Abstract
Objective
Hepatocellular carcinoma (HCC) represents a typical inflammation-associated cancer. Tissue resident innate lymphoid cells (ILCs) have been suggested to control tumor surveillance. Here we studied how the local cytokine milieu controls ILCs in HCC.
Design
We performed bulk RNA sequencing of HCC tissue as well as flow cytometry and single-cell RNA sequencing of enriched ILCs from non-tumor liver, margin and tumor core derived from 48 HCC patients. Simultaneous measurement of protein and RNA expression at the single-cell level (AbSeq) identified precise signatures of ILC subgroups. In-vitro culturing of ILCs was used to validate findings from in-silico analysis. Analysis of RNA-sequencing data from large HCC cohorts allowed stratification and survival analysis based on transcriptomic signatures.
Results
RNA sequencing of tumor, non-tumor and margin identified tumor-dependent gradients of which were not only associated with poor survival but also control ILC plasticity. Single-cell RNA sequencing and flow cytometry of ILCs from HCC livers identified NK-like cells in the non-tumor tissue, losing their cytotoxic profile as they transitioned into tumor ILC1 and NK-like-ILC3 cells. Tumor ILC composition was mediated by cytokine gradients that directed ILC plasticity towards activated tumor ILC2s. This was liver-specific and not seen in ILCs from PBMC. Patients with high ILC2/ILC1 ratio expressed IL-33 in the tumor that promoted ILC2 generation which was associated with better survival.
Conclusion
Our results suggest that the tumor cytokine milieu controls ILC composition and HCC outcome. Specific changes of cytokines modify ILC composition in the tumor by inducing plasticity and alter ILC function.
Keywords: Innate Lymphoid Cells, Cytokine Gradients, Tumor Microenvironment, Plasticity
INTRODUCTION
Hepatocellular Carcinoma (HCC) is the fourth most common cause of cancer related death with rising incidence (www.iarc.fr). One factor in the development of HCC is chronic inflammation in the liver due to infection with hepatitis B and C viruses as well as alcoholic and non-alcoholic fatty liver disease. Cytokines play a major role in regulating immune processes during liver inflammation and HCC development [1]. Innate immune cells contribute to the cytokine milieu and are regulated by cytokines. Innate immune cells lack antigen-specific receptors present in cells of the adaptive immune system but overexpress specific cytokine receptors [2]. Innate lymphoid cells (ILCs) are a heterogenous cell population of the innate immune system and regulators of inflammation, mucosal defenses and tissue repair of many disease processes including cancer. ILCs are partitioned into three groups based on transcription factor expression and cytokine signatures that resemble various T helper (Th) cell subsets. Group 1 ILCs consist of two subsets, EOMES-positive natural killer (NK) cells, a counterpart to cytotoxic T cells, and T-bet-positive helper ILC1s, characterized by Th1-like immunity. ILC2s express the transcription factor GATA-3 and secrete Th2 associated cytokines. RORγt-dependent ILC3s secrete IL-22 and IL-17 characteristic of Th17 and Th22 helper T cells [3].
A previous study of hepatic ILCs suggests that all ILC subsets contribute to the regulation of hepatitis B infection and liver fibrosis [4]. Characterization of ILCs in fetal and adult human livers showed that NKp44− ILC3s were the dominant ILC population within adult livers and a positive correlation between ILC2 frequency and liver fibrosis exists [5]. Cytokines, including IL-33 and IL-6, induce a Th2-like response in hepatic ILC2s affecting pathogenesis of immune-mediated hepatitis, hepatic inflammation and fibrosis [6].
The role of ILCs in cancer remains largely unknown. To date, studies of ILCs in cancer have been mainly descriptive in nature and yielded contradictory results. Some suggest that ILCs are involved in tumor progression while others suggest that ILCs confer anti-tumor properties [7, 8]. Detailed human ILC studies are rare due to challenges caused by the low abundance of ILCs. To date, ILC models have been oversimplified by limiting their focus on only one ILC subgroup or manipulating cells with non-physiological concentrations of cytokines, missing the complexity of the ILC network and its regulating cytokines. Thus, a thorough analysis of human tumor ILCs is required to understand their roles in tumor immunology.
We performed bulk tissue RNA sequence analysis from tumor, margin and non-tumor tissue samples of HCC patients and analyzed differentially expressed cytokines, cytokine receptors and growth factors. Expression analysis revealed gradual changes of ILC-controlling cytokines from non-tumor to tumor which was associated with poor survival in the liver cancer TCGA cohort. Trajectory analysis demonstrated that increased frequency of ILC2 in tumor were partly due to ILC1s and ILC3s changing into an ILC2 phenotype in the tumor. Plasticity was orchestrated by a cytokine gradient in the tumor microenvironment (TME) of increased inflammatory cytokines including TGFβ, IL-6 and IL-8 and reduced cytokines specifically of the common-γ chain family. Here, we describe an intricate picture of how the cytokine gradients in the tumor microenvironment orchestrate ILC diversity and plasticity in the liver associated with patient survival.
Methods
Human HCC Specimens
Fresh HCC samples as well as peripheral blood from patients undergoing liver transplantation or HCC resection in the Medstar Georgetown University Hospital were collected after informed consent in a collaboration between the Georgetown University Medical Center (IRB #2017-0365) and the National Institutes of Health. Tumor, non-tumor as well as margin tissue were collected. All flow cytometry and scRNA-seq experiments have been conducted using fresh samples without freezing. Frozen cells were used for in vitro studies. This study was approved by an Institutional Review Board of the National Institutes of Health. Detailed patient demographics can be found in Table S2A. Matched samples were used for several analysis whenever possible. Flow cytometry data is derived from 32 matched samples for NT, M, T, 12 matched samples for NT and M and 4 NT samples (48 patients total). Cytokine data in Figure 1A is derived from 11 matched samples for NT, M, and T, 11 matched samples for NT and M and 3 NT samples (26 patients total). Cytokine data presented in Figure 1B are also derived from 6 completely matched sample sets and 2 additional tumor samples. 6 completely matched sample sets have been used for scRNA-seq. All of these samples have also been included in the flow cytometry analysis.
Figure 1: Cytokines in the tumor environment of patients with HCC.
A. Heatmap showing expression of cytokines, cytokine receptors and growth factors by individual region analyzed by bulk tissue RNA sequencing from liver and tumor tissue of patients with HCC. Cytokine expression data is derived from 59 samples from a total of 25 patients. Matched non-tumor (NT), margin (M) and tumor (T) samples were available from 11 patients, matched NT and M from 11 patients and NT only from 3 patients.
B. Multiplex cytokine analysis of supernatants derived from NT (black) or T tissue (red). Cytokine concentration in supernatants harvested from culturing of minced non-tumor or tumor tissue, incubated for 6h and cleared from residual cells. Adjusted for weight of tissue piece. 6 matched NT and T samples with two additional T samples. Bar graphs presenting median with 95% confidence interval.
C. Expression of TGFB2 comparing NT, M and T in the HCC cohort.
D. Expression of IL33 comparing NT, M and T in the HCC cohort.
E. Expression of IL1B comparing NT, M and T in the HCC cohort.
F. Survival prediction analysis of TCGA liver cancer cohort using expression of IL33. Patients were split at median.
Survival Analysis
To define gene signatures of interest, we used Monocle to identify DEGs between each cell type, such as ILC1, in the tumor core compared to all other cell types in all patients and all regions combined. The “signature” is defined to contain all genes overexpressed in the cell type of interest at a fold change of at least 1.5 and q-value < 1e-04, except that genes not measured in TCGA were necessarily excluded. We split the two patients into two approximately equal-sized groups according to median values of a test statistic and then used the R survival and survminer packages [9] to compare either survival in the two groups and to visualize the Kaplan-Meier curves. The test statistics we used are based on single sample Gene Set Enrichment Scores calculated using GSVA [10].
Survival analysis using the TCGA LIHC cohort was performed using Kaplan Meier-plotter web-based online tool [11]. Patients were split by median expression of DEGs identified as a set of single markers. Overall survival or progression-free survival were analyzed using the complete cohort.
Ex vivo co-culture experiments
Non-tumor tissue and liver tissue were manually minced using a sterile blade. Tissue was resuspended in a 1:5 tissue to media ratio (1g tissue resuspended in 5ml media) using X-VIVO™ 15 (Lonza) with 1% human serum. The tissue-media suspension was incubated in a 6-well plate for 6h at 37° C and 5% CO2.
Supernatants were filtered through a 40μm sterile filter. To pellet residual cells, the supernatant was further centrifuged. Supernatant derived from non-tumor tissue was referred to as non-tumor-supernatant (NT-SN). Supernatant derived from tumor tissue was referred to as tumor-supernatant (T-SN).
NT-SN and T-SN were separately diluted in 1:1 ratio with fresh X-VIVO™ 15 (Lonza) with 1% human Serum. 1.5x106 mononuclear cells (MNCs) isolated from non-tumor tissue were incubated in 24 well plate with either 1ml media only (X-VIVO™ 15 (Lonza)+ 1% human Serum=Media only), 1ml SN (500ul NT-SN + 500ul fresh media) or 1ml T-SN (500ul TSN + 500ul fresh media) overnight (12h-16h) at 37°C and 5% CO2. For NK cell subgroup culturing, liveCD45+CD56dimCD3−, liveCD45+CD56brightCD3−, or liveCD45+lin−CD127− NK-like cells were isolated using FCM sorting. Antibody titration for saturation of cells and additional gating for CD94 confirmed CD94 negativity in NK-like cells. Sorted cells were incubated with 300ul media only or T-SN in a 96 well plate for 72hours. In both experiments, cells were finally stained for ILC markers for FCM analysis.
Trajectory Analysis
For pseudotime trajectory analysis, we used the DDRTree [12] algorithm which was best supported in Monocle 2. Trajectory plots, Branched expression analysis modeling (BEAM) heatmaps, and single gene trajectories were produced using methods provided by Monocle 2.
RESULTS
The Expression of Cytokines in HCC-Environment is Associated with Survival in the TCGA Hepatocellular Carcinoma Cohort.
To explore the expression pattern of cytokines, cytokine receptors and growth factors in the microenvironment of liver tissue, we performed bulk tissue RNA sequencing of samples from tumor, tumor margin and non-tumor tissue from HCC patients (Figure S1A). Samples passing quality control were analyzed for differential gene expression. Differentially expressed genes (DEGs) comparing non-tumor and tumor tissue and filtering for cytokines, cytokine receptors, chemokines and growth factors resulted in a list of genes upregulated in the tumor, which were associated with poor survival in the TCGA LIHC (hepatocellular carcinoma) cohort (Figure S1B, DEGs in Table S1). Unbiased clustering revealed genes that showed gradual expression changes from non-tumor over margin to tumor tissue, with strong differences between non-tumor and tumor tissues (Figure 1A). To study changes of cytokines on a protein level, we used the same tissue from non-tumor liver and tumor of selected patients to generate supernatants. Concentrations of fourteen cytokines differed between non-tumor and tumor tissue supernatant at the protein level (Figure 1B). The concentrations of common-γ chain cytokines as well as IL-1β, IL-23, IL-17A, soluble CD40 ligand and IL-33 were lower in tumor-derived supernatants. Higher TGFβ, IL-6, IL-8 and IL-10 concentrations were found in tumor-derived supernatant in line with RNA sequencing data (Figure 1B). A signature of the corresponding transcripts was associated with survival in the TCGA LIHC cohort (Figure S1C and S1D). Survival analyses indicated that patients with high expression of TGFB2, IL6, IL8 and IL10 showed poor survival (Figure S1C). Patients with low expression of cytokines IL1B, IL23A, IL17A, CD40LG, IL2, IL15, IL12A, IL9, IL33 and IL4 demonstrated poor overall survival (Figure S1D). This indicates that the HCC tumor is characterized by up- and downregulated immune-regulatory cytokines that induce an immunosuppressive environment (Figure S1E).
