Abstract
Attention Deficit Hyperactivity Disorder (ADHD) is a common neurodevelopmental disorder that, in addition to inattention, excessive activity, or impulsivity, makes it difficult for children to process facial emotions and thus to interact with their peers. Here we analyze neuronal networks of children with this disorder by means of the phase-locking value (PLV) method. In particular, we determine the level of phase synchronization between 62 EEG channels of 22 healthy boys and 22 boys with ADHD, recorder whilst observing facial emotions of anger, happiness, neutrality, and sadness. We construct neuronal networks based on the gamma sub-band, which according to previous studies, shows the highest response to emotional stimuli. We find that the functional connectivity of the frontal and occipital lobes in the ADHD group is significantly (P-value < 0.01) higher than in the healthy group. More functional connectivity in these lobes shows more phase synchronization between the neurons of these brain regions, representing some problems in the brain emotional processing center in the ADHD group. The shortest path lengths in these lobes are also significantly (P-value < 0.01) higher in the ADHD group than in the healthy group. This result indicates less efficiency of information transmission and segregation in occipital and frontal lobes of ADHD neuronal networks, responsible for visual and emotional processing in the brain, respectively. We hope that our approach will help obtain further insights into ADHD with methods of network science.
Keywords: ADHD, Phase locking value, Functional connectivity, EEG, Facial emotions
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is one of the most common childhood disorders which creates many problems in children’s lives and usually lasts into adulthood. It is associated with low social and educational functioning. This behavioral disorder, which is more common in males, affects about 8–10% of school-age children and is characterized by inattention, hyperactivity, and impulsive behavior (Rubia 2018). The exact causes of ADHD are not yet known. Some of this disorder’s physiological factors include decreased dopamine in the brain’s striated and anterior regions as well as a connectivity disorder in the cerebral cortex’s frontal areas. Therefore, it can be said that behavioral disturbances in these children are not only due to abnormalities in different regions of the brain but also due to abnormal changes in the structural and functional connectivity of these areas (Ahmadlou and Adeli 2010). Another effect of ADHD is the difficulty in recognizing others’ emotions, which leads to social unrest in this group. Most children who suffer from this disorder experience depression, anxiety, and a lack of self-confidence in life. In this regard, recognizing non-verbal cues (such as facial expressions) that convey others’ feelings is especially important because it helps them behave towards others (Razavi et al. 2017).
In recent years, much attention has been paid to the dynamical analyses of neural processes. These dynamical analyses can be divided into three main categories: analysis of neural processes based on (1) neuronal signals, (2) neuronal models, and (3) a combination of these two methods (Parastesh et al. 2019; Zhang et al. 2013). One of the techniques used to investigate ADHD disorder is to measure the degree of synchronization between individuals’ neuronal signals with this disorder. Several studies have used measuring the synchronization between neuronal signals of healthy and ADHD individuals using different modalities such as MEG, fMRI, and EEG. According to these studies, the EEG signal can be considered as a better choice to examine the ADHD disorder, especially in children, due to its non-invasiveness and high temporal resolution (An et al. 2013; Dockstader et al. 2008; Yu 2013).
One well-known approach that researchers have considered in recent years to study ADHD disorder is to measure the synchronization in dynamical neuronal networks, modeled with the help of neuronal signals such as EEG signals of both healthy and ADHD groups. There are many methods to estimate neuronal networks with the help of EEG signals, including Pearson correlation coefficient, coherence coefficient, phase lag index (PLI), and phase-locking value (PLV) (Wang et al. 2020). Linear analysis based on Pearson correlation coefficient and coherence coefficients methods is useful for estimating stationary signals. However, EEG signals are practically nonstationary during emotional processing, so analysis based on these methods is limited (Wang et al. 2020). Among the techniques for measuring brain signals’ synchronization, PLV can reflect the amount of phase interactions between time series and has a low computational cost (Dasdemir et al. 2017). So far, this method is used in several studies to investigate brain behavior. For instance, Dasdemir et al. conducted a study to examine the brain’s functional connectivity under positive and negative emotions using the phase-locking value. In this study, three different emotional stimulation types, audio-only, video-only, and audio-video, were used. The results showed a significant difference (at a significance level of 0.01) between phase-locking values, only under negative and neutral emotions for all types of video-only and audio-only stimuli (Dasdemir et al. 2017). Wang et al. used a combination of functional integration and functional segregation to analyze individuals’ neuronal networks under positive and negative emotions using the phase-locking value. This study showed that phase synchronization between EEG signals under negative emotions is higher than positive emotions. According to the results of this study, the gamma frequency band has shown the highest response during emotional stimulation of the face (Wang et al. 2020). In a study conducted by Gong et al., the neuronal networks of professional and non-athlete shooters in the delta, theta, alpha, and beta frequency bands were examined using the phase-locking value. The results showed significant differences in clustering coefficient and small-world characteristics of professional athletes compared to non-professional ones (at a significant level of 0.01) (Gong et al. 2019). Many studies have reported gamma frequency band activity in the study of facial emotion perception (Razavi et al. 2017; Balconi and Pozzoli 2009). Sato et al. reported more gamma band activity in response to emotional faces than in neutral ones (Sato et al. 2011).
