Abstract
Study Objectives:
To assess the impact of coronavirus disease 2019 (COVID-19)–related restrictions on narcolepsy type 1 (NT2), narcolepsy type 2 (NT2), and idiopathic hypersomnia (IH).
Methods:
Participants with NT1, NT2, and IH followed in a university hospital completed an online 78-question survey assessing demographic, clinical, and occupational features of the population during the first COVID-19–related lockdown.
Results:
A total of 219 of 851 (25.7%) respondents of the survey reported a mean increase of 1.2 ± 1.9 hours (P < .001) in night sleep time and a mean decrease of 1.0 ± 3.4 points (P < .001) on the Epworth Sleepiness Scale during lockdown. Bedtime was delayed by 46.1% of participants and wakeup time was delayed by 59.6%, driven primarily by participants with IH. Teleworkers (but not in-person workers) reported a mean increase of 0.9 ± 1.2 hours in night sleep (P < .001) and a mean decrease in sleepiness score of 1.6 ± 3.1 (P < .001). Cataplexy improved in 54.1% of participants with NT1. Sleepiness correlated with psychological wellness (r = .3, P < .001). As many as 42.5% enjoyed the lockdown, thanks to reallocation of time usually spent commuting toward longer sleep time, hobbies, and family time, and appreciated a freer napping schedule. Conversely, 13.2% disliked the lockdown, feeling isolation and psychological distress.
Conclusions:
Extended sleep time, circadian delay (in patients with IH), and teleworking resulted in decreased symptoms of central hypersomnias. These findings suggest that people with IH, NT1, and NT2 may benefit from a decrease in social and professional constraints on sleep-wake habits, and support advocacy efforts aimed at facilitating workplace and schedule accommodations for this population.
Citation:
Nigam M, Hippolyte A, Dodet P, et al. Sleeping through a pandemic: impact of COVID-19–related restrictions on narcolepsy and idiopathic hypersomnia. J Clin Sleep Med. 2022;18(1):255–263.
Keywords: narcolepsy, hypersomnia, COVID-19, SARS-CoV-2, lockdown, confinement, teleworking
BRIEF SUMMARY
Current Knowledge/Study Rationale: The first lockdown during the COVID-19 pandemic constituted a real-world experiment on the impact of modifications of social and occupational constraints on sleep. We evaluated the impact of these changes via a survey in participants with narcolepsy and idiopathic hypersomnia.
Study Impact: Participants reported a freer napping schedule, circadian realignment, decreased commuting time, increased night sleep time and decreased sleepiness (particularly in teleworkers), decreased cataplexy, increased quality time, and improved well-being. These results advocate for more frequent teleworking days and workplace scheduling accommodations in patients with central hypersomnias.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) worldwide pandemic has forced countries around the world to adopt stringent measures aimed at curbing the spread of the highly infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. France was first put under such a lockdown from March 17 to May 11, 2020. Specific measures put in place included prohibitions on traveling, outdoor activities, and in-person schooling and working. Work-from-home (teleworking) was mandated for all but essential workers (eg, health care workers, grocers, and transportation workers).1 Much interest has been generated within the sleep medicine community on the effects of the COVID-19 pandemic and associated lockdown measures on the sleep health of the general public and many have reported increased insomnia and circadian rhythm changes.2–4
The experience of people living with central hypersomnias during COVID-19–related restrictions is of particular interest. Narcolepsy is characterized by excessive daytime sleepiness, short and refreshing naps, and rapid eye movement (REM) sleep state dissociation. Narcolepsy type 1 (NT1) is further distinguished from narcolepsy type 2 (NT2) by cataplexy and hypocretin deficiency. Idiopathic hypersomnia (IH) is characterized by sleepiness despite normal or prolonged sleep time, long non-refreshing naps, and sleep drunkenness. A key difficulty faced by this population lies in the reconciliation of their particular sleep needs (eg, increased total sleep time or frequent napping) with their daytime obligations (eg, work, schooling, etc). This conflict accounts for a worsened quality of life and may contribute to excessive daytime sleepiness.5–7 We seized upon the opportunity of lockdown to understand how changes in social and professional constraints on sleep-wake habits affect people with central hypersomnias, using a survey sent to patients followed in our academic unit.
