Abstract
Study Objectives:
This review aimed to summarize current knowledge about disrupted nighttime sleep (DNS) and sleep instability in narcolepsy, including self-reported and objective assessments, potential causes of sleep instability, health consequences and functional burden, and management.
Methods:
One hundred two peer-reviewed publications from a PubMed search were included.
Results:
DNS is a key symptom of narcolepsy but has received less attention than excessive daytime sleepiness and cataplexy. There has been a lack of clarity regarding the definition of DNS, as many sleep-related symptoms and conditions disrupt sleep quality in narcolepsy (eg, hallucinations, sleep paralysis, rapid eye movement sleep behavior disorder, nightmares, restless legs syndrome/periodic leg movements, nocturnal eating, sleep apnea, depression, anxiety). In addition, the intrinsic sleep instability of narcolepsy results in frequent spontaneous wakings and sleep stage transitions, contributing to DNS. Sleep instability likely emerges in the setting of orexin insufficiency/deficiency, but its exact pathophysiology remains unknown. DNS impairs quality of life among people with narcolepsy, and more research is needed to determine its contributions to cardiovascular risk. Multimodal treatment is appropriate for DNS management, including behavioral therapies, counseling on sleep hygiene, and/or medication. There is strong evidence showing improvement in self-reported sleep quality and objective sleep stability measures with sodium oxybate, but rigorous clinical trials with other pharmacotherapies are needed. Treatment may be complicated by comorbidities, concomitant medications, and mood disorders.
Conclusions:
DNS is a common symptom of narcolepsy deserving consideration in clinical care and future research.
Citation:
Maski K, Mignot E, Plazzi G, Dauvilliers Y. Disrupted nighttime sleep and sleep instability in narcolepsy. J Clin Sleep Med. 2022;18(1):289–304.
Keywords: orexin/hypocretin, disrupted nighttime sleep, narcolepsy, polysomnography, sleep instability
BRIEF SUMMARY
Current Knowledge/Study Rationale: Narcolepsy is best known as a disorder of excessive daytime sleepiness, hence its categorization as a hypersomnolence condition. Disrupted nighttime sleep (DNS) is another key feature of narcolepsy that has received less attention; therefore, this manuscript reviews the definition of DNS, self-reported symptoms of DNS, objective markers of sleep instability, consequences of DNS/sleep instability on quality of life and health, and management of DNS.
Study Impact: This review will alert clinical providers to the importance of DNS and prompt further discussion with patients about its symptoms and treatment. Gaps in the literature regarding the health consequences of DNS and pathophysiology of sleep instability are highlighted to encourage future research.
INTRODUCTION
Narcolepsy is a disabling sleep disorder characterized by a pentad of symptoms: excessive daytime sleepiness (EDS), cataplexy, sleep-related hallucinations (hypnagogic and hypnopompic), sleep paralysis, and disrupted/disturbed nighttime sleep (DNS).1 Not all people with narcolepsy experience all 5 symptoms, but all experience EDS.1 Narcolepsy type 1 (NT1) is a lifelong disorder characterized by the presence of cataplexy and/or orexin deficiency (cerebrospinal fluid [CSF] orexin-A, also called hypocretin-1: ≤ 110 pg/mL).1 In narcolepsy type 2 (NT2), cataplexy is absent and CSF orexin levels are typically normal or mildly reduced.1 NT2 may sometimes be challenging to distinguish from other hypersomnolence disorders.2 Symptoms of narcolepsy mainly begin in adolescence, but diagnosis is often delayed.3
DNS has historically been an underrecognized symptom of narcolepsy,4 as the clinical focus has been EDS and cataplexy management. Narcolepsy was previously described as a dyssomnia due to the severity of sleep disturbances, but is now considered a disorder of hypersomnolence.5 Due to variability in definition, assessment methods, and exclusion of comorbid sleep disorders, the estimated prevalence of DNS in narcolepsy ranges widely, from 30% to 95%.6 Fifty-three percent to 78% of pediatric patients with NT1 and their caregivers report DNS.7 In a study using the Narcolepsy Severity Scale (NSS)8 to assess individual symptoms of narcolepsy, 60.8% of untreated patients and 40.3% of treated patients reported experiencing nighttime sleep disturbance.9
In 2013, an expert panel reviewed the literature and detailed the evidence that DNS is a common feature of narcolepsy that differs in certain key respects from sleep disruption in other disorders.6 Subsequently, the American Academy of Sleep Medicine (AASM) added DNS as an associated feature of narcolepsy in the International Classification of Sleep Disorders, third edition (ICSD-3), diagnostic criteria.1 Since the publication of the consensus characterization and the update to the classification, which focused additional attention on DNS in narcolepsy, there have been advancements in understanding of this condition.
The aim of this review is to summarize the current state of knowledge about DNS and, more specifically, the intrinsic sleep instability unique to narcolepsy. Topics covered include assessment of self-reported DNS, objective assessment of sleep instability, potential causes of sleep instability in narcolepsy, health consequences and the functional burden of DNS, and available evidence regarding DNS management. The newer literature since the publication of the 2013 Roth et al6 review will be emphasized. No new data were generated or analyzed in support of this research.
METHODS
Literature search
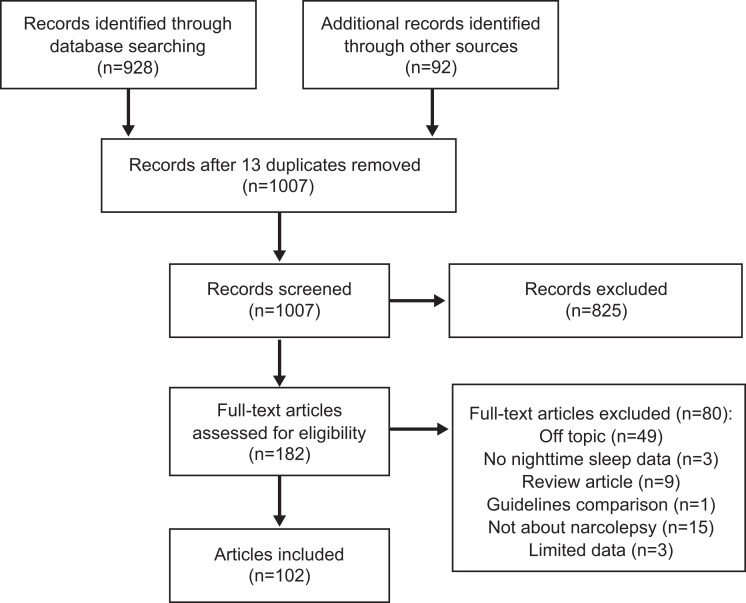
To identify peer-reviewed publications on nighttime sleep, sleep instability, and DNS in narcolepsy, PubMed was searched (June 23, 2020), without limits on date or language, for articles on narcolepsy that also included the terms disrupted/disturbed nighttime sleep, sleep stability, sleep instability, insomnia, nighttime sleep, polysomnography (PSG), REM, fragmentation, fragmented, disrupted, sleep transitions, transitional sleep, sleep architecture, arousals, actigraphy, actigraphic, hypocretin, orexin, cardiovascular, obesity, puberty, or mood, and were not about studies in rats, mice, or dogs (canines). A total of 894 items were returned (Figure 1). Additional literature was identified from the bibliographies of articles from the initial search, further targeted searching, and the collections of the authors. After reviewing for relevance, a total of 102 articles were included in this report. Characteristics of clinical studies that assessed nighttime sleep in participants with narcolepsy are summarized in Table 1 and Table 2. The term(s) used to describe the patient population in the original article were used to describe the patient population in the current report (ie, “NT1” or “narcolepsy with cataplexy” [NwC], “NT2” or “narcolepsy without cataplexy” [NwoC]). If the narcolepsy subtype was not defined in the original article or the population consisted of a mix of the subtypes, then “narcolepsy (mixed population)” was used in the current report.
Figure 1. Literature search results.
Table 1.
Summary of studies involving PSG assessment of nocturnal sleep architecture in narcolepsy.
