag. 2005;30:493–495. doi: 10.1016/j.jpainsymman.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Ware MA, Daeninck P, Maida V. A review of nabilone in the treatment of chemotherapy-induced nausea and vomiting. Ther Clin Risk Manag. 2008;4:99. doi: 10.2147/TCRM.S1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasik A, Almestrand S, Wang X, Hultenby K, Dackland Å-L, Andersson P, Kimby E, Christensson B, Sander B. WIN55, 212–2 induces cytoplasmic vacuolation in apoptosis-resistant MCL cells. Cell Death Dis. 2011;2:e225–e225. doi: 10.1038/cddis.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Seto SW, Chang D, Kiat H, Razmovski-Naumovski V, Chan K, Bensoussan A. Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front Pharmacol. 2016 doi: 10.3389/fphar.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutt M, Hänssle H, Emmert S, Neumann C, Kretschmer L. Dronabinol for supportive therapy in patients with malignant melanoma and liver metastases. Hautarzt. 2006;57:423–427. doi: 10.1007/s00105-005-1063-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.