METHODS
Participants
Eligible participants were patients diagnosed with NT1, NT2, or IH as per International Classification of Sleep Disorders, third edition, criteria8 and followed at the National Reference Center for Narcolepsy and Rare Hypersomnias at Pitié-Salpétrière Hospital’s Sleep Disorders Unit. Approximately 1600 such patients are followed at our center, of whom approximately one-half have given their email address (at the time of this study) for participation in research projects. Participants who responded to an email containing the survey were included in the study. The sole exclusion criterion was noncompletion of the survey. All participants gave consent for use of their responses in the present publication, in accordance with French research ethics laws.
Questionnaire
A 78-question survey was devised by a team of sleep medicine experts using the SurveyMonkey software (SurveyMonkey Inc, San Mateo, CA). The questionnaire assessed participants’ demographic features, occupational status, COVID-19 status, sleep habits, hypersomnia symptoms, Epworth Sleepiness Scale (ESS) score, treatments, and any changes to the above that occurred as a result of the COVID-19–related lockdown measures in France. The complete questionnaire in its original French and translated into English is available in the supplemental material. Changes in sleepiness were assessed by asking participants to first complete an ESS questionnaire retrospectively assessing their pre-lockdown somnolence (ESS scores just preceding the lockdown were not available in most participants’ charts), followed by an ESS questionnaire assessing current somnolence. Similarly, participants were asked to first estimate their night sleep time prior to lockdown, followed by a report of their contemporary night sleep time. The majority of questions contained in the survey were closed, multiple-choice or yes/no questions (eg, “Was “x” increased, decreased or unchanged during lockdown?), assessing changes in sleep-related symptoms and habits, psychological symptoms, and general perceptions related to the lockdown. The questionnaire concluded with 2 open-ended questions pertaining to participants’ global appreciation of the lockdown: “If you liked/disliked the lockdown, why/why not?” The survey was sent to prospective participants 1 week prior to the end of the first COVID-19–related lockdown in May 2020 and was available for completion during a 6-day window.
Statistical analysis
Significance was set at P < .05. All statistical analyses were computed using R version 4.0.3 (2020) from the R Foundation for Statistical Computing (Vienna, Austria). Three group comparisons of quantitative variables were performed using one-way analysis of variance. Three group comparisons of binary categorical variables were performed using chi-square (presented as χ2, degrees of freedom [df], P) or Fisher’s exact test (presented as odd ratio, 95% confidence interval, P), depending on sample size. Ordinal categorical variables were assessed using ordinal logistic regression. Comparisons of teleworkers to in-person workers and within-group comparisons for measures assessed before and during lockdown were performed using Student’s t test. Correction for multiple testing was done using Bonferroni’s adjustments (obtained P values were multiplied by the number of comparisons done for a given hypothesis test).
Using the “FactoMineR” package in R, multiple correspondence analysis (MCA) was performed as an exploratory technique to assess additional relationships between qualitive measures related to psychological well-being and sleep quality. MCA permits the representation of a large number of qualitative measures graphically in multidimensional space and identifies those dimensional axes that explain the largest variability within the dataset (percentage of inertia). Further assessment was performed to determine which qualitative measures were the principal determinants of the identified axes (measures with R2 score ≥ 0.3 were considered important). Finally, supplemental quantitative and qualitative variables expected to relate to these dimensions (telework, changes in sleep time, ESS, and others) were assessed for correlation to the principal dimensional axes.
Inductive thematic analysis
In order to extract meaningful information from participants’ open-ended responses to the questions specified above, inductive thematic analysis was employed. This subtype of reflexive thematic analysis is a common methodology in qualitative research used to identify patterns of meaning across a dataset (ie, open-ended responses, texts, speeches). This is achieved through a structured process of data familiarization, coding of data extracts, development, and naming of themes and revision. Inductive thematic analysis differs from deductive thematic analysis in that codes are induced based on the data rather than predetermined based on the author’s research questions. In the present study, inductive thematic analysis was performed using the step-by-step framework set forth by Braun and Clarke.9 Data familiarization was first achieved by repetitive reading of participants’ responses. Codes were then induced from the data extracts by recognizing patterns of semantic (explicit) and latent (implicit) meaning (see Table S1 in the supplemental material for examples of the coding process). Coding was done manually, without the use of specialized software. Using a conceptual map, codes were then grouped together into encompassing themes. Themes were then reviewed and named during a group discussion with all coauthors until all were in agreement. Representative data extracts (participant citations) are presented to justify and support the identified themes. Participant responses were translated from French to English by the first author of this paper, a bilingual native English speaker.