To identify immune cells that contribute to the cytokine milieu, we performed in-silico deconvolution using the bulk tissue RNA sequencing data of tumor, margin and non-tumor. We estimated the abundance of major immune cells in every region using the LM22 signature and the CibersortX algorithm [13, 14, 15]. In margin and tumor compared to non-tumor, M0 macrophages showed significantly higher frequencies. M2 macrophages showed high frequencies in all regions of the HCC tumor liver, unlike M1 macrophages that were low in frequency, which is in accordance with previous research [16] (Figure S1F). Top differentially expressed genes included the macrophage markers MARCO and SPP1 (Figure S1G, S1H), both transcripts that have been recently described in single cell analysis of HCC and colorectal cancer tissue to identify subsets of tumor associated macrophages [17, 18]. Interestingly, among the differentially expressed cytokine genes were three cytokines (IL1β, IL-33 and TGF-β) that have been described to affect plasticity and function of ILCs. These three cytokines are produced by myeloid cells and macrophages. TGFβ has been shown to affect plasticity from NK-cells to ILC1s, IL-33 has been shown to activate ILC2s and IL1β is involved in plasticity of ILC3s to ILC1s [19, 20] (Figures 1C-E). All three cytokines contributed to the gradual cytokine changes in the microenvironment and were differentially expressed between tumor and non-tumor tissue. A differential expression of these cytokines could also be confirmed in the TCGA cohort (Figures S1I-K). Most importantly, TGFβ or IL1β did not predict survival but high expression of the ILC2-activating cytokine, IL-33 was significantly associated with better overall survival in the TCGA cohort. (Figures 1F, S1L & S1M) [21]. Both in silico as well as previous single-cell analyses of tumors could not detect rare innate immune cell populations such as innate lymphoid cells (ILCs), although they can functionally contribute to the cytokine environment [22]. These findings prompted us to evaluate further the role of ILCs in HCC.
The Distribution of ILCs in Liver Tumor, Tumor Margin and Non-Tumor Liver Tissue
To assess the frequency, phenotype and distribution pattern of ILCs in HCC, we analyzed liver samples from 48 patients with HCC (Table S2A). Mononuclear cells (MNCs) from non-tumor liver, tumor margin and core of the tumor as well as peripheral blood were isolated. CD1a, CD34, CD3, TCRβ/β, TCRγ/δ, CD14, CD19, CD16, CD94, CD123, BDCA2, FCeR1a staining was used to identify lineage (lin) positive cells (T cells, typical NK cells, myeloid cells, B cells, dendritic cells, mast cells and hematopoietic stem cells). The remaining lin− cells were stained for ILC surface markers CD127, CD117 (c-Kit), CD294 (CRTH2), CD336 (NKp44) [23, 24]. ILC1s were defined as lin−CD127+CRTH2−c-Kit−NKp44−; ILC2s as lin−CD127+CRTH2+c-Kit−/+ and ILC3s as lin−CD127+CRTH2−c-Kit+NKp44−/+ [3, 25, 26] (Figure S2A). ILCs expressed immune-checkpoints cytotoxic T-lymphocyte-associated Protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) but barely programmed cell death protein ligand 1 (PD-L1) in accordance with previous reports [27]. There was a significant difference in CTLA-4 expression on ILC3s comparing non-tumor and tumor and margin and tumor (Figure S2B). Besides the CD127+ helper ILC population in the liver, we also identified a CD127− population within lin− cells (Figure S2A). t-SNE analysis revealed that CD127− cells formed a separate distinct cluster in close contact with ILC1s, NKp44− ILC3 cells and c-Kit− ILC2s (Figure 2A). CD127− cells were similarly distributed between tumor and non-tumor but were sparse in peripheral blood mononuclear cells (PBMCs) (Figure S2C). Lin−CD127− cells did not express CD94, CD16 and CD34 but shared characteristics of both NK cells and ILC1s. Therefore, we named them “NK-like” cells [28, 29]. NK-like cells expressed the transcription factor EOMES and Tbet+ similar to ILC1s (Figure 2C & S2D). Most NK-like cells expressed CD56 with its expression decreasing in the tumor (p=0.004). In addition, we detected c-Kit+ (CD117+) NK-like cells, at an increased frequency in the tumor (p=0.0016) (Figure 2B). The profile of the here identified NK-like cells in the HCC liver represents a mixture of liver-resident CD16−CD56+CD94+/− NK cells and CD16− CD34−c-Kit+CD94−NK cell progenitors, indicating a phenotype with potential for differentiation into ILC1s and ILC3s, as previously described [30]. Increased Tbet and c-Kit expression of NK-like cells in the tumor indicate their overlap with ILC1s and ILC3s (Figure S2D).
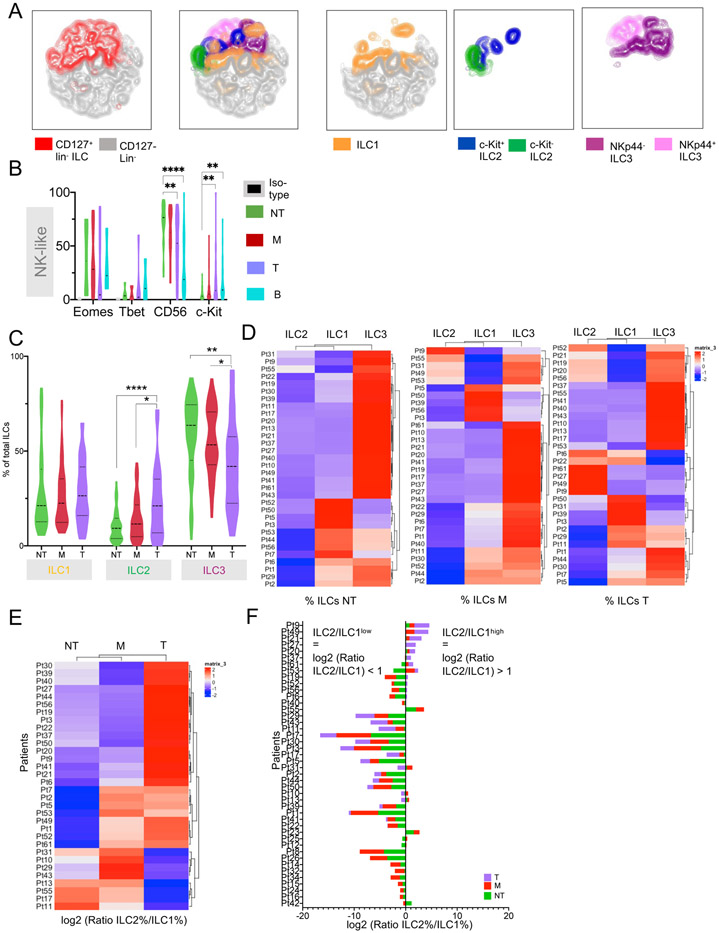
Figure 2. Distribution of ILCs in liver tumor, margin and non-tumor liver tissue in patients with HCC.
A. t-SNE plot of FCM analysis of lin− cells merged from NT, M and T of 48 HCC patients. Left: Distribution of CD127+ total ILCs and CD127− cells within all lin− cells. Middle: t-SNE plot showing distribution of helper ILC subgroups within total ILCs. Right: Distribution of ILCs split by subgroup. Flow cytometry data is derived from 48 patients. Matched NT, M, T were available from 32 patients, matched NT, M were available from 12 patients, only NT samples were available from 4 patients.
B. FCM showing expression of EOMES, Tbet, CD56 and c-Kit (CD117) on CD127− ILCs across all liver regions and PBMCs.
C. FCM analysis of ILC groups. Violin plots with frequencies of ILCs by region. N=48 patients. 4 patients only NT and 12 patients with no tumor sample available resulting in NT=48, M=44, T=32 samples. Distribution of matched samples see also Figure 2A.
D. Heatmap showing clustering by ILC1, ILC2 and ILC3 frequencies by individual patient and region.
E Heatmap showing clustering by log2 (ILC2 frequency (%) / ILC1 frequency (%)) ratio by individual region from individual patients.
F. Stacked bar plots showing log2 (ILC2% / ILC1%) ratio in NT, M and T region within individual patients ranked from high to low tumor log2 (ILC2 / ILC1) ratio.
All graphs. Where indicated: *p<0.05, **p<0.01, ****p<0.0001. Dotted line = median.
FCM analysis demonstrated that the frequency of CD127+ helper ILCs differed in non-tumor, margin and tumor tissue in the liver of HCC patients. ILC2 frequency increased from non-tumor liver to tumor tissue whereas ILC3 frequency decreased (Figure 2C). Specifically, the frequency of NKp44− ILC3s decreased and c-Kit+ ILC2s increased (Figure S2E). Differential analysis of ILC frequency changes between tumor and non-tumor (ΔT-NT) by patient and ILC subset demonstrated a significant increase in frequency of ILC2s from non-tumor to tumor in the majority of analyzed patients (Figure S2F). Clustering of relative ILC frequencies from individual patients by region revealed a pattern that the majority of ILCs in the non-tumor liver were ILC3s with increasing proportions of ILC1s and ILC2s in the margin and tumor. (Figure 2D). More in-depth analysis demonstrated that the ratio of ILC2s to ILC1s increased from non-tumor to tumor tissue in the majority of the patients (Figure 2E). Although ILC2 frequencies increased towards the tumor, only a minority of the patients showed an ILC2/ILC1 ratio > 1 (Figure 2F) and we labeled this cohort “ILC2/ILC1high” Interestingly, only ILC2/ILC1high patients showed significant changes in their ILC composition in the tumor compared to non-tumor with a significant increase of ILC2s and a reduced frequency of ILC3s (Figures S2G & S2H). Additionally, a high ILC2/ILC1 ratio correlated with larger tumors (Table S2B). IL-33 expression in the tumor was not significantly associated with any clinical patient characteristics (Table S2C). Furthermore, patients have been grouped based on the highest ILC subgroup frequency (based on FCM analysis) in the tumor or margin (if tumor sample was not available). Individual heatmaps showing expression of cytokines, cytokine receptors and growth factors by region were studied. A cytokine gradient specifically for the ILC-influencing cytokines could be confirmed in the tumor and also margin samples compared to non-tumor tissue in all three patient groups (Figure S2I).
