Abstract
Globally, 2.2 billion people live with some form of vision impairment and/or eye disease. To date, most systematic reviews examining associations have focused on a single eye disease and there is no systematic evaluation of the relationships between eye diseases and diverse physical and mental health outcomes. Moreover, the strength and reliability of the literature is unclear. We performed an umbrella review of observational studies with meta analyses for any physical and/or mental comorbidities associated with eye disease. For each association, random-effects summary effect size, heterogeneity, small-study effect, excess significance bias and 95% prediction intervals were calculated, and used to grade significant evidence from convincing to weak. 34 studies were included covering 58 outcomes. No outcomes yielded convincing evidence, six outcomes yielded highly suggestive results (cataract positively associated with type 2 diabetes, open-angled glaucoma positively associated with myopia and diabetes, diabetic retinopathy positively associated with cardiovascular disease and cardiovascular mortality, and retinopathy of prematurity positively associated with chorioamnionitis), eight outcomes yielded suggestive results (diabetic retinopathy positively associated with all-cause mortality and depression, diabetic macular oedema positively associated with dyslipidaemia, cataract positively associated with gout, nuclear sclerosis positively associated with all-cause mortality, open angled glaucoma positively associated with migraine and hypertension, and age-related macular degeneration positively associated with diabetes), and 18 outcomes yielded weak evidence. Results show highly suggestive or suggestive evidence for associations between several types of eye diseases with several comorbid outcomes. Practitioners and public health policies should note these findings when developing healthcare policies.
Subject terms: Risk factors, Outcomes research
摘要
全球22亿人患有视力障碍和/或各种眼疾。到目前为止, 大多数系统综述都是针对眼病而进行的, 没有系统地评价眼病与各种生理和心理健康结果之间的关系。此外, 文献的影响力和可靠性尚不清楚。我们对与眼病相关的任何全身和/或精神共患疾病的meta分析的观察性研究进行了伞形评价。对于每项研究, 我们都计算了随机效应的总效应量、异质性、小型研究效应、显著性偏差和95%的可信区间, 并将显著性证据等级从信服到较弱进行了划分。共纳入34项研究, 包括58项结果。其中没有一项结果的证据等级达到信服, 6个结果的证据等级达到高度有意义 (白内障与2型糖尿病正相关, 开角型青光眼与近视和糖尿病正相关, 糖尿病视网膜病变与心血管疾病和心血管死亡率正相关, 早产儿视网膜病变与绒毛膜羊膜炎呈正相关, 8项结果的证据等级为有意义 (糖尿病视网膜病变与全因死亡率和抑郁呈正相关, 糖尿病黄斑水肿与血脂异常呈正相关, 白内障与痛风呈正相关, 核硬化与全因死亡率呈正相关, 开角型青光眼与偏头痛和高血压呈正相关, 年龄相关性黄斑变性与糖尿病呈正相关, 18项结果的证据不足。结果显示多种眼病与多种共患疾病之间的相关性为高度有意义或有意义等级的证据。相关人员在制定公共卫生保健政策时应注意这些发现。
Introduction
Globally, it is estimated that ~2.2 billion people live with some form of vision impairment and/or eye disease, with at least 1 billion of these having preventable visual impairment [1, 2]. The leading causes of visual impairment include several eye diseases, including cataract, glaucoma, and diabetic retinopathy [3], with prevalence rates accelerating over the last 10 years due to population growth and ageing. There are also large differences in eye disease prevalence depending on geographic location, with the greatest prevalence being in low income countries [3].
A large body of literature reports that those with eye disease may be at a higher risk of physical and mental health complications when compared to those who are normally sighted (e.g. mobility limitations [4], chronic kidney disease [5], gout [6], obstructive sleep apnoea [7], depression [8], lower cognitive function [9], and suicidal behaviour [10]) and, importantly, increased risk of cardiovascular disease mortality [11, 12].
Given the incidence, morbidity, and mortality rates associated with eye disease, numerous systematic reviews and meta-analyses have attempted to quantify this disparate literature. To date, most systematic reviews have focused on a single eye disease end point and there has not been a systematic evaluation of the relationships between eye disease and diverse physical and mental health outcomes. Moreover, the strength and reliability of the relationships reported in the literature is unclear. In order to address the breadth of the literature of complex conditions and comorbid outcomes, an increasing number of studies have used an ‘umbrella review’ approach (i.e., the syntheses of existing systematic reviews with meta-analyses, to capture the breadth of outcomes associated with a given exposure) [13, 14].
Therefore, the aim of the present study is to assess the strength and credibility of the evidence on eye disease and associated health outcomes derived from meta-analyses of observational studies using an umbrella review approach, aiming to the answer the following questions:
Which comorbid outcomes are associated with eye diseases?
What is the epidemiological credibility of the relationships between eye diseases and comorbid outcomes?
Methods
An umbrella review was carried out following standardised procedures [13, 15]. The protocol for the present umbrella review was preregistered with PROSPERO (registration number CRD42018093358).
Search strategy and selection criteria
We searched PsycINFO, Medline, CINAHL, and Embase databases (from inception to 15/03/2021) to identify systematic reviews with meta-analyses, pooling observational (cross-sectional, case-control, cohort) studies to examine any association between eye disease and any comorbidity/medical condition. The following search key was used:
“(meta-analysis or meta-anal* or systematic review) AND (vision OR visual* impair* OR eyesight OR blindness OR macular degeneration OR retinopathy OR cataract OR glaucoma OR corneal opacit* OR trachoma OR onchocerciasis)”.
Two independent reviewers (MT, DP) searched titles/abstracts for eligibility, and then evaluated the full text of those articles surviving title/abstract phase. A third reviewer resolved any potential conflict (LS). When more than one meta-analysis assessed the same risk factor or the same outcome, we only included the one with the greatest number of included studies [16–18]. Exclusion criteria were: 1) meta-analyses of randomised controlled trials (RCTs); 2) studies published in languages other than English, 3) meta-analyses reporting only one study for an outcome, since no meta-analysis was possible.
Data extraction
Data was independently extracted by two investigators (MT, DP) into a pre-prepared spreadsheet. For each meta-analysis, we extracted PMID/DOI, first author, publication year, population included in the study, study design, number of included studies, the total sample size and number of cases, i.e. people having the outcome of interest. The methodological quality of each included meta-analysis was assessed with the Assessment of multiple systematic reviews (AMSTAR) 2 tool (available at https://amstar.ca/Amstar-2.php), which is a recent update of AMSTAR [19], by two independent investigators (MT, DP). The AMSTAR2 tool was chosen because it has been used in several similar umbrella reviews [20–22].