RESULTS
Participants
The questionnaire was sent to 851 participants with NT1, NT2 and IH. A total of 225 responded to the survey (return rate: 26.5%). Among participants with IH, 77/88 (87.5%) had a phenotype with long sleep time. The mean time to completion of the survey was 24 minutes and 11 seconds. Six participants were excluded for incomplete surveys and thus 219 participants were included in the final analysis. Table 1 summarizes the main clinical and demographic features of our participants grouped by hypersomnia type. Of the respondents, 73 had NT1, 58 had NT2, and 88 had IH. There were no between-group differences, with the exception of sex (women being more common in the IH group compared with NT1, but not NT2).
Table 1.
Demographic, occupational and clinical features of participants with NT1, NT2, and IH.
| Participants | Total (n = 219) | NT1 (n = 73) | NT2 (n = 58) | IH (n = 88) |
|---|---|---|---|---|
| Age, mean (SD), y | 37.0 (13.2) | 37.5 (14.1) | 40.2 (14.6) | 34.8 (11.1) |
| Sex female | 70.8 (155) | 58.7 (44) | 66.1 (39) | 81.8 (72)* |
| Age at diagnosis, mean ± SD, y | 28.1 ± 11.2 | 25.9 ± 11.9 | 28.7 ± 10.9 | 29.8 ± 10.7 |
| Disease course, mean ± SD, y | 18.8 ± 13.0 | 18.0 ± 11.8 | 22.1 ± 15.6 | 17.7 ± 12.8 |
| Employment status at the beginning of lockdown | ||||
| Full-time worker | 48.4 (106) | 43.8 (32) | 43.1 (25) | 55.7 (49) |
| Part-time worker | 14.6 (32) | 12.3 (9) | 17.2 (10) | 14.8 (13) |
| Student | 12.8 (28) | 16.4 (12) | 12.3 (9) | 8.0 (7) |
| Unemployed | 24.2 (53) | 27.4 (20) | 24.1 (14) | 21.6 (19) |
| Commuting time, mean (SD), min | 66.1 (50.3) | 58.4 (42.6) | 67.6 (50.0) | 70.5 (57.5) |
| Use of stimulants | 79.7 (175) | 86.7 (65) | 83.1 (49) | 70.50 (62) |
| Stimulants | ||||
| Modafinil | 38.8 (85) | 45.2 (33) | 36.2 (21) | 35.2 (31) |
| Methylphenidate | 28.3 (62) | 30.1 (22) | 24.1 (14) | 29.5 (26) |
| Pitolisant | 20.5 (45) | 20.5 (15) | 24.1 (14) | 18.2 (16) |
| Sodium oxybate | 6.4 (14) | 13.7 (10) | 5.2 (3) | 1.1 (1) |
| Dextroamphetamine | 1.8 (4) | 2.7 (2) | 1.7 (1) | 1.1 (1) |
| Confirmed COVID-19 | 6.8 (15) | 4.1 (3) | 5.2 (3) | 10.2 (9) |
Data are displayed as % (n) unless otherwise indicated. *More women were present in the IH group compared with NT1 (P < .008). COVID-19 = coronavirus disease 2019, IH = idiopathic hypersomnia, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2.
Impact of lockdown measures
The impact of lockdown measures on nighttime sleep habits and symptoms is displayed in Table 2. Night sleep time increased in all groups, and no difference was observed between hypersomnia subgroups. Insomnia was reported at some point during lockdown by 51.8% of participants. Participants with NT1 were more likely to report insomnia than those with IH (χ2 = 5.8, df = 1, P = .004), but not NT2 (χ2 = 0.8, df = 1, P = 1). Overall, 46.1% of participants reported delaying bedtime and 59.6% reported delaying wakeup time. This trend toward sleep phase delay was driven by participants with IH, who were more likely to delay both bedtime and wakeup time compared with participants with NT1 (P < .01 and P < .01 for bedtime and wakeup time, respectively) and NT2 (P < .001, P < .01).
Table 2.