| Reference | Comparison | Adult or Pediatric Population | Group Sizes | TST | WASO | Awakenings | Arousals | N1 | N2 | N3 (S3/S4; SWS) | REM Sleep | Sleep Efficiency |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Narcolepsy vs controls | ||||||||||||
| Narcolepsy (mixed population) | ||||||||||||
| Jiménez-Correa 200922 | N vs HC | Adult | N: 23 HC: 23 | ns | ns (“wakefulness”) | ↑ (< 3 min) | ↑ | ↑ (%) (S1) | ↑ (%) (S2) | ↓ (%) (SWS) | ↓ (%) | ns (index) |
| Narcolepsy with cataplexy/NT1 | ||||||||||||
| Mukai 200325 | NwC vs HC | Adult | NwC: 8 HC: 8 | ns | ↑ | – | – | ↑ (%) | ns (%) (↓P = .06) | ↓ (%) (S3+S4) | ns (%) | ns (%) |
| Dauvilliers 200726 | NwC vs HC | Adult | NwC: 16 HC: 16 | ns | – | – | – | ns (%) | ↓ (%) | ns (%) (SWS) | ns (%) | ↓ |
| Khatami 200723 | NwC vs HC | Adult | NwC: 11 HC: 11 | ↓ | ↑ | – | – | ↑ (min) | ↓ (min) | ns (min) (SWS) | ns (min) | ↓ (%) |
| Khatami 200824 | NwC vs HC | Adult | NwC: 6 HC: 6 | ↓ | ↑ | – | – | ↑ (min) | ↓ (min) | ns (min) (SWS) | ns (min) | ↓ (%) |
| Frauscher 201127 | NwC vs C | Mixed | NwC: 6 Con: 6 | ns | ns | – | – | ns (%) | ↓ (%) | ns (%) (S3/S4) | ns (%) | ns (%) |
| Antelmi 201728 | NT1 vs HC | Pediatric | NT1: 40 HC: 22 | ns | ↑ | – | – | ↑ (%) | ↓ (%) | ns (%) (N3) | ns (%) | ns (%) |
| Walacik-Ufnal 201729 | NT1 vs HC | Adult | NT1: 15 HC: 15 | ns | ↑ | – | – | ↑ (%) | ↓ (%) | ↓ (%) (S3+S4) | ns (%) | ns (%) |
| Vandi 201930 | NT1 vs C | Pediatric | NT1: 27 C: 19 | ns | ↑ | – | – | ↑ (%) | ns (%) | ns (%) (↓P = .07) | ns (%) | ns (%) |
| Pizza 201531 | NT1 vs sHS | Adult | NT1: 79 sHS: 52 | ns | ↑ | ↑ | – | ↑ (%) ↑ (min) | ↓ (%) ↓ (min) | ↓ (%) ns (min) | ns (%) ns (min) | ↓ (%) |
| Maski 202132 | NT1 vs SSS | Pediatric | NT1: 46 SSS: 48 | ns | ↑ | – | ↑ (index) | ↑ (%) | ↓ (%) | ns (%) | ns (%) | ↑ (%) |
| Narcolepsy without cataplexy/NT2 | ||||||||||||
| Pizza 201531 | NT2 vs sHS | Adult | NT2: 22 sHS: 52 | ns | ns | ns | – | ns (%) | ns (%) | ns (%) | ns (%) | ns (%) |
| Maski 202132 | NT2 vs SSS | Pediatric | NT2: 12 SSS: 48 | ns | ns | – | ns (index) | ns (%) | ns (%) | ns (%) | ↑ (%) | ↑ (%) |
| Narcolepsy with cataplexy/NT1 vs narcolepsy without cataplexy/NT2 | ||||||||||||
| Harsh 200015 | NwC vs NwoC | Adult | NwC: 430 NwoC: 100 | ↓ | – | ↑ (index) | ↑ (index) | ↑ (%) | ↓ (%) | ns (%) (SWS) | ns (%) | ↓ |
| Takei 201234 | NwC vs NwoC | Adult | NwC: 52 NwoC: 62 | ns | ↑ | – | ↑ (index) | ↑ (%) | ↓ (%) | ns (%) (3/4) | ns (%) | ↓ (%) |
| DelRosso 201335 | NwC vs NwoC | Adult | NwC: 4 NwoC: 4 | ns | ↑ | – | – | ns (%) | ns (%) | ns (%) | ns (%) | ns |
| Sorensen 201336 | NwC vs NwoC | Adult | NwC: 43 NwoC: 20 | ns | – | – | – | ↑ (%) | ns (%) | ns (%) | ns (%) | ns (%) |
| Pizza 201531 | NT1 vs NT2 | Adult | NT1: 79 NT2: 22 | ns | ↑ | ↑ | – | ↑ (%) ↑ (min) | ↓ (%) ↓ (min) | ns (%) ns (min) | ns (%) ns (min) | ↓ (%) |
| Maski 202132 | NT1 vs NT2 | Pediatric | NT1: 46 NT2: 12 | ns | ↑ | – | ↑ (index) | ns (%) | ns (%) | ns (%) | ns (%) | ns (%) |
| Presence vs absence of DQB1*0602 allele in patients with NwC or NwoC | ||||||||||||
| Hong 200038 | NwC/ DQB1*0602 positive vs NwC/DQB1*0602 negative | Adult | NwC/ DQB1*0602 positive: 312 NwC/DQB1*0602 negative: 114 | ns | ↑ (%) | – | – | ↑ (%) | ↓ (%) | ns (%) (S3, S4) | ↓ (%) | ↓ (%) |
| Hong 200038 | NwoC/ DQB1*0602 positive vs NwoC/DQB1*0602 negative | Adult | NwoC/ DQB1*0602 positive: 52 NwoC/DQB1*0602 negative: 36 | ns | ↑ (%) | – | – | ↑ (%) | ns (%) | ns (%) | ns (%) | ns (%) |
| Narcolepsy vs other disorders of hypersomnolence | ||||||||||||
| Narcolepsy (mixed population) | ||||||||||||
| DelRosso 201335 | N vs IH | Adult | N: 8 IH: 8 | ↓ | ns | – | – | ns (%) | ns (%) | ns (%) | ↑ (%) | ns |
| Narcolepsy with cataplexy/NT1 | ||||||||||||
| Takei 201234 | NwC vs IH w/o LST | Adult | NwC: 52 IH w/o LST: 50 | ns | ↑ | – | ↑ (index) | ↑ (%) | ↓ (%) | ns (%) (3/4) | ns (%) | ns (%) |
| DelRosso 201335 | NT1 vs IH | Adult | NT1: 4 IH: 8 | ns (P = .06) | ns | – | – | ns (%) | ns (%) | ns (%) | ns (%) | ns (%) |
| Pizza 201531 | NT1 vs IH | Adult | NT1: 79 IH: 22 | ns | ↑ | ↑ | – | ↑ (%) ↑ (min) | ↓ (%) ↓ (min) | ns (%) ns (min) | ns (%) ns (min) | ↓ (%) |
| Walacik-Ufnal 201729 | NT1 vs Psych | Adult | NT1: 15 Psych: 14 | ns | ↑ | – | – | ↑ (%) | ↓ (%) | ↓ (%) (3/4) | ns (%) | ns (%) |
| Maski 202132 | NT1 vs IH | Pediatric | NT1: 46 IH: 18 | ↓ | ↑ | – | ↑ (index) | ↑ (%) | ↓ (%) | ↑ (%) | ns (%) | ns |
| Narcolepsy without cataplexy/NT2 | ||||||||||||
| DelRosso 201335 | NT2 vs IH | Adult | NT2: 4 IH: 8 | ns | ns | – | – | ns (%) | ns (%) | ns (%) | ↑ (%) | ns |
| Pizza 201531 | NT2 vs IH | Adult | NT2: 22 IH: 22 | ns | ns | ns | – | ns (%) ns (min) | ns (%) ns (min) | ns (%) ns (min) | ns (%) ns (min) | ns (%) |
| Maski 202132 | NT2 vs IH | Pediatric | NT2: 12 IH: 18 | ns | ns | – | ns | ns (%) | ↓ (%) | ns (%) | ns (%) | ns |
C = control, HC = healthy control, IH = idiopathic hypersomnia, LST = long sleep time, N = narcolepsy (mixed population), N1/2/3 = stage 1/2/3 non-REM sleep, ns = not significant, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, NwC = narcolepsy with cataplexy, NwoC = narcolepsy without cataplexy, PSG = polysomnography, psych = psychiatric disorder, REM = rapid eye movement, sHS = subjective hypersomnolence, SSS = subjectively sleepy subjects, SWS = slow-wave sleep, TST = total sleep time, w/o = without, WASO = wake after sleep onset.
Table 2.
Summary of studies involving PSG assessment of wake-sleep stages (duration, number, or transitions) during nocturnal sleep in narcolepsy.
| Narcolepsy vs Controls | NwC/NT1 vs NwoC/NT2 | Narcolepsy vs Other Disorders of Hypersomnolence | CSF hcrt-1 Levels | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antelmi 201728 | Vandi 201930 | Pizza 201531 | Pizza 201531 | Maski 202132 | Maski 202132 | Sorensen 201336 | Pizza 201531 | Maski 202132 | Pizza 201531 | Pizza 201531 | Maski 202039 | Maski 202132 | Maski 202132 | Barateau 202060 | Sorensen 201336 | |
| Comparison | NT1 vs HC | NT1 vs C | NT1 vs sHS | NT2 vs sHS | NT1 vs SSS | NT2 vs SSS | NwC vs NwoC | NT1 vs NT2 | NT1 vs NT2 | NT1 vs IH | NT2 vs IH | NT1 vs NT2/ IH/ SSS | NT1 vs IH | NT2 vs IH | CSF hcrt-1 ≤ 110 pg/mL vs CSF hcrt-1 > 110 pg/mL | CSF hcrt-1 < 129 pg/mL vs CSF hcrt-1 ≥ 129 pg/mL |
| Population | Ped | Ped | Adult | Adult | Primarily ped (5–21 y) | Primarily ped (5–21 y) | Adult | Adult | Primarily ped (5–21 y) | Adult | Adult | Ped | Primarily ped (5–21 y) | Primarily ped (5–21 y) | Adult (n = 229) and ped (n = 71) | Adult |
| Group sizes | NT1: 40 HC: 22 | NT1: 27 C: 19 | NT1: 79 sHS: 52 | NT2: 22 sHS: 52 | NT1: 46 SSS: 48 | NT2: 12 SSS: 48 | NwC: 43 NwoC: 20 | NT1: 79 NT2: 22 | NT1: 46 NT2: 12 | NT1: 79 IH: 22 | NT2: 22 IH: 22 | NT1: 150 NT2: 22 IH: 27 SSS: 117 | NT1: 46 IH: 18 | NT2: 12 IH: 18 | CSF hcrt-1 ≤ 110 pg/mL: 164 CSF hcrt-1 > 110 pg/mL: 136 | CSF hcrt-1 < 129 pg/mL: 37 CSF hcrt-1 ≥ 129 pg/mL: 20 |
| Transitions between/among wake, sleep, sleep stages (bidirectional) | ||||||||||||||||
| Wake, sleep | ↑a | – | ↑a | nsa | – | – | ↑b | ↑a | – | ↑a | nsa | – | – | – | – | ↑b |
| Wake, NREM sleep, REM sleep | ↑a | – | ↑a | nsa | – | – | – | ↑a | – | ↑a | nsa | – | – | – | – | – |
| Wake, N1, N2/N3, REM sleep | ↑a | – | ↑a | nsa | – | – | – | ↑a | – | ↑a | nsa | – | – | – | – | – |
| REM sleep, NREM sleep | – | – | – | – | – | – | nsb | – | – | – | – | – | – | – | – | nsb |
| N1, N2, N3 | – | – | – | – | – | – | nsb | – | – | – | – | – | – | – | – | nsb |
| N1, N2, N3, REM | – | – | – | – | – | – | nsb | – | – | – | – | – | – | – | – | nsb |
| Wake, N1, N2, N3, REM sleep | – | ns (↑ P = .07)c | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Transitions from sleep to wake (directional) | ||||||||||||||||
| Sleep to wake | – | – | – | – | – | – | – | – | – | – | – | ↑d | – | – | ↑ | – |
| REM sleep to wake | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| REM sleep to N1/wake | – | – | – | – | – | – | – | – | – | – | – | ↑d | – | – | – | – |
| NREM sleep to wake | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| N1 to wake | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – | |
| N2 to wake | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| N3 to wake | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| N2/N3 to N1/wake | – | – | – | – | – | ↑d | – | – | – | |||||||
| N2/N3/REM sleep to N1/wake | – | – | – | – | – | – | – | – | – | – | – | ↑d | – | – | – | – |
| Sleep to wake and N2/N3/REM sleep to N1 | – | – | – | – | – | – | – | – | – | – | – | ↑d | – | – | – | – |
| Transitions from wake to sleep (directional) | ||||||||||||||||
| Wake to sleep | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| Wake to REM sleep | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| Wake to NREM sleep | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| Wake bouts | ||||||||||||||||
| Wake bouts, total number | – | – | – | – | ↑ | ns | – | – | ↑ | – | – | – | ↑ | ns | ↑ | – |
| Wake bouts/h of wake time, index | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↓ | – |
| Wake bouts of 30 s, % | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| Wake bouts of 1 min 30 s, % | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| Sleep bouts | ||||||||||||||||
| Sleep bouts, total number | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| Sleep bouts/h of sleep time, index | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| Sleep bouts of ≤14 min (2–5 min), % | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↑ | – |
| Sleep bouts of >32 min 30 s, number | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ↓ | – |
| N1 bouts, number | ↑ | ns | ↑ | ↑ | ns | |||||||||||
| N2 bouts, number | ↑ | ns | ↑ | ns | ns | |||||||||||
| N3 bouts, number | ns | ns | ns | ns | ns | |||||||||||
| REM sleep bouts, number | ↑ | ns | ns | ↑ | ns | |||||||||||
Number of transitions between states divided by time in bed (in hours). bNumber of transitions per hour of sleep of the investigated sleep stage. cNumber of transitions per hour between the start and the end of nocturnal sleep. dNumber of transitions per hour of total sleep time. C = control, CSF = cerebrospinal fluid, HC = healthy control, hcrt-1 = hypocretin-1, IH = idiopathic hypersomnia, N1/2/3 = stage 1/2/3 non-REM sleep, NREM = nonrapid eye movement, ns = not significant, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, NwC = narcolepsy with cataplexy, NwoC = narcolepsy without cataplexy, ped = pediatric, PSG = polysomnography, REM = rapid eye movement, sHS = subjective hypersomnolence, SSS = subjectively sleepy subjects.