In the present study, the complex dynamics of the neuronal network of ADHD children are investigated during facial emotion processing. EEG signals are recorded from 22 healthy boys and 22 age and sex-matched ADHD children while observing four types of emotions anger, happiness, neutrality, and sadness. After dividing the signal’s frequency band into five frequency sub-bands, all analyses are performed on the gamma band as an appropriate band according to previous studies (Razavi et al. 2017; Balconi and Pozzoli 2009; Sato et al. 2011). Based on the results of these studies, the gamma band is more sensitive to facial emotional stimuli. Then the PLVs between every electrode channel pairs are used to construct neuronal networks. Graph characteristics, including clustering coefficient and shortest path length, are extracted from the weighted neuronal networks. At last, using the Repeated Measure ANOVA test, significant differences in functional connectivity and graph features between two healthy and ADHD groups in four types of emotion are examined.
Materials and methods
Participants
In this study, EEG signals recorded from 22 untreated boys diagnosed with ADHD (7 to 11 years old) as the experimental group and 22 healthy children of the same age and sex as the control group are investigated. All participants are assessed using criteria such as the Conner’s Parent Rating Scale Questionnaire (CPRS) (Conners et al. 1998) and the Child Symptoms Inventory-4 Questionnaire (CSI-4) (Gadow and Sprafkin 1997) for initial screening of ADHD. Also, clinical evaluations are performed based on the DSM-V criterion, and finally, individuals are assigned to each of the two groups.
Data collection and experimental procedure
In this study, recorded signals in (Razavi et al. 2017) are used. Signals were obtained by a 62-channel electrode cap based on the standard 10–20 system with a sampling frequency of 512 Hz. The recording process was such that the participant sat in a chair in front of the monitor. Four facial expressions (anger, happiness, neutrality, and sadness) were displayed on the screen. In this phase, to assess the individual’s ability to understand the face’s emotions and then recognize and provide the answer, the participant, after observing the stimulus, presented his response by pressing the relevant key. A total of 80 images, including four facial expressions (anger, happiness, neutrality, and sadness), were selected from the Cohn-Kanade Database (Lucey et al. 2010). The pictures were shown to forty healthy children, then each of them determined their response using a questionnaire. Finally, 24 images (including both men and women) were selected. The recording protocol was shown by displaying the word ”start” on the screen for 2000 ms. Each trial consisted of an image of a facial expression that was randomly selected from four facial expressions. A time interval of 1500–1700ms was considered between the trials. Therefore, the duration of each trial was msms.
Preprocessing
After recording the EEG signals, a third-order Butterworth filter with cut-off frequencies of 1 and 80 Hz is used to remove the low and high-frequency artifacts. A notch filter with a cut-off frequency of 50 Hz is applied to remove the power line noise. To remove ocular artifacts, the Independent Component Analysis (ICA) method is used. Considering the stationary assumption, the signal is held in each trial for 1 second before and 2 seconds after visual stimulation. Finally, epochs with statistical characteristics more than five standard deviations far from the rest of the epochs are identified. At the expert’s diagnosis, inappropriate epochs are discarded as outlier data, and the remaining epochs are used for further analysis. The entire signal frequency band is subdivided into five sub-bands, delta, theta, alpha, beta, and gamma, using the Daub7 wavelet transform.
Volume conduction effect
One of the fundamental issues in studying brain connectivity using EEG signals is the volume conduction effect. Due to this effect, each neural source’s activity is recorded by electrodes at different locations on the scalp and causes fake connectivity between different channel signals. To degrade the impact of volume conduction, the current source density (CSD) method is used. In the CSD method, each channel’s spatial properties are obtained using the second spatial derivative while ignoring the effect of other channels. In this research, the CSD toolbox is used to reduce the volume conduction effect (Perrin et al. 1989).
Functional connectivity
This section aims to investigate ADHD disorder by constructing neuronal networks of both healthy and ADHD groups in the gamma band under four types of emotions. The number of nodes, edges, and the weights of each edge should be determined to this end. In this study, the number of EEG recording electrodes shows the number of the network’s nodes. There are many different methods to characterize the number of network edges and to calculate the weights between each node. The phase-locking value is one of the most common approaches to determine the weights between each node using the phase synchronization between EEG signals (Kong et al. 2017). Fluctuations in brain signals are expected to contain more important information than their amplitude. By establishing the relationship between the phase synchronization of EEG signals and the neuronal network’s physiological characteristics, it is possible to analyze the phase synchronization between all possible pairs of electrode channels and form a neuronal connectivity matrix (Kong et al. 2019).
In this study, the phase-locking value method is used to measure the phase synchronization between EEG signals, which show a kind of chaotic behavior. This method is based on calculating the instantaneous phase difference between two signals regardless of their amplitude. As a result, this method is sensitive to signal fluctuations and is not based on the signal’s stationary assumption, so fewer restrictions are on its usage. An unavoidable phenomenon in real signals is the effect of noise on the signal, which mainly affects the signal amplitude. Since the PLV method is based on the phase difference of the signals, it is much more resistant to noise, and the resulting estimate is more accurate than the methods based on the signal amplitude. This method is also simple and has a low computational cost, so it is especially suitable for calculating whole brain connectivity (Kong et al. 2019; Lowet et al. 2016). The PLV between two time series is calculated based on the following definition:
| 1 |
The Hilbert transform is applied to calculate the instantaneous phase of a time series. In this study, the PLV is extracted between all electrodes’ pairs under the gamma band with a time window of 3 seconds. Then the PLV matrix is formed. Each EEG electrode channel of this experiment consists of sixty epochs (), so the overall PLV is the mean of N PLV corresponding to its epochs. The average PLV is calculated as
| 2 |
where is the phase difference between and , i.e., and is the number of the epochs. The value of the calculated PLV between two electrode channels is between 0 and 1, which indicates the lack of synchronization and complete synchronization of the two signals, respectively. Since the PLV is the absolute value of the mean phase difference between two signals, any PLV value lying in the interval (0, 1) shows the degree of phase synchronization between the two signals. In other words, a large PLV value in the interval (0, 1) indicates that the instantaneous phase difference between the two signals over time is more constant, and as a result, the two signals are more synchronous. Conversely, a small PLV value represents less constancy of the instantaneous phase difference between the two signals over time and results in less synchronization between the two signals.