Nighttime sleep habits and symptoms during lockdown.
| Total | NT1 | NT2 | IH | Pa | |
|---|---|---|---|---|---|
| Mean (SD) night sleep time, h | |||||
| Pre-lockdown | 8.3 (1.4) | 7.8 (1.7) | 7.9 (1.7) | 8.3 (1.4) | .3 |
| Lockdown | 9.1 (2.1) | 8.5 (1.8) | 8.5 (2.4) | 9.6 (2.1) | |
| Δ Sleep time | 1.2 (1.9) | 0.9 (0.7) | 0.7 (1.7) | 1.3 (1.9) | |
| Pb | < .001 | .001 | .004 | < .001 | |
| Insomnia during lockdown | 51.8 (113) | 60.0 (45) | 50.8 (30) | 43.2 (38) | .04 |
| Change in bedtime during lockdown | |||||
| Later | 47.6 (101) | 37.5 (27) | 39.3 (22) | 61.9 (52) | .002 |
| No change | 39.1 (83) | 47.2 (34) | 39.3 (22) | 32.1 (27) | |
| Earlier | 13.2 (28) | 15.3 (11) | 21.4 (12) | 6.0 (5) | |
| Change in wakeup time during lockdown | |||||
| Later | 63.1 (130) | 46.5 (33) | 60.0 (33) | 80.0 (64) | < .001 |
| No change | 25.2 (52) | 36.6 (26) | 21.8 (12) | 17.5 (14) | |
| Earlier | 11.7 (24) | 16.9 (12) | 18.2 (10) | 2.5 (2) |
Data are displayed as % (n) unless otherwise indicated. aSignificance testing for between-group comparisons. bSignificance testing for within-group comparison before and during lockdown. IH = idiopathic hypersomnia, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2.
Changes in daytime symptoms, fatigue, concentration, naps, sleep attacks (sudden-onset lapses into sleep with little warning), and stimulant dosages during the lockdown are shown in Table 3. Participants reported a mean decrease in ESS score of 1 ± 3.4, with no difference between hypersomnia subgroups (P = .6). Within-group comparisons showed that this ESS decrease was significant in the IH and NT2 groups, and tended to be significant in the NT1 group (P = .08). Fifty-five (25.1%) participants reported a > 3-point decrease in ESS. On average, 32% of participants reported less fatigue, 27.3% reported better concentration, and 30.1% reported a decrease in sleep attack frequency, despite the fact that 35.7% had decreased their dosage stimulants. There were no further between-group differences in fatigue, concentration, number of naps per day, sleep attacks, or stimulant dose adjustments.
Table 3.
Daytime sleep habits and symptoms during lockdown.
| Total (n = 219) | NT1 (n = 73) | NT2 (n = 58) | IH (n = 88) | |
|---|---|---|---|---|
| Mean (SD) ESS | ||||
| Before lockdown | 14.4 (4.8) | 15.5 (4.8) | 14.3 (4.9) | 13.4 (4.5) |
| During lockdown | 13.4 (5.3) | 14.7 (5.6) | 12.9 (5.2) | 12.5 (4.8) |
| Δ ESS | −1.0 (3.4) | −0.8 (3.3) | −1.4 (3.8) | −0.9 (2.9) |
| Pa | <.001 | .08 | <.001 | <.01 |
| Less fatigue | 32.0 (70) | 37.0 (27) | 25.9 (15) | 31.8 (28) |
| Better concentration | 27.3 (60) | 23.3 (17) | 24.1 (14) | 33.0 (29) |
| Naps per day, mean (SD) | 1.2 (1.0) | 0.8 (1.1) | 1.4 (1.0) | 1.1 (0.6) |
| Sleep attack frequency | ||||
| Decreased | 30.1 (66) | 38.4 (28) | 31.0 (18) | 22.7 (20) |
| Unchanged | 54.3 (119) | 42.5 (31) | 62.1 (36) | 59.1 (52) |
| Increased | 15.5 (34) | 19.1 (14) | 7.9 (4) | 18.2 (16) |
| Stimulant dose adjustments | ||||
| Reduced dose | 35.7 (66) | 29.6 (21) | 36.9 (24) | 42.9 (21) |
| Same dose | 61.6 (114) | 63.4 (45) | 63.1 (41) | 57.1 (28) |
| Increased dose | 2.7 (5) | 7.0 (5) | 0 | 0 |
Data are displayed as % (n) unless otherwise indicated. aSignificance testing for within-group comparison before and during lockdown. ESS = Epworth Sleepiness Scale, IH = idiopathic hypersomnia, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2.