The Transcriptional Signatures of ILCs in HCC Liver
To better understand the differences in ILC composition in HCC, we studied their transcriptomic profile (Figure S3A). High-throughput analysis of all ILC subgroups by single-cell RNA sequencing (scRNA-seq) in human cancers has not been possible to date due to the low abundance of ILCs [17]. Therefore, enrichment is needed to study ILC heterogeneity and cellular trajectory. We first evaluated ILC-specific transcriptomic profiles on the BD Rhapsody™ platform using a targeted immune panel of 405 transcripts (Table S3A). Additionally, we connected transcript data with surface protein expression of ILC marker using scRNA-seq and oligo-tagged antibodies (AbSeq, Table S3B) [31]. AbSeq analysis was performed using lin+ and lin− FACS sorted PBMCs (Figure S3B). There was good separation of the lin− and lin+ cells (Figure S3C) with the major immune cell populations of B cells, CD4+, CD8+, γδ -T cells, monocytes and conventional NK cells identified within the lin+fraction [32] (Figure S3D, Table S4). Within the lin− fraction, the main ILC clusters were identified by previously described surface markers. ILC2s and ILC3s formed two distinct clusters and showed high expression of the subgroup-specific markers PTDGR2 with the corresponding surface marker CRTH2 and KIT with the corresponding surface marker c-Kit, respectively. CD6 was expressed by ILC1s and T cells. Only expression of surface CD3 could clearly distinguish T cells from ILC1s (Figure S3E). Distinct CD4+ and CD8+ ILC1 clusters were identified, confirming the existence of previously described CD4+ and CD8+ ILC1s in PBMCs (Roan et al., 2016) (Figure S3E, Table S4). Additionally, we performed single-cell whole transcriptome analysis (WTA) of lin− cells from liver ILCs of HCC patients using 10x Genomics Chromium platform and compared it with the targeted immune response panel of the BD Rhapsody™ platform. We used a prediction score to identify ILC subgroups based on markers from the literature [3]. ILCs could be identified in both platforms. Nevertheless, signatures from the targeted panel resulted in a more specific classification of ILC subgroups, since WTA analysis showed high scores for ILC2s in all cluster, indicating background signal resulting in less specific classification of cells. (Figures S3F & S3G). This shows that our pipeline along with the targeted immune panel robustly identified ILC subpopulations using a combination of ILC-specific transcripts.
Next, we performed scRNA-seq of freshly isolated MNCs from tumor, margin, and non-tumor liver of six HCC patients (Figure S3A). ILCs were enriched by fluorescence activated cell sorting (FACS), gating for lin− cells (Figure S3B). No further enrichment for ILC subsets was performed, avoiding exclusion of unknown subpopulations. Lin−, FACS-sorted cells were subjected to scRNA-seq using the BD Rhapsody™ platform [31, 32, 33]. The Rhapsody data were merged across all tissues and patients. After filtering, a total of 16,515 cells were sequenced with an average of 98,000 reads/cell and average detection of 554 molecules/cell (Tables S3B and S3C).
The ILC profile of the six patients analyzed by scRNA-seq matched the distribution of ILCs in the complete cohort when analyzed by FCM. Similar to what we had seen by FCM (Figure 2C and E) four patients (Patients 6, 19, 20 and 21) showed increases in ILC2 frequency from non-tumor to tumor and belonged to the group with high ILC2/ILC1 ratio. Two patients (Patients 10 and 17) showed low ILC2/ILC1 ratios (Figures 3A-C).
Figure 3. Transcriptional signature of ILCs in HCC liver.
A. Heatmap showing clustering of ILC2/ILC1 ratio from 6 patients with matched NT, M and T samples used for scRNA-seq.
B. Frequency of ILC subgroups within individual patients used for scRNA-seq along different regions of NT, M or T. All samples matched.
C. Frequency of Lin−, NK-like and total ILCs of live lymphocytes or ILC1, ILC2 and ILC3 within total ILCs of 6 patients used for scRNA-seq.
D. Unsupervised clustering and t-SNE analysis of scRNA-seq of lin− cells from HCC liver including NT, M and T. t-SNE showing ILC1 (IL7R+, CD6+/−, PTGDR2−, KIT−) ILC2 (IL7R+, CD6−, PTGDR2+, KIT+/−), ILC3 (IL7R+, CD6−, PTGDR2−, KIT+) and ILCs with multiple marker expression ILC1/2 (IL7R+, CD6+, PTGDR2+, KIT−) or ILC1/3 (IL7R+, CD6+, PTGDR2−, KIT+). NK-like cells defined as IL7R−. Total number of cells=16,515; Il7R+=4,680; IL7R−=11,835
E: Violin plots showing expression of main ILC transcripts by clusters. Y-axis = molecules (mols) per cell.
F: t-SNE as in 3A showing ILC 12 clusters after unbiased clustering. (defined in Figure 3D).
G.-I. Violin plots showing expression of selected transcripts of clusters in 3F. Cutoffs: p<0.05, log2FC>0.58 (=FC>1.5). Y-axis in all violin plots = molecules (mols) per cell
G. ILC1-specific transcripts.
H. ILC2-specific transcripts.
I. ILC3-specific transcripts.
J. Selected NK-like-specific transcripts.
K. Selected markers of cytotoxicity.
L. Bar graphs comparing mean mols per cell of selected transcripts defining NK-like cells between all clusters ranked from high to low expression.
M. Violin plot showing DEGs comparing NK-like cells from T vs NT.
Clustering and t-SNE analysis of liver lin− cells merged from these 6 patients identified the main ILC populations (Figure 3D). Expression of IL7R (encoding for CD127) and NKG7 was used to differentiate between helper ILCs (IL7R+, NKG7+/−) and NK-like cells (IL7R−, NKG7+/−) (Figures 3E). We identified major subtypes of ILCs using the expression of ILC-specific transcripts analogous to FCM and AbSeq analysis (Figures 3D and 3E, S3D). Although there is no positive marker specific to helper ILC1s [3, 25, 34], we identified CD6 to be expressed in some PTGDR2−, KIT− clusters in the liver [24]. We also identified PTGDR2−, KIT−, CD6− ILC1s. Cells with markers of both ILC1/2 or ILC1/3 were sparse and did not form distinct clusters. However, we identified PTGDR2+KIT+ cells, the correlate of c-Kit+ ILC2s, which formed intermediate clusters (Figures 3D and 3E, Table S5).
Within ILC populations, unbiased clustering identified a total of 12 clusters (Figure 3F, Table S5). In the two-dimensional t-SNE rendering, these 12 clusters separate into 4 larger meta-clusters distinguishing ILC1, ILC2, ILC3 and NK-like cells from each other. Figure S3H displays the distribution of cells from each patient across the clusters. As expected, some clusters were dominated by individual patients, which could be due to the different numbers of cells sequenced from every patient. We also calculated the proportion of cells from each patient that fell within each meta-cluster/cell type. As shown in Figure S3I, there was a solid representation from each patient across all of the meta-clusters/cell types. Since further analysis is focused on differences between meta-clusters/cell types as opposed to individual clusters, the representation of each patient in all 4 meta-clusters minimizes patient-specific effects.
Cluster 6 identified as CD6+ ILC1 (Lin−-ILC1-cl6). Distinctive to Lin−-ILC1-cl6 were T-cell receptor associated transcripts like CD3E, CD4 and CD8, as seen also by AbSeq [24, 35] (Figure 3G). We identified two ILC2 clusters (Lin−-ILC2-cl5 and Lin−-ILC2-cl10) which also expressed KLRG1, NCR3 and TNFSF10 (TRAIL), markers shown to be involved in ILC2-ILC3 plasticity (Figure 3H) [36]. We also identified two ILC3 clusters (Lin−-ILC3-cl1 and Lin−-ILC3-cl11). Lin−-ILC3-cl1 showed RORC and NCR2 (NKp44). Both, ILC3 clusters expressed higher levels of pro-angiogenic VEGFA, identifying ILC3s as a potential source for this growth factor in the HCC liver [37] (Figure 3I).
We characterized seven IL7R− NK-like clusters (Lin−-NK-cl2, cl3, cl4, cl7, cl8, cl9, cl12) (Figure 3F). We detected NK-like cells expressing a combination of NK cell-associated genes such as NKG7, CD160, EOMES, TBX21, KLRK1 and KLRF1, confirming the mixed NK-like - ILC1 phenotype (Figure 3J). NK-like clusters expressed markers of cytotoxicity including PRF1 and CCL5 and IFNG (Figure 3K). We identified a gradient of these NK-specific markers as well as cytotoxic molecules within NK-like cluster decreasing from Lin−-NK-cl2, −4 and −12 towards Lin−-NK-cl7 and cl9 clusters (Figure 3L). When comparing NK-like cells from tumor and non-tumor tissue, non-tumor NK-like cells showed enriched transcripts of ILC3s including KIT, VEGFA, and CCR6. This indicates the abundance of an NK-like-ILC3 subtype found in the non-tumor region, showing a profile compatible with a previously described NK-like progenitor cell (Figure 3M) [30]. In summary, these data indicate a transition of NK-like cells towards a helper-like ILC1 population with decreasing cytotoxicity profile and identified an NK-like ILC3 subtype. In the non-tumor tissue with progenitor function.
Analyzing the contribution of the individual regions of non-tumor, margin and tumor to the different clusters, this identifies clusters 3, 5 and 10 to be mainly derived from tumor tissue, cluster 11 to be mainly derived from margin and clusters 1, 2, 4, 7, 8, 9 and 12 to be mainly derived from non-tumor tissue. As indicated by flow cytometry analysis, ILC2 clusters (5, 10) and NK-like clusters (3) were mainly derived from tumor tissue, NK-like cells with more typical NK-markers were mainly present in non-tumor tissue (3, 4, 12) and the margin showed a mix of several populations. ILC3s as the dominating ILC helper subgroup was present in all regions (Figure 3N).
Patients with High or Low ILC2/ILC1 Ratio Show Differences in Their Transcriptomic ILC Profile
Since we have identified patients with either high or low ILC2/ILC1 ratios (Figure 2E), we analyzed ILCs from these two groups of patients separately. Unbiased clustering of IL7R+ ILCs from ILC2/ILC1high patients revealed heterogeneity within ILCs, forming 13 clusters (Figure 4A, Table S5), consisting of 3 ILC1 clusters (2, 5, 13), 2 ILC2 clusters (1, 4), 4 ILC3 clusters (3, 9, 11, 12) and 5 intermediate clusters (6, 7, 8, 10) (Figure 4B). Pairwise comparison of ILCs between tumor and non-tumor tissue in the ILC2/ILC1high subset, revealed that ILC1s showed increased expression of typical helper ILC1s markers (CD3D, TRBC2, ZNF683) (Figure 4C). ILC2s in the tumor showed an activated phenotype with high expression of IL32, IL13, KLRB1, ICOS, KRLG1 (specifically KIT+ ILC2s) and NCR3 (Figure 4D). ILC3s showed high angiogenesis promoting VEGFA in the non-tumor tissue (Figure 4E).
Figure 4. Transcriptional signature of ILCs in ILC2/ILC1 high and ILC2/ ILC1 low patients.
A. t-SNE plot of IL7R+ ILCs from patients 6, 19, 20 and 21, previously identified to have a high ratio of ILC2/ILC1. Unbiased clustering reveals 13 clusters from 2,744 cells.
B. Same t-SNE plot of IL7R+ ILCs from patients 6, 19, 20 and 21 colored by ILC subgroups
C. Volcano plot showing pairwise comparison of ILC1s (CD6+/−) from ILC2/ILC1 high patients comparing tumor and non-tumor.
D. Volcano plot showing pairwise comparison of ILC2s (KIT−/+) from ILC2/ILC1 high patients comparing tumor and non-tumor.
E. Volcano plot showing pairwise comparison of ILC3s from ILC2/ILC1 high patients comparing tumor and non-tumor.
F. t-SNE plot of IL7R+ ILCs from patients 7 and 10 previously identified to have a low ratio of ILC2/ILC1. Unbiased clustering reveals 11 clusters from 1,936 cells.
G. Same t-SNE plot of IL7R+ ILCs from patients 7 and 10 colored by ILC subgroups
H. Volcano plot showing pairwise comparison of ILC1s (CD6+/−) from ILC2/ILC1 low patients comparing tumor and non-tumor.
I. Volcano plot showing pairwise comparison of ILC2s (KIT−/+) from ILC2/ILC1 low patients comparing tumor and non-tumor.
J. Volcano plot showing pairwise comparison of ILC3s from ILC2/ILC1 low patients comparing tumor and non-tumor.
Volcano plots in C., D., E., H., I., J. red = number of genes significantly upregulated in T; blue = number of genes significantly upregulated in NT; grey = genes not being significantly different between regions.