Topological feature extracting
Two principal features are extracted from the weighted, not thresholded neuronal networks to compare two ADHD and healthy groups. These features include the clustering coefficient and shortest path length, representing two neuronal network integration and segregation characteristics, respectively. Each group’s clustering coefficient is calculated based on the average of the edges connected to one node and each other two nodes that form a triangle with that node. Using the clustering coefficient, it is possible to understand how the network separates information or how subnets are composed of a general network. The shortest path length is calculated by averaging all path lengths between every two nodes in the networks. This feature helps to understand how information is disseminated in neuronal networks (Rubinov and Sporns 2010).
Statistical analysis
The data of this study are divided into two general categories of healthy and ADHD children. EEG signals are recorded under four different types of emotion. This research’s first independent variable is ”disease”, which has two categories: ADHD and healthy. Another independent variable is ”emotion,” which has four compartments: anger, happiness, neutrality, and sadness. The PLVs and graph features are the characteristics extracted based on functional connectivity analysis in this study. In this research, the significant differences in functional connectivity and extracted graph features are investigated between ADHD and healthy groups. Due to the dependence of different groups in this study, a Repeated Measure ANOVA test is used to evaluate the effect of ”disease” regardless of the ”emotion” types and the interaction effects of ”emotion disease.” These analyzes include both whole-brain analysis and investigation of different brain regions. In this research, all figures are obtained using MATLAB software version 2020.
Results
Functional connectivity analysis
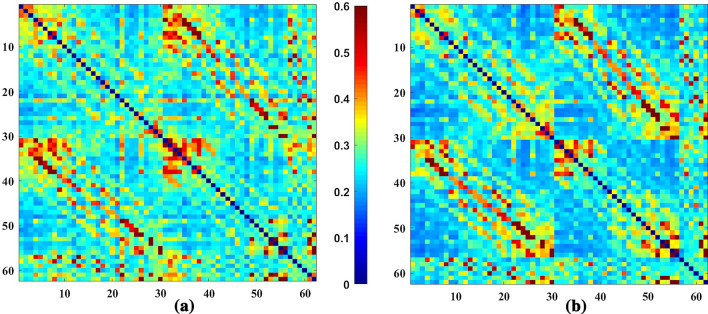
Figure 1 shows the average gamma band’s connectivity matrix for happy emotion in Healthy and ADHD groups. The Repeated Measure ANOVA test is used to find pairs of channels with significant differences between groups. The main effect of ”disease” and the interaction effect of ”diseaseemotion” are investigated using Dunn-Sidak post hoc tests. Table 1 shows 12 pairs of common channels in the gamma band that show a significant difference in terms of the main effect ”disease” as well as the interaction effect ”diseaseemotion” in healthy and ADHD groups. In this table, the columns ”diff ” show the differences between the mean connectivity of the related channel pairs in two ADHD and healthy groups (ADHD-healthy). The columns ”P” represent the P-value results from the Dun-Sidak post hoc test showing significant differences between the connectivity of the relevant channel pairs in two ADHD and healthy groups. These pairs of significant channels are found mainly in the Frontal and Parietal-Occipital regions of the brain. There are also significant differences between the channels in the Frontal and Occipital regions (AF3-PO5) between the two groups. In this table, except for the pairs of (CP6-C6) and (P3-P1) channels, the connectivity is significantly higher in the ADHD than in the healthy group in other pairs. Figure 2 shows the pairs of channels that were significantly different in the two groups in the study of the main effect ”disease” and the interaction effect ”diseaseemotion”. Finally, the whole-brain connectivity is calculated by averaging all the connectivity matrix elements related to each group’s individuals. The ANOVA test results do not show any significant differences in whole-brain connectivity analysis in all four types of emotion between the two groups of healthy and ADHD, as shown in Fig. 3. The mean, standard deviation, and calculated P-values for whole-brain analysis are listed in Table 2.
Fig. 1.
Average PLV matrices in the gamma band of Happy emotion in a ADHD and b Healthy groups. The figures are 62 62 matrices which dimensions show the number of electrode channels for recording EEG signals. According to the color bar, the color of each matrix element expresses the level of phase synchronization between the relevant pairs of electrodes. In these matrices, regions with warm colors show high values of PLV and, as a result, high phase synchronization between channel pairs. Cold color areas indicate low PLV values and low phase synchronization. Comparison of figures (a) and (b) shows more mean phase synchronization in the ADHD group than in the healthy one in some channel pairs
Table 1.