Among the 61 participants with NT1 who typically had cataplexy pre-lockdown, 54.1% reported a decrease in or disappearance of cataplexy, while 14.8% reported increased cataplexy and 31.1% reported no change. Sleep-related hallucinations decreased in 35.0% of participants with NT1, increased in 10.7%, and were unchanged in 54.3%. In participants with NT2, 34.5% experienced a decrease in sleep-related hallucinations, 1.7% reported an increase, and 63.8% reported no change. In participants with IH, 71.6% reported difficulty getting out of bed and 22.7% did not during lockdown. However, this percentage was not contrasted with pre-lockdown measures.
Measures of psychological wellness changes during lockdown are shown in Table S2. Although mood, irritability, and stress worsened and improved similarly in participants, 42.5% of them appreciated the lockdown period, 13.2% disliked this period, and the remaining 44.3% had a neutral opinion. There were no further group differences in mood changes, stress level, irritability, and overall appreciation of the lockdown.
Comparisons of teleworkers to in-person workers
At the beginning of the lockdown, 138 participants were employed on a full- or part-time basis. Of those, 74 (53.6%) transitioned to teleworking, while 24 (17.4%) continued to work at their usual workplace (in-person workers), and the remaining 40 (29.0%) were placed on temporary paid leave as a result of pandemic-related social assistance protecting citizens from loss of employment. Table 4 summarizes the differences between teleworkers and in-person workers. Hypersomnia subtypes were present in similar proportions in each group. There were more white-collar workers among teleworkers (odds ratio: 6.2; 95% confidence interval: 1.5–28.4). As expected, commuting time (total duration per day) changed to 0 in teleworkers and was probably unchanged in in-person workers (we only asked for usual commuting time). There was no difference for typical workday start time between teleworkers and in-person workers. Within-group testing revealed a prolongation of night sleep time and a decrease in ESS scores (on average, −1.6) during lockdown among teleworkers, but not in-person workers. Between-group comparison revealed a trend among teleworkers toward larger improvements in ESS scores and greater likelihood to report decreased fatigue (odds ratio: 3.11; 95% confidence interval: 0.99–11.74; P = .04).
Table 4.
Outcomes in teleworkers vs in-person workers.
| Teleworkers (n = 74) | In-Person Workers (n = 24) | Pa | |
|---|---|---|---|
| Hypersomnia type | |||
| NT1 | 28.4 (21) | 33.3 (8) | .5 |
| NT2 | 27.0 (20) | 16.7 (4) | |
| IH | 44.6 (33) | 50.0 (12) | |
| Occupation | |||
| White collar | 91.9 (68) | 62.5 (15) | .005 |
| Blue collar | 6.8 (5) | 29.2 (7) | |
| Commuting time before and during lockdown, mean (SD), min | |||
| Before | 71.2 (48.9) | 55.6 (51.8) | n/a |
| During | 0 | 55.6 (51.8) | |
| Workday start time, mean (SD), hh:mm | 9:10 (1.1) | 8:39 (1.8) | .2 |
| Mean (SD) night sleep time before and during lockdown, h | |||
| Before | 7.9 (1.4) | 8.0 (1.6) | .17 |
| During | 8.8 (1.8) | 8.5 (2.1) | |
| Δ Sleep time | 0.9 (1.2) | 0.6 (1.4) | |
| Pb | < .001 | .3 | |
| Mean (SD) ESS before and during lockdown | |||
| Before | 14.0 (4.2) | 13.4 (3.7) | .08 |
| During | 12.6 (5.1) | 13.3 (4.4) | |
| Δ ESS | −1.6 (3.1) | −0.1 (3.7) | |
| Pa | < .001 | .7 | |
| Less fatigue | 47.2 (35) | 20.8 (5) | .04 |
| Insomnia | 50.0 (37) | 58.3 (14) | .63 |
| Naps per day, mean (SD) | 0.9 (0.8) | 1.2 (0.9) | .22 |
Data are displayed in % (n) unless otherwise indicated. Commuting time during lockdown was deduced on the assumption that teleworkers reduced their commuting time to 0, with approximately unchanged commuting time in teleworkers. aSignificance testing for between-group comparisons. bSignificance testing for within-group comparison before and during lockdown. ESS = Epworth Sleepiness Scale, IH = idiopathic hypersomnia, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, n/a = not applicable.