RESULTS
DNS terminology
There is still no single agreed-upon definition of DNS in narcolepsy. The AASM ICSD-3 guidelines describe DNS as “an inability to maintain continuous sleep.”1 However, there are many nocturnal symptoms of narcolepsy that may disrupt sleep continuity, and thus the term DNS could be conceptualized in a broad sense to encompass underlying narcolepsy-related sleep symptoms and disorders. Hypnagogic/hypnopompic hallucinations and/or sleep paralysis are found in 33%–80% of patients with NT11 and may lead to sleep disturbances if they cause distress and difficulty initiating sleep or maintaining sleep when they occur in the middle of the night. Narcolepsy (mixed population) is associated with sleep-related movement disorders, including rapid eye movement (REM) sleep behavior disorder (RBD), restless legs syndrome, and periodic limb movements of sleep (PLMS).10 RBD presents as REM sleep without atonia and abnormal movements during REM sleep related to acting out dreams, and is reported in up to 61% of people with NwC.11 Such REM sleep abnormalities have been less studied in NT2. In a small pediatric cohort, patients with NT2 had numerically higher REM sleep without atonia density than subjectively sleepy controls, and 2 of the 6 NT2 patients demonstrated vocalizations and/or movements consistent with RBD.12 PLMS involves repetitive movements of the legs that occur during REM and non-REM (NREM) sleep13 and is reported in up to 75% of people with narcolepsy (mixed population).14 These abnormal movements during sleep may be associated with sleep disruption through frequent awakenings or arousals (narcolepsy [mixed population]),15 but the degree of sleep disturbance they cause is unknown. Patients with narcolepsy (mixed population) report problematic nightmares and nocturnal eating that may also contribute to sleep disruption.16 Furthermore, obesity is more common among patients with narcolepsy (mixed population) and likely contributes to frequent reporting of obstructive sleep apnea (OSA), which may further fragment sleep.10,17–19 Last, the degree to which comorbidities such as anxiety and depression or use of wake-promoting medications such as traditional stimulants may contribute to problems of sleep continuity is unclear (narcolepsy [mixed population]).20
The expert panel convened in 2011 to refine the definition of DNS that may exist independently of comorbid conditions such as OSA, RBD, and PLMS; review the literature; and formulate a consensus characterization of DNS in narcolepsy based on self-reported and objective data.6 The panel concluded that patients with narcolepsy (mixed population) typically describe frequent, usually brief, nocturnal awakenings and generally poor sleep quality, and that objective measures of their sleep problems included increased arousals, increased wake after sleep onset (WASO) time, frequent shifts to wake or increased N1 sleep with reduction of N3/N4, and overall decreased sleep efficiency. The authors proposed that DNS in narcolepsy (mixed population) is “a stand-alone and specific symptom that is not associated with other sleep disorders,”6 but this claim has not yet been substantiated against other disorders such as primary OSA.
While this panel highlighted important nocturnal sleep continuity impairment in narcolepsy, the term DNS continues to be used inconsistently in the subsequent literature. Most important, when patients with narcolepsy are queried about DNS or sleep disruptions, they may be reporting the effects of the broad range of sleep disorders that commonly occur in narcolepsy. In this review, we favor using DNS as an umbrella term for all sleep symptoms/conditions common to narcolepsy as well as the intrinsic sleep instability that produces spontaneous wakings, arousals, and excessive sleep stage transitions.
Characteristics of DNS in narcolepsy
Researchers have described DNS in narcolepsy using objective tools such as PSG and actigraphy, as well as self-reported measures obtained by clinical interviews, sleep diaries, and questionnaires. Patients with common narcolepsy-related sleep disorders/conditions such as PLMS and RBD have not typically been excluded from clinical studies; thus, it is not possible to say whether findings are a reflection of sleep instability intrinsic to narcolepsy.
Objective DNS measures reported in the literature include standard (static) measures of sleep continuity, such as time spent asleep (total sleep time [TST]), time awake after sleep onset (WASO), number of awakenings, arousal index, time spent (or percentage of TST spent) in sleep stages (NREM sleep, NREM sleep stages [N1, N2, N3], slow-wave sleep [SWS], and REM sleep), and sleep efficiency, as well as dynamic measures of sleep architecture and stability, which relate to the frequency and nature of transitions among sleep states and between wake and sleep states. Transitions have been quantified in terms of raw numbers (counts), by transitional indices (eg, number of transitions per hour of sleep), sleep and wake bout numbers, or sleep and wake bout median durations. Techniques including sophisticated statistical analyses and computer scoring algorithms have been used to examine the diagnostic utility of sleep stage dynamics.21
Although an extensive body of research in this field is lacking, trends in objective and self-reported measures of sleep instability are observable in comparisons of narcolepsy (including NT1 or NwC, NT2 or NwoC, and mixed populations of both narcolepsy groups) with controls (including healthy controls [HCs], subjectively sleepy controls [people with self-reported daytime sleepiness but normal PSG and Multiple Sleep Latency Test results], and people with other central nervous system disorders of hypersomnolence), and comparisons between narcolepsy subtypes (NT1/NwC and NT2/NwoC).
Objective assessment of sleep instability: PSG
Narcolepsy vs healthy controls or subjectively sleepy controls
Static measures:
Findings related to static measures of sleep architecture in people with narcolepsy vs HCs or subjectively sleepy controls (subjectively sleepy subjects [SSS] or subjective hypersomnolence [sHS], defined here as people with self-reported daytime sleepiness but who do not meet the clinical criteria for NT1, NT2, or a different disorder1) are summarized in Table 1.
Narcolepsy (mixed population): In a single study in people with narcolepsy (mixed population) vs HCs, there was no significant between-group difference in TST, WASO (“wakefulness”), or sleep efficiency; however, the patient population had increased awakenings and arousals, increased N1 and N2 percentage, and decreased SWS and REM sleep percentage.22
NwC/NT1: In 10 studies comparing people with NwC or NT1 vs controls (HC or sHS), TST was reduced significantly in people with NwC/NT1 in 2 studies23,24 but did not differ between groups in the remaining 8 studies.25–32 WASO was increased significantly in people with NwC/NT1 in 8 of 9 studies examining WASO.23–25,28,29,31,32 One study reported no significant difference in WASO between NwC and controls, although the study only included a total of 12 participants and thus may have been underpowered to detect differences.27 Of 10 studies, 8 reported that participants with NwC/NT1 had significant increases in N1 sleep (N1 percentage or total duration of N1)23–25,28–32 and decreases in N2 sleep (N2 percentage or total duration of N2) compared with controls;23,24,26–29,31,32 however, N3 sleep and REM sleep were generally similar between groups, with only 3 of 10 studies reporting a significant decrease in N3 percentage25,29,31 and no studies reporting a significant group difference in REM sleep percentage or duration.23–32 Four of the 10 studies reported significantly decreased sleep efficiency in NT1/NwC vs controls23,24,26,31 and 1 reported significantly increased sleep efficiency in NT1 vs subjectively sleepy controls.32
NwoC/NT2: In 2 studies comparing participants with NT2 vs sHS or SSS, there were no group differences in TST, WASO, awakenings, N1, N2, or N3.31,32 In one of these studies, in pediatric participants, REM sleep and sleep efficiency were increased in NT2 compared with subjectively sleepy controls,32 but in the other, in adults, there were no differences in these parameters.31
Dynamic measures:
Three studies examined wake and sleep stage transitions in NT1 vs HC or sHS (Table 2).28,30,31 In a study by Antelmi et al28 involving drug-naïve children and adolescents with NT1 vs HC, transitions between wake and sleep or sleep stages (calculated as sleep transitional indexes, or frequency of transitions between combinations of sleep stages) were significantly increased in NT1, including transitions between wake and sleep; transitions among wake, NREM sleep, and REM sleep; and transitions among wake, N1, N2/N3, and REM sleep. Vandi et al30 compared nocturnal sleep stage transitions using a frameshift index (calculated as number of transitions between states [wake, N1, N2, N3, REM sleep]) in children and adolescents with NT1 vs subjectively sleepy controls. The NT1 frameshift index was numerically higher in the NT1 group; however, results neared but did not meet statistical significance. The results of the 2 studies are not necessarily conflicting, as they assessed transitions with different measures and differed in comparator groups (HCs vs subjectively sleepy people). Pizza, Vandi, et al31 examined adults with NT1 or NT2 vs sHS in terms of transitions between sleep and wake; among wake, NREM sleep, and REM sleep; and among wake, N1, N2/N3, and REM sleep and found increases in all 3 types of transitions in people with NT1 vs sHS but no significant group differences between NT2 and sHS.
Maski, Colclasure, and colleagues32 compared young people (5–21 years of age) with NT1, NT2, idiopathic hypersomnia (IH), or SSS in terms of nocturnal wake and sleep stage (N1, N2, N3, and REM sleep), bout duration, bout number, and survival of bouts using nocturnal PSG. Findings suggested different sleep phenotypes for each group. Compared with SSS, the NT1 group showed evidence of sleep fragmentation with unstable REM sleep and N2 sleep, the NT2 group showed comparable sleep with the exception of longer N1 bouts, and the IH group showed increased N2 sleep stability and shorter N3 bout duration.