On the other hand, the ILC2/ILC1low group showed 11 clusters (Figure 4F, Table S5) consisting of 3 ILC1 clusters (3, 6, 8), 1 ILC2 cluster (2), 5 ILC3 clusters (1, 4, 5, 9, 10) and 2 intermediate clusters (7, 11) (Figure 4G). Interestingly, ILC1s in the non-tumor tissue of ILC2/ILC1low patients showed higher expression of NK cell markers NKG7, KLRB1 and CST7 as well as markers of cytotoxicity PRF1, IFNG and GZMK indicating their intermediate stage between NK-like cells and helper ILC1s (Figure 4H). Both, ILC2s and ILC3s, showed fewer genes specifically upregulated in the tumor compared to the ILC2/ILC1high group. ILC3s expressed pro-angiogenic VEGFA and pro-fibrotic IL32 in the non-tumor liver [38] (Figures 4I and 4J). In summary, ILC2/ILC1high patients showed activation in all ILC subgroups in the tumor. This was particularly evident in ILC2s, which had a more activated phenotype expressing markers like KLRB1 and IL13.
Additionally, we studied ILCs in PBMCs of patients with HCC (Figures S4A-E). Lin− PBMCs formed distinct clusters and did not merge with liver ILCs. (Figure S4A and S4B). PBMC-ILCs showed few intermediate clusters with minimal overlap between the ILC populations, indicating no plasticity (Figure S4C). ILC1s and NK-like cells were on the opposite sides of branches, pointing to their different transcriptomic profiles (Figure S4D). Pairwise comparison of signatures of PBMC ILCs to tumor or non-tumor ILCs showed little overlap, confirming that PBMC-ILCs were distinct from liver ILCs (Figure S4E, Table S5).
HCC Mediates ILC Plasticity That Can Be Induced by Cytokines Ex-Vivo
Next, we performed trajectory analysis on all lin− cells to identify relationships between ILC subgroups and intermediate populations. Trajectory analysis was done on cell clusters described in Figure 3D. The trajectory plot showed that NK-like cells transitioned into CD6+/− helper ILC1s, which finally divided into ILC2, ILC3s (branch 2) and CD6 ILC1 and ILC3s (branch 3) (Figure 5A). ILC3s were split between branch 2 and branch 3, indicating their potential to transition to both, ILC2 and ILC1s (Figure 5B). Importantly, trajectory analysis of the six individual patients revealed similar results as shown in Figure 5A (Figure S4F) indicating that the findings are not skewed by any individual patient.
Figure 5. Trajectory analysis of ILCs reveals ILC2-directed plasticity mediated by tumor cytokines as confirmed by ex-vivo study.
A. Trajectory analysis of all lin− cells showing distribution of main ILC subgroups along trajectory (subgroups defined in Figure 3D).
B. Same trajectory as in 4A now showing distribution of the 12 clusters of ILCs as identified by unbiased clustering in Figure 3F.
C-E. Trajectory analysis of lin− ILCs from individual regions. Distribution of main ILC groups (defined in Figure 3D) along the trajectory.
C. Trajectory of lin− cells from NT.
D. Trajectory of lin− cells from M.
E. Trajectory of lin− cells from T.
F. Trajectory analysis of lin− cells from ILC2/ILC1high patients 6, 19, 20 and 21. Distribution of main ILC subgroups along trajectory (subgroups defined in figure 3D).
G. Trajectory analysis of lin− cells from ILC2/ILC1low patients 10 and 17. Distribution of main ILC subgroups along trajectory (subgroups defined in figure 3D).
H. Trajectory analysis of IL7R+ cells from ILC2/ILC1high patients. Distribution of main ILC subgroups along trajectory.
I. Trajectory analysis of IL7R+ cells from ILC2/ILC1high patients. Distribution of clusters along trajectory.
J. Trajectory analysis of IL7R+ cells from ILC2/ILC1low patients. Distribution of main ILC subgroups along trajectory.
K. Trajectory analysis of IL7R+ cells from ILC2/ILC1low patients. Distribution of clusters along trajectory.
L. Scheme of ex vivo analysis of influence of supernatant (SN) derived from T or NT tissue on MNCs isolated from matched NT. Same supernatant was initially analyzed for cytokine concentrations as shown in Figure 1B. Representative plot from FCM analysis of ILC subgroups in MNCs after incubation with NT- or T-derived SN (NT-SN or T-SN, respectively) overnight from an ILC2/ILC1 high patient compared to MNCs incubated with media only.
M. Ex vivo analysis of ILCs upon incubation with supernatant. ILCs were isolated from NT and incubated overnight with non-tumor supernatant (NT-SN), tumor supernatant (T-SN) or media only. FCM analysis of incubated cells. Bar graphs showing frequency of ILC subgroups. Ex vivo experiments repeated from liver samples of selected HCC patients with increase in tumor ILC2s. (#Media only= 8, #NT-SN=6 due to limited tissue availability, #T-SN=8).
Trajectory analysis of cells from non-tumor tissue confirmed heterogeneity in the NK-like population and beginning plasticity from NK-like cells to CD6+ ILC1s (Figures 5C). NK-like cells from the margin showed even more diversity, with several branches (Figure 5D). Trajectory analysis of margin cells revealed beginning heterogeneity within ILCs with ILC1s and ILC3s split into two distinct populations (Figures 5D). Within tumor ILCs we found ILC1s and ILC3s contributing to ILC2s as the potential endpoint of the plasticity (Figure 5E). In summary, trajectory and branch point analysis showed intricate plasticity between NK like cells and ILC subsets from non-tumor to tumor tissue directed towards ILC1s and ILC2s in the tumor.
Next, we studied cellular trajectory of ILCs from ILC2/ILC1 high and ILC2/ILC1 low patients separately. This analysis revealed distinct differences between the 2 cohorts with ILC2/ILC1 high patients displaying more transcriptional changes and plasticity within IL7R+ ILCs whereas ILC2/ILC1 low patients showed more diversity in NK-like cells (Figure 5F and 5G).
This was confirmed in the trajectory analysis of IL7R+ cells from the two patient groups (Figures 5H & 5I). Trajectory and clustering of branch dependent genes (BEAM) in the ILC2/ILC1 high group revealed clear intermediate clusters between ILC3s and ILC2s as well as ILC3s and CD6+ ILC1s (Figure 5H & S5A). Specifically, cluster 4 of the ILC2/ILC1 high group expressed both PTGDR2 and KIT supporting its intermediate profile between ILC2s and ILC3s (Figure 5I). Cluster 7 showed high expression of ZNF683, also called HOBIT, and formed the end of the ILC2 branch (Figures 5I & 4A with corresponding DEGs in Table S5). HOBIT is a transcription factor required for ILC1 development, indicating that Cluster 7 is an ILC2 cluster derived from plastic transition of ILC1s [39, 40]. Both of these clusters could not be found in the ILC2/ILC1 low group (Figures 5J & 5K). In this group, intermediate clusters were limited and mainly associated with CD6− ILC1s and ILC2s (Figures 5J, 5K & S5B).
To validate our findings and identify the origin(s), we studied ILC plasticity ex vivo. We used the supernatants previously analyzed for cytokine gradients (Figure 1B). Non-tumor and tumor tissues were minced and incubated with media for 6h. The supernatant was harvested and incubated with isolated bulk mononuclear cells (MNCs) derived from non-tumor liver (Figure 5L). Non-tumor supernatant (NT-SN) induced an increase in ILC1s and a decrease in ILC3s compared to culturing in media only. In contrast, tumor supernatant (T-SN) induced an increase in c-Kit+ ILC2s. In parallel, the frequency of ILC3s and ILC1s was reduced upon 6-hour culture with T-SN (Figure 5L & 5M). These changes were not seen when the cells were cultured with either the non-tumor supernatant or media only (Figures 5M). Additionally, tumor supernatant from an ILC2/ILC1 low patient failed to induce a strong ILC2 response and the frequency of ILC1s remained higher in comparison to ILC2s (Figure S5C). Overall absolute cell numbers did not change significantly in the co-culture, indicating a relative change rather than preferential proliferation or death of one subgroup (Figure S5D). Additionally, the proliferation marker MKI67 was not expressed in any of the lin− clusters indicating that proliferation of a specific cell population did not cause the changes in relative ILC subset frequencies seen Figure S5E. Next, we repeated the in vitro studies using defined cell populations rather than unsorted MNCs. Conventional CD56dimCD3− and CD56brightCD3− NK cells as well as lin−CD127− NK-like cells from non-tumor liver were isolated and stimulated for 72 hours with tumor supernatant (T-SN) or control media. Incubation of mature CD56dim NK cells with T-SN increased the frequency of lin−CD127− cells (Figure S5F). Additionally, c-Kit expression increased after incubation with T-SN in all three cell populations tested and CD56 expression decreased when liver derived, sorted NK-like cells were incubated with T-SN, confirming our findings from flow cytometry (Figure S5G, Figure 2B). In summary, the ex vivo experiments corroborated the trajectory analysis showing that lin−CD127− NK-like cells undergo T-SN induced plasticity into a less mature state with c-Kit expression and potential to convert into ILC3s.
To characterize ILCs from tumor and non-tumor liver even further, we also measured ILC cytokines by intracellular cytokine staining. Frequencies of IL-5- (p=0.03) and IL-13-producing (p=0.02) ILC2s increased in the tumor. ILC1s showed more TNF-α producing cells than IFN-γ producing cells in both non-tumor and tumor. TNF-α positive cells were significantly increased in NK-like cells in the tumor. Interestingly, there was also an increase in IL-5 and IL-13 in ILC1s, ILC3s and NK-like cells, indicating a mixed population with type 2-like immune response in the tumor (Figure S5H). ILC3s, NK-like cells and ILC1s cells showed higher expression of the ILC2 transcription factor GATA3 in the tumor, confirming their transition towards ILC2 cells (Figure S5I). In summary trajectory, in-vitro analysis of ILCs and analysis of ILC transcription factors provided evidence of an ILC2-directed plasticity.
ILC-associated cytokines and mature ILC2 Signature are Associated with better Survival
Differences between ILC2/ILC1high and ILC2/ILC1low patients indicate that only ILC2/ILC1high patients undergo full plasticity towards mature tumor ILC2s. Thus, we asked what might drive the different plasticity and composition of ILCs in the two groups.
First, we analyzed bulk RNA-sequencing data of ILC2/ILC1high and ILC2/ILC1low patients separately using again the CibersortX algorithm to identify frequencies of main immune subsets (Figure S6A). There was a trend towards increased CD8+ T cells and decreased Treg in margin and tumors of ILC2/ILC1high compared to ILC2/ILC1low patients (Figure S6A). A high ILC2/ILC1 ratio in tumor and margin was significantly associated with less M0 macrophages resulting in a significantly higher M2/M0 ratio (Figures S6B and S6C). This indicates a different composition of macrophages in ILC2/ILC1high and ILC2/ILC1low patients, which might affect ILC2 plasticity. IL-4 is not only involved in macrophage polarization but can also induce ILC2-directed plasticity [20]. We found high expression of IL-4 in the tumor margin (Figure S6D). IL-4 receptor was highly expressed on tumor margin ILC3s and ILC1s specifically in ILC2/ILC1low patients indicating their potential to transition to ILC2s. (Figure S6E).
