Abstract
Study Objectives:
The impact of the COVID-19 outbreak on sleep of participants with autism spectrum disorder (ASD) was assessed.
Methods:
Parents of 111 children and adolescents with ASD filled out an anonymous online survey shared via social media, investigating the sleep patterns and disturbances before and during the lockdown.
Results:
The lockdown changed significantly the bedtime on weekdays in 57.8% of participants with ASD (56.9% delayed; 0.9% advanced) and the rise time in 69.2% (61.7% delayed and 7.5% advanced). Sleep duration varied on weekdays in 49.1% (24.1% increased; 25% decreased). During the lockdown, participants with ASD showed a significant increase of sleep disturbances, compared to the preceding period, especially falling asleep (35.1% vs 22.5%), anxiety at bedtime (22.5% vs 10.8%), sleep terrors (5.4% vs 0%), and daytime sleepiness (14.4% vs 3.6%).
Conclusions:
Lockdown affected sleep of patients with ASD, showing an increase of difficulty in falling asleep, anxiety at bedtime, sleep terrors, and daytime sleepiness. The outbreak of COVID-19 significantly worsened sleep disturbances of children with ASD.
Citation:
Bruni O, Melegari MG, Breda M, et al. Impact of COVID-19 lockdown on sleep in children with autism spectrum disorders. J Clin Sleep Med. 2022;18(1):137–143.
Keywords: COVID-19, sleep patterns, sleep disturbances, autism
BRIEF SUMMARY
Current Knowledge/Study Rationale: COVID-19 experience has caused significant changes in the lifestyle of children and adolescents. Children with autism spectrum disorder are known to be characterized by a high vulnerability to routine changes.
Study Impact: Our findings underline how home confinement due to the COVID-19 pandemic affected the sleep schedule and increased sleep problems of children with autism spectrum disorder, reporting an increase of difficulty in falling asleep, anxiety at bedtime, sleep terrors, and daytime sleepiness. These symptoms probably reflect a psychological distress condition linked to extreme changes in routine and might further affect daytime functioning in children and adolescents with autism spectrum disorder.
INTRODUCTION
Several studies carried out during the last year all over the world have reported that the impact of COVID-19 experience has created dramatic psychological sequelae and caused significant changes in the lifestyle of children and adolescents and their parents. Although several specific guidelines were developed for managing children with neurodevelopmental disorders1,2 during the COVID-19 period, only a few studies have been conducted in children and adolescents with autism spectrum disorders (ASD). Children with ASD are known to be characterized by a high vulnerability to lifestyle changes and restrictions because of their difficulty to tolerate extreme changes in routine, such as those caused by the lockdown due to COVID-19.3,4 Again, social distancing measures and interruption of school activities and peer interactions represent risk factors for a worsening of ASD problems and for a lower chance to develop essential skills.4 It is well documented that the limitation of activities due to the restrictions have caused important changes in sleep-wake rhythms and sleep disturbances in a large percentage of the whole world population, independently by age and health conditions.5–7 Since children with autism face difficulties in understanding the complexity of the pandemic situation and in communicating their internal states, it is likely that they experience even higher distress linked to enforced restrictions, with variations of their sleep/wake patterns and sleep disturbance. A higher prevalence of sleep disorders in children with ASD compared to typically developing children has been reported by several previous publications, which have suggested that these disorders may not only worsen daytime behaviors and core symptoms of ASD but also contribute to increased parental stress levels.8–10 Therefore, exacerbated sleep disturbance during the lockdown could be an important hallmark of distress caused by conditions of restriction in this clinical population. Moreover, the detailed description of changes in sleep patterns caused by the lockdown in children with ASD, as well as in children with other neurodevelopmental disorders,11 might represent the optimal way to address sleep-related challenges in different clinical populations. A recent study showed that 44% of 87 participants with ASD (age range 3–29 years) changed their sleep with a significant worsening of sleep disturbance, sleep duration, and sleep quality.12 Similarly, another study from Turkey also found increased sleep disturbances in 46 children with ASD, such as more difficulties falling asleep, increased delay in falling asleep, and more night awakenings during lockdown, while no differences were found for sleep anxiety, parasomnias, sleep breathing disorders, and daytime sleepiness.13 Conversely, other authors did not report changes in sleep duration, but only 9 adolescents with ASD were included.14,15
Therefore, the aim of the study described here is to evaluate the impact of the lockdown on sleep patterns and sleep disturbances, with respect to the preceding conditions of each individual, in a relatively large sample of children and adolescents with ASD.