Data analysis
For each association of meta-analyses providing individual study data, we extracted effect sizes (ESs) of individual studies and re-performed the meta-analysis calculating the pooled effect size and the 95% confidence intervals (CIs), with random-effects models [23]. Heterogeneity was assessed with the I2 statistic [24]. Additionally, we calculated the 95% prediction intervals (PIs) for the summary random ESs providing the possible range in which the ESs of future studies is expected to fall [25].
We also tested the presence of small-study effect bias [16, 26–28], which is deemed to be present in case of both pooled estimates larger than the individual largest study, and publication bias (Egger’s regression asymmetry test p < 0.10). We then assessed the existence of excess significance bias by evaluating whether the observed number of studies with nominally statistically significant results (p < 0.05) was different from the expected number of studies with statistically significant results (significance threshold set at p < 0.10) [28, 29], a test designed to assess whether the published meta-analyses comprise an over-representation of false positive findings [28].
Assessment of the credibility of the evidence
Credibility of meta-analyses providing individual study data was assessed according to stringent criteria based on previously published umbrella reviews [18, 20, 26, 27, 30, 31]. In brief, associations that presented nominally significant random-effects summary effect sizes (p < 0.05) were ranked as convincing, highly suggestive, suggestive, and weak evidence based on number of events, strength of the association, and the presence of several biases (criteria available in Supplementary Table 1).
Results
Search
The flow diagram of search, selection and inclusion process is fully reported in Supplementary Fig. 1. Out of 9239 hits initially identified, after duplicate removal, 4508 were assessed at title/abstract level. Finally, 34 systematic reviews and meta-analyses were included examining a total of 58 independent outcomes [5–7, 32–62].
Findings from the case-control and cross-sectional studies
Overall, 41 outcomes were assessed by case-control or cross-sectional studies. The most common outcome examined was modifiable risk factors (n = 14), followed by mental health/cognition outcomes (n = 12), disease outcomes (n = 11), pregnancy related condition (n = 2), and visual impairment (n = 2). The median number of studies was 7 and the median number of participants was 3865. Full information can be found in Table 1 and Fig. 1.
Table 1.
Main findings of the case-control and cross-sectional studies.
| Visual impairment type | Outcome | Type of metric | No. of studies | Cases | Sample size | Effect size (95% CI) | P | I2 | Small study effect | Excess significance bias | Largest study significant | PI | Level of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diseases | |||||||||||||
| Open-angle glaucoma | Diabetes | OR | 13 | 11,472 | 3,480,114 | 1.46 (1.27–1.68) | <0.001 | 70.8 | No | Yes | Yes | 0.76–1.67 | II |
| AMD | Chlamydia pneumoniae | OR | 7 | 758 | 1395 | 1.11 (0.78–1.57) | 0.570 | 40.3 | No | No | No | −0.89–0.26 | NS |
| Diabetes | OR | 11 | NA | 175,305 | 1.30 (1.13–1.49) | <0.001 | 73.3 | No | NA | Yes | −28.02–46.18 | III | |
| Early AMD | Chronic kidney disease | OR | 14 | NA | 299,374 | 1.44 (1.24–1.68) | <0.001 | 69.9 | No | NA | Yes | NA | IV |
| Glaucoma | Diabetes | RR | 29 | NA | NA | 1.48 (1.29–1.71) | <0.001 | 82.6 | No | NA | NA | 1.02–3.60 | IV |
| Obstructive sleep apnoea | OR | 18 | 651,335 | 9,179,644 | 1.48 (1.26–1.75) | <0.001 | 83.8 | Yes | Yes | No | 0.81–2.70 | IV | |
| Nonarthritic ischemic optic neuropathy | Obstructive sleep apnoea | OR | 13 | 905 | 1332 | 3.8 (2.36–6.13) | <0.001 | 49.7 | Yes | Yes | Yes | 0.88–1.77 | IV |
| Any cataract | Gout | OR | 20 | NA | 56,248 | 1.47 (1.29–1.68) | <0.001 | 0.0 | Yes | NA | No | 0.98–1.55 | III |
| Type 2 Diabetes | OR | 23 | NA | 66,718 | 1.64 (1.42–1.88) | <0.001 | 60.9 | Yes | NA | Yes | 0.86–4.54 | II | |
| Diabetic retinopathy (T1D) | Metabolic syndrome | OR | 13 | NA | 10,651 | 1.38 (0.99–1.91) | 0.060 | 71.4 | Yes | NA | No | −27.14–64.37 | NS |
| Diabetic retinopathy | Non-alcoholic fatty liver disease | OR | 9 | NA | 7170 | 0.94 (0.51–1.72) | 0.810 | 96.3 | Yes | NA | Yes | 0.10–8.79 | NS |
| Mental health/cognition | |||||||||||||
| Diabetic retinopathy | Depression | OR | 20 | 4912 | 16,553 | 1.43 (1.21–1.69) | <0.001 | 81.8 | Yes | Yes | Yes | 1.15–2.63 | III |