Pseudotime analysis of IL-33 receptor transcript IL1RL1 shows that intermediate c-Kit+ ILC2s but not mature c-Kit− ILC2s in the tumor express this subunit (Figure S6F, related to trajectory Figure 5E). This indicates that IL-33 is required to promote the final step of ILC2-directed plasticity specifically to induce mature c-Kit− ILC2s. DEGs comparing tumors from the ILC2/ILC1high and ILC2/ILC1low groups revealed a list of cytokines, cytokine receptors and growth factors including IL-33 that were relatively higher in tumors of ILC2/ILC1high patients (Figure 6A, Table S6). Interestingly, high expression of these genes was associated with better progression-free survival, which was not the case for DEGs upregulated in the tumor of ILC2/ILC1low patients (Figures 6B & S6G, Table S6).
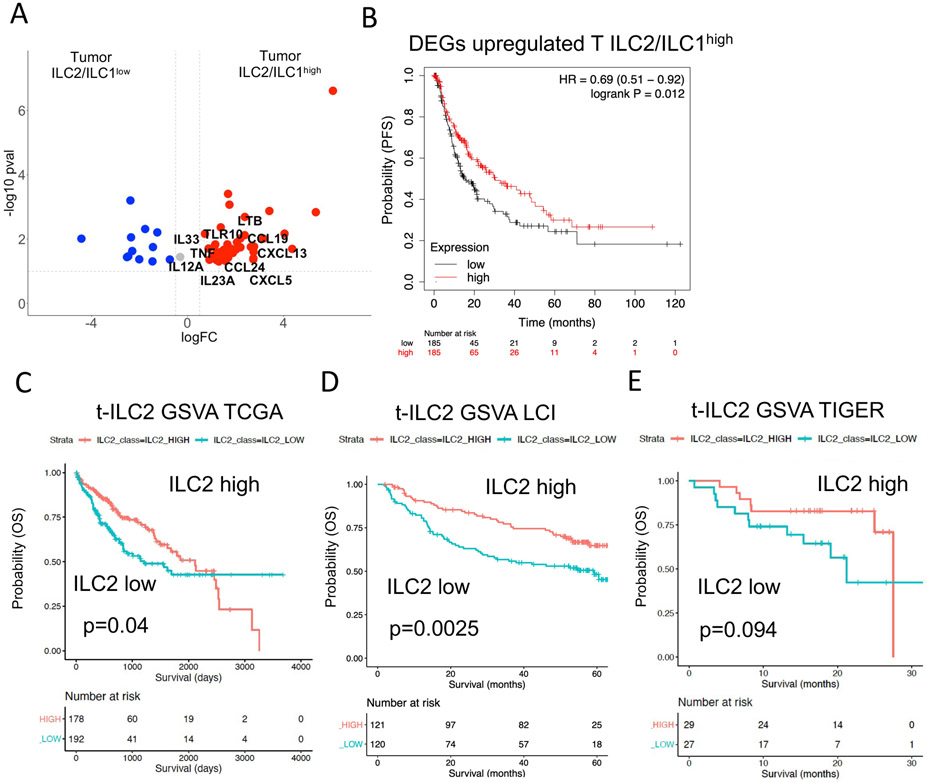
Figure 6. Survival analysis using large HCC cohorts reveals better survival in patients with high tumor ILC2 signature mediated by IL-33.
A. Volcano plot showing DEGs from comparison of bulk RNA sequencing data from tumors of ILC2/ILC1high vs tumors of ILC2/ILC1low patients filtered for cytokines, cytokine receptors and growth factors.
B. Survival risk prediction using significantly upregulated DEGs (p<0.05, logFC>0.58) in tumors of ILC2/ILC1high patients. Progression-free survival (PFS) risk probability based on high or low expression in TCGA liver cancer cohort split by median.
C. Geneset variance analysis (GSVA) using a signature of tumor c-Kit− ILC2s derived from single-cell sequencing analysis (Table S6). Analysis of survival prediction within TCGA liver cancer cohort.
D. Same analysis as in Figure 6C, now analyzing patients of the LCI liver cancer cohort.
E. Same analysis as in Figure 6C, now analyzing patients of the TIGER liver cancer cohort.
Finally, we used the 11-gene signature of mature c-Kit− ILC2s from the tumor (IL32, CD52, PTGDR2, KLRB1, IL7R, DPP4, CTSW, TNFSF10, IL13, LTB, IL2RA; Table S6, FDR<0.05, log2FC>0.58) derived from scRNA-seq to predict overall survival in large HCC cohorts (TCGA, LCI, TIGER). Gene set analysis revealed better overall survival in patients with high expression of the transcriptomic signature of mature c-Kit− ILC2s in three different cohorts of HCC (Figures 6C-E). This data underlines the significance of tumor infiltrating ILC2 for patient outcome. The high ILC2/ILC1 ratio in the tumor identifies patients with a better survival. This introduces new options to finetune the tumor microenvironment to improve anti-tumor immunity.
DISCUSSION
Here, we provide a comprehensive molecular and phenotypic analysis of tumor ILCs in HCC. We describe a complex network of interplay between ILC subsets and extensive plasticity in the context of cancer mediated by tumor-induced cytokine gradients. Due to the low frequency of ILCs, previous studies using scRNA-seq on bulk CD45+ cells failed to characterize all ILC subsets in healthy or HCC livers [17, 44]. We established a protocol using fresh tumor samples as well as a targeted sequencing approach of rare cells that allowed for isolation and sequencing of 16,515 ILCs from HCC livers and PBMCs with minimal ex vivo manipulation. This required large tissue samples of several grams each to gain reasonable numbers of ILCs. Clustering analysis separated ILCs into 12 clusters, which further amalgamated into 4 larger meta-clusters which we identified as the cell types ILC1, ILC2, ILC3 and NK-like based on marker gene expression. While some individual clusters were dominated by cells from individual patients, meta-clusters were more evenly composed of cells from all patients, eliminating the impact of any patient-specific batch effect on our down-stream analysis. Indeed, comparing differentially expressed genes between clusters that all fall within a single meta-cluster or cell type would yield only a small list of genes.
Multiple transcriptional states of ILCs in non-tumor, tumor-margin, and tumor were characterized reflecting plasticity as induced by the HCC environment. Additionally, we identified a CD127− NK-like cell population present in the HCC microenvironment with intermediate status between NK cells and ILC1s, a contributing source of ILC plasticity within liver tumor. Phenotypic and transcriptomic analyses of NK-like cells showed that they shared characteristics of both NK cells and ILC1s. The NK-like cells showed decreased expression of transcripts associated with cytotoxicity and anti-tumor function such as PRF1, GZMA, IFNG as they transitioned into ILC1 cells.
The transcriptomic profile of NK-like cells with decreased CD56 expression and an increased expression of c-Kit suggests a less mature NK-like state with potential precursor function. Conversion between CD117+ NK cells and CD117+ ILC3s has been shown to stem from CD56+ ILC precursors (ILCPs) [30]. ILC2s, however, do not develop from CD56+ ILCP. It has been shown that CD34− CD117+ ILCPs can differentiate into NK-cells and all ILC populations under IL-1 β, IL-2, IL-7, IL-15 and IL-23 stimulation (Chen et al.2018; Lim et al. 2016). Ex vivo analysis identified a gradient of proinflammatory cytokines in the HCC environment regulating this ILC plasticity from non-tumor liver to tumor liver. We detected IL-1β, IL-23 and IL-15 in the non-tumor liver and margin, which might promote development of all ILC-lineages from NK-like cells. TGFβ has been shown to mediate the conversion of NK cells to ILC1 in a murine tumor model to evade the immune system [45]. We found high levels of TGF-β in HCC liver that could mediate the conversion of NK-like cells into ILC1 with reduced cytotoxic function within tumor. The NK-like to ILC1 plasticity was mainly seen on a transcriptomic level reflected by multiple intermediate cell populations. Although there was a trend towards lower NK-like cells in the tumor specifically in the ILC2/ILC1high group, this did not result in significant changes in the overall NK-like frequencies. Other sources for plastic transition to NK-like cells like typical NK-cells or other stages of NK-development cannot be excluded and might affect NK-like frequency [46].
Our data also suggest direct conversions of ILC3s to ILC2s and ILC1s to ILC2s, transitions that have not previously been described in detail. IL-4 is a potential cytokine to drive conversion of ILC3s as well as ILC1s to ILC2s [20]. IL4R was not expressed on tumor ILC2s but on ILC3s and on intermediate ILC1s. It is not clear currently if there is bi-directional conversion between the ILC1 and ILC2 cells in HCC tumors. ILβ, IL-23 and TGF-β have been shown to drive plasticity of ILC2s towards IL-17 producing ILC3s, but IL-6 reversed ILC2-ILC3 plasticity [47]. We detected a mixed cytokine profile with high IL-6 and TGF-β in the tumor, which leads to transition of ILC3s to cKit+ ILC2s and c-Kit− ILC2s in the tumor
Identification of specific gene signatures for ILCs in HCC tumors allowed us to test whether this signature would correlate with survival. Specifically, we noted better overall survival in patients with higher tumor ILC2 signature. On the contrary, higher ILC2/ILC1 ratio was associated with larger tumors in our study. Considering that ILC2s are a counterpart of type 2 helper CD4+ T cells (Th2 cells), whose absence have been described to positively correlate with patient survival [48], it remains to be studied if there are other cells involved which contribute to anti-tumor immunity or if ILC2s counter-regulate Th2 cells. In this context, ILC2s have been reported to promote anti-tumor immunity in pancreatic cancer by mediating CCL5 producing dendritic cells subsequently inducing infiltration of cytotoxic CD8+ T cells [21]. Our CibersortX analysis indicates that dendritic cells were not significantly different, but there was a trend towards increased CD8+ T cells and decreased Treg in margin and tumors of ILC2/ILC1high compared to ILC2/ILC1low patients. We found that ILC2/ILC1low patients show low M2/M0 macrophages ratio in the margin and tumor, indicating a different composition of these cytokine producing cells, which can also contribute to antigen presentation. Specifically, M2 macrophages have been shown to produce IL-33 [49]. Additionally, IL-4 can be produced by ILC2s and M2 macrophages, but also induces M2 macrophage polarization [50]. Certain levels of IL-4 and IL-33 derived from macrophages may be required to induce a strong ILC2 directed plasticity. M2 macrophages have been shown to be detrimental for anti-tumor immunity and are associated with poor survival. We have shown that IL-33 is decreased from non-tumor to tumor, which was associated with poor survival. High IL-33 in the tumor micromilieu inducing high frequencies of ILC2s might counterbalance this phenomenon. Furthermore, ILC2s have been shown to be activated during the early phase of type 2 responses and can subsequently initiate Th2 response [51, 52, 53], indicating that the effect on pro- and anti-tumor immune responses in the tumor microenvironment are time sensitive and might change during tumor progression.
We show that plasticity originates in non-tumor liver and margin indicating a primed environment driving transition between ILC subtypes with cytokines as one of the main potential effectors. It seems that patients with a low ILC2/ILC1 signature have not yet undergone a complete process of ILC2 directed plasticity.