METHODS
Participants
Parents of children and adolescents with ASD completed an anonymous online survey, shared via social media, for a limited time window (from May 7 to June 15, 2020), targeting children aged 1 to 18 years followed and diagnosed by a child and adolescent psychiatrist of the Child and Adolescent Mental Health Services before the survey. The survey was developed and conducted following the guidelines set by the Checklist for Reporting Results of Internet E-Surveys (CHERRIES).16
There was no monetary or credit compensation for participating in the study. The study protocol was approved by the Ethics Committee of Sapienza University and was conducted in accordance with the Declaration of Helsinki.
The total sample of children and adolescents with ASD comprised 111 participants: 18 females (16.22%) and 93 males (83.78%). The age distribution was: 1–3 years: 11 participants (9.91%); 4–5 years: 24 participants (21.62%); 6–12 years: 47 participants (42.34%); 13–18 years: 29 participants (26.13%).
Measures
A specific questionnaire was arranged for the survey. The first section of the survey was devoted to the collection of demographic data (age, sex, caregiver education, region of Italy). A second section was organized to gather information on sleep arrangement and schedule during weekdays and during weekend (bedtime, rise time, sleep latency, sleep duration, napping, cosleeping). All these questions were asked to evaluate differences between before and during the lockdown period. A third section of the survey was related to family composition, work of parents during the lockdown, online lessons for children and adolescents, screen time excluding the hours for lessons, cosleeping, and use of over-the-counter or prescription drugs for sleep.
Caregivers completed a modified version of the Sleep Disturbance Scale for Children (SDSC).17
Retrospective questions were used to estimate perceived changes across 2 time periods: from “before the lockdown” (ie, in the last month before the outbreak) to “during the lockdown” (ie, in the 7 days prior to filling out the survey).
The SDSC was originally validated on a sample of 6-year-old to 16-year-old healthy children from the general population but was also used for younger children. We grouped questions related to sleep-disordered breathing into 1 question and selected, in total, 13 items to facilitate the compilation by parents.
A final specific question was related to factors that influenced the child’s sleep changes (ie, more time at home with the family, not waking up too early in the morning, more time available for social media use or video games or TV, decreased physical/sport activity).
Data analysis
Descriptive statistics were applied to characterize sociodemographic variables, sleep patterns, and sleep disturbances. Data were reported as frequencies and percentages. MacNemar chi-square tests were conducted to compare changes in sleep patterns, sleep schedule, and sleep disturbances before and during lockdown. For all comparisons, P values < .05 were considered to be statistically significant. Statistical analyses were performed using SPSS software release 17.0 (SPSS Inc., Chicago, IL).
RESULTS
Demographics of the sample are reported in Table 1. Of the 111 parents providing data on their child’s sleep habits, 100 (91.0%) were mothers. As for the education level, 100 (90.1%) of compilers had a graduate or high school degree. Most families (86; 77.5%) had a middle income.
Table 1.
Demographics of the sample (n = 111).
| Sex | Total |
|---|---|
| Female | 18 (16.22%) |
| Male | 93 (83.78%) |
| Region | |
| COVID-19 region* | 57 (51.35%) |
| No COVID-19 region | 54 (48.65%) |
| Compiler | |
| Mother | 101 (90.99%) |
| Father | 10 (9.01%) |
| Education level | |
| Graduation | 43 (38.74%) |
| High schools | 57 (51.35%) |
| Middle schools | 9 (8.11%) |
| Elementary schools | 2 (1.80%) |
| Family income | |
| Low | 21 (18.92%) |
| Middle | 86 (77.48%) |
| High | 4 (3.60%) |
| Siblings | |
| Only child | 44 (40.00%) |
| 2 children | 49 (44.55%) |
| 3 children | 15 (13.64%) |
| > 4 children | 2 (1.82%) |
Lombardy, Piedmont, Veneto, and Emilia Romagna.