| Open-angle glaucoma | Migraine | RR | 11 | NA | 467,008 | 1.23 (1.11–1.36) | <0.001 | 42.2 | No | NA | Yes | 0.44–4.27 | III |
| AMD | Cognitive dysfunction - measured by MMSE | Standard mean difference | 5 | NA | 1566 | −0.32 (−0.51; −0.13) | 0.001 | 51.6 | No | NA | Yes | −12.22–19.76 | IV |
| Cognitive dysfunction - measured by TMT-A | Standard mean difference | 2 | NA | 435 | 0.32 (0.13–0.51) | 0.001 | 0.0 | NA | No | Yes | −3.24–0.96 | IV | |
| Cognitive dysfunction - measured by TMT-B | Standard mean difference | 2 | NA | 435 | 0.10 (−0.10–0.29) | 0.330 | 0.0 | NA | No | No | −1.85–0.69 | NS | |
| Wet-AMD | Cognitive dysfunction - measured by MMSE | Standard mean difference | 3 | NA | 543 | −0.58 (−0.78; −0.38) | <0.001 | 0.0 | No | NA | Yes | 0.51–33.81 | IV |
| Cognitive dysfunction - measured by TMT-A | Standard mean difference | 2 | NA | 435 | 0.76 (0.13–1.39) | 0.020 | 78.5 | NA | No | Yes | 0.53–1.50 | IV | |
| Cognitive dysfunction - measured by TMT-B | Standard mean difference | 2 | NA | 435 | 0.32 (−0.04–0.69) | 0.080 | 44.9 | NA | No | Yes | 0.94–2.85 | NS | |
| Dry-AMD | cognitive dysfunction - measured by MMSE | Standard mean difference | 3 | NA | 543 | −1.16 (−1.72; −0.60) | <0.001 | 44.2 | No | NA | No | 0.53–3.52 | IV |
| Cognitive dysfunction - measured by TMT-A | Standard mean difference | 2 | NA | 435 | 1.22 (−0.18–2.62) | 0.090 | 91.8 | NA | NA | Yes | 0.72–1.87 | NS | |
| Cognitive dysfunction - measured by TMT-B | Standard mean difference | 2 | NA | 435 | 0.22 (−0.16–0.61) | 0.250 | 0.0 | NA | NA | No | NA | NS | |
| Onchocerciasis | Epilepsy | RR | 9 | NA | 5293 | 1.47 (1.04–2.09) | 0.030 | 81.0 | Yes | NA | No | 0.90–1.08 | IV |
| Modifiable risk factors | |||||||||||||
| Diabetic Macular Oedema | Dyslipidaemia - overall CHL | Standard mean difference | 7 | NA | 1125 | 30.08 (21.15–39.02) | <0.001 | 99.7 | No | NA | Yes | 0.66–2.80 | III |
| Dyslipidaemia - LDL levels | Standard mean difference | 7 | NA | 1125 | 18.62 (5.73–31.51) | 0.008 | 99.9 | No | NA | No | 0.79–7.41 | IV | |
| Triglyceride levels | Standard mean difference | 7 | NA | 1125 | 24.82 (9.21–40.42) | 0.002 | 99.8 | No | NA | No | 0.77–2.64 | IV | |
| Dyslipidaemia - HDL levels | Standard mean difference | 7 | NA | 1125 | 2.24 (−0.18–4.67) | 0.070 | 99.9 | No | NA | No | 0.18–59.90 | NS | |
| Diabetic retinopathy (T2D) | Dyslipidaemia - LDL levels | Mean difference | 4 | NA | 3465 | 3.74 (0.13–7.35) | 0.040 | 19.7 | No | NA | No | −23.18–72.80 | IV |
| Dyslipidaemia - overall CHL levels | Mean difference | 6 | NA | 4032 | 3.77 (−2.45–9.99) | 0.240 | 41.0 | No | NA | No | −8.71–4.43 | NS | |
| Dyslipidaemia - HDL levels | Mean difference | 5 | NA | 3698 | −1.14 (−2.43–0.15) | 0.080 | 0.0 | No | NA | No | 0.81–2.44 | NS | |
| Triglyceride levels | Mean difference | 7 | NA | 4366 | 9.08 (−4.20–22.36) | 0.180 | 64.6 | No | NA | No | 0.71–1.96 | NS | |
| Blood pressure | OR | 6 | NA | 7408 | 1.37 (0.96–1.95) | 0.080 | 45.5 | No | NA | No | 1.28–1.70 | NS | |
| Diabetic Retinopathy | BMI - overweight | OR | 6 | NA | 23,830 | 0.89 (0.75–1.07) | 0.210 | 65.5 | No | NA | No | NA | NS |
| BMI - obese | OR | 6 | NA | 23,830 | 0.97 (0.73–1.30) | 0.860 | 72.6 | No | NA | No | 0.47–1.64 | NS | |
| Intraoperative floppy iris syndrome | Hypertension | OR | 2 | NA | 1399 | 2.2 (1.15–4.19) | 0.020 | 0 | NA | NA | Yes | 0.41–2.30 | IV |
| Diabetes | OR | 4 | NA | 3281 | 1.26 (0.71–2.21) | 0.430 | 0.0 | No | NA | No | NA | NS | |
| Open-angle glaucoma | Hypertension | OR | 17 | NA | 60,084 | 1.25 (1.09–1.43) | 0.001 | 29.3 | No | NA | No | −6.94–14.42 | III |
| Pregnancy related conditions | |||||||||||||
| Retinopathy of prematurity | Hyperglycaemia | OR | 7 | 323 | 1211 | 4.15 (2.08–8.28) | <0.001 | 65.4 | Yes | Yes | Yes | 1.28–4.15 | IV |
| Pre-eclampsia | OR | 7 | 4356 | 32,890 | 1.29 (0.81–2.05) | 0.280 | 84.5 | No | Yes | Yes | NA | NS | |
| Visual impairment | |||||||||||||
| Open-angle glaucoma | Myopia | OR | 11 | NA | 43,958 | 1.92 (1.54–2.38) | <0.001 | 53.0 | Yes | NA | Yes | 0.32–5.64 | II |
| Diabetic retinopathy | Myopia | OR | 7 | NA | 27,638 | 0.83 (0.66–1.04) | 0.100 | 36.7 | No | NA | No | 1.08–1.20 | NS |
PI prediction interval, AMD advanced macular degeneration, T2D Type 2 diabetes, T1D Type 1 diabetes, CHL cholesterol, LDL low-density lipoprotein, HDL high-density lipoprotein, BMI Body mass index, MMSE mini-mental state examination, TMT-A Trial making test part A, TMT-B Trial making test part B, OR Odds ratio, RR Risk ratio, NS Non-significant.