Common channel pairs with significant connectivity differences (ADHD-Healthy) in all types of emotions
| Channel | Angry | Happy | Neutral | Sad | ||||
|---|---|---|---|---|---|---|---|---|
| pairs | diff | P | diff | P | diff | P | diff | P |
| FP2-AF8 | 0.1185 | 0.0056 | 0.1230 | 0.0048 | 0.1175 | 0.0057 | 0.1202 | 0.0051 |
| FP2-FC4 | 0.1020 | 0.0029 | 0.0974 | 0.0033 | 0.0890 | 0.0072 | 0.0989 | 0.0026 |
| FC1-AF3 | 0.1241 | 0.0053 | 0.1239 | 0.0049 | 0.1240 | 0.0056 | 0.1210 | 0.0049 |
| CP6-C6 | − 0.1724 | 0.0018 | − 0.1761 | 0.0020 | − 0.1713 | 0.0027 | − 0.1699 | 0.0022 |
| P3-P1 | − 0.1710 | 0.0016 | − 0.1818 | 0.0006 | − 0.1769 | 0.0007 | − 0.1777 | 0.0007 |
| AF3-PO5 | 0.1274 | 0.0040 | 0.1288 | 0.0038 | 0.1229 | 0.0054 | 0.1256 | 0.0036 |
| AF3-PO6 | 0.1283 | 0.0050 | 0.1392 | 0.0022 | 0.1286 | 0.0043 | 0.1315 | 0.0033 |
| AF3-PO7 | 0.1362 | 0.0026 | 0.1446 | 0.0017 | 0.1378 | 0.0026 | 0.1372 | 0.0020 |
| AF3-PO8 | 0.1352 | 0.0020 | 0.1385 | 0.0016 | 0.1325 | 0.0023 | 0.1340 | 0.0018 |
| AF8-FC4 | 0.1216 | 0.0022 | 0.1181 | 0.0036 | 0.1132 | 0.0051 | 0.1199 | 0.0020 |
| FC5-PO4 | 0.1596 | 0.0010 | 0.1565 | 0.0024 | 0.1554 | 0.0016 | 0.1566 | 0.0020 |
| PO3-PO7 | 0.1711 | 0.0052 | 0.1770 | 0.0013 | 0.1681 | 0.0007 | 0.1696 | 0.0031 |
Fig. 2.
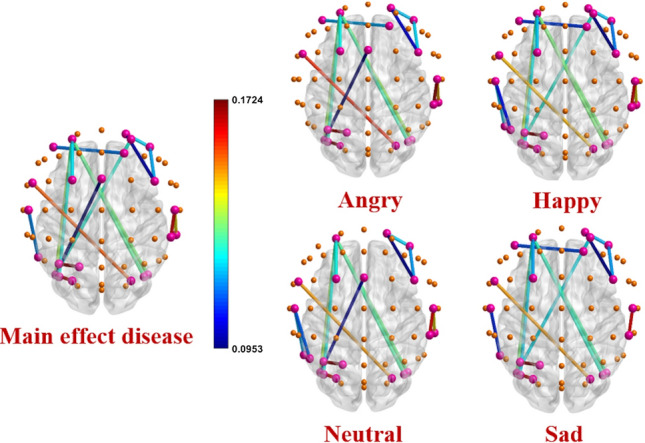
Channel pairs with significant connectivity differences in main effect disease and interaction effect ”diseaseemotion” for all types of emotions. The spheres represent 62 electrode channels in different brain areas. The pink spheres show electrode channels with significant connections in functional connectivity between the two ADHD and healthy groups. The other spheres show the electrode channels that do not have significant connections. The colored lines represent the significant edges between the pairs of electrode channels. The corresponding colors express the level of PLV differences between two healthy and ADHD groups. Edges with warm colors show connections with high connectivity differences between two ADHD and healthy groups. Cold color edges indicate low connectivity differences between the two groups. Details of common edges in all four emotional types are presented in Table 1. All these connections (except CP6-C6 and P3-P1) are significantly (P-value) stronger in the ADHD group than in the healthy one representing more phase synchronization of the related channel pairs in ADHD neuronal networks < 0.01
Fig. 3.
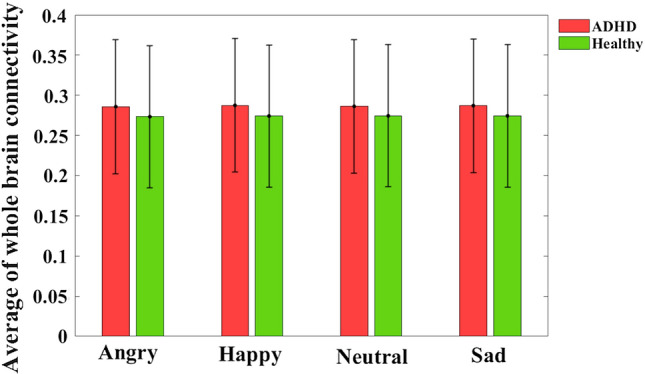
Average of whole brain connectivity in all types of emotions. In each bar graph, the center of the drawn error bar is the average of the functional connectivity. The lines drawn with the same length above and below the center of the error bar show the standard deviation related to each group in different types of emotions. Details of the bar graphs are presented in Table 2. No significant differences are found in all emotional types between two ADHD and healthy groups (P-value > 0.01)
Table 2.