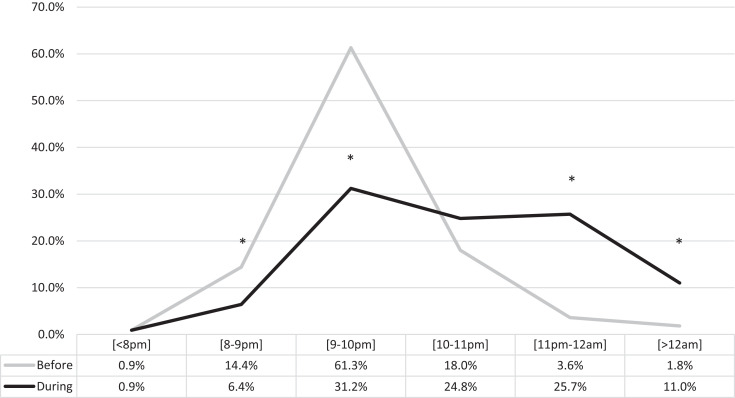
Children with autism changed their habitual sleep schedule: 57.8% of participants with ASD modified the bedtime on weekdays (56.9% delayed; 0.9% advanced), with a decrease in the percentage of children going to bed at 8:00–9:00 pm and 9:00–10:00 pm and an increase for the time slots 11:00 pm–12:00 am and > 12:00 am (Figure 1; Table S1 in the supplemental material).
Figure 1. Changes in bedtime on weekdays before and during lockdown.
*Significant at P < .05.
During the weekdays, the bedtime delay was evident in all age groups (Table S1a), with the majority of participants going to bed after 10:00 pm. Bedtime after 10:00 pm in the 1–3 years group was 9.1% before vs 44.4% during; in the 4–5 years group, 20.8% before vs 70.9% during; in the 6–12 years group, 8.5% before vs 53.2% during; in the 13–18 years group, 55.1% before vs 72.4% during.
On weekend, 49.1% of participants with ASD changed the bedtime (44.4% delayed and 4.6% advanced), with a decrease in the percentage of children going to bed at 9:00–10:00 pm and an increase for the time slots 11:00 pm–12:00 am and > 12:00 am (Table S1).
During the weekend, the delay in bedtime was evident in all age groups but less pronounced (Table S1a), with the majority of participants having bedtime after 10:00 pm. Weekend bedtime after 10:00 pm in the 1–3 years group was 27.3% before vs 66.5% during; in the 4–5 years group, 54.1% before vs 70.8% during; in the 6–12 years group, 45.6% before vs 76.5% during; in the 13–18 years group, 65.4% before vs 82.7%% during.
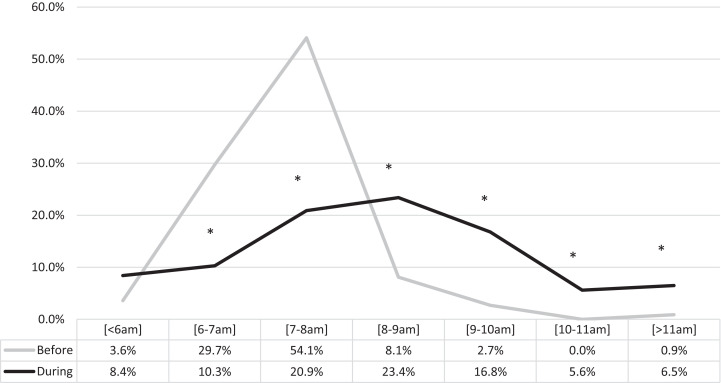
Rise time on weekdays changed in 69.2% of children (61.7% delayed and 7.5% advanced), with a decreased percentage of children waking up at 6:00–7:00 am and 7:00–8:00 am and an increased percentage of participants waking up at 8:00–9:00 am, 9:00–10:00 am, 10:00–11:00 am, and > 11:00 am (Figure 2 and Table S2). During the weekdays, the delay in rise time was evident in all age groups (Table S2a), with the majority of participants reporting rise time after 8:00 am. Rise time after 8:00 am in the 1–3 years group was 18.2% before vs 60% during; in the 4–5 years group, 16.7% before vs 69.2% during; in the 6–12 years group, 2.1% before vs 40.4% during; in the 13–18 years group, 20.6% before vs 55.5% during.