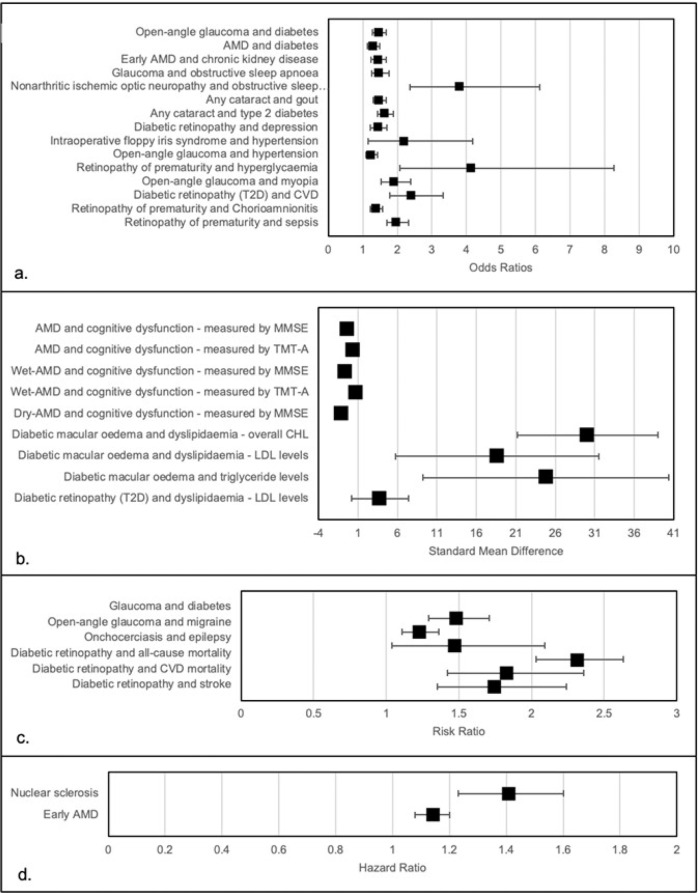
Fig. 1. Significant associations between various eye diseases and health outcomes.
a odds ratios; b standard mean difference; c risk ratio; d hazard ratio.
The p value for effect-size, under a random effects model, was <0.05 in 24/41 outcomes, and three reported a p value < 1*10−6. Among the 41 outcomes, 18 reported low heterogeneity (I2 < 50%), 11 moderate heterogeneity (I2 between 50 and 75%) and 12 high heterogeneity. Small study effect affected 10/41 outcomes, whilst 6/41 had excess significance bias (see Table 1). The largest study, in terms of participants, for each outcome was significant in 19 associations. For five outcomes, the PIs excluded the null value.
Using the criteria to grade the quality of the evidence, no outcome reached a convincing evidence (class I), three outcomes reached highly suggestive evidence (class II), six reached suggestive evidence (class III), 15 a weak strength of evidence (class IV), and 17 outcomes had no statistical significance. Regarding the class II evidence, open-angle glaucoma was associated with a myopia (n = 11 studies; OR = 1.92; 95% CI: 1.54–2.38) and with diabetes (n = 13 studies; OR = 1.46; 95% CI: 1.27–1.68); and any cataract was associated with a higher presence of type 2 diabetes (OR = 1.64; 95% CI:1.42–1.88) (see Table 1).
Findings from cohort studies
Overall, 17 outcomes were explored in prospective and retrospective designs. Mortality was the most explored outcome (n = 9), followed by pregnancy conditions (n = 4), disease outcomes (n = 3), and modifiable risk factors (n = 1). The median number of studies was 10, and the median number of participants was 30,118. Full information can be found in Table 2 and Fig. 1.
Table 2.
Main findings of the prospective and retrospective studies.
| Visual impairment type | Outcome/Type of comorbidity | Type of metric | No. of studies | Cases | Sample size | Effect size (95% CI) | P | I2 | Small study effects | Excess significance bias | Largest study significant | PI | Level of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | |||||||||||||
| Nuclear sclerosis | All-cause mortality | HR | 23 | 13,463 | 86,160 | 1.41 (1.23–1.60) | <0.001 | 78.2 | Yes | NA | No | 0.52–4.2 | III |
| Diabetic retinopathy | All-cause mortality | RR | 38 | NA | 29,647 | 2.31 (2.03–2.63) | <0.001 | 68.2 | Yes | NA | No | 5.69–169.00 | III |
| CVD mortality | RR | 10 | NA | 11,239 | 1.83 (1.42–2.36) | <0.001 | 76.3 | No | NA | No | 0.81–4.13 | IV | |
| Diabetic retinopathy (T2D) | CVD | OR | 12 | NA | 16,787 | 2.42 (1.77–3.32) | <0.001 | 81.2 | Yes | NA | Yes | 0.99–2.16 | II |
| Early AMD | All-cause mortality | HR | 26 | 3294 | 12,284 | 1.14 (1.08–1.20) | <0.001 | 0.0 | No | NA | No | 0.93–15.44 | IV |
| Cancer mortality | HR | 6 | 1024 | 20,329 | 1.07 (0.86–1.34) | 0.55 | 37.9 | No | No | Yes | NA | NS | |
| CVD mortality | HR | 11 | NA | NA | 1.16 (0.97–1.39) | 0.10 | 42.3 | No | NA | NA | 0.61–1.88 | NS | |
| AMD | CVD mortality | RR | 5 | NA | 17,250 | 1.18 (0.98–1.43) | 0.09 | 33.6 | No | NA | Yes | 0.41–2.86 | NS |
| Open-angle glaucoma | All-cause mortality | RR | 9 | NA | 2,636 | 1.13 (0.97–1.31) | 0.12 | 50.6 | No | NA | NA | 0.72–2.00 | NS |
| Diseases | |||||||||||||
| Diabetic retinopathy | Stroke | RR | 5 | NA | 7,727 | 1.74 (1.35–2.24) | <0.001 | 0.0 | No | NA | Yes | 0.47–1.44 | IV |
| AMD | Diabetes | RR | 5 | NA | 139,200 | 1.06 (0.99–1.13) | 0.10 | 5.3 | No | NA | Yes | 0.94–1.78 | NS |
| Stroke | OR | 9 | NA | 1,420,978 | 1.08 (0.81–1.43) | 0.59 | 96 | No | NA | Yes | 0.9–2.31 | NS | |
| Pregnancy related conditions | |||||||||||||
| Retinopathy of prematurity | Chorioamnionitis | OR | 71 | NA | 49,710 | 1.38 (1.21–1.57) | <0.001 | 62.5 | Yes | NA | Yes | 0.36–4.35 | II |
| Retinopathy of prematurity | Sepsis | OR | 42 | 16,286 | 79,408 | 1.98 (1.69–2.33) | <0.001 | 80.4 | Yes | Yes | Yes | 0.99–1.65 | II |
| Retinopathy of prematurity | Gestational hypertensive disorder | OR | 7 | 4356 | 32,890 | 1.35 (0.88–2.08) | 0.17 | 83.8 | No | Yes | Yes | 0.93–1.20 | NS |
| Retinopathy of prematurity | Pre-eclampsia | OR | 7 | 4356 | 32,890 | 1.29 (0.81–2.05) | 0.28 | 84.5 | No | Yes | Yes | NA | NS |
| Modifiable risk factors | |||||||||||||
| Diabetic retinopathy | BMI (as a continuous variable) | OR | 23 | NA | 30,588 | 0.99 (0.97–1.00) | 0.22 | 78.5 | No | NA | No | NA | NS |
PI prediction interval, AMD advanced macular degeneration, T2D Type 2 diabetes, BMI Body mass index, CVD Cardio-vascular disease, OR Odds ratio, RR Risk ratio, HR Hazard ratio, NS Non-significant.