ILCs secrete cytokines both in steady state and under inflammatory conditions and also respond to cytokines released by myeloid cells [19, 54]. Potential sources of the cytokines observed in our study include the ILCs themselves, tumor cells as well as macrophages. Furthermore, IL-5 and IL-13 secretion by ILC2s has been shown to attract eosinophils with anti-tumor properties [55]. IL-33 concentration in the HCC environment was reduced in the tumor tissue compared to non-tumor liver. We identified relatively higher expression of a group of cytokines including IL-33 in the tumor microenvironment in patients with high ILC2 frequency. High IL-33 was independently associated with better overall and progression-free survival but has also been shown to activate ILC2s [21, 56]. Whether one can target these cytokine gradients and plastic ILC populations therapeutically towards a more anti-tumor like ILC environment also needs to be tested. Moreover, the impact of tumor progression on cytokine gradients and ILC plasticity should also be considered, but current clinical treatment strategies provide access to tissue only at one timepoint, which makes addressing these questions more difficult [57]. ILCs in the blood might be easier to access, but only partly reflect the phenomena in the liver. Nevertheless, advanced disease or systemic treatment might influence ILCs in the blood so that they could be used diagnostically.
We provide the first comprehensive and detailed account of how ILC composition as instructed by the cytokines in HCC liver can control HCC patient survival. We have identified several plastic transitions of ILCs that are controlled by tumor-induced cytokine gradients. Further studies are required to explore the contribution of adaptive immune cells to ILC plasticity and function in tumor settings. Although the tumor-induced cytokine environment promotes tumor development and inhibits anti-tumor immunity, we provide a first comprehensive analysis of associations that can be used to plan new treatment strategies to improve anti-tumor immunity. IL-33 seems to be a candidate to alter the tumor microenvironment in a favorable way by promoting ILC2s in the tumor, which might improve survival. We have shown that the complex network of ILCs is very sensitive for alterations induced by the tumor and should be considered in every analysis when studying the tumor immune environment.
Supplementary Material
Figure S1. Characterization of ILCs in Tumor and Non-Tumor Liver from HCC patients, Related to Figure 1
A. Representative pictures with 10x or 20x magnification of H&E staining of liver tissue from non-tumor (NT) liver, tumor margin (M, red line) and HCC tumor. Scale bar = 100 mm
B. Survival prediction analysis of TCGA liver cancer cohort using expression of DEGs between NT and T. DEG list in Table S1 with cut off of FDR<0.05 and log2FC>0.58. OS = overall survival.
C. Survival prediction analysis of TCGA liver cancer cohort using expression of transcripts of cytokines TGFB2, IL6, IL8, IL19 that have shown significant increase in concentration in supernatants derived from non-tumor vs tumor. OS = overall survival.
D. Survival prediction analysis of TCGA liver cancer cohort using expression of transcripts of cytokines IL1B, IL23A, IL17A, CD40LG, IL2, IL15, IL12A, IL9, IL33, and IL4 that have shown significant decrease in concentration in supernatants derived from non-tumor vs tumor. OS = overall survival.
E. Scheme of cytokine gradient in the microenvironment showing changes from NT over M to T associated with survival.
F. CibersortX analysis of samples used in Figure 1A showing abundance of main immune cell populations defined in signature LM22 in regions of liver cancer. Bar plots showing relative frequency of cell populations within individual region. **p<0.01; ****p<0.0001
G. Expression of MARCO comparing NT, M and T in the HCC cohort.
H. Expression of SPP1 comparing NT, M and T in the HCC cohort.
I. Expression of TGFB2 comparing NT and T in the TCGA LIHC cohort.
J. Expression of IL33 comparing NT and T in the TCGA LIHC cohort.
K. Expression of IL1B comparing NT and T in the TCGA LIHC cohort.
L. Survival prediction analysis of TCGA LIHC cohort using expression of TGFB2. Patients were split at median.
M. Survival prediction analysis of TCGA LIHC cohort using expression of IL1B. Patients were split at median.
Figure S2. Characterization of ILCs in Tumor and Non-Tumor Liver from HCC patients, Related to Figure 2
A. FCM gating strategy for identification of CD127−, lin− cells and CD127+, lin− helper ILCs and ILC subgroups including ILC1, c-Kit+/− ILC2 and NKp44+/− ILC3. Lin markers: CD1a, CD34, CD3, TCRαβ, TCRγδ, CD14, CD19, CD16, CD94, CD123, BDCA2, FCeR1a
B. FCM showing expression of CTLA-4, PD-1 and PD-L1 on ILC subgroups across all liver regions and PBMCs.
C. Violin plots showing frequencies of all lin−, CD127−, lin− and CD127+, lin− total ILCs of live lymphocytes.
D. FCM showing expression of EOMES, Tbet and CD56 on ILC1s and ILC3s across all liver regions and PBMCs.
E. FCM analysis of ILCs from 48 HCC patients. Violin plots showing frequencies of ILC subgroups of KIT+/− ILC2 and NKp44+/−ILC3 by region.
F. Frequency of ILCs in NT was subtracted from frequency of ILCs in T (ΔT-NT). Heatmap showing clustering ΔT-NT of ILC1, ILC2 and ILC3. Delta was created from 32 matched samples.
G-H. Bar graph showing frequencies of ILC subgroups by region. S2F: Frequencies of ILC subgroups in ILC2/ILC1high group. S2G: Frequencies of ILC subgroups in ILC2/ILC1low group.
I. Heatmaps showing expression of cytokines, cytokine receptors and growth factors by individual region analyzed by bulk tissue RNA sequencing from liver and tumor tissue of patients with HCC. Patients have been grouped based on the highest ILC subgroup frequency (based on FCM analysis) in the tumor or margin (if tumor sample was not available). ILC1 high patients NT=5, M=4, T=3; ILC2 high patients NT=4, M=4, T=3; ILC3 high patients NT=15, M=14, T=5.
All graphs. Where indicated: *p<0.05, **p<0.01, ****p<0.0001. Dotted line = median.
Figure S3. Transcriptional Characterization of ILCs, Related to Figure 3
A. Analysis pipeline of ILCs from NT, M and T as well as peripheral blood mononuclear cells (PBMCs, B) from HCC patients. Cells were isolated using percoll gradient and analyzed or sorted using FCM. ILC-enriched cells underwent scRNA-seq analysis using BD Rhapsody™ platform.
B. Gating strategy for enrichment of ILCs in lineage+ (lin+) and lineage− (lin−) fraction.
C. t-SNE plot showing distribution of cells by sample of either lin+ or lin− sorted cells.
D. Same t-SNE plot as in C, showing clusters of main immune cells and ILCs within lin+ and lin− sorted PBMCs, identified by expression of main transcriptomic and AbSeq markers (DEGs in Table S4).
E. t-SNE plot as shown in (C), showing expression of individual ILC or T cell specific transcripts or AbSeq-antibody expression of surface markers.
F. BD Rhapsody scRNA-seq analysis of liver ILCs from HCC patient. The cell type for each cluster was identified by the generalized linear model (glm) analysis of NK/ILC cell markers [58] and single cell gene expression profile.
G. Same analysis pipeline as in S3F, now using the 10x Genomics Chromium scRNA-seq platform for analysis of liver ILCs from HCC patient. Again, the cell type for each cluster was identified by the generalized linear model (glm) analysis of NK/ILC cell markers [58] and single cell gene expression profile to compare both scRNA-seq platforms for analysis of liver ILCs.
H. Same t-SNE plot as in Figure 3D and 3F. Cells colored by patient samples.
I. Representation of each patient across meta-clusters/cell types.
Figure S4. Transcriptional signature of ILCs in ILC2/ILC1 high and ILC2/ILC1 low patients. Plots of individual patients. Related to Figure 4.
A-B. t-SNE plot showing merged cells from NT, M and T as wells as PBMCs after unbiased clustering colored by origin of cells by region (A) and main ILC subgroups (B). Number of cells=16.515.
C. t-SNE plot of lin− cells from PBMCs only, showing main ILC groups.
D. Trajectory analysis of lin− cells from PBMCs only.
E. Venn Diagram showing overlap of DEGs from comparison of PBMC in blood (=B) ILCs with tissue ILCs from different regions by ILC subgroup.
F. Trajectory analysis of lin− cells showing distribution of main ILC subgroups along trajectory in individual patients.
Figure S5. BEAM Heatmap analysis and ex-vivo culturing confirms ILC2-directed plasticity in the tumor. Related to Figure 5.
A. BEAM Heatmap showing clustering of branchpoint DEGs from trajectory of IL7R+ ILCs of ILC2/ILC1high patients related to figure 5H. Branch including ILC3s (left) or branch including ILC1s (right) were set as origin.
B. BEAM Heatmap showing clustering of branchpoint DEGs from trajectory of IL7R+ ILCs of ILC2/ILC1low patients related to figure 5j. Branch including ILC3s (left) or branch including ILC1s (right) were set as origin.
C. Representative plot from FCM analysis of ILC subgroups in MNCs after incubation with NT- or T-derived SN (NT-SN or T-SN, respectively) from an ILC2/ILC1low patient compared to MNCs incubated with media only.
D. Ex vivo analysis of ILCs upon incubation with supernatant. Bar graphs showing frequency of ILC subgroups within all live lymphocytes and absolute numbers. Ex vivo experiments repeated from liver samples of selected HCC patients with increase in ILC2s (#Media only= 8, #NT-SN=6 due to limited tissue availability, #T-SN=8).
E. Expression of proliferation marker MKI67 in 12 clusters of all lin− cells (as shown in Figure 3F).
F. FCM analysis of isolated CD56dimCD3−, CD56brightCD3− cells or lin−CD127− NK-like ILCs isolated from NT liver tissue incubated for 72 hours with x-vivo control media (upper row) or T-SN T-SN from matched tumor samples (lower row) for 72 hours. After 72 hours cells were stained for ILC markers. Expression of lin markers and CD127 are shown on gated CD45+ lymphocytes.
G. CD117 (c-Kit) and CD56 analysis on live CD45+lin−CD127− cells from experiment described in S5F.
H. Intracellular cytokine staining of NK-like cells and ILC subgroups by region. Frequency of cells expressing IL-5, IL-13, IFN-γ or TNF-α.
I. Intracellular staining of transcription factor GATA3 of NK-like cells and ILC subgroups by region.
Figure S6. ILCs are mediated by tumor antigens and macrophage cytokines both promoting ILC2 directed plasticity and ILC2 activation in the HCC tumor. Related to Figure 6.
A. CibersortX analysis of samples used in Figure S1F now split by NT or T+M samples from either ILC2/ILC1high or ILC2/ILC1low patients. Abundance of main immune cell populations defined in signature LM22 in regions of liver cancer. Barplots showing relative frequency of cell population within individual region.
B. Correlation of frequency of M0 macrophages determined by in-silico deconvolution or bulk tissue RNA-sequencing using CibersortX with ILC2/ILC1 frequency ratio measured by flow cytometry.
C. Ratio of frequency of M2 vs M0 macrophages from CibersortX analysis comparing ILC2/ILC1 high and ILC2/ILC1 low patients.
D. Expression of IL4 comparing NT, M and T in this HCC cohort.
E. Volcano plot showing DEGs from comparison of T and M of CD6− ILC1s and ILC3s from ILC2/ILC1low patients.
F. Pseudotime analysis of trajectory from tumor ILCs (Figure 5F) of IL-33 receptor IL1RL1, starting at branch 2 (NK-like cells) as origin.
G. Survival risk prediction using significantly upregulated DEGs (p<0.05, logFC>0.58) in tumors of ILC2/ILC1low patients. Progression-free survival (PFS) risk probability based on high or low expression in TCGA liver cancer cohort split by median.
Table S1. DEGs of Cytokines, Cytokine Receptors and Growth Factors Comparing T and NT. Related to Figures 1A and S1B. Cut off for survival analysis FDR>0.05, log2FC>0.58.
Tables S2. A. Clinical Characteristics of HCC Patients Involved in This Study. B. Comparison of Clinical Characteristics of ILC2/ILC1high and ILC2/ILC1low Patients. C. Comparison of Clinical Characteristics of Patients stratified by IL-33 expression.