Figure 2. Changes in rise time on weekdays before and during lockdown.
*Significant at P < .05.
On weekends, rise time changed in 44.0% of children (31.2% delayed and 12.8% advanced), with a decrease for the time slots 7:00–8:00 am and 9:00–10:00 am and an increase at 8:00–9:00 am and > 11:00 am (Table S2). During the weekend, the delay in rise time was not particularly relevant in all age groups with the exception of the 6–12 years group (Table S2a). Rise time after 8:00 am in the 1–3 years group was 63.6% before vs 66.6% during; in the 4–5 years group, 41.6% before vs 66.6% during; in the 6–12 years group, 38.3% before vs 48.9% during; in the 13–18 years group, 58.5% before vs 62.0% during.
A change in sleep duration was observed on weekdays in 49.1% of children with ASD (24.1% increased; 25% decreased) with no significant difference, except for a decrease of children sleeping 7–8 hours per night. During the weekdays, sleep duration did not change significantly in all age groups (Table 2). Sleep duration less than 8 hours in the 1–3 years group was 27.3% before vs 30% during; in the 4–5 years group, 45.8% before vs 33.3% during; in the 6–12 years group, 39.1% before vs 38.3% during; in the 13–18 years group, 64.3% before vs 64.3% during (Table S3).
Table 2.
Changes in sleep duration before and during lockdown.
| < 6 h | 6–7 h | 7–8 h | 8–9 h | 9–10 h | 10–11 h | > 11 h | Total | |
| Weekdays | ||||||||
| Before | 0 | 12 (11.0%) | 38 (34.9%) | 32 (29.4%) | 18 (16.5%) | 9 (8.3%) | 0 | 109 |
| During | 4 (3.7%) | 19 (17.4%) | 24 (22.0%) | 29 (26.6%) | 24 (22.0%) | 7 (6.4%) | 2 (1.8%) | 109 |
| Weekend | ||||||||
| Before | 0 | 9 (8.1%) | 29 (26.1%) | 38 (34.2%) | 22 (19.8%) | 12 (10.8%) | 1 (0.9%) | 111 |
| During | 4 (3.7%) | 17 (15.6%) | 22 (20.2%) | 33 (30.3%) | 21 (19.3%) | 8 (7.3%) | 4 (3.7%) | 109 |
Significant differences at P < .05 are in bold.
Similarly, on weekends, sleep duration varied in 43.1% (24.8% decreased; 18.3% increased) with no significant difference, with the exception of an increase of children with ASD sleeping 6–7 hours per night (Table 2).
Similarly, during the weekend, sleep duration did not change significantly in all age groups (Table S3). Sleep duration less than 8 hours in the 1–3 years group was 18.2% before vs 33.3% during; in the 4–5 years group, 41.7% before vs 29.1% during; in the 6–12 years group, 23.4% before vs 36.2% during; in the 13–18 years group, 51.7% before vs 55.2% during. The 6–12 years age group showed a different trend with a tendency to increase their sleep duration on the weekend.
Regarding sleep latency, there was an increase in the percentage of participants who took 15–30 minutes, 30–60 minutes, and more than 60 minutes to fall asleep during weekdays and who took more than 60 minutes to fall asleep on weekends, paralleled by a decrease in the number of participants who took 5–15 minutes to fall asleep, during both weekdays and weekends (Table 3).
Table 3.
Changes in sleep latency before and during lockdown.
| 5–15 min | 15–30 min | 30–60 min | > 60 min | Total | |
| Weekdays | |||||
| Before | 33 (37.5%) | 30 (34.1%) | 20 (22.7%) | 5 (5.7%) | 88 |
| During | 16 (14.7%) | 34 (31.2%) | 37 (33.9%) | 22 (20.2%) | 109 |
| Weekend | |||||
| Before | 32 (28.8%) | 49 (44.1%) | 24 (21.6%) | 6 (5.4%) | 111 |
| During | 16 (14.5%) | 36 (32.7%) | 33 (30.0%) | 25 (22.7%) | 110 |
Significant differences at P < .05 are in bold.