Almost half (8/17) of the associations included were statistically significant under a random-effects model, with three outcomes having a p value < 1*10−6. Among the 17 outcomes included, six were of low heterogeneity (I2 < 50%), three were of moderate heterogeneity (I2 between 50 and 75%) and eight were of high heterogeneity. Small study effects were present in five outcomes, and three outcomes showed excess significance bias (see Table 2). The largest study, in terms of participants, for each outcome was significant in 10/17 outcomes.
Using the criteria to grade the quality of the evidence, no outcome reached a convincing evidence (class I), three reached highly suggestive evidence (class II), two reached suggestive evidence (class III) and three showed weak strength of evidence (class IV). Regarding class II evidence, retinopathy of prematurity was associated with a higher incidence of chorioamnionitis (n = 71 studies; OR = 1.38; 95% CI: 1.3–1.57) and a higher risk of sepsis (n = 42; OR = 1.98; 95% CI: 1.69–2.33), and diabetic retinopathy was positively associated with incident cardiovascular disease (n = 12; OR = 2.42; 95% CI: 1.77–3.32).
Study quality
The majority of meta-analyses scored critically low (n = 31/34) on AMSTAR2, and three scored low (see Table 3). The main reasons for the critically low scoring was that most studies failed to report an explicit statement that the review methods were established prior to the conduct of the review (AMSTAR2 question 2; 3/34 studies satisfied this criteria) and failed to provide a list of excluded studies and justify the exclusions (AMSTAR2 question 7; 1/34 studies satisfied this criteria).
Table 3.
AMSTAR2 results.
| Author of meta-analysis | Year of meta-analysis | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | AMSTAR 2 rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akbari et al. | 2009 | Yes | No | Yes | Yes | Yes | Yes | No | No | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Marcus et al. | 2011 | Yes | No | Yes | Partial Yes | Yes | No | No | Partial Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Critically low |
| Li et al. | 2014 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Zhou et al. | 2014 | Yes | No | Yes | Partial Yes | NO | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Chen et al. | 2014 | Yes | No | Yes | Partial Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Critically low |
| Bae et al. | 2014 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | No | No | Yes | No | No | No | Yes | Yes | Critically low |
| Zhau et al. | 2015 | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Song et al. | 2014 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Critically low |
| Shi et al. | 2016 | Yes | No | Yes | No | Yes | No | No | Partial Yes | Yes | No | Yes | No | No | Yes | Yes | No | Critically low |
| Au et al. | 2015 | Yes | No | Yes | Partial Yes | No | No | No | Partial Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Das et al. | 2015 | Yes | Yes | Yes | Partial Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Fernandez et al. | 2015 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | No | No | Yes | No | No | Yes | No | Yes | Critically low |
| Zhou et al. | 2016 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | No | No | Yes | No | No | No | No | Yes | Critically low |
| Chan et al. | 2016 | Yes | No | Yes | Partial Yes | No | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Critically low |
| Wang et al. | 2016 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes | Critically low |
| Zhu et al. | 2017 | Yes | No | Yes | Partial Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | No | No | Yes | No | Critically low |
| McGuinness et al. | 2017 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Critically low |
| Zhou et al. | 2017 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | Yes | No | Yes | No | No | No | Yes | Yes | Critically low |
| Luo et al. | 2017 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Xu et al. | 2018 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Critically low |
| Zhou et al. | 2018 | Yes | No | Yes | Partial Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | No | No | Yes | Critically low |
| Zhou et al. | 2018 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | Yes | No | Yes | No | No | No | No | Yes | Critically low |
| Villamor-Martinez | 2018 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Critically low |
| Chen et al. | 2018 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | Partial Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Huang et al. | 2019 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Critically low |
| Huon et al. | 2016 | Yes | No | Yes | Partial Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | Yes | Critically low |
| Druet-Cabanac et al. | 2004 | Yes | No | Yes | No | Yes | No | No | No | No | No | Yes | Yes | No | Yes | Yes | No | Critically low |
| Wu and You | 2018 | Yes | No | Yes | Partial Yes | Yes | Yes | No | No | NO | No | No | No | No | Yes | Yes | No | Critically low |
| Xin et al. | 2018 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | No | Critically low |
| Wang et al. | 2016 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Partial Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Guo et al. | 2016 | Yes | No | Yes | Partial Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Critically low |
| Chatziralli and Sergentanis | 2011 | Yes | No | Yes | No | Yes | Yes | No | No | No | No | Yes | No | No | Yes | Yes | Yes | Critically low |
| Song et al. | 2020 | Yes | No | Yes | Partial yes | Yes | Yes | No | Partial yes | Yes | No | Yes | No | No | No | Yes | Yes | Critically low |
| Xu et al. | 2020 | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Low |
AMSTAR@ Questions: Q1: Did the research questions and inclusion criteria for the review include the components of PICO?; Q2: Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol?; Q3: Did the review authors explain their selection of the study designs for inclusion in the review?; Q4: Did the review authors use a comprehensive literature search strategy?; Q5: Did the review authors perform study selection in duplicate?; Q6: Did the review authors perform data extraction in duplicate?; Q7: Did the review authors provide a list of excluded studies and justify the exclusions?; Q8: Did the review authors describe the included studies in adequate detail?; Q9: Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review?; Q10: Did the review authors report on the sources of funding for the studies included in the review?; Q11: If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results?; Q12: If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis?; Q13: Did the review authors account for RoB in individual studies when interpreting/ discussing the results of the review?; Q14: Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review?; Q15: If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review?; Q16: Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?.