A detailed examination of nocturnal sleep stage sequences and transitions (due to complexity of analyses, not included in Table 2) was conducted by Ferri and colleagues,33 who found that the sleep stage transition pattern was significantly different between people with NT1 vs sHS, NT2, and IH. Comparisons of between-stage transition probabilities (derived from standardized sleep stage transition matrices containing the probability of stage transitions, rather than the absolute number of transitions, and confirmed using Markovian analysis of group matrices) indicated a higher probability of transitions from REM sleep to wake, wake to N1, N1 to N2, and wake to REM sleep and a lower probability of transitions from N2 to N1, N3 to N2, REM sleep to N2, and N2 to REM sleep in people with NT1 vs sHS, NT2, or IH.33 The lower probability of the REM sleep to N2 transition was the most reliably observed of these, leading the authors to propose that frequent alternation between REM and NREM sleep may represent a PSG fingerprint of orexin-deficient hypersomnia, and thus may be useful as a diagnostic tool.33
NT1 vs NT2
Static measures:
Relatively little information is available regarding sleep instability in NT1 vs NT2 because both are rare disorders and thus small sample sizes are typically reported in existing studies. Based on the evidence that does exist, the overall impression is one of greater sleep instability in NT1 compared with NT2.
Findings relating to static measures of sleep architecture are summarized in Table 1. Six studies compared participants with NwC/NT1 vs NwoC/NT2. In 5 of these studies, no group difference was noted in TST,31,32,34–36 but Harsh et al15 reported decreased TST in participants with NwC vs NwoC. All 4 studies that examined WASO showed increased WASO among participants with NwC/NT1 vs NwoC/NT2.31,32,34,35 Participants with NwC/NT1 had increased N1 sleep in 4 of 6 studies15,31,34,36 and decreased N2 sleep in 3 of 6 studies vs NwoC/NT2.15,31,34 No significant group differences were detected in N3 or REM sleep.15,31,32,34,35,37 Sleep efficiency was decreased significantly in NwC/NT1 vs NwoC/NT2 in 3 of 6 studies.15,31,34
Hong et al38 compared participants with NwC or NwoC categorized by the presence or absence of the human leukocyte antigen (HLA) DQB1*06:02 allele (Table 1). They found that the presence of the allele in participants with NwC was associated with increased WASO and N1 sleep percentage and decreased N2 sleep and REM sleep percentage and sleep efficiency, but no group differences in TST or N3 sleep, compared with the absence of the allele. In participants with NwoC, the presence of the allele was associated with increased WASO and N1 sleep percentage but no significant difference (compared with the absence of the allele) in TST; N1, N2, N3, or REM sleep percentage; or sleep efficiency.38
Dynamic measures:
Two studies, both in adults, examined wake and sleep stage transitions in NT1 vs NT2 (Table 2). Sorensen, Knudsen, and Jennum36 reported that transitions (quantified as “number of transitions per hour of sleep of the investigated sleep stage”) between wake and sleep were significantly increased in participants with NT1 vs NT2, but transitions between REM and NREM sleep; transitions to/from N1, N2, and N3; and transitions between all sleep stages were not significantly different between groups. Pizza, Vandi, et al31 reported that transitions between wake and sleep; transitions among wake, REM sleep, and NREM sleep; and transitions among wake, N1, N2/N3, and REM sleep (calculated as sleep transitional indexes per hour of sleep) were significantly higher in drug-naïve adults with NT1 vs NT2. The apparent differences in the results of these studies may be related to differences in the specific transitions examined (eg, between NREM and REM sleep in the study by Sorensen, Knudsen, Petersen, et al37 vs wake, NREM sleep, and REM sleep in the study by Pizza, Vandi, et al31), in addition to the relatively small sample size in the study by Sorensen, Knudsen, and Jennum36 compared with Pizza, Vandi, et al.31 In a cohort aged 5–21 years, Maski, Colclasure, and colleagues32 reported more sleep fragmentation in the NT1 group than in the NT2 group, with more wake, N1 sleep, N2 sleep, and REM sleep bouts in the NT1 group compared with NT2.
Narcolepsy vs other disorders of hypersomnolence
Static measures:
Several studies compared people with narcolepsy vs other disorders of hypersomnolence, including IH and hypersomnolence associated with psychiatric disorders. Results relating to static measures of sleep architecture are summarized in Table 1.
In a study by DelRosso and colleagues35 comparing participants with narcolepsy (mixed population) vs IH, the authors reported no significant group differences in WASO, NREM (N1, N2, or N3) sleep percentage, or sleep efficiency. This study reported that people with narcolepsy (mixed population of NT1 and NT2) had decreased TST and increased REM sleep percentage vs IH; however, the group sizes were small (narcolepsy, n = 8; IH, n = 8). Takei et al;34 Pizza, Vandi, et al;31 and Maski, Colclasure, et al32 compared people with NwC/NT1 with people with IH. Takei et al34 and Pizza, Vandi, et al31 reported no group differences in TST or N3 sleep, whereas Maski, Colclasure, et al32 reported a decrease in TST and an increase in N3 sleep in NT1 vs IH. All 3 sets of investigators reported no group difference in REM sleep, increased WASO and N1 sleep, and decreased N2 sleep in people with NwC/NT1 compared with IH.31,32,34 Pizza, Vandi, et al31 reported decreased sleep efficiency in NT1 vs IH, whereas Takei et al34 and Maski, Colclasure, et al32 reported no significant group difference in sleep efficiency. Walacik-Ufnal and colleagues29 compared participants with NT1 vs hypersomnolence associated with a psychiatric disorder (including dysthymic disorder, major depressive disorder, mixed anxiety and depressive disorder, somatoform disorders, and dissociative disorders) and found that NT1 participants had increased WASO and N1 sleep percentage, decreased N2 and N3 sleep percentage, and no significant group differences in TST, REM sleep percentage, or sleep efficiency compared with those with psychiatric disorders.
Three studies compared participants with NT2 vs IH; none reported differences between the groups on TST, WASO, N1 sleep percentage, N3 sleep percentage, or sleep efficiency. Maski, Colclasure, et al32 reported decreased N2 sleep percentage in NT2 compared with IH, whereas neither of the other studies reported a group difference in this parameter. DelRosso and colleagues35 reported increased REM sleep percentage in NT2 compared with IH, whereas Pizza, Vandi, and colleagues31 and Maski, Colclasure, and colleagues32 reported no difference in REM sleep percentage in NT2 vs IH.
Dynamic measures:
Few studies have compared dynamic measures of sleep architecture in people with narcolepsy vs other disorders of hypersomnolence (Table 2). Maski, Pizza, et al39 reported that children and adolescents with NT1 demonstrated increases in all sleep state transitions studied, compared with children and adolescents with IH. Pizza, Vandi, et al31 reported significantly increased transitions between wake and sleep; among wake, NREM, and REM; and among wake, N1, N2/N3, and REM in drug-naïve adults with NT1 vs IH but not NT2 vs IH.
Using measures of bout number and bout duration as metrics of sleep instability in a cohort aged 5–21 years, Maski, Colclasure, and colleagues32 reported that the NT1 group had more wake, N1, and REM sleep bouts compared with IH, but no differences in the number of N2 or N3 sleep bouts. In people with NT2, N1 sleep bouts were longer compared with people with IH, but wake and sleep stage bout number was comparable.
Objective assessment of sleep instability: actigraphy
Actigraphy is another technique for objectively assessing nocturnal sleep instability in narcolepsy. Actigraphy measures rest/activity and allows for the approximation of sleep/wake cycles and nighttime sleep parameters, including TST, number of awakenings, and sleep efficiency, with the help of specialized computer scoring algorithms.40 Compared with PSG, actigraphy is much less costly and can be performed longitudinally over days and weeks at home but provides less information, is prone to artifacts, and does not always reliably distinguish quiet wake from sleep, even in healthy individuals.40,41
Results of clinical studies using actigraphy to assess nighttime sleep are consistent with PSG profiles of sleep quality, demonstrating poorer sleep in people with NT1 compared with HCs or people with insomnia. Filardi, Pizza, Martoni, and colleagues42 found that estimated TST and sleep efficiency were significantly lower in adults with NT1 compared with HCs, and mean WASO, number of awakenings, number of awakenings longer than 5 minutes, and mean sleep motor activity were significantly higher. Results from a pediatric study were similar.43 Likewise, Leger and colleagues44 found a higher sleep fragmentation index in adults with NT1 compared with primary insomnia in a study using actigraphy.
Despite the consistency of actigraphy and PSG in describing sleep quality measures, recent data suggest that actigraphy tends to underestimate TST and sleep efficiency in patients with narcolepsy (both NT1 and NT2, with greater underestimation in patients with NT1).45 Additionally, Alakuijala, Sarkanen, Jokela, and colleagues45 pointed out that patients with NT1 may have abnormal motor behavior during REM sleep. Actigraphy could erroneously interpret these movements as wake, leading to an underestimation of TST and sleep efficiency. In general, actigraphy also tends to underestimate sleep latency, likely because individuals often lie immobile for some time prior to falling asleep. However, in patients with NT1 or NT2, actigraphic and PSG sleep onset latency were concordant, likely because patients with narcolepsy tend to fall asleep quickly rather than lie awake immobile.45 Formal validation has not been reported in the literature for actigraphy vs polysomnography as a measure of nocturnal sleep instability in narcolepsy.
Self-reported description of DNS
DNS has been described or assessed subjectively through clinical interviews as part of patient care and self-report measures in a research setting. Bruck and Parkes46 examined nighttime sleep parameters using self-report questionnaires and sleep diaries (completed for 3 consecutive days) and found that people with NwC reported significantly more nocturnal awakenings and increased duration of nocturnal wakefulness compared with people with IH. Patient-reported nocturnal sleep latency, nocturnal time in bed, and nocturnal TST did not differ between the groups.