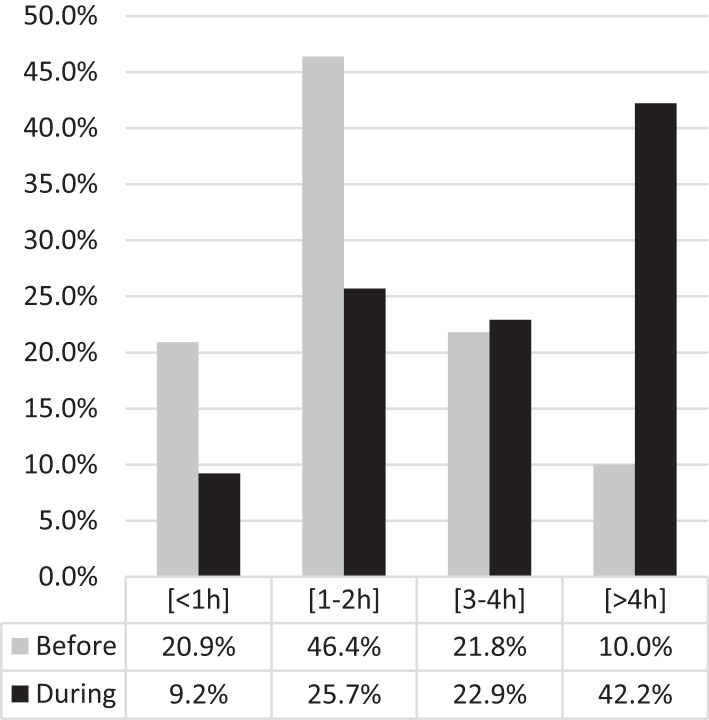
The amount of leisure screen time increased during the lockdown, with 42.2% of children with ASD spending more than 4 hours in activities requiring exposure to screens (excluding online lessons), compared to only 10.0% before. In parallel, there was a decrease in the number of those who spent less than 1 hour (20.9% before vs 9.2% during) and 1–2 hours (46.4% before vs 25.7% during) in leisure screen time (Figure 3 and Table S4). The analysis by age group showed that the increase of screen time was evident in all age groups, with high percentages of children using screens for more than 4 hours: 9.1% before vs 45.5% during, in the 1–3 years group; 8.7% before vs 47.8% during, in the 4–5 years group; 4.3% before vs 31.9% during, in the 6–12 years group; 20.7% before vs 55.2% during, in the 13–18 years group Table S4a.
Figure 3. Changes in screen time before and during lockdown.
During lockdown the amount of leisure screen time importantly increased in children with autism spectrum disorder.
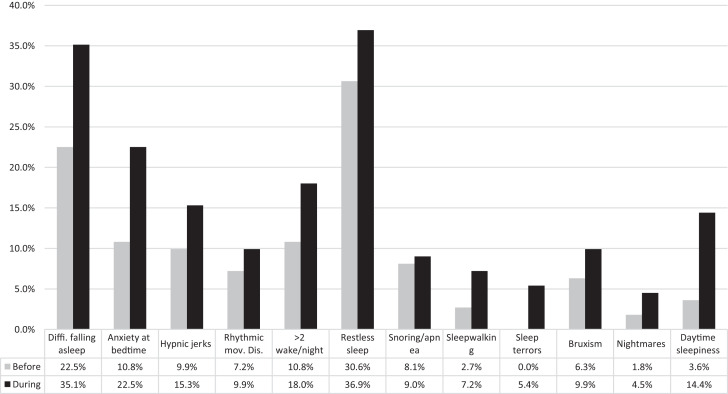
Based on the SDSC, during the lockdown, participants with ASD showed a significant (P < .05) increase of sleep disturbances compared to the preceding period, especially difficulty falling sleep (35.1% vs 22.5%), anxiety at bedtime (22.5% vs 10.8%), sleep terrors (5.4% vs 0%), and daytime sleepiness (14.4% vs 3.6%). No significant changes were found in the percentage of participants having hypnic jerks, rhythmic movement disorder, night awakenings, restless sleep, snoring/apnea, sleepwalking, bruxism, and nightmares (Figure 4 and Table S5).