Discussion
The present review, including 34 studies and 58 outcomes associated with varying eye diseases, no convincing (Class I) evidence for any comorbidity across all eye diseases was found. Highly suggestive levels of evidence (Class II) for cohort, case-control and cross-sectional studies showed that people with diabetic retinopathy were nearly 2.5 times more likely to suffer from cardiovascular diseases, and 1.8 times more likely to suffer CVD mortality. Diabetic retinopathy is a microvascular disease and it is not surprising that cardiovascular diseases will have a significant effect on the eye, with sepsis and chorioamnionitis being significant risk factors for retinopathy of prematurity [63]. Furthermore, babies with retinopathy of prematurity are nearly twice as likely to suffer from sepsis [53]. Retinopathy of prematurity is a vasoproliferative disease that affects the retinal vascular system in premature babies. As infection is a significant risk factor for neonatal brain damage, and sepsis is the key cause of neonatal inflammation, this could be the reason why the strong association with retinopathy of prematurity has been found. The foetal inflammatory response induced by chorioamnionitis [64], leads to proinflammatory cytokines having a substantial effect on retinal angiogenesis and subsequent development of the retina [65, 66], which could lead to retinopathy of prematurity.
Our analysis shows people suffering from open angle glaucoma are twice as likely to suffer from diabetes. Diabetes is a serious condition and its effects on macrovascular and micro vascular structures are well documented [67, 68]. While the strong association of diabetes and cataract is well known, the link with open angle glaucoma has been open to debate. Our analysis shows highly suggestive evidence of the link between diabetes and open angle glaucoma. One possible mechanism could be because long standing hyperglycaemia increases the risk of neural injury and the reduced capacity for auto-regulation of blood in diabetes could have an effect on the optic nerve and nerves in the eye. Furthermore, diabetes affects nerves in the body (neuropathy) and research has shown diabetes having a negative effect on ganglion cells in the eye [69].
Myopia also yielded a highly suggestive (Class II) association with open angle glaucoma. One possible mechanism is the biomechanical stress induced by increased axial length and oxidative stress, although this needs further investigation. The increasing global prevalence of myopia would have significant consequences on the global burden of eye diseases beyond just refractive error, and may explain, to a certain extent, the increasing prevalence of open angle glaucoma worldwide.
Suggestive levels of evidence (Class III) include cataract (including nuclear sclerosis) being associated with all-cause mortality and gout, diabetic retinopathy with depression, and open angle glaucoma with hypertension and migraine. Weaker strength of evidence (Class IV) links AMD with cognitive function, and glaucoma with sleep apnoea. Further studies need to be carried out to strengthen and confirm possible association between these conditions and the eye diseases.
Umbrella reviews provide top-tier evidence and important insights, however there are a number of limitations. Although we measured for heterogeneity, the meta-analyses included in this study included differing study designs, methods of measuring VI and eye diseases and populations. Furthermore, meta-analyses have inherent limitations [70]: their findings are dependent on estimates that are selected from each primary study and how they are applied in the meta-analysis. Finally, almost all of the studies included scored ‘critically low’ in quality control. Some studies were scored low as they had missed quality indicators such as confirming review methods or details about excluded studies. It is important that all the quality indicators are included in order to assure confidence in the data presented.
Conclusion
Our results show highly suggestive evidence for associations between diabetic retinopathy and cardiovascular disease, open angle glaucoma and diabetes, myopia and open angle glaucoma. Furthermore, we found suggestive evidence for associations between cataract and all-cause mortality and gout, depression and diabetic retinopathy, and hypertension and migraine for open angle glaucoma. Clinicians should take note of these and consider these associations in the delivery of care. Furthermore, public health policies should reflect and accommodate these associations in healthcare policies, practices and guidelines.
Summary table
What this study adds
This is the first study to examine the credibility of evidence against strict statistical criteria of eye disease and all types of health outcomes.
Six significant associations were classified as ‘highly suggestive’, including cataract and type 2 diabetes; open-angled glaucoma, myopia and diabetes; diabetic retinopathy, cardiovascular disease, and cardiovascular mortality; and retinopathy of prematurity and chorioamnionitis.
Eight significant associations were classified as ‘suggestive’, including diabetic retinopathy, all-cause mortality, and depression; diabetic macular oedema and dyslipidaemia; cataract and gout; nuclear sclerosis and all-cause mortality; open angled glaucoma, migraine, and hypertension; age-related macular degeneration and diabetes.
18 significant associations were classified as ‘weak’.
Study limitations
The risk of bias of included meta-analyses was high.
This study included only meta-analyses of observation studies, which carry inherent limitations.
Supplementary information
Author contributions
MT: conceptualisation, data collection, writing manuscript. LS: conceptualisation, supervision, critical appraisal of manuscript and revision writing; NV: conceptualisation, data analysis, writing manuscript, revision of manuscript; DP: conceptualisation, data analysis, writing manuscript, revision of manuscript; YB: conceptualisation, writing manuscript, critical appraisal; TG: conceptualisation, writing manuscript, critical appraisal; SP: conceptualisation, supervision, critical appraisal of manuscript and revision writing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-021-01684-x.
References
- 1.World Health Organization. Blindness and vision impairment: The key facts. 2019. https://www.who.int/en/news-room/fact-sheets/detail/blindness-and-visual-impairment. Accessed May 6, 2020.