Since this study, validated tools have been developed and/or utilized to assess DNS. The NSS is a 15-item narcolepsy-specific measure for quantifying the severity of the 5 key narcolepsy symptoms, including DNS.8,9 The NSS has 1 question on DNS (question number 15) that asks patients about the degree of nighttime sleep disturbance (“Currently, how disrupted is your nighttime sleep?”).9 It should be noted that this question may not be interpreted in the same way by every patient, and responses may reflect broad sleep problems, as previously noted (parasomnias or comorbid conditions such as OSA, RBD, or PLMS). Similarly, validated sleep quality instruments such as the Insomnia Severity Index and Pittsburgh Sleep Quality Index have been used to assess DNS. The Insomnia Severity Index is a 7-item instrument that includes questions relating to difficulty staying asleep, early awakenings, and sleep dissatisfaction,47 which may capture concerns about DNS. In untreated patients with NT1, Insomnia Severity Index scores were positively correlated with DNS question scores on the NSS.9 The Pittsburgh Sleep Quality Index includes 19 self-rated questions grouped into components relating to self-reported sleep quality, sleep duration, habitual sleep efficiency, and sleep disruptions, among others.48 These surveys may be useful to clinicians to assess the severity of DNS, but further validation is needed in people with narcolepsy.
Association between objective measures of sleep instability and self-reported descriptions of DNS
Conversely, it is unclear which objective measure of sleep instability best matches self-reported sleep problems in people with narcolepsy, as there are few data directly comparing self-reports of nighttime sleep with PSG or actigraphic measures. In 1 study in which 79% of participants had NwC, participants who reported “trouble sleeping” on the questionnaire also reported reduced TST, increased number of awakenings, and reduced sleep efficiency, compared with participants identified as having “no trouble sleeping.”49 On PSG, participants with “trouble sleeping” had significantly increased “wake time during sleep,” increased N1 sleep percentage, and decreased N2 sleep percentage, compared with those without trouble sleeping, but there was no difference between groups on TST, number of awakenings, sleep efficiency, SWS percentage, or REM sleep percentage. A limitation of this study was that participants were mailed the questionnaire years after PSG was performed, and it is unclear whether DNS may vary over time or in different environments. In a posthoc analysis of a phase 3 clinical trial of sodium oxybate (SXB) in participants with NwC, decrease in frequency of sleep stage shifts (from stages N2/N3/REM sleep to stage N1/wake, or from stages N2/N3 to stage N1/wake) following 8 weeks of SXB use was moderately correlated with improvement in patient-reported quality of nocturnal sleep (rated using a unique 4-point Likert-type scale [0 = excellent; 1 = good; 2 = fair; 3 = poor]).50 In a recent study in children and adolescents with NT1, NT2, IH, or self-reported sleepiness, there were significant associations between nocturnal PSG parameters (sleep efficiency, arousal index, percentage N1 sleep, wake index, wake/N1 index, and N2/N3 to wake/N1 index) and self-reported sleep continuity (on a categorical question asking about any difficulty staying asleep).39 Across these studies, the number of sleep stage transitions in both NREM and REM sleep that occur across the night was associated with self-reported symptoms of DNS.
Potential causes of sleep instability in narcolepsy
The etiology of intrinsic sleep instability in narcolepsy is unknown, but research supports a strong association with orexin loss/deficiency. Switching mechanisms (flip/flop switches, which are based on mutually inhibitory circuits) are hypothesized to regulate transitions between sleep/wake states and NREM/REM sleep stages.51,52 Destabilization of these switches would be expected to lead to an increase in the number of transitions between states, as well as switching at inappropriate times.51,52 Orexin is a neuropeptide that helps maintain wakefulness during the day due to excitatory input to monoaminergic activity; hence, loss of orexin neurons, as in narcolepsy, results in daytime lapses into sleep and excessive daytime sleepiness.52 Based on mathematical modeling of the sleep/wake switch, orexin is also critical for stabilizing sleep state continuity. Fulcher et al53 showed that during sleep, the ventrolateral preoptic neurons of the hypothalamus inhibited both orexin and monoaminergic neurons to produce sustained sleep, and subsequent reduction in orexin input to the monoaminergic neurons indirectly sustained sleep episodes. Simulating a loss of orexin neurons, the model produced sleep dynamics characteristic of narcolepsy measures among patients with PSG, including frequent transitions between states.53
Preclinical studies have also demonstrated that loss of orexin alters sleep continuity. For example, targeted deletion of the orexin gene in mice results in a phenotype similar to that of human narcolepsy, including disruption of sleep/wake patterns and reduced REM sleep latency.54 In mouse models of orexin neuronal degeneration, there is a progressive increase in the number of wake bouts that emerge during the light and dark periods from baseline prior to orexin neuronal loss.55
In humans, there has been ongoing research to establish a link between orexin levels and DNS. In a retrospective chart review including adults with NwoC, Andlauer and colleagues56 found no significant differences in PSG assessments, including sleep efficiency and time spent in N1, when comparing participants who had CSF orexin levels that were low (≤ 110 pg/mL), intermediate (> 110 to ≤ 200 pg/mL), or normal (> 200 pg/mL), other than a significant increase in TST in participants with intermediate vs low CSF orexin levels. This study showed an increase in self-reported number of nocturnal awakenings in participants with intermediate vs normal CSF orexin levels but did not assess PSG nocturnal awakenings.56 Alakuijala, Sarkanen, and Partinen57 observed an association between lower CSF orexin levels and increased sleep fragmentation based on actigraphy (decreased sleep latency, decreased sleep efficiency, increased number of immobile phases of 1 minute, and increased movement + fragmentation index) in a mixed population (pediatric and adult) of participants with NT1. Sorensen, Knudsen, and Jennum36 compared people with narcolepsy (with or without cataplexy) with low CSF orexin levels (< 30% of normal mean, which in this sample was < 129 pg/mL58) vs normal CSF orexin levels and found that transitions between wake and sleep and between REM and NREM sleep were significantly increased in people with narcolepsy with low orexin. There were no significant differences in terms of transitions to/from N1, N2, and N3 and transitions among all sleep stages.36 Finally, Brink-Kjaer and colleagues59 reported that increased CSF orexin levels were associated with decreased cortical arousal frequency (arousal index) on nocturnal PSG in people with NT1 and NT2 and controls.
More recently, Barateau and colleagues60 examined the association between CSF orexin levels and markers of sleep instability (wake bouts, sleep bouts, and sleep stage transitions) on nocturnal PSG in 300 drug-free participants (adults and children) with hypersomnolence (including 167 with NT1, 51 with NT2, and the remainder with IH or EDS without a central disorder of hypersomnolence; Table 2). In participants with CSF orexin deficiency (≤ 110 pg/mL) compared with normal CSF orexin levels (> 110 pg/mL), there was an increased number of wake bouts and sleep bouts across the sleep period, reflecting sleep fragmentation.60 During sleep, participants with CSF orexin deficiency had a lower percentage of short wake bouts (30 seconds) and a higher percentage of long wake bouts (1 minute, 30 seconds) than those with normal CSF orexin levels.60
Altogether, these results suggest that lack of orexin may disrupt sleep continuity.60 However, this notion may be challenged by the recent development of orexin antagonists. Orexin antagonists are used to improve sleep continuity and do not cause an increase in wake bouts at the doses used to manage primary insomnia.61 Arguably, the low clinical dosing of orexin antagonists likely does not fully suppress orexin effects and produce a narcolepsy phenotype. Pizza, Francheschini, et al62 found that in children developing NwC, total 24-hour sleep time is first increased and then returns to normal as the disease progresses, suggesting adaptation. Secondary contributors to disrupted sleep continuity in narcolepsy, such as changes in histaminergic neurons63 (despite discrepant reporting of CSF levels of histamine and tele-methylhistamine in people with narcolepsy64,65) and/or imbalance of monoaminergic transmission,52 may be at play.
Burden of DNS in narcolepsy
Medical and economic consequences associated with narcolepsy have been well documented,66 but few studies have examined the burden associated with specific narcolepsy symptoms.
DNS has a negative impact on daytime functioning. In a large, cross-sectional study of people with narcolepsy (mixed population) using a specially designed survey, DNS was identified by about one-third of participants as one of the top 1 to 3 symptoms with the most significant negative impact on their life.67 There is modest evidence suggesting a relationship between nighttime sleep quality and daytime sleepiness in people with narcolepsy (mixed population). In some studies, PSG measures of sleep architecture, continuity, or transitions among sleep stages were associated with self-reported (Epworth Sleepiness Scale68) and/or objective (Multiple Sleep Latency Test and Maintenance of Wakefulness Test) measures of daytime sleepiness.22,39,50,69 The effects of sleep disturbances and cognitive and executive functioning have been well studied in other sleep disorders, such as insomnia and OSA, but less is known about narcolepsy. Lecendreux et al70 reported an association between self-reported sleep disturbance and attention-deficit/hyperactivity disorder symptoms among children with narcolepsy (mixed population). Interestingly, this association persisted even when patients took psychostimulants (most commonly modafinil) and had improvement in EDS.70
Narcolepsy is also associated with increased risk of cardiovascular and cardiometabolic comorbidities, obesity, and all-cause mortality.10,18,71 For example, the nondipping blood pressure phenotype, in which the normal nighttime decreases in systolic and diastolic blood pressure are reduced or absent, is overrepresented among people with NwC72,73 and associated with increased cardiovascular risk and risk of mortality in the general population.74,75 One study in pediatric NT1 showed no relationship between a measure of cardiovascular autonomic function and sleep architecture alterations or muscle overactivity during sleep.30 It has yet to be studied whether DNS contributes to the failure of this nondipping pattern in NwC.
Management of DNS in narcolepsy
The AASM recommends that treatment of patients with narcolepsy (mixed population) include the goal of controlling DNS, if it is present and troublesome, and recommends SXB as a standard of care.5 Older guidelines from the European Federation of Neurological Societies recommend several treatment options for poor sleep: benzodiazepines, nonbenzodiazepine hypnotics, and SXB.76 Currently, no medications are indicated by the U.S. Food & Drug Administration and the European Medicines Agency for the treatment of DNS in patients with narcolepsy, and few clinical trials have included DNS as an outcome measure. The European Federation of Neurological Societies and AASM guidelines do not report on behavioral or cognitive-behavioral therapy treatments for DNS management.