Figure 4. Sleep disturbances before and during lockdown.
Lockdown affected sleep of patients with autism spectrum disorder showing an increase of difficulty in falling asleep, anxiety at bedtime, sleep terrors, and daytime sleepiness.
The analysis by age group showed that the increase of difficulties falling asleep was evident in all age groups, while the increase of anxiety at bedtime was relevant in the first age groups but not in adolescents. The increase of sleep terrors was evident in the 1–3 years age group but also in the 6–12 years group. Daytime sleepiness increased in all age groups (Table S5 and Table S5a).
Cosleeping did not change during lockdown in ASD children (32.4% before vs 30.6% during) as well as the use of over-the-counter or prescription drugs for sleep (23.4% before vs 24.3% during).
Parents also described the main factors that influenced the changes of their child’s sleep: 32.4% of parents reported lack of obliged rise time, 45.0% lack of sports activities, 22.5% more leisure screen time, 35.1% more time spent with family, and 4.5% change of routine. When analyzed by age group, no qualitative difference was found in the presence of these factors, although lack of obligated rise time (χ2 = 6.830; P = .033) was more present in children aged 6–12 years, whereas more time spent with family (χ2 = 7.800; P = .020), change in routine (χ2 = 6.682; P = .035), and lack of sports activities (χ2 = 6.096; P = .047) were found to be more frequent among adolescents. Finally, no differences were found between the age groups for leisure screen time.
DISCUSSION
Bedtime, rise time, and sleep duration on both weekdays and weekends changed significantly in children and adolescents with ASD during the COVID-19 lockdown. As reported in other studies in typically developing children6,7,18,19 and in adults,5 sleep was one of the domains mostly impaired by lockdown, independently by mental health condition and age.
There are very few studies on the influence of COVID-19 lockdown on sleep in participants with ASD. The worsening of sleep patterns and disturbance found in our study is supported by the results of Mutluer et al12 and Türkoğlu et al.13 However, while Mutluer et al12 reported that participants with ASD decreased their sleep duration, we did not find a significant decrease in sleep duration, in agreement with other authors.14 Regarding sleep disturbances, our results are in agreement with the Turkish study in 46 children with ASD showing an increase in difficulties falling asleep and delay in falling asleep; in contrast, we did not find an increase of falling asleep anxiety and night awakenings, but we observed an increase of sleep terrors and daytime sleepiness that was not reported earlier. Data, however, are not completely comparable, since the Turkish study used the scores of the CSHQ and not the frequencies.
Based on parental reports, the limitations due to the COVID-19 restrictions, such as lack of obligations, critical reduction of leisure and sport activities, and consequently increased screen exposure time, mostly affected weekday sleep habits, in agreement with previous studies in children and adolescents.4,6,7
In children and teenagers with autism, the reason for the disruption of circadian rhythms might be connected with the extreme importance of routine in their everyday life; even very minor disruptions to a routine can cause a child diagnosed with autism to feel distress and confusion and affect sleep-wake pattern; in addition, these children have difficulties in coping with unexpected changes in routine.
A nonnegligible role in the changes of sleep habits might be played by the screen-exposure time. A significant increase in daily screen exposure was reported in our children with ASD, who spent more than 4 hours/day (excluding online lessons) in screen activities. Several reports, conducted in both children and adults from the general population,5,20,21 highlighted that the delay of bedtime and increase of sleep latency are positively related to screen time exposure at night.
Several studies showed increased levels of stress, anxiety, and life disruption in individuals with ASD and their caregivers during the COVID-19 pandemic;22–24 these issues can represent additional challenges for individuals to cope with during the COVID-19 pandemic and may play a role in sleep habits disruption.25
It is relevant to note that very few differences have been found between age groups with respect to the changes of bedtime, rise time, sleep duration, and sleep latency, as well as the use of screen before and during lockdown.