Tables S3A-C. A: BD Rhapsody 405 Gene Panel Used in This Study. B. Raw Metrics of scRNAseq with AbSeq, Related to Figures S3C-E: Raw Metrics of scRNAseq, Related to Figures 3D and 3F, S3F and S3G, 4, S4.
Table S4. DEGs of Immune Cell Subsets Identified by scRNAseq with AbSeq, Related to Figures S3D and S3E.
Table S5. DEGs of Clusters from t-SNE Analysis of All lin− cells, Related to Figures 3D and 3F. DEGs of Clusters Identified in Figure 4A and Figure 4E. DEGs from Comparison of NK-like cells and ILCs from Blood with NK-like cells and ILCs from Non-Tumor or Tumor Tissue, Related to Figure S4E.
Table S6. Cytokine DEGs comparing T ILC2/ILC1high vs T ILC2/ILC1low. Related to Figures 6A, 6B and S6G. DEGs of Tumor ILC2s Used as Signature for Survival Prediction Analysis. Related to Figures 6C-E.
SUMMARY.
What is already known?
Previous studies identified the contribution of ILC2s to the development of liver fibrosis in mice. The biological function of ILCs in human HCC remains largely unknown. Little is known about the dynamics of ILC-controlling cytokines in the HCC tumor environment.
What are the new findings?
ILC-controlling cytokines are differentially expressed in non-tumor and tumor tissue and are associated with survival in the TCGA HCC cohort. Trajectory analysis of ILCs can be used to identify transcriptomic and phenotypic transition of non-tumor ILCs towards ILC2s and non-cytotoxic ILC1s in the tumor. Cytokines derived from HCC tumor can be used to induce ILC2-directed plasticity in-vitro. Composition of ILCs in the tumor can be used to stratify HCC patients. Patients with higher IL-33 expression in the tumor show a high ILC2/ILC1 ratio, which is associated with better survival.
How might it impact on clinical practice in the foreseeable future?
Manipulation of ILC-controlling cytokine gradients could be used to improve anti-tumor immune responses in HCC.
Acknowledgements
We thank Dr. Xiaolin Wu and David Sun from CCR Genomic Technology Laboratory, Frederick National Laboratory for Cancer Research (FNLCR), for single cell sequencing on BD Rhapsody platform. We thank Dr. Mariam Malik and Dr. David Goldstein from the Office of Science and Technology Resources. We thank Dr. Jon Inglefield and Yanyu Wang from Lymphokine Testing Section, Clinical Services Program, FNLCR, for performing multiplex cytokine analysis. We thank Dr. Jay Berzofsky, and Dr. Jeff Zhu for helpful discussions and comments.
Funding
T.F.G., E.R. and X.W.W. were supported by the Intramural Research Program of the NIH, NCI (ZIA BC 011344, ZIA BC 011870). Alexander Kroemer is supported by the National Institute of Allergy and Infectious Diseases (R01AI132389; R21AI130800). This work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Abbreviations:
- HCC
hepatocellular carcinoma
- ILC
innate lymphoid cells
- RNA
ribonucleic acid
- NK
natural killer
- PBMCs
peripheral blood mononuclear cells
- TCGA
the cancer genome atlas
- scRNA-seq
single-cell RNA sequencing
- CTLA-4
cytotoxic T-lymphocyte-associated Protein 4
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death protein ligand 1
- t-SNE
t-distributed stochastic neighbor embedding
- DEGs
differentially expressed genes
Footnotes
Competing Interests
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. Journal of leukocyte biology 2006;80:1197–213. [DOI] [PubMed] [Google Scholar]
- 2.Hou J, Zhang H, Sun B, Karin M. The immunobiology of hepatocellular carcinoma in humans and mice: Basic concepts and therapeutic implications. Journal of hepatology 2020;72:167–82. [DOI] [PubMed] [Google Scholar]
- 3.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell 2018;174:1054–66. [DOI] [PubMed] [Google Scholar]
- 4.Liu M, Zhang C. The role of innate lymphoid cells in immune-mediated liver diseases. Front Immunol 2017;8:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forkel M, Berglin L, Kekalainen E, Carlsson A, Svedin E, Michaelsson J, et al. Composition and functionality of the intrahepatic innate lymphoid cell-compartment in human nonfibrotic and fibrotic livers. European journal of immunology 2017;47:1280–94. [DOI] [PubMed] [Google Scholar]
- 6.Neumann K, Karimi K, Meiners J, Voetlause R, Steinmann S, Dammermann W, et al. A proinflammatory role of type 2 innate lymphoid cells in murine immune-mediated hepatitis. Journal of immunology (Baltimore, Md : 1950) 2017;198:128–37. [DOI] [PubMed] [Google Scholar]
- 7.Trabanelli S, Chevalier MF, Derre L, Jandus C. The pro- and anti-tumor role of ILC2s. Seminars in immunology 2019;41:101276. [DOI] [PubMed] [Google Scholar]
- 8.Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 2016;164:365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Therneau T, Grambsch P. Modeling survival data. Extending the Cox model. 2000 by Springer-Verlag New York. Inc, 2000. [Google Scholar]
- 10.Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 2013; 14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menyhart O, Nagy A, Gyorffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R Soc Open Sci 2018;5:181006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, et al. Reversed graph embedding resolves complex single-cell trajectories. Nature methods 2017;14:979–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nature biotechnology 2019;37:773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods in molecular biology (Clifton, NJ) 2018;1711:243–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nature methods 2015;12:453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohr-Udilova N, Klinglmuller F, Schulte-Hermann R, Stift J, Herac M, Salzmann M, et al. Deviations of the immune cell landscape between healthy liver and hepatocellular carcinoma. Scientific reports 2018;8:6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 2019;179:829–45 e20. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O'Brien SA, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 2020; 181:442–59 e29. [DOI] [PubMed] [Google Scholar]
- 19.Mortha A, Burrows K. Cytokine networks between innate lymphoid cells and myeloid Cells. Front Immunol 2018;9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bal SM, Golebski K, Spits H. Plasticity of innate lymphoid cell subsets. Nature reviews Immunology 2020;20:552–65. [DOI] [PubMed] [Google Scholar]
- 21.Moral JA, Leung J, Rojas LA, Ruan J, Zhao J, Sethna Z, et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 2020;579:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattiola I, Diefenbach A. Innate lymphoid cells and cancer at border surfaces with the environment. Seminars in immunology 2019;41:101278. [DOI] [PubMed] [Google Scholar]
- 23.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nature immunology 2013;14:221–9. [DOI] [PubMed] [Google Scholar]
- 24.Bjorklund AK, Forkel M, Picelli S, Konya V, Theorell J, Friberg D, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nature immunology 2016;17:451–60. [DOI] [PubMed] [Google Scholar]
- 25.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews Immunology 2013;13:145–9. [DOI] [PubMed] [Google Scholar]
- 26.Hazenberg MD, Spits H. Human innate lymphoid cells. Blood 2014;124:700–9. [DOI] [PubMed] [Google Scholar]
- 27.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood 2009;113:5488–96. [DOI] [PubMed] [Google Scholar]
- 28.Jiao Y, Huntington ND, Belz GT, Seillet C. Type 1 innate lymphoid cell biology: Lessons learnt from Natural Killer cells. Front Immunol 2016;7:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colonna M Innate lymphoid cells: Diversity, plasticity, and unique functions in immunity. Immunity 2018;48:1104–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Youssef Y, Robinson C, Ernst GF, Carson MY, Young KA, et al. CD56 expression marks human group 2 innate lymphoid cell divergence from a shared NK cell and group 3 innate lymphoid cell developmental pathway. Immunity 2018;49:464–76 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shum EY, Walczak EM, Chang C, Christina Fan H. Quantitation of mRNA transcripts and proteins using the BD Rhapsody™ single-cell analysis system. In: Suzuki Y, ed. Single Molecule and Single Cell Sequencing. Singapore: Springer Singapore, 2019:63–79. [DOI] [PubMed] [Google Scholar]
- 32.Fan HC, Fu GK, Fodor SP. Expression profiling. Combinatorial labeling of single cells for gene expression cytometry. Science (New York, NY) 2015;347:1258367. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen A, Khoo WH, Moran I, Croucher PI, Phan TG. Single cell RNA sequencing of rare immune cell populations. Front Immunol 2018;9:1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernink JH, Mjosberg J, Spits H. Human ILC1: To be or not to be. Immunity 2017;46:756–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roan F, Stoklasek TA, Whalen E, Molitor JA, Bluestone JA, Buckner JH, et al. CD4+ Group 1 innate lymphoid cells (ILC) form a functionally distinct ILC subset that is increased in systemic sclerosis. Journal of immunology (Baltimore, Md : 1950) 2016;196:2051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagasawa M, Heesters BA, Kradolfer CMA, Krabbendam L, Martinez-Gonzalez I, de Bruijn MJW, et al. KLRG1 and NKp46 discriminate subpopulations of human CD117(+)CRTH2(−) ILCs biased toward ILC2 or ILC3. The Journal of experimental medicine 2019;216:1762–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 2016;166:1231–46 e13. [DOI] [PubMed] [Google Scholar]
- 38.Moschen AR, Fritz T, Clouston AD, Rebhan I, Bauhofer O, Barrie HD, et al. Interleukin-32: a new proinflammatory cytokine involved in hepatitis C virus-related liver inflammation and fibrosis. Hepatology (Baltimore, Md) 2011;53:1819–29. [DOI] [PubMed] [Google Scholar]
- 39.Lunemann S, Martrus G, Goebels H, Kautz T, Langeneckert A, Salzberger W, et al. Hobit expression by a subset of human liver-resident CD56(bright) Natural Killer cells. Scientific reports 2017;7:6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Post M, Cuapio A, Osl M, Lehmann D, Resch U, Davies DM, et al. The transcription factor ZNF683/HOBIT regulates human NK-Cell development. Front Immunol 2017;8:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol 2018;9:1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scoville SD, Freud AG, Caligiuri MA. Modeling Human Natural Killer Cell Development in the Era of Innate Lymphoid Cells. Front Immunol 2017;8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duerr CU, Fritz JH. Regulation of group 2 innate lymphoid cells. Cytokine 2016;87:1–8. [DOI] [PubMed] [Google Scholar]
- 44.MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun 2018;9:4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao YL, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nature immunology 2017;18:1004-+. [DOI] [PubMed] [Google Scholar]
- 46.Stamatiades EG, Li MO. Tissue-resident cytotoxic innate lymphoid cells in tumor immunosurveillance. Seminars in immunology 2019;41:101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golebski K, Ros XR, Nagasawa M, van Tol S, Heesters BA, Aglmous H, et al. IL-1beta, IL-23, and TGF-beta drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nat Commun 2019;10:2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foerster F, Hess M, Gerhold-Ay A, Marquardt JU, Becker D, Galle PR, et al. The immune contexture of hepatocellular carcinoma predicts clinical outcome. Scientific reports 2018;8:5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furukawa S, Moriyama M, Miyake K, Nakashima H, Tanaka A, Maehara T, et al. Interleukin-33 produced by M2 macrophages and other immune cells contributes to Th2 immune reaction of IgG4-related disease. Scientific reports 2017;7:42413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casella G, Garzetti L, Gatta AT, Finardi A, Maiorino C, Ruffini F, et al. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J Neuroinflammation 2016;13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014;41:283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halim TY, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014;40:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurram RK, Zhu J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cellular & molecular immunology 2019;16:225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015;517:293–301. [DOI] [PubMed] [Google Scholar]
- 55.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, et al. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. Journal of immunology (Baltimore, Md : 1950) 2012;188:703–13. [DOI] [PubMed] [Google Scholar]
- 56.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. The Journal of experimental medicine 2013;210:2939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghaedi M, Ohashi PS. ILC transdifferentiation: roles in cancer progression. Cell research 2020;30:562–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nature reviews Immunology 2018;18:671–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Characterization of ILCs in Tumor and Non-Tumor Liver from HCC patients, Related to Figure 1
A. Representative pictures with 10x or 20x magnification of H&E staining of liver tissue from non-tumor (NT) liver, tumor margin (M, red line) and HCC tumor. Scale bar = 100 mm
B. Survival prediction analysis of TCGA liver cancer cohort using expression of DEGs between NT and T. DEG list in Table S1 with cut off of FDR<0.05 and log2FC>0.58. OS = overall survival.