Nonmedication treatments for DNS among narcolepsy patients
Behavioral interventions (eg, exercise, sleep hygiene, managing naps during the day) are generally recommended as part of a treatment plan for narcolepsy. Evidence for a beneficial effect of exercise comes from a study of children and adolescents with NT1, in which patients who engaged in leisure-time physical activities demonstrated increased nighttime TST, increased sleep efficiency, and reduced WASO duration as assessed using actigraphy, compared with patients who were more sedentary; however, nocturnal awakenings were increased in the active vs sedentary patients.77 Reduction in screen time should be a priority as well, as increasing evidence implicates screen time in disruption of nighttime sleep, including difficulty falling asleep and waking up too early.78 Daytime napping is often used to manage EDS, but nap timing and nap duration should be considered so as not to further disrupt nighttime sleep.79,80
Medications for DNS
Among available narcolepsy treatments, the most is known about SXB in terms of its effects on DNS and sleep instability, based primarily on analyses of PSG data from 2 pivotal trials in adults with narcolepsy (NwC and/or NwoC). In the first trial,81 8 weeks of SXB treatment in participants with NwC resulted in increased time spent in SWS, increased delta power, decreased N1 sleep, and fewer nocturnal awakenings compared with baseline.82 These improvements in sleep were dose dependent.82 In additional analyses from this trial, SXB showed a dose-dependent reduction in shifts from deeper to lighter stages of sleep or wake (N2/N3/REM sleep to N1/wake, N2/N3 to N1/wake, and REM sleep to N1/wake) and improvement in patient-reported sleep quality (measured using a 4-point Likert-type scale).50 In the second study,83 treatment groups of participants with narcolepsy (mixed population) taking 8 weeks of SXB (with or without concurrent modafinil) also showed improvement in self-reported sleep quality assessed by the Pittsburgh Sleep Quality Index and objective DNS measures.84,85 In participants receiving SXB, there was significantly less time spent in N1 or REM sleep, more time spent in SWS, increased delta power, and fewer nocturnal awakenings, compared with baseline.84 Groups receiving SXB or SXB + modafinil had a reduction in shifts from deeper to lighter stages of sleep or wake, including transitions from N2/N3/REM sleep to N1/wake and REM sleep to N1/wake.85 Finally, in a study using actigraphy, Filardi, Pizza, Antelmi, Ferri, and colleagues86 reported changes in the sleep/wake cycle of children with NT1 following 1 year of SXB treatment, with improvements seen in all nighttime actigraphic parameters examined (increases in TST, longest sleep time, and sleep efficiency; decreases in awakenings, time in WASO, and sleep motor activity). SXB is associated with adverse events, including nausea, dizziness, vomiting, somnolence, enuresis, and tremor, and in adolescents and children with headache, decreased weight, and decreased appetite.87 SXB is a part of a Risk Evaluation and Mitigation Strategy safety program because of rare but serious adverse effects, including sleep-disordered breathing and psychiatric complications as well as potential for abuse or misuse that could result in seizure, respiratory depression, decreased consciousness, coma, or even death.87 Screening for OSA is necessary prior to initiating SXB treatment as SXB can aggravate sleep apnea.87
By comparison, little is known about the effects on DNS of other medications used for the treatment of narcolepsy. Baclofen has beneficial effects on sleep in some people with NT1, including those with inadequate response to other therapies.88,89 In a clinical study in healthy volunteers, baclofen administered prior to nighttime sleep during each of 5 weekly experimental sessions improved (increased) nocturnal SWS relative to placebo, as did SXB.90 A small retrospective study assessed nocturnal temazepam use among people with NwC; the authors reported improvement in daytime sleepiness with nocturnal temazepam use, but changes in sleep measures were not measured.91 In a small, single-blind, within-patient study conducted to examine the effect of triazolam on nighttime sleep in people with NwC, a beneficial effect of triazolam vs placebo was noted on TST, sleep efficiency, percentage N1 sleep, and WASO, but not other PSG parameters (percentage N2 or REM sleep and arousals).92 There have been no randomized clinical trials of benzodiazepines to treat DNS in people with narcolepsy. Finally, mixed results have been reported with ritanserin, a serotonin 5-HT2 receptor antagonist. In a randomized, double-blind, placebo-controlled trial in people with narcolepsy (mixed population), ritanserin administered for 4 weeks increased SWS duration and decreased WASO but did not affect the number of awakenings or sleep stage shifts, compared with placebo.93 Furthermore, the results were not replicated.94 The compound is not commercially available.
It is not known to what degree treatment for other sleep disorders, including RBD, restless legs syndrome, or PLMS, improves self-reported or objective measures of DNS. There is mixed evidence with regard to an effect of continuous positive airway pressure therapy on daytime sleepiness, measured by Epworth Sleepiness Scale score, in people with narcolepsy (mixed population) and sleep-disordered breathing or OSA, with 1 study reporting significant improvement and another reporting no effect in a majority of patients.95,96
Effects of wake-promoting medications on DNS
Stimulants and wake-promoting agents are commonly used on-label or off-label for the treatment of EDS in narcolepsy, but evidence suggests that they are associated with either no improvement in, or a disruption of, nighttime sleep. For example, in a phase 3 clinical trial examining SXB and modafinil in people with narcolepsy (mixed population; described above), modafinil was found to have no effect on objective or patient-reported sleep quality (assessed with PSG and Pittsburgh Sleep Quality Index, respectively) as a standalone treatment.85 Pitolisant, a histamine-3 receptor antagonist/inverse agonist used to treat EDS and/or cataplexy in adults with narcolepsy, did not meaningfully impact sleep architecture (assessed using overnight PSG) in a sample of 14 patients with narcolepsy (mixed population).97 High-dose methamphetamine treatment for EDS in patients with NwC has been linked to lower sleep efficiency, longer REM sleep latency, and less REM sleep time.98 Additional evidence for an effect of stimulants on sleep quality comes from studies of attention-deficit/hyperactivity disorder, for which stimulants are a standard of care.99 In a meta-analysis of 7 studies examining the effect of stimulant treatment on sleep in children with attention-deficit/hyperactivity disorder, stimulants were associated with significantly longer sleep latency, poorer sleep efficiency, and shorter TST.100
DISCUSSION
Although progress has been made in recent years in terms of characterizing and addressing DNS and sleep instability in narcolepsy, additional research is necessary. First, the use of a standardized instrument such as the NSS to quantify self-reporting of DNS separately from other symptoms of narcolepsy will be helpful moving forward.8 Further validated measures of DNS, including sleep instability, are needed. There are many PSG measures of sleep instability that have been used, including N1%, WASO, sleep efficiency, sleep maintenance, number of waking periods from sleep relative to TST, sleep stage transitions, and number and duration of wake and sleep stage bouts, but no consensus on the best measure. Actigraphy measures, including sleep efficiency, number of awakenings, and WASO, also reflect DNS, but validation with PSG is needed. It is important to validate any self-reported assessment with objective measures of nighttime sleep, and this relationship may help elucidate pathophysiology. Furthermore, nighttime sleep characteristics that define aspects of DNS could potentially be used to refine diagnostic criteria and simplify clinical diagnostic protocols to using nocturnal PSGs alone. Specific sleep architecture and DNS characteristics derived by sophisticated machine learning from large PSG datasets have shown a role as an avenue for improving diagnosis.21,101,102 Second, more DNS data among people with NT2 are needed to discern potential differences from NT1. Third, a better understanding of the impact of DNS (specifically sleep instability) on patient health, cognition, mood, metabolism, cardiovascular health, and health-related quality of life is necessary to further justify sleep treatments in the routine care of patients with narcolepsy. Finally, clinical trials to establish efficacious DNS treatments are needed, as are prospective studies to determine the effect of daytime wake-promoting medications on nocturnal sleep architecture.
Currently, management of DNS in patients with narcolepsy may be based on a number of considerations. The first of these is to recognize that DNS is a common complaint in patients with narcolepsy. As such, it is important for health care providers to review sleep symptoms/disorders common to narcolepsy that may be contributing to poor sleep quality and/or if disruption is spontaneous and a reflection of intrinsic sleep instability. Sleep disorders such as OSA may require further testing for diagnosis and treatment via surgery, an oral appliance, or positive airway pressure management. Second, measures of DNS in patients with narcolepsy should be reported in PSG testing using terminology relating to sleep continuity measures (sleep efficiency, WASO, arousal index, number of awakenings, amount of N1, and sleep stage transitions) as well as PSG presence of REM sleep without atonia, parasomnia behavior, and periodic limb movements resulting in arousal. Self-reported DNS should be regularly monitored in the course of narcolepsy via clinical interview and use of dedicated questionnaires such as the NSS,8 as it is possible that DNS increases with age, comorbidities, and/or treatment. Overall, intrinsic sleep instability improves with SXB in clinical trials but SXB risks and benefits should be discussed with patients. Other sleep aids have yet to be vigorously studied.
Future research could also address the many questions remaining regarding intrinsic sleep instability, pathophysiology, and interaction between other sleep symptoms and disorders common to narcolepsy (eg, PLMS, RBD, nightmares). There are still basic gaps in knowledge regarding sleep instability changes with age and whether sleep instability prevalence and severity differs between NT1 and NT2 as well as between NT2 and IH.