Increased disordered sleep symptoms probably reflect a psychological distress condition linked to extreme and unprecedented changes in routine and lifestyles of children with ASD. Our study showed a significant increase after the disruption of life habits due to the pandemic of children with ASD having difficulties falling asleep, anxiety at bedtime, sleep terrors, and daytime sleepiness, although no significant changes in the incidence of nightmares was found. It is probable that, due to their limitation in communication skills, children with ASD are not able to report nightmares and these might be misinterpreted by parents.
The analysis of sleep disturbances by age confirmed the age-related presentation of symptoms with anxiety at bedtime in the first age groups and sleep terrors that were more prevalent (and increased during lockdown) in the 1–3 years group. Interestingly, an increase of daytime sleepiness was evident in all age groups, probably reflecting the impact of lockdown on sleep quality.
Several limitations of this study need to be considered. The main limitations are the sample size (although relatively large for this condition) and sampling only respondents from a single country; thus, results may not be fully generalizable to other countries. Furthermore, most self-reported elements of the SDSC should be interpreted with caution due to potential parental misinterpretation in children with ASD, who are often nonverbal. Moreover, we did not screen family members for psychopathology and we were unable to do postconfinement follow-up with participants. Finally, we should consider that self-selection bias is inherent with the online survey methodology employing nonprobability sampling. Nevertheless, the accurate analyses of changes before and during lockdown in each patient with ASD support the reliability of the results.
In conclusion, home confinement due to the COVID-19 pandemic affected the sleep schedule and increased sleep problems of children with ASD that commonly worsen internalizing (social withdrawal, anxiety, and depression) and externalizing symptoms (hyperactivity, aggression, and irritability).26,27 Therefore, in the continuing conditions of home confinement and social isolation caused by the COVID-19 pandemic, it is imperative to treat sleep problems in children with ASD to improve the quality of life of these children and their families.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Sapienza University. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Oliviero Bruni: conceptualization and study design, data analysis, data interpretation; writing and revision of the manuscript; approved the final manuscript as submitted. Maria Grazia Melegari: conceptualization and study design; data analysis, data collection and interpretation; writing and revision of the manuscript; approved the final manuscript as submitted. Maria Breda: data analysis and interpretation; writing and revision of the manuscript; approved the final manuscript as submitted. Alessandra Cedrone: data analysis and interpretation; writing and revision of the manuscript; approved the final manuscript as submitted. Elena Finotti: conceptualization and study design, data collection; revision of the manuscript; approved the final manuscript as submitted. Emanuela Malorgio: conceptualization and study design, data collection; revision of the manuscript; approved the final manuscript as submitted. Mattia Doria: data collection; revision of the manuscript; approved the final manuscript as submitted. Raffaele Ferri: conceptualization and study design, revision of the manuscript; approved the final manuscript as submitted.
ABBREVIATIONS
- ASD
autism spectrum disorder
- SDSC
Sleep Disturbance Scale for Children
REFERENCES
- 1. Cortese S, Asherson P, Sonuga-Barke E, et al. European ADHD Guidelines Group. ADHD management during the COVID-19 pandemic: guidance from the European ADHD Guidelines Group. Lancet Child Adolesc Health. 2020; 4( 6): 412– 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UNICEF. Children with autism and COVID-19. Tips for establishing a daily routine during the coronavirus epidemic. https://www.unicef.org/serbia/en/children-autism-and-covid-19. Published 2020. Accessed August 2, 2021.