The statistics (Mean ± standard deviation) of calculated whole brain connectivity, shortest path length, and clustering coefficient in all types of emotions
| Angry | Happy | Neutral | Sad | ||
|---|---|---|---|---|---|
| ADHD | 0.2859 ± 0.0834 | 0.2875 ± 0.0831 | 0.2863 ± 0.0830 | 0.2871 ± 0.0832 | |
| connectivity | Healthy | 0.2734 ± 0.0885 | 0.2742 ± 0.0886 | 0.2745 ± 0.0884 | 0.2744 ± 0.0885 |
| P value | 0.6959 | 0.6780 | 0.7116 | 0.6869 | |
| ADHD | 4.1503 ± 0.8817 | 4.1348 ± 0.8809 | 4.1494 ± 0.8786 | 4.1314 ± 0.8778 | |
| L | Healthy | 4.1519 ± 0.8841 | 4.1504 ± 0.8855 | 4.1409 ± 0.8812 | 4.1407 ± 0.8791 |
| P value | 0.9960 | 0.9618 | 0.9793 | 0.9770 | |
| ADHD | 0.2704 ± 0.0142 | 0.2721 ± 0.0145 | 0.2707 ± 0.0139 | 0.2716 ± 0.0141 | |
| C | Healthy | 0.2564 ± 0.0088 | 0.2570 ± 0.0088 | 0.2574 ± 0.0088 | 0.2573 ± 0.0088 |
| P value | 0.6760 | 0.6568 | 0.6931 | 0.6676 |
Feature analysis of neuronal networks
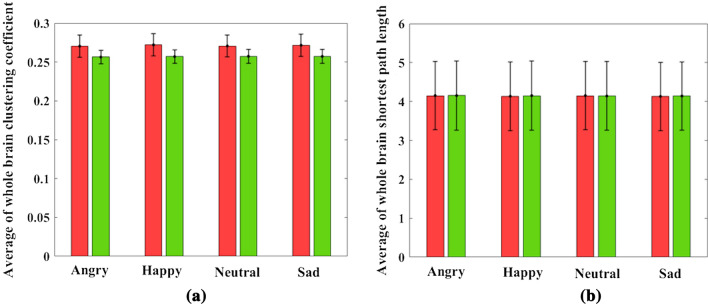
As mentioned in the previous sections, the shortest path length (L) between two nodes is calculated by averaging all the edges between the two nodes. The clustering coefficient (C) for each node is obtained by averaging all the edges connected to that node and every two other nodes that form a triangle with that node. Finally, for each participant, a matrix of 6262 and a vector of 621 are calculated for the features of L and C, respectively. Table 3 shows 12 pairs of common channels for L that show a significant difference in terms of the main effect ”disease” and the interaction effect ”diseaseemotion” in the two groups of healthy and ADHD. These pairs of channels are located mainly in the Frontal and Parietal lobes of the brain. In this table, except for three pairs of (CZ-F5), (F5-FC3), and (PO3-PO7) channels, in the other pairs of channels, the value of L in the ADHD group is significantly higher than the healthy group. Figure 4 shows the pairs of channels that are significantly different in the two groups in the study of the main effect ”disease” and the interaction effect ”disease emotion” for all types of emotions. This figure represents that the shortest path length in the ADHD group is higher, particularly in the occipital and occipital-central brain connections. Also, it is shown in the figure that P4-PO7 and P3-F1 channel pairs have the highest value of the shortest path length differences in all emotional types, which are related to the parietal-occipital and parietal-frontal connections of the brain, respectively. Then by averaging L’s value in all pairs of channels in each group, the whole brain’s shortest path length is calculated. As shown in Fig. 5b and Table 2, the results do not show any significant differences in terms of whole-brain shortest path length between the healthy and ADHD groups. All similar calculations are performed to investigate the main effect ”disease,” and the interaction effect ”diseaseemotion” on the clustering coefficient, and results (Fig. 5a and Table 2) show no significant differences in the four types of emotion between the two groups of healthy and ADHD.
Table 3.
Common channel pairs with significant differences (ADHD-Healthy) of shortest path length in all types of emotions
| Channel | Angry | Happy | Neutral | Sad | ||||
|---|---|---|---|---|---|---|---|---|
| pairs | diff | P | diff | P | diff | P | diff | P |
| CZ-F5 | − 1.2617 | 0.0015 | − 1.2894 | 0.0011 | − 1.2373 | 0.0027 | − 1.3285 | 0.0009 |
| CP1-FC4 | 1.0895 | 0.0014 | 1.0608 | 0.0014 | 1.0398 | 0.0015 | 1.0044 | 0.0016 |
| CP6-P1 | 1.2913 | 0.0059 | 1.3371 | 0.005 | 1.2844 | 0.0062 | 1.3449 | 0.0072 |
| P7-OZ | 1.4137 | 0.0026 | 1.4079 | 0.0021 | 1.3844 | 0.0028 | 1.3537 | 0.0046 |
| P7-O2 | 1.1269 | 0.0030 | 1.1341 | 0.0026 | 1.0851 | 0.0029 | 1.1176 | 0.0028 |
| P7-P2 | 1.4397 | 0.0051 | 1.5692 | 0.0042 | 1.5381 | 0.0040 | 1.5532 | 0.0045 |
| P3-F1 | 1.6374 | 0.0026 | 1.6849 | 0.0021 | 1.5918 | 0.0032 | 1.5857 | 0.0051 |
| P4-F1 | 0.7058 | 0.0021 | 0.7599 | 0.0008 | 0.7445 | 0.0009 | 0.7119 | 0.0011 |
| P4-PO7 | 1.6991 | 0.0037 | 1.7460 | 0.0033 | 1.7473 | 0.0034 | 1.6650 | 0.0041 |
| F5-FC3 | − 1.1634 | 0.0091 | − 1.3216 | 0.0025 | − 1.1952 | 0.0065 | − 1.1398 | 0.0099 |
| PO3-PO7 | − 1.1026 | 0.0053 | − 1.1610 | 0.0041 | − 1.0906 | 0.0066 | − 1.0463 | 0.0089 |
| F3-OZ | 1.2087 | 0.0033 | 1.2013 | 0.0029 | 1.1439 | 0.0040 | 1.1730 | 0.0014 |
Fig. 4.