In conclusion, DNS is a broad term that reflects a constellation of sleep symptoms and disorders common to narcolepsy, as well as the intrinsic sleep instability that likely occurs in the setting of orexin insufficiency/deficiency. Although this article is not a meta-analysis, DNS is more commonly reported and more severe among patients with NT1 compared with controls and hypersomnolence disorders based on literature review. The self-reported and objective measures of DNS described in this review may represent a step forward to discussion of DNS in the clinic, NT1 diagnostic criteria, and inclusion of more sophisticated outcome measures in this area for future clinical trials.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was funded by Jazz Pharmaceuticals. Under the direction of the authors, Michael J. Theisen, PhD, and Karyn Liu, PhD, employees of Peloton Advantage, LLC, an OPEN Health company, provided medical writing assistance funded by Jazz Pharmaceuticals; editorial assistance in formatting, proofreading, copyediting, and fact-checking was also provided by Peloton Advantage. Kiran Maski has been a consultant for Takeda Pharmaceuticals, Jazz Pharmaceuticals, Alkermes, and Harmony Biosciences. She has received research funding from Jazz Pharmaceuticals. She receives salary support from NINDS of the National Institutes of Health under award number K23 NS104267-01A1. Emmanuel Mignot has received research support from Apple, Jazz Pharmaceuticals, Merck, GlaxoSmithKline, and Sunovion; is a consultant for Dreem, Alairion, Orexia, and Inexia; and is on the speakers’ bureau for Vox Media. Giuseppe Plazzi has received consultancy fees from UCB Pharma, Jazz Pharmaceuticals, and Bioprojet. Yves Dauvilliers is a consultant for and has participated in advisory boards for Jazz Pharmaceuticals, UCB Pharma, Avadel Technologies, Idorsia, Takeda, Theranexus, and Bioprojet.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- CSF
cerebrospinal fluid
- DNS
disturbed nighttime sleep
- EDS
excessive daytime sleepiness
- HC
healthy control
- IH
idiopathic hypersomnia
- NSS
Narcolepsy Severity Scale
- NT1
narcolepsy type 1
- NT2
narcolepsy type 2
- NwC
narcolepsy with cataplexy
- NwoC
narcolepsy without cataplexy
- OSA
obstructive sleep apnea
- PLMS
periodic limb movements of sleep
- PSG
polysomnography
- RBD
REM sleep behavior disorder
- REM
rapid eye movement
- sHS
subjective hypersomnolence
- SSS
subjectively sleepy subjects
- SWS
slow-wave sleep
- SXB
sodium oxybate
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1. American Academy of Sleep Medicine. International Classification of Sleep Disorders . 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2. Ruoff C, Pizza F, Trotti LM, et al. The MSLT is repeatable in narcolepsy type 1 but not narcolepsy type 2: a retrospective patient study. J Clin Sleep Med . 2018; 14( 1): 65– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med . 2014; 15( 5): 502– 507. [DOI] [PubMed] [Google Scholar]
- 4. Guilleminault C, Dement WC, Passouant P. Narcolepsy. In: Proceedings of the First International Symposium on Narcolepsy , July 1975, Montpellier, France. New York: Spectrum Publishing; 1976. [Google Scholar]
- 5. Morgenthaler TI, Kapur VK, Brown T, et al. ; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep . 2007; 30( 12): 1705– 1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med . 2013; 9( 9): 955– 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pizza F, Peltola H, Sarkanen T, Moghadam KK, Plazzi G, Partinen M. Childhood narcolepsy with cataplexy: comparison between post-H1N1 vaccination and sporadic cases. Sleep Med . 2014; 15( 2): 262– 265. [DOI] [PubMed] [Google Scholar]
- 8. Dauvilliers Y, Beziat S, Pesenti C, et al. Measurement of narcolepsy symptoms: the Narcolepsy Severity Scale. Neurology . 2017; 88( 14): 1358– 1365. [DOI] [PubMed] [Google Scholar]
- 9. Dauvilliers Y, Barateau L, Lopez R, et al. Narcolepsy Severity Scale: a reliable tool assessing symptom severity and consequences. Sleep . 2020; 43( 6):zsaa009. [DOI] [PubMed] [Google Scholar]
- 10. Black J, Reaven NL, Funk SE, et al. Medical comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study. Sleep Med . 2017; 33: 13– 18. [DOI] [PubMed] [Google Scholar]
- 11. Dauvilliers Y, Jennum P, Plazzi G. Rapid eye movement sleep behavior disorder and rapid eye movement sleep without atonia in narcolepsy. Sleep Med . 2013; 14( 8): 775– 781. [DOI] [PubMed] [Google Scholar]
- 12. Bin-Hasan S, Videnovic A, Maski K. Nocturnal REM sleep without atonia is a diagnostic biomarker of pediatric narcolepsy. J Clin Sleep Med . 2018; 14( 2): 245– 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plazzi G, Ferri R, Franceschini C, et al. Periodic leg movements during sleep in narcoleptic patients with or without restless legs syndrome. J Sleep Res . 2012; 21( 2): 155– 162. [DOI] [PubMed] [Google Scholar]
- 14. Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med . 2013; 9( 8): 805– 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harsh J, Peszka J, Hartwig G, Mitler M. Night-time sleep and daytime sleepiness in narcolepsy. J Sleep Res . 2000; 9( 3): 309– 316. [DOI] [PubMed] [Google Scholar]
- 16. Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy—clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol . 2019; 15( 9): 519– 539. [DOI] [PubMed] [Google Scholar]
- 17. Cohen A, Mandrekar J, St Louis EK, Silber MH, Kotagal S. Comorbidities in a community sample of narcolepsy. Sleep Med . 2018; 43: 14– 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jennum P, Ibsen R, Knudsen S, Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep . 2013; 36( 6): 835– 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inocente CO, Lavault S, Lecendreux M, et al. Impact of obesity in children with narcolepsy. CNS Neurosci Ther . 2013; 19( 7): 521– 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry . 2017; 78( 2): 171– 176. [DOI] [PubMed] [Google Scholar]
- 21. Christensen JA, Carrillo O, Leary EB, et al. Sleep-stage transitions during polysomnographic recordings as diagnostic features of type 1 narcolepsy. Sleep Med . 2015; 16( 12): 1558– 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiménez-Correa U, Haro R, Obdulia González R, Velázquez-Moctezuma J. Correlations between subjective and objective features of nocturnal sleep and excessive diurnal sleepiness in patients with narcolepsy. Arq Neuropsiquiatr . 2009; 67( 4): 995– 1000. [DOI] [PubMed] [Google Scholar]
- 23. Khatami R, Landolt HP, Achermann P, et al. Insufficient non-REM sleep intensity in narcolepsy-cataplexy. Sleep . 2007; 30( 8): 980– 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khatami R, Landolt HP, Achermann P, et al. Challenging sleep homeostasis in narcolepsy-cataplexy: implications for non-REM and REM sleep regulation. Sleep . 2008; 31( 6): 859– 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mukai J, Uchida S, Miyazaki S, Nishihara K, Honda Y. Spectral analysis of all-night human sleep EEG in narcoleptic patients and normal subjects. J Sleep Res . 2003; 12( 1): 63– 71. [DOI] [PubMed] [Google Scholar]
- 26. Dauvilliers Y, Rompré S, Gagnon JF, Vendette M, Petit D, Montplaisir J. REM sleep characteristics in narcolepsy and REM sleep behavior disorder. Sleep . 2007; 30( 7): 844– 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frauscher B, Gschliesser V, Brandauer E, et al. Motor disturbances during non-REM and REM sleep in narcolepsy-cataplexy: a video-polysomnographic analysis. J Sleep Res . 2011; 20( 4): 514– 521. [DOI] [PubMed] [Google Scholar]
- 28. Antelmi E, Pizza F, Vandi S, et al. The spectrum of REM sleep-related episodes in children with type 1 narcolepsy. Brain . 2017; 140( 6): 1669– 1679. [DOI] [PubMed] [Google Scholar]
- 29. Walacik-Ufnal E, Piotrowska AJ, Wołyńczyk-Gmaj D, et al. Narcolepsy type 1 and hypersomnia associated with a psychiatric disorder show different slow wave activity dynamics. Acta Neurobiol Exp (Wars) . 2017; 77( 2): 147– 156. [DOI] [PubMed] [Google Scholar]
- 30. Vandi S, Rodolfi S, Pizza F, et al. Cardiovascular autonomic dysfunction, altered sleep architecture, and muscle overactivity during nocturnal sleep in pediatric patients with narcolepsy type 1. Sleep . 2019; 42( 12): zsz169. [DOI] [PubMed] [Google Scholar]
- 31. Pizza F, Vandi S, Iloti M, et al. Nocturnal sleep dynamics identify narcolepsy type 1. Sleep . 2015; 38( 8): 1277– 1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maski KP, Colclasure A, Little E, et al. Stability of nocturnal wake and sleep stages defines CNS disorders of hypersomnolence. Sleep . 2021. ; 44 ( 7 ):zsab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferri R, Pizza F, Vandi S, Iloti M, Plazzi G. Decreased sleep stage transition pattern complexity in narcolepsy type 1. Clin Neurophysiol . 2016; 127( 8): 2812– 2819. [DOI] [PubMed] [Google Scholar]
- 34. Takei Y, Komada Y, Namba K, et al. Differences in findings of nocturnal polysomnography and Multiple Sleep Latency Test between narcolepsy and idiopathic hypersomnia. Clin Neurophysiol . 2012; 123( 1): 137– 141. [DOI] [PubMed] [Google Scholar]
- 35. DelRosso LM, Chesson AL Jr, Hoque R. Characterization of REM sleep without atonia in patients with narcolepsy and idiopathic hypersomnia using AASM Scoring Manual criteria. J Clin Sleep Med . 2013; 9( 7): 675– 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sorensen GL, Knudsen S, Jennum P. Sleep transitions in hypocretin-deficient narcolepsy. Sleep . 2013; 36( 8): 1173– 1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sorensen GL, Knudsen S, Petersen ER, et al. Attenuated heart rate response is associated with hypocretin deficiency in patients with narcolepsy. Sleep . 2013; 36( 1): 91– 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hong SC, Hayduk R, Lim J, Mignot E. Clinical and polysomnographic features in DQB1*0602 positive and negative narcolepsy patients: results from the modafinil clinical trial. Sleep Med . 2000; 1( 1): 33– 39. [DOI] [PubMed] [Google Scholar]
- 39. Maski K, Pizza F, Liu S, et al. Defining disrupted nighttime sleep and assessing its diagnostic utility for pediatric narcolepsy type 1. Sleep . 2020; 43 ( 10):zsaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Zambotti M, Cellini N, Goldstone A, Colrain IM, Baker FC. Wearable sleep technology in clinical and research settings. Med Sci Sports Exerc . 2019; 51( 7): 1538– 1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev . 2011; 15( 4): 259– 267. [DOI] [PubMed] [Google Scholar]
- 42. Filardi M, Pizza F, Martoni M, Vandi S, Plazzi G, Natale V. Actigraphic assessment of sleep/wake behavior in central disorders of hypersomnolence. Sleep Med . 2015; 16( 1): 126– 130. [DOI] [PubMed] [Google Scholar]
- 43. Filardi M, Pizza F, Bruni O, Natale V, Plazzi G. Circadian rest-activity rhythm in pediatric type 1 narcolepsy. Sleep . 2016; 39( 6): 1241– 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leger D, Gauriau C, Tonetti L, et al. Using actigraphy to assess sleep and wake rhythms of narcolepsy type 1 patients: a comparison with primary insomniacs and healthy controls. Sleep Med . 2018; 52: 88– 91. [DOI] [PubMed] [Google Scholar]