- 3. Bellomo TR, Prasad S, Munzer T, Laventhal N. The impact of the COVID-19 pandemic on children with autism spectrum disorders. J Pediatr Rehabil Med. 2020; 13( 3): 349– 354. [DOI] [PubMed] [Google Scholar]
- 4. Lee J. Mental health effects of school closures during COVID-19. Lancet Child Adolesc Health. 2020; 4( 6): 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cellini N, Canale N, Mioni G, Costa S. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J Sleep Res. 2020; 29( 4): e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moore SA, Faulkner G, Rhodes RE, et al. Impact of the COVID-19 virus outbreak on movement and play behaviours of Canadian children and youth: a national survey. Int J Behav Nutr Phys Act. 2020; 17( 1): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pietrobelli A, Pecoraro L, Ferruzzi A, et al. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity (Silver Spring). 2020; 28( 8): 1382– 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, and possible biopsychosocial aetiologies. Sleep Med Rev. 2009; 13( 6): 403– 411. [DOI] [PubMed] [Google Scholar]
- 9. Schreck KA, Mulick JA, Smith AF. Sleep problems as possible predictors of intensified symptoms of autism. Res Dev Disabil. 2004; 25( 1): 57– 66. [DOI] [PubMed] [Google Scholar]
- 10. Singh K, Zimmerman AW. Sleep in autism spectrum disorder and attention deficit hyperactivity disorder. Semin Pediatr Neurol. 2015; 22( 2): 113– 125. [DOI] [PubMed] [Google Scholar]
- 11. Bruni O, Giallonardo M, Sacco R, Ferri R, Melegari MG. The impact of lockdown on sleep patterns of children and adolescents with ADHD. J Clin Sleep Med. 2021; 17( 9): 1759– 1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mutluer T, Doenyas C, Aslan Genc H. Behavioral implications of the Covid-19 process for autism spectrum disorder, and individuals’ comprehension of and reactions to the pandemic conditions. Front Psychiatry. 2020; 11: 561882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Türkoğlu S, Uçar HN, Çetin FH, Güler HA, Tezcan ME. The relationship between chronotype, sleep, and autism symptom severity in children with ASD in COVID-19 home confinement period. Chronobiol Int. 2020; 37( 8): 1207– 1213. [DOI] [PubMed] [Google Scholar]
- 14. Garcia JM, Lawrence S, Brazendale K, Leahy N, Fukuda D. Brief report: the impact of the COVID-19 pandemic on health behaviors in adolescents with Autism Spectrum Disorder. Disabil Health J. 2021; 14( 2): 101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000; 23( 8): 1043– 1051. [PubMed] [Google Scholar]
- 16. Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res. 2004; 6( 3): e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996; 5( 4): 251– 261. [DOI] [PubMed] [Google Scholar]
- 18. Bruni O, Malorgio E, Doria M, et al. Changes in sleep patterns and disturbances in children and adolescents in Italy during the Covid-19 outbreak [published online ahead of print, 2021 February 9]. Sleep Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Z, Tang H, Jin Q, et al. Sleep of preschoolers during the coronavirus disease 2019 (COVID-19) outbreak. J Sleep Res. 2021; 30( 1): e13142. [DOI] [PubMed] [Google Scholar]
- 20. Guan H, Okely AD, Aguilar-Farias N, et al. Promoting healthy movement behaviours among children during the COVID-19 pandemic. Lancet Child Adolesc Health. 2020; 4( 6): 416– 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiang M, Zhang Z, Kuwahara K. Impact of COVID-19 pandemic on children and adolescents’ lifestyle behavior larger than expected. Prog Cardiovasc Dis. 2020; 63( 4): 531– 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manning J, Billian J, Matson J, Allen C, Soares N. Perceptions of families of individuals with autism spectrum disorder during the COVID-19 crisis. J Autism Dev Disord. 2021; 51( 8): 2920– 2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asbury K, Fox L, Deniz E, Code A, Toseeb U. How is COVID-19 affecting the mental health of children with special educational needs and disabilities and their families? J Autism Dev Disord. 2021; 51( 5): 1772– 1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amorim R, Catarino S, Miragaia P, Ferreras C, Viana V, Guardiano M. The impact of COVID-19 on children with autism spectrum disorder. Rev Neurol. 2020; 71( 8): 285– 291. [DOI] [PubMed] [Google Scholar]
- 25. Eshraghi AA, Li C, Alessandri M, et al. COVID-19: overcoming the challenges faced by individuals with autism and their families. Lancet Psychiatry. 2020; 7( 6): 481– 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henderson JA, Barry TD, Bader SH, Jordan SS. The relation among sleep, routines, and externalizing behavior in children with an autism spectrum disorder. Res Autism Spectr Disord. 2011; 5( 2): 758– 767. [Google Scholar]
- 27. Johnson CR, Smith T, DeMand A, et al. Exploring sleep quality of young children with autism spectrum disorder and disruptive behaviors. Sleep Med. 2018; 44: 61– 66. [DOI] [PMC free article] [PubMed] [Google Scholar]