- 2.Bourne RR, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e888–e897. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 3.Fricke TR, Tahhan N, Resnikoff S, Papas E, Burnett A, Ho SM, et al. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology. 2018;125:1492–9. doi: 10.1016/j.ophtha.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Swenor BK, Simonsick EM, Ferrucci L, Newman AB, Rubin S, Wilson V, et al. Visual impairment and incident mobility limitations: the health, aging and body composition study. J Am Geriatr Soc. 2015;63:46–54. doi: 10.1111/jgs.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y-J, Yeung L, Sun C-C, Huang C-C, Chen K-S, Lu Y-H. Age-related macular degeneration in chronic kidney disease: a meta-analysis of observational studies. Am J Nephrol. 2018;48:278–91. doi: 10.1159/000493924. [DOI] [PubMed] [Google Scholar]
- 6.Luo C, Chen X, Jin H, Yao K. The association between gout and cataract risk: a meta-analysis. PloS One. 2017;12:e0180188. doi: 10.1371/journal.pone.0180188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Liu P, Guan J, Lu Y, Su K. Association between glaucoma and obstructive sleep apnea syndrome: a meta-analysis and systematic review. PloS One. 2015;10:e0115625–e0115625. doi: 10.1371/journal.pone.0115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi HG, Lee MJ, Lee S-M. Visual impairment and risk of depression: a longitudinal follow-up study using a national sample cohort. Sci Rep. 2018;8:2083. doi: 10.1038/s41598-018-20374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong T, Mitchell P, Burlutsky G, Liew G, Wang JJ. Visual impairment, hearing loss and cognitive function in an older population: longitudinal findings from the Blue Mountains Eye Study. PLoS One. 2016;11:e0147646. doi: 10.1371/journal.pone.0147646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer-Rochow VB, Hakko H, Ojamo M, Uusitalo H, Timonen M. Suicides in visually impaired persons: a nation-wide register-linked study from finland based on thirty years of data. PloS One. 2015;10:e0141583–e0141583. doi: 10.1371/journal.pone.0141583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajala U, Pajunpää H, Koskela P, Keinänen-Kiukaanniemi S. High cardiovascular disease mortality in subjects with visual impairment caused by diabetic retinopathy. Diabetes Care. 2000;23:957. doi: 10.2337/diacare.23.7.957. [DOI] [PubMed] [Google Scholar]
- 12.McCarty CA, Nanjan MB, Taylor HR. Vision impairment predicts 5 year mortality. Br J Ophthalmol. 2001;85:322–6. doi: 10.1136/bjo.85.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181:488–93. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ioannidis J. Next-generation systematic reviews: prospective meta-analysis, individual-level data, networks and umbrella reviews. Br J Sports Med. 2017;51:1456–8. doi: 10.1136/bjsports-2017-097621. [DOI] [PubMed] [Google Scholar]
- 15.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Health. 2015;13:132–40. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 16.Radua J, Ramella‐Cravaro V, Ioannidis JP, Reichenberg A, Phiphopthatsanee N, Amir T, et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry. 2018;17:49–66. doi: 10.1002/wps.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145:1719–30. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 18.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veronese N, Solmi M, Caruso MG, Giannelli G, Osella AR, Evangelou E, et al. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr. 2018;107:436–44. doi: 10.1093/ajcn/nqx082. [DOI] [PubMed] [Google Scholar]
- 21.Veronese N, Demurtas J, Pesolillo G, Celotto S, Barnini T, Calusi G, et al. Magnesium and health outcomes: an umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur J Nutr. 2020;59:263–72. doi: 10.1007/s00394-019-01905-w. [DOI] [PubMed] [Google Scholar]
- 22.Machado MO, Veronese N, Sanches M, Stubbs B, Koyanagi A, Thompson T, et al. The association of depression and all-cause and cause-specific mortality: an umbrella review of systematic reviews and meta-analyses. BMC Med. 2018;16:1–13. doi: 10.1186/s12916-018-1101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 26.Bortolato B, Köhler CA, Evangelou E, León‐Caballero J, Solmi M, Stubbs B, et al. Systematic assessment of environmental risk factors for bipolar disorder: an umbrella review of systematic reviews and meta‐analyses. Bipolar Disord. 2017;19:84–96. doi: 10.1111/bdi.12490. [DOI] [PubMed] [Google Scholar]
- 27.Dragioti E, Evangelou E, Larsson B, Gerdle B. Effectiveness of multidisciplinary programmes for clinical pain conditions: an umbrella review. J Rehabil Med. 2018;50:779–91. doi: 10.2340/16501977-2377. [DOI] [PubMed] [Google Scholar]
- 28.Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4:245–53. doi: 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- 29.Ioannidis JP. Clarifications on the application and interpretation of the test for excess significance and its extensions. J Math Psychol. 2013;57:184–7. [Google Scholar]
- 30.Dragioti E, Karathanos V, Gerdle B, Evangelou E. Does psychotherapy work? An umbrella review of meta‐analyses of randomized controlled trials. Acta Psychiatr Scand. 2017;136:236–46. doi: 10.1111/acps.12713. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, et al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:j2376. doi: 10.1136/bmj.j2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akbari M, Akbari S, Pasquale LR. The association of primary open-angle glaucoma with mortality: a meta-analysis of observational studies. Arch Ophthalmol. 2009;127:204–10. doi: 10.1001/archophthalmol.2008.571. [DOI] [PubMed] [Google Scholar]
- 33.Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–94.e2. doi: 10.1016/j.ophtha.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Wan X, Zhao G. Meta-analysis of the risk of cataract in type 2 diabetes. BMC Ophthalmol. 2014;14:94. doi: 10.1186/1471-2415-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Jhanji V, Chen C, Chen H. Serological association of Chlamydia pneumoniae infection with age-related macular degeneration: a systematic review and meta-analysis. PloS One. 2014;9:e103466. doi: 10.1371/journal.pone.0103466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou M, Wang W, Huang W, Zhang X. Diabetes mellitus as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. PloS One. 2014;9:e102972. doi: 10.1371/journal.pone.0102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bae HW, Lee N, Lee HS, Hong S, Seong GJ, Kim CY. Systemic hypertension as a risk factor for open-angle glaucoma: a meta-analysis of population-based studies. PloS One. 2014;9:e108226. doi: 10.1371/journal.pone.0108226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015;122:72–78. doi: 10.1016/j.ophtha.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 39.Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan C-W. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PloS One. 2014;9:e112054. doi: 10.1371/journal.pone.0112054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Au SCL, Tang S-M, Rong S-S, Chen L-J, Yam JCS. Association between hyperglycemia and retinopathy of prematurity: a systemic review and meta-analysis. Sci Rep. 2015;5:9091. doi: 10.1038/srep09091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das R, Kerr R, Chakravarthy U, Hogg RE. Dyslipidemia and diabetic macular edema: a systematic review and meta-analysis. Ophthalmology. 