- 45. Alakuijala A, Sarkanen T, Jokela T, Partinen M. Accuracy of actigraphy compared to concomitant ambulatory polysomnography in narcolepsy and other sleep disorders. Front Neurol . 2021; 12: 629709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bruck D, Parkes JD. A comparison of idiopathic hypersomnia and narcolepsy-cataplexy using self report measures and sleep diary data. J Neurol Neurosurg Psychiatry . 1996; 60( 5): 576– 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep . 2011; 34( 5): 601– 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep . 2006; 29( 9): 1155– 1173. [DOI] [PubMed] [Google Scholar]
- 49. Rosenthal LD, Merlotti L, Young DK, et al. Subjective and polysomnographic characteristics of patients diagnosed with narcolepsy. Gen Hosp Psychiatry . 1990; 12( 3): 191– 197. [DOI] [PubMed] [Google Scholar]
- 50. Roth T, Dauvilliers Y, Guinta D, Alvarez-Horine S, Dynin E, Black J. Effect of sodium oxybate on disrupted nighttime sleep in patients with narcolepsy. J Sleep Res . 2017; 26( 4): 407– 414. [DOI] [PubMed] [Google Scholar]
- 51. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci . 2001; 24( 12): 726– 731. [DOI] [PubMed] [Google Scholar]
- 52. Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron . 2010; 68( 6): 1023– 1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fulcher BD, Phillips AJ, Postnova S, Robinson PA. A physiologically based model of orexinergic stabilization of sleep and wake. PLoS One . 2014; 9( 3): e91982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell . 1999; 98( 4): 437– 451. [DOI] [PubMed] [Google Scholar]
- 55. Branch AF, Navidi W, Tabuchi S, et al. Progressive loss of the orexin neurons reveals dual effects on wakefulness. Sleep . 2016; 39( 2): 369– 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andlauer O, Moore H 4th, Hong SC, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep . 2012; 35( 9): 1247– 1255F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alakuijala A, Sarkanen T, Partinen M. Hypocretin-1 levels associate with fragmented sleep in patients with narcolepsy type 1. Sleep . 2016; 39( 5): 1047– 1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Knudsen S, Gammeltoft S, Jennum PJ. Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency. Brain . 2010; 133( Pt 2): 568– 579. [DOI] [PubMed] [Google Scholar]
- 59. Brink-Kjaer A, Christensen JAE, Cesari M, Mignot E, Sorensen HBD, Jennum P. Cortical arousal frequency is increased in narcolepsy type 1. Sleep . 2021; 44( 5): zsaa255. [DOI] [PubMed] [Google Scholar]
- 60. Barateau L, Lopez R, Chenini S, et al. Association of CSF orexin-A levels and nocturnal sleep stability in patients with hypersomnolence. Neurology . 2020; 95( 21): e2900– e2911. [DOI] [PubMed] [Google Scholar]
- 61. Herring WJ, Connor KM, Ivgy-May N, et al. Suvorexant in patients with insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry . 2016; 79( 2): 136– 148. [DOI] [PubMed] [Google Scholar]
- 62. Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain . 2013; 136( Pt 12): 3787– 3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Valko PO, Gavrilov YV, Yamamoto M, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol . 2013; 74( 6): 794– 804. [DOI] [PubMed] [Google Scholar]
- 64. Dauvilliers Y, Delallée N, Jaussent I, et al. Normal cerebrospinal fluid histamine and tele-methylhistamine levels in hypersomnia conditions. Sleep . 2012; 35( 10): 1359– 1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kanbayashi T, Kodama T, Kondo H, et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep . 2009; 32( 2): 181– 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thorpy MJ, Hiller G. The medical and economic burden of narcolepsy: implications for managed care. Am Health Drug Benefits . 2017; 10( 5): 233– 241. [PMC free article] [PubMed] [Google Scholar]
- 67. Maski K, Steinhart E, Williams D, et al. Listening to the patient voice in narcolepsy: diagnostic delay, disease burden, and treatment efficacy. J Clin Sleep Med . 2017; 13( 3): 419– 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep . 1991; 14( 6): 540– 545. [DOI] [PubMed] [Google Scholar]
- 69. Harsh JR, Hayduk R, Rosenberg R, et al. The efficacy and safety of armodafinil as treatment for adults with excessive sleepiness associated with narcolepsy. Curr Med Res Opin . 2006; 22( 4): 761– 774. [DOI] [PubMed] [Google Scholar]
- 70. Lecendreux M, Lavault S, Lopez R, et al. Attention-deficit/hyperactivity disorder (ADHD) symptoms in pediatric narcolepsy: a cross-sectional study. Sleep . 2015; 38( 8): 1285– 1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ohayon MM, Black J, Lai C, Eller M, Guinta D, Bhattacharyya A. Increased mortality in narcolepsy. Sleep . 2014; 37( 3): 439– 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dauvilliers Y, Jaussent I, Krams B, et al. Non-dipping blood pressure profile in narcolepsy with cataplexy. PLoS One . 2012; 7( 6): e38977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grimaldi D, Calandra-Buonaura G, Provini F, et al. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep . 2012; 35( 4): 519– 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension . 2007; 49( 6): 1235– 1241. [DOI] [PubMed] [Google Scholar]
- 75. Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens . 2002; 20( 11): 2183– 2189. [DOI] [PubMed] [Google Scholar]
- 76. Billiard M, Bassetti C, Dauvilliers Y, et al. ; EFNS Task Force. EFNS guidelines on management of narcolepsy. Eur J Neurol . 2006; 13( 10): 1035– 1048. [DOI] [PubMed] [Google Scholar]
- 77. Filardi M, Pizza F, Antelmi E, Pillastrini P, Natale V, Plazzi G. Physical activity and sleep/wake behavior, anthropometric, and metabolic profile in pediatric narcolepsy type 1. Front Neurol . 2018; 9: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mireku MO, Barker MM, Mutz J, et al. Night-time screen-based media device use and adolescents’ sleep and health-related quality of life. Environ Int . 2019; 124: 66– 78. [DOI] [PubMed] [Google Scholar]
- 79. Mullington J, Broughton R. Scheduled naps in the management of daytime sleepiness in narcolepsy-cataplexy. Sleep . 1993; 16( 5): 444– 456. [DOI] [PubMed] [Google Scholar]
- 80. Rogers AE, Aldrich MS. The effect of regularly scheduled naps on sleep attacks and excessive daytime sleepiness associated with narcolepsy. Nurs Res . 1993; 42( 2): 111– 117. [PubMed] [Google Scholar]
- 81. Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med . 2005; 1( 4): 391– 397. [PubMed] [Google Scholar]
- 82. Black J, Pardi D, Hornfeldt CS, Inhaber N. The nightly use of sodium oxybate is associated with a reduction in nocturnal sleep disruption: a double-blind, placebo-controlled study in patients with narcolepsy. J Clin Sleep Med . 2010; 6( 6): 596– 602. [PMC free article] [PubMed] [Google Scholar]
- 83. Black J, Houghton WC. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep . 2006; 29( 7): 939– 946. [DOI] [PubMed] [Google Scholar]
- 84. Black J, Pardi D, Hornfeldt CS, Inhaber N. The nightly administration of sodium oxybate results in significant reduction in the nocturnal sleep disruption of patients with narcolepsy. Sleep Med . 2009; 10( 8): 829– 835. [DOI] [PubMed] [Google Scholar]
- 85. Dauvilliers Y, Roth T, Guinta D, Alvarez-Horine S, Dynin E, Black J. Effect of sodium oxybate, modafinil, and their combination on disrupted nighttime sleep in narcolepsy. Sleep Med . 2017; 40: 53– 57. [DOI] [PubMed] [Google Scholar]
- 86. Filardi M, Pizza F, Antelmi E, Ferri R, Natale V, Plazzi G. In-field assessment of sodium oxybate effect in pediatric type 1 narcolepsy: an actigraphic study. Sleep . 2018; 41( 6): zxy050. [DOI] [PubMed] [Google Scholar]
- 87. Xyrem [prescribing information] (sodium oxybate), Palo Alto, CA: Jazz Pharmaceuticals. US. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021196s030lbl.pdf . Accessed September 14, 2021. [Google Scholar]
- 88. Morse AM, Kelly-Pieper K, Kothare SV. Management of excessive daytime sleepiness in narcolepsy with baclofen. Pediatr Neurol . 2019; 93: 39– 42. [DOI] [PubMed] [Google Scholar]
- 89. Huang YS, Guilleminault C. Narcolepsy: action of two gamma-aminobutyric acid type B agonists, baclofen and sodium oxybate. Pediatr Neurol. 2009 Jul;41(1):9–16 10.1016/j.pediatrneurol.2009.02.008 . PMID: 19520267. [DOI] [PubMed]
- 90. Vienne J, Lecciso G, Constantinescu I, et al. Differential effects of sodium oxybate and baclofen on EEG, sleep, neurobehavioral performance, and memory. Sleep . 2012; 35( 8): 1071– 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kansagra S, Walter R, Vaughn B. Nocturnal temazepam in the treatment of narcolepsy. J Clin Sleep Med . 2013; 9( 5): 499– 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Thorpy MJ, Snyder M, Aloe FS, Ledereich PS, Starz KE. Short-term triazolam use improves nocturnal sleep of narcoleptics. Sleep . 1992; 15( 3): 212– 216. [DOI] [PubMed] [Google Scholar]
- 93. Lammers GJ, Arends J, Declerck AC, Kamphuisen HA, Schouwink G, Troost J. Ritanserin, a 5-HT2 receptor blocker, as add-on treatment in narcolepsy. Sleep . 1991; 14( 2): 130– 132. [PubMed] [Google Scholar]
- 94. Mayer G. Ritanserin improves sleep quality in narcolepsy. Pharmacopsychiatry . 2003; 36( 4): 150– 155. [DOI] [PubMed] [Google Scholar]
- 95. Sansa G, Iranzo A, Santamaria J. Obstructive sleep apnea in narcolepsy. Sleep Med . 2010; 11( 1): 93– 95. [DOI] [PubMed] [Google Scholar]
- 96. Pataka AD, Frangulyan RR, Mackay TW, Douglas NJ, Riha RL. Narcolepsy and sleep-disordered breathing. Eur J Neurol . 2012; 19( 5): 696– 702. [DOI] [PubMed] [Google Scholar]
- 97. Kallweit U, Triller A. Effects of pitolisant on nighttime sleep. Sleep . 2019; 42( Suppl 1): A244. [Google Scholar]
- 98. Mitler MM, Hajdukovic R, Erman MK. Treatment of narcolepsy with methamphetamine. Sleep . 1993; 16( 4): 306– 317. [PMC free article] [PubMed] [Google Scholar]
- 99. Wolraich ML, Hagan JF Jr, Allan C, et al. ; Subcommittee on Children and Adolescents With Attention-Deficit/Hyperactive Disorder. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics . 2019; 144( 4): e20192528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kidwell KM, Van Dyk TR, Lundahl A, Nelson TD. Stimulant medications and sleep for youth with ADHD: a meta-analysis. Pediatrics . 2015; 136( 6): 1144– 1153. [DOI] [PubMed] [Google Scholar]
- 101. Christensen JA, Munk EG, Peppard PE, et al. The diagnostic value of power spectra analysis of the sleep electroencephalography in narcoleptic patients. Sleep Med . 2015; 16( 12): 1516– 1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Stephansen JB, Olesen AN, Olsen M, et al. Neural network analysis of sleep stages enables efficient diagnosis of narcolepsy. Nat Commun . 2018; 9( 1): 5229. [DOI] [PMC free article] [PubMed] [Google Scholar]