C. Survival prediction analysis of TCGA liver cancer cohort using expression of transcripts of cytokines TGFB2, IL6, IL8, IL19 that have shown significant increase in concentration in supernatants derived from non-tumor vs tumor. OS = overall survival.
D. Survival prediction analysis of TCGA liver cancer cohort using expression of transcripts of cytokines IL1B, IL23A, IL17A, CD40LG, IL2, IL15, IL12A, IL9, IL33, and IL4 that have shown significant decrease in concentration in supernatants derived from non-tumor vs tumor. OS = overall survival.
E. Scheme of cytokine gradient in the microenvironment showing changes from NT over M to T associated with survival.
F. CibersortX analysis of samples used in Figure 1A showing abundance of main immune cell populations defined in signature LM22 in regions of liver cancer. Bar plots showing relative frequency of cell populations within individual region. **p<0.01; ****p<0.0001
G. Expression of MARCO comparing NT, M and T in the HCC cohort.
H. Expression of SPP1 comparing NT, M and T in the HCC cohort.
I. Expression of TGFB2 comparing NT and T in the TCGA LIHC cohort.
J. Expression of IL33 comparing NT and T in the TCGA LIHC cohort.
K. Expression of IL1B comparing NT and T in the TCGA LIHC cohort.
L. Survival prediction analysis of TCGA LIHC cohort using expression of TGFB2. Patients were split at median.
M. Survival prediction analysis of TCGA LIHC cohort using expression of IL1B. Patients were split at median.
Figure S2. Characterization of ILCs in Tumor and Non-Tumor Liver from HCC patients, Related to Figure 2
A. FCM gating strategy for identification of CD127−, lin− cells and CD127+, lin− helper ILCs and ILC subgroups including ILC1, c-Kit+/− ILC2 and NKp44+/− ILC3. Lin markers: CD1a, CD34, CD3, TCRαβ, TCRγδ, CD14, CD19, CD16, CD94, CD123, BDCA2, FCeR1a
B. FCM showing expression of CTLA-4, PD-1 and PD-L1 on ILC subgroups across all liver regions and PBMCs.
C. Violin plots showing frequencies of all lin−, CD127−, lin− and CD127+, lin− total ILCs of live lymphocytes.
D. FCM showing expression of EOMES, Tbet and CD56 on ILC1s and ILC3s across all liver regions and PBMCs.
E. FCM analysis of ILCs from 48 HCC patients. Violin plots showing frequencies of ILC subgroups of KIT+/− ILC2 and NKp44+/−ILC3 by region.
F. Frequency of ILCs in NT was subtracted from frequency of ILCs in T (ΔT-NT). Heatmap showing clustering ΔT-NT of ILC1, ILC2 and ILC3. Delta was created from 32 matched samples.
G-H. Bar graph showing frequencies of ILC subgroups by region. S2F: Frequencies of ILC subgroups in ILC2/ILC1high group. S2G: Frequencies of ILC subgroups in ILC2/ILC1low group.
I. Heatmaps showing expression of cytokines, cytokine receptors and growth factors by individual region analyzed by bulk tissue RNA sequencing from liver and tumor tissue of patients with HCC. Patients have been grouped based on the highest ILC subgroup frequency (based on FCM analysis) in the tumor or margin (if tumor sample was not available). ILC1 high patients NT=5, M=4, T=3; ILC2 high patients NT=4, M=4, T=3; ILC3 high patients NT=15, M=14, T=5.
All graphs. Where indicated: *p<0.05, **p<0.01, ****p<0.0001. Dotted line = median.
Figure S3. Transcriptional Characterization of ILCs, Related to Figure 3
A. Analysis pipeline of ILCs from NT, M and T as well as peripheral blood mononuclear cells (PBMCs, B) from HCC patients. Cells were isolated using percoll gradient and analyzed or sorted using FCM. ILC-enriched cells underwent scRNA-seq analysis using BD Rhapsody™ platform.
B. Gating strategy for enrichment of ILCs in lineage+ (lin+) and lineage− (lin−) fraction.
C. t-SNE plot showing distribution of cells by sample of either lin+ or lin− sorted cells.
D. Same t-SNE plot as in C, showing clusters of main immune cells and ILCs within lin+ and lin− sorted PBMCs, identified by expression of main transcriptomic and AbSeq markers (DEGs in Table S4).
E. t-SNE plot as shown in (C), showing expression of individual ILC or T cell specific transcripts or AbSeq-antibody expression of surface markers.
F. BD Rhapsody scRNA-seq analysis of liver ILCs from HCC patient. The cell type for each cluster was identified by the generalized linear model (glm) analysis of NK/ILC cell markers [58] and single cell gene expression profile.
G. Same analysis pipeline as in S3F, now using the 10x Genomics Chromium scRNA-seq platform for analysis of liver ILCs from HCC patient. Again, the cell type for each cluster was identified by the generalized linear model (glm) analysis of NK/ILC cell markers [58] and single cell gene expression profile to compare both scRNA-seq platforms for analysis of liver ILCs.
H. Same t-SNE plot as in Figure 3D and 3F. Cells colored by patient samples.
I. Representation of each patient across meta-clusters/cell types.
Figure S4. Transcriptional signature of ILCs in ILC2/ILC1 high and ILC2/ILC1 low patients. Plots of individual patients. Related to Figure 4.
A-B. t-SNE plot showing merged cells from NT, M and T as wells as PBMCs after unbiased clustering colored by origin of cells by region (A) and main ILC subgroups (B). Number of cells=16.515.
C. t-SNE plot of lin− cells from PBMCs only, showing main ILC groups.
D. Trajectory analysis of lin− cells from PBMCs only.
E. Venn Diagram showing overlap of DEGs from comparison of PBMC in blood (=B) ILCs with tissue ILCs from different regions by ILC subgroup.
F. Trajectory analysis of lin− cells showing distribution of main ILC subgroups along trajectory in individual patients.
Figure S5. BEAM Heatmap analysis and ex-vivo culturing confirms ILC2-directed plasticity in the tumor. Related to Figure 5.
A. BEAM Heatmap showing clustering of branchpoint DEGs from trajectory of IL7R+ ILCs of ILC2/ILC1high patients related to figure 5H. Branch including ILC3s (left) or branch including ILC1s (right) were set as origin.
B. BEAM Heatmap showing clustering of branchpoint DEGs from trajectory of IL7R+ ILCs of ILC2/ILC1low patients related to figure 5j. Branch including ILC3s (left) or branch including ILC1s (right) were set as origin.
C. Representative plot from FCM analysis of ILC subgroups in MNCs after incubation with NT- or T-derived SN (NT-SN or T-SN, respectively) from an ILC2/ILC1low patient compared to MNCs incubated with media only.
D. Ex vivo analysis of ILCs upon incubation with supernatant. Bar graphs showing frequency of ILC subgroups within all live lymphocytes and absolute numbers. Ex vivo experiments repeated from liver samples of selected HCC patients with increase in ILC2s (#Media only= 8, #NT-SN=6 due to limited tissue availability, #T-SN=8).
E. Expression of proliferation marker MKI67 in 12 clusters of all lin− cells (as shown in Figure 3F).
F. FCM analysis of isolated CD56dimCD3−, CD56brightCD3− cells or lin−CD127− NK-like ILCs isolated from NT liver tissue incubated for 72 hours with x-vivo control media (upper row) or T-SN T-SN from matched tumor samples (lower row) for 72 hours. After 72 hours cells were stained for ILC markers. Expression of lin markers and CD127 are shown on gated CD45+ lymphocytes.
G. CD117 (c-Kit) and CD56 analysis on live CD45+lin−CD127− cells from experiment described in S5F.
H. Intracellular cytokine staining of NK-like cells and ILC subgroups by region. Frequency of cells expressing IL-5, IL-13, IFN-γ or TNF-α.
I. Intracellular staining of transcription factor GATA3 of NK-like cells and ILC subgroups by region.
Figure S6. ILCs are mediated by tumor antigens and macrophage cytokines both promoting ILC2 directed plasticity and ILC2 activation in the HCC tumor. Related to Figure 6.
A. CibersortX analysis of samples used in Figure S1F now split by NT or T+M samples from either ILC2/ILC1high or ILC2/ILC1low patients. Abundance of main immune cell populations defined in signature LM22 in regions of liver cancer. Barplots showing relative frequency of cell population within individual region.
B. Correlation of frequency of M0 macrophages determined by in-silico deconvolution or bulk tissue RNA-sequencing using CibersortX with ILC2/ILC1 frequency ratio measured by flow cytometry.
C. Ratio of frequency of M2 vs M0 macrophages from CibersortX analysis comparing ILC2/ILC1 high and ILC2/ILC1 low patients.
D. Expression of IL4 comparing NT, M and T in this HCC cohort.
E. Volcano plot showing DEGs from comparison of T and M of CD6− ILC1s and ILC3s from ILC2/ILC1low patients.
F. Pseudotime analysis of trajectory from tumor ILCs (Figure 5F) of IL-33 receptor IL1RL1, starting at branch 2 (NK-like cells) as origin.
G. Survival risk prediction using significantly upregulated DEGs (p<0.05, logFC>0.58) in tumors of ILC2/ILC1low patients. Progression-free survival (PFS) risk probability based on high or low expression in TCGA liver cancer cohort split by median.
Table S1. DEGs of Cytokines, Cytokine Receptors and Growth Factors Comparing T and NT. Related to Figures 1A and S1B. Cut off for survival analysis FDR>0.05, log2FC>0.58.
Tables S2. A. Clinical Characteristics of HCC Patients Involved in This Study. B. Comparison of Clinical Characteristics of ILC2/ILC1high and ILC2/ILC1low Patients. C. Comparison of Clinical Characteristics of Patients stratified by IL-33 expression.
Tables S3A-C. A: BD Rhapsody 405 Gene Panel Used in This Study. B. Raw Metrics of scRNAseq with AbSeq, Related to Figures S3C-E: Raw Metrics of scRNAseq, Related to Figures 3D and 3F, S3F and S3G, 4, S4.
Table S4. DEGs of Immune Cell Subsets Identified by scRNAseq with AbSeq, Related to Figures S3D and S3E.
Table S5. DEGs of Clusters from t-SNE Analysis of All lin− cells, Related to Figures 3D and 3F. DEGs of Clusters Identified in Figure 4A and Figure 4E. DEGs from Comparison of NK-like cells and ILCs from Blood with NK-like cells and ILCs from Non-Tumor or Tumor Tissue, Related to Figure S4E.
Table S6. Cytokine DEGs comparing T ILC2/ILC1high vs T ILC2/ILC1low. Related to Figures 6A, 6B and S6G. DEGs of Tumor ILC2s Used as Signature for Survival Prediction Analysis. Related to Figures 6C-E.