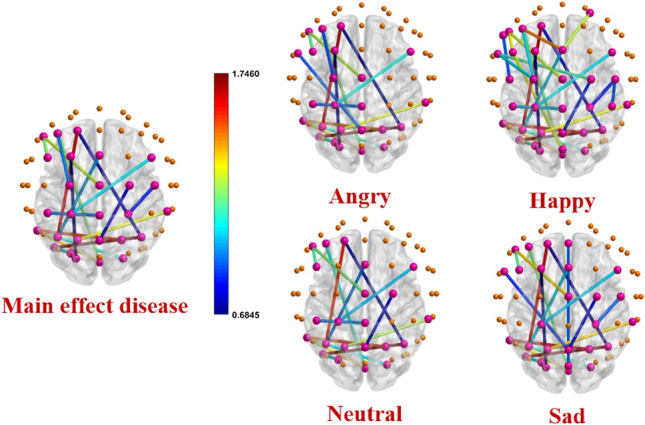
Channel pairs with significant differences in shortest path length in main effect disease and interaction effect ”diseaseemotion” for all emotions. The spheres represent 62 electrode channels in different brain regions. The pink spheres show electrode channels with significant connections in the shortest path length between the two ADHD and healthy groups. The other spheres show the electrode channels that do not have significant connections. The colored lines represent the significant edges between the pairs of electrode channels. The corresponding colors express the level of shortest path length differences between two healthy and ADHD groups. Edges with warm colors show high shortest path length differences between two ADHD and healthy groups. Cold color edges represent connections with low differences. Details of the common significant connections are presented in Table 3. The shortest path length of all these connections (except CZ-F5, F5-FC3, and PO3-PO7) is higher in the ADHD group than the healthy one indicating less segregation of information in the related channel pairs in ADHD neuronal networks
Fig. 5.
Average of whole-brain a clustering coefficient and b shortest path length in all types of emotions. In each bar graph, the center of the drawn error bar is the average of the clustering coefficient/shortest path length. The lines drawn with the same length above and below the center of the error bar show the standard deviation related to each group in different types of emotions. Details of the bar graphs are presented in Table 2. No significant differences are found in all types of emotions between two ADHD and healthy groups (P-value )
Discussion
In this study, the EEG signals of 22 children diagnosed with ADHD and 22 healthy children while observing facial emotions of anger, happiness, neutrality, and sadness were examined. After the preprocessing stage and removing noise and artifacts from the raw signals, the CSD method was applied to the signals to reduce the volume conduction effect. The signal was then divided into the delta, theta, alpha, beta, and gamma sub-bands using the Daub7 wavelet transform. The gamma-band was selected as an appropriate frequency band during facial emotion processing based on previous studies (Razavi et al. 2017; Balconi and Pozzoli 2009; Sato et al. 2011). As a result, all subsequent analyses were performed on the gamma band. Graph features, including clustering coefficient and shortest path length, and the connectivity feature, including PLV for all participants in four different emotional groups, were calculated. To investigate significant differences in the main effect ”disease” and the interaction effect ”diseaseemotion”, a Repeated Measure ANOVA test was used. Finally, using graph features as well as functional connectivity and comparing them between different groups, the following results were obtained:
In this study, stronger functional connectivity of ADHD children in the Frontal lobe of the brain was observed, which is consistent with the results observed in (Barttfeld et al. 2014; Dini et al. 2020).
Significant differences were observed in the connectivity of the central regions to the occipital areas of the brain (FC5-PO4) in all types of emotions, consistent with the results obtained in (Barttfeld et al. 2014).
The significant differences observed in the brain connectivity of healthy and ADHD individuals during facial emotion processing in gamma-band oscillations within the brain’s occipital regions (PO3-PO7) are consistent with the findings of (Razavi et al. 2017; Garcia-Garcia et al. 2010). It has been reported that the activity of the occipital areas of the brain in gamma-band oscillations is higher than other regions of the brain while observing facial emotions in two groups of healthy and ADHD.
In this study, significant differences were observed in the left regions of the occipital lobe and the right and left areas of the brain’s parietal lobe, which is accordant with the findings in (Ahmadlou and Adeli 2011). There was a significant difference on the occipital lobe’s left side and the right side of the brain’s parietal lobe. Still, there was no significant difference on the left side of the parietal lobe between the two groups.
Findings in (Dini et al. 2020; Ahmadlou and Adeli 2011) indicated a defect in transferring of information from the occipital lobe to the brain’s frontal lobe in the ADHD group. The present study results also showed that the shortest path length in ADHD is significantly higher than healthy individuals in the pair of channels related to the occipital-frontal lobe (OZ-F3).