2015;122:1820–7. doi: 10.1016/j.ophtha.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez AB, Panza GA, Cramer B, Chatterjee S, Jayaraman R, Wu W-C. Age-related macular degeneration and incident stroke: a systematic review and meta-analysis. PloS One. 2015;10:e0142968. doi: 10.1371/journal.pone.0142968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L-X, Sun C-L, Wei L-J, Gu Z-M, Lv L, Dang Y. Lower cognitive function in patients with age-related macular degeneration: a meta-analysis. Clin Interv Aging. 2016;11:215–23. doi: 10.2147/CIA.S102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan PYL, Tang S-M, Au SCL, Rong S-S, Lau HHW, Ko STC, et al. Association of gestational hypertensive disorders with retinopathy of prematurity: a systematic review and meta-analysis. Sci Rep. 2016;6:30732. doi: 10.1038/srep30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Xue Y, Thapa S, Wang L, Tang J, Ji K. Relation between age-related macular degeneration and cardiovascular events and mortality: a systematic review and meta-analysis. BioMed Res Int. 2016;2016:8212063. doi: 10.1155/2016/8212063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X-R, Zhang Y-P, Bai L, Zhang X-L, Zhou J-B, Yang J-K. Prediction of risk of diabetic retinopathy for all-cause mortality, stroke and heart failure: Evidence from epidemiological observational studies. Medicine. 2017;96:e5894. doi: 10.1097/MD.0000000000005894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGuinness MB, Karahalios A, Finger RP, Guymer RH, Simpson JA. Age-related macular degeneration and mortality: a systematic review and meta-analysis. Ophthalmic Epidemiol. 2017;24:141–52. doi: 10.1080/09286586.2016.1259422. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y, Zhang Y, Shi K, Wang C. Body mass index and risk of diabetic retinopathy: a meta-analysis and systematic review. Medicine. 2017;96:e6754. doi: 10.1097/MD.0000000000006754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu C, Li J, Li Z, Mao X. Migraine as a risk factor for primary open angle glaucoma: a systematic review and meta-analysis. Medicine. 2018;97:e11377. doi: 10.1097/MD.0000000000011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Wang C, Shi K, Yin X. Relationship between dyslipidemia and diabetic retinopathy: a systematic review and meta-analysis. Medicine. 2018;97:e12283. doi: 10.1097/MD.0000000000012283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Wang C, Shi K, Yin X. Relation of metabolic syndrome and its components with risk of diabetic retinopathy: a meta-analysis of observational studies. Medicine. 2018;97:e12433. doi: 10.1097/MD.0000000000012433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villamor-Martinez E, Cavallaro G, Raffaeli G, Mohammed Rahim OMM, Gulden S, Ghazi AMT, et al. Chorioamnionitis as a risk factor for retinopathy of prematurity: An updated systematic review and meta-analysis. PloS One. 2018;13:e0205838. doi: 10.1371/journal.pone.0205838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J, Tang Y, Zhu T, Li Y, Chun H, Qu Y, et al. Cumulative evidence for association of sepsis and retinopathy of prematurity. Medicine. 2019;98:e17512. doi: 10.1097/MD.0000000000017512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huon L-K, Liu SY-C, Camacho M, Guilleminault C. The association between ophthalmologic diseases and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2016;20:1145–54. doi: 10.1007/s11325-016-1358-4. [DOI] [PubMed] [Google Scholar]
- 55.Druet-Cabanac M, Boussinesq M, Dongmo L, Farnarier G, Bouteille B, Preux PM. Review of epidemiological studies searching for a relationship between onchocerciasis and epilepsy. Neuroepidemiology. 2004;23:144–9. doi: 10.1159/000075958. [DOI] [PubMed] [Google Scholar]
- 56.Wu C, You Z. Meta-analysis of the relationship between depression and diabetic retinopathy. Biomed Res. 2018;29:0970–938X. [Google Scholar]
- 57.Xin X, Sun Y, Li S, Xu H, Zhang D. Age-related macular degeneration and the risk of all-cause and cardiovascular mortality: a meta-analysis of cohort studies. Retina. 2018; 38. https://journals.lww.com/retinajournal/Fulltext/2018/03000/AGE_RELATED_MACULAR_DEGENERATION_AND_THE_RISK_OF.7.aspx. [DOI] [PubMed]
- 58.Wang X, Tang L, Gao L, Yang Y, Cao D, Li Y. Myopia and diabetic retinopathy: a systematic review and meta-analysis. Diabetes Res Clin Pr. 2016;111:1–9. doi: 10.1016/j.diabres.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Guo VY, Cao B, Wu X, Lee JJW, Zee BC. Prospective association between diabetic retinopathy and cardiovascular disease—a systematic review and meta-analysis of cohort studies. J Stroke Cerebrovasc Dis. 2016;25:1688–95. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Chatziralli IP, Sergentanis TN. Risk factors for intraoperative floppy iris syndrome: a meta-analysis. Ophthalmology. 2011;118:730–5. doi: 10.1016/j.ophtha.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 61.Song D, Li C, Wang Z, Zhao Y, Shen B, Zhao W. Association of non‐alcoholic fatty liver disease with diabetic retinopathy in type 2 diabetic patients: a meta‐analysis of observational studies. J Diabetes Investig. 2020. [DOI] [PMC free article] [PubMed]
- 62.Xu X-H, Sun B, Zhong S, Wei D-D, Hong Z, Dong A-Q. Diabetic retinopathy predicts cardiovascular mortality in diabetes: a meta-analysis. BMC Cardiovasc Disord. 2020;20:1–8. doi: 10.1186/s12872-020-01763-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63:618–37. doi: 10.1016/j.survophthal.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh J-W, Park C-W, Moon KC, Park JS, Jun JK. The relationship among the progression of inflammation in umbilical cord, fetal inflammatory response, early-onset neonatal sepsis, and chorioamnionitis. PloS One. 2019;14:e0225328–e0225328. doi: 10.1371/journal.pone.0225328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolinger MT, Antonetti DA. Moving past anti-VEGF: novel therapies for treating diabetic retinopathy. Int. J. Mol. Sci. 2016;17:1498. doi: 10.3390/ijms17091498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsai T, Kuehn S, Tsiampalis N, Vu M-K, Kakkassery V, Stute G, et al. Anti-inflammatory cytokine and angiogenic factors levels in vitreous samples of diabetic retinopathy patients. PloS One. 2018;13:e0194603–e0194603. doi: 10.1371/journal.pone.0194603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Domingueti CP, Dusse LMS, das Graças Carvalho M, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complicat. 2016;30:738–45. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 68.Donnelly R, Emslie-Smith AM, Gardner ID, Morris AD. Vascular complications of diabetes. BMJ. 2000;320:1062–6. doi: 10.1136/bmj.320.7241.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586:4401–8. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ioannidis JPA. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 2016;94:485–514. doi: 10.1111/1468-0009.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.