Studies (Lin et al. 2014; Liu et al. 2015) based on fMRI and EEG data have shown that the L feature is significantly higher in the ADHD group than in healthy individuals, which is consistent with the findings of this study.
In several studies, the functional and structural connectivity analysis patterns have shown that healthy human neuronal networks follow the small-world characteristics. In small-world networks, the amount of information dissemination and segregation is efficient (Liao et al. 2017). High clustering coefficient (C) and low shortest path length (L) are considered as two main hallmarks of small-worldness in a healthy brain. Some studies have claimed these two features change abnormally under different brain disorders, and deficiency in small-world networks is related to brain function defects (Ahmadlou and Adeli 2010; Barttfeld et al. 2014; Ahmadlou and Adeli 2011). The present study showed no significant differences in the average of whole-brain clustering coefficient and shortest path length between ADHD and healthy groups in gamma-band oscillations, as shown in Fig. 5 and Table 2). However, the Repeated Measure ANOVA test results represented significant differences between the two groups in terms of shortest path length for some channel pairs that are mainly related to the brain occipital, occipital-central, and occipital-frontal connections. These results may indicate less information propagation in the ADHD neuronal networks, resulting in high L as a criterion of small worldness. More research is needed to evaluate deficiency in small worldness and other features with specific tools and more special task designs.
Conclusions
Numerous studies have reported the influential role of gamma-band oscillations in analyzing brain signals during facial emotion processing ( Balconi and Pozzoli 2009; Garcia-Garcia et al. 2010). According to these studies’ findings, the occipital lobe activity in this band is higher than other brain areas during facial emotion processing, which is consistent with the present study’s findings. More functional connectivity in the occipital lobe of the brain of the ADHD group indicates more phase synchronization of the brain neurons in these areas. The occipital lobe is the center of visual processing (Pitcher et al. 2011), and defects in these areas’ connectivity indicate a brain function defect during visual processing. This can be due to excessive inattention of ADHD children to visual stimuli. The whole-brain analysis results did not show significant brain connectivity, and the extracted graph features (L and C) differences in healthy and ADHD groups. However, a separate examination of different brain areas showed that in some areas, especially the Frontal lobe, the brain connectivity in the ADHD group is significantly stronger than in the Healthy group. Also, the analysis of the graph features showed that the shortest path length in these areas is significantly higher in ADHD, which is consistent with the findings in (Lin et al. 2014; Liu et al. 2015). The frontal lobe is responsible for the processing of human emotions (Stuss and Knight 2013). The present study results showed that the phase synchronization of neurons in this lobe is significantly higher in the ADHD group than the healthy one, indicating a defect of the brain performance in this lobe. This result can be interpreted as more functional connectivity is associated with defects in facial emotion processing in individuals with ADHD. Higher L in the ADHD group, especially in the occipital-frontal region’s connectivity, may indicate a defect in transferring information from the visual center to the emotional processing center. This study used the method, phase-locking value (PLV), between EEG signals to analyze the brain’s functional connectivity in two healthy and ADHD groups. Methods based on measuring the phase interactions between EEG signals may better analyze the brain’s complex dynamics. This research can help better understand the brain’s behavior and its dysfunction in individuals with ADHD.
Acknowledgements
Matjaž Perc was supported by the Slovenian Research Agency (Grant Nos.P1-0403 and J1-2457).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee of the Iran University of Medical Sciences (Number: IR.IUMS.REC.1394.92133070).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmadlou M, Adeli H. Wavelet-synchronization methodology: A new approach for eeg-based diagnosis of adhd. ClinEEG Neurosci. 2010;41:1–10. doi: 10.1177/155005941004100103. [DOI] [PubMed] [Google Scholar]
- Ahmadlou M, Adeli H. Fuzzy synchronization likelihood with application to attention-deficit/hyperactivity disorder. Clin EEG Neurosci. 2011;42:6–13. doi: 10.1177/155005941104200105. [DOI] [PubMed] [Google Scholar]
- An L, Cao QJ, Sui MQ, Sun L, Zou QH, Zang YF, Wang YF. Local synchronization and amplitude of the fluctuation of spontaneous brain activity in attention-deficit/hyperactivity disorder: a resting-state fmri study. Neurosci Bull. 2013;29:603–613. doi: 10.1007/s12264-013-1353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi M, Pozzoli U. Arousal effect on emotional face comprehension: Frequency band changes in different time intervals. Physiol Behav. 2009;97:455–462. doi: 10.1016/j.physbeh.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Barttfeld P, Petroni A, Báez S, Urquina H, Sigman M, Cetkovich M, Torralva T, Torrente F, Lischinsky A, Castellanos X, et al. Functional connectivity and temporal variability of brain connections in adults with attention deficit/hyperactivity disorder and bipolar disorder. Neuropsychobiol. 2014;69:65–75. doi: 10.1159/000356964. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised conners’ parent rating scale (cprs-r): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–268. doi: 10.1023/A:1022602400621. [DOI] [PubMed] [Google Scholar]
- Dasdemir Y, Yildirim E, Yildirim S. Analysis of functional brain connections for positive-negative emotions using phase locking value. Cogn Neurodyn. 2017;11:487–500. doi: 10.1007/s11571-017-9447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini H, Ghassemi F, Sendi MS. Investigation of brain functional networks in children suffering from attention deficit hyperactivity disorder. Brain Topogr. 2020;33:733–750. doi: 10.1007/s10548-020-00794-1. [DOI] [PubMed] [Google Scholar]
- Dockstader C, Gaetz W, Cheyne D, Wang F, Castellanos FX, Tannock R. Meg event-related desynchronization and synchronization deficits during basic somatosensory processing in individuals with adhd. Behav Brain Funct. 2008;4:1–13. doi: 10.1186/1744-9081-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Child symptom inventory 4: CSI. NY, NewYork: Checkmate Plus Stony Brook; 1997. [Google Scholar]
- Garcia-Garcia M, Yordanova J, Kolev V, Domínguez-Borràs J, Escera C. Tuning the brain for novelty detection under emotional threat: The role of increasing gamma phase-synchronization. Neuroimage. 2010;49:1038–1044. doi: 10.1016/j.neuroimage.2009.07.059. [DOI] [PubMed] [Google Scholar]
- Gong A, Liu J, Lu L, Wu G, Jiang C, Fu Y. Characteristic differences between the brain networks of high-level shooting athletes and non-athletes calculated using the phase-locking value algorithm. Biomed Signal Process Control. 2019;51:128–137. doi: 10.1016/j.bspc.2019.02.009. [DOI] [Google Scholar]
- Kong W, Zhou Z, Jiang B, Babiloni F, Borghini G. Assessment of driving fatigue based on intra/inter-region phase synchronization. Neurocomput. 2017;219:474–482. doi: 10.1016/j.neucom.2016.09.057. [DOI] [Google Scholar]
- Kong W, Wang L, Xu S, Babiloni F, Chen H. Eeg fingerprints: Phase synchronization of eeg signals as biomarker for subject identification. IEEE Access. 2019;7:121165–121173. doi: 10.1109/ACCESS.2019.2931624. [DOI] [Google Scholar]
- Liao X, Vasilakos AV, He Y. Small-world human brain networks: Perspectives and challenges. Neurosci Biobehav Rev. 2017;77:286–300. doi: 10.1016/j.neubiorev.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Lin P, Sun J, Yu G, Wu Y, Yang Y, Liang M, Liu X. Global and local brain network reorganization in attention-deficit/hyperactivity disorder. Brain Imaging Behav. 2014;8:558–569. doi: 10.1007/s11682-013-9279-3. [DOI] [PubMed] [Google Scholar]
- Liu T, Chen Y, Lin P, Wang J. Small-world brain functional networks in children with attention-deficit/hyperactivity disorder revealed by eeg synchrony. Clin EEG Neurosci. 2015;46:183–191. doi: 10.1177/1550059414523959. [DOI] [PubMed] [Google Scholar]
- Lowet E, Roberts MJ, Bonizzi P, Karel J, De Weerd P. Quantifying neural oscillatory synchronization: A comparison between spectral coherence and phase-locking value approaches. PLoS ONE. 2016;11:1–37. doi: 10.1371/journal.pone.0146443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey P, Cohn JF, Kanade T, Saragih J, Ambadar Z, Matthews I (2010) The extended cohn-kanade dataset (ck+): A complete dataset for action unit and emotion-specified expression. In: 2010 IEEE Computer Society Conference on Computer Vision and Pattern Recognition - Workshops, pp 94–101
- Parastesh F, Azarnoush H, Jafari S, Hatef B, Perc M, Repnik R. Synchronizability of two neurons with switching in the coupling. Appl Math Comput. 2019;350:217–223. [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier J. Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Duchaine B. The role of the occipital face area in the cortical face perception network. Exp Brain Res. 2011;209:481–493. doi: 10.1007/s00221-011-2579-1. [DOI] [PubMed] [Google Scholar]
- Razavi MS, Tehranidoost M, Ghassemi F, Purabassi P, Taymourtash A. Emotional face recognition in children with attention deficit/hyperactivity disorder: Evidence from event related gamma oscillation. Basic Clin Neurosci. 2017;8:419. doi: 10.18869/nirp.bcn.8.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K. Cognitive neuroscience of attention deficit hyperactivity disorder (adhd) and its clinical translation. Front Hum Neurosci. 2018;12:100. doi: 10.3389/fnhum.2018.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Matsuda K, Usui K, Inoue Y, Toichi M. Rapid amygdala gamma oscillations in response to fearful facial expressions. Neuropsychologia. 2011;49:612–617. doi: 10.1016/j.neuropsychologia.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of frontal lobe function. Oxford: Oxford University Press; 2013. [Google Scholar]
- Wang Z, Zhou R, He Y, Guo X (2020) Functional integration and separation of brain network based on phase locking value during emotion processing. IEEE T Cogn Dev Syst pp 1–1
- Yu D. Additional brain functional network in adults with attention-deficit/hyperactivity disorder: A phase synchrony analysis. PLoS ONE. 2013;8:1–10. doi: 10.1371/annotation/2212a861-273a-4c34-816a-ead5d0d8a7f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang Q, Perc M, Chen G. Synaptic plasticity induced transition of spike propagation in neuronal networks. Commun Nonlinear Sci Numer Simul. 2013;18:601–615. doi: 10.1016/j.cnsns.2012.08.009. [DOI] [Google Scholar]