Multiple correspondence analysis
MCA identified 3 principal dimensional axes, accounting collectively for 45.9% of the variability within the dataset (Table S3). The name of each axis was based on the principal variables that determined them. Axis 1 was of most interest, accounting for 23.7% of the variance within the dataset. It was determined by variables associated with neuropsychological wellness (decreased stress and irritability, better mood, increased concentration, decreased fatigue, and increased appreciation of the lockdown). Axis 2, accounting for 11.5% of variability, was mainly explained by a decreased irritability and stress. Axis 3, accounting for 10.7% of variability, was mainly explained by delayed sleep phase (delayed bedtime and wakeup time). Supplementary qualitative and quantitative variables hypothesized to be related to axis 1 were identified and tested for correlation. Axis 1 was weakly associated with improving ESS score (R2 = 0.3, P < .0001) and was not associated with age, night sleep time, number of naps per day, commuting time, hypersomnia type, or teleworking status.
Thematic analysis of participants’ overall assessment of the lockdown
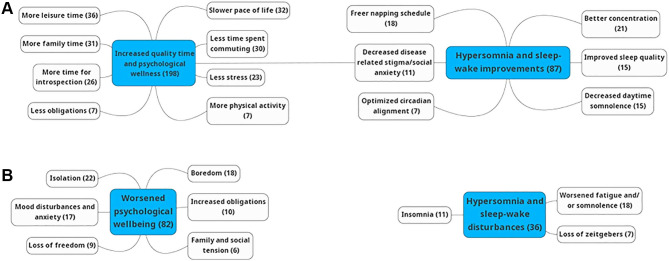
Inductive thematic analysis of participants’ responses to 2 open-ended questions assessed reasons why participants liked or disliked the lockdown. Seventeen codes were identified from those relating a positive experience of the lockdown and 11 codes from those relating a negative experience. These codes were grouped into overarching themes (Figure 1): increased quality time and improved psychosocial well-being, worsened psychosocial well-being, and improved/worsened sleep-wake quality and hypersomnia symptoms. Representative data extracts are shown in Table 5. Among those who noted an improvement in the quality of sleep and alertness, many alluded to a pace of life more compatible with their sleep disorder, allowing increased time in bed and a freer napping schedule as a mitigating factor for somnolence during lockdown. Several participants also expressed relief at reduced exposure to social stigma in public settings related to their disorder. Many participants reported that the lockdown measures allowed them to reallocate time (often spent commuting) toward increased quality time (more time with family and practicing hobbies), leading to improvement of their psychological and physical wellness. Among those who disliked the lockdown, several participants reported that the loss of regularly scheduled zeitgebers disrupted their circadian rhythm. Commonly, participants described feelings of isolation, loss of freedom, boredom, and anxiety, which often caused disturbances in sleep.
Figure 1. Inductive thematic analysis codes grouped into overarching themes.
Codes identified from participant responses and grouped by theme concerning reasons for (A) liking or (B) disliking the lockdown. Values in parenthesis signify the number of times a code or theme was evoked. A single statement could evoke multiple codes or themes.
Table 5.
Representative participant citations identified during inductive thematic analysis.
| Codes/Themes | Representative Extracts |
|---|---|
| Enjoyed lockdown | |
| Improved sleep schedule | “For once in my life, it was as if society was moving at my own pace […] a less constrained schedule increased my joie de vivre.” |
| “I slept in one hour later and napped as needed in the afternoon, which allowed me to be more attentive during afternoon classes.” | |
| “[…] My bed is always accessible with no obstacles to napping [….] This allows me a better sleep hygiene and improved work efficiency.” | |
| Decreased social stigma | “[…] I don’t have to constantly hide my somnolence, fatigue, yawning and need to nap. Not being told ‘you look tired!’” |
| “[…] I am less stressed by social interactions, as I no longer have to apologize for being tired.” | |
| Quality time and improved psychological wellness | “Not having to commute to work allowed me to get up later and spend more time pursuing hobbies that I don’t usually have time for.” |
| “Having less obligations and a freer schedule allows me to spend precious time with my child and husband.” | |
| Disliked lockdown | |
| Perturbed schedule | “The loss of structure and temporal cues was difficult. Although waking up early to get my children to school is difficult, it helps me to have a structured daily rhythm.” |
| “Staying at home is the worst thing possible for my sleep. I had great difficulty maintaining a regular schedule and sleep/wake times.” | |
| Mood and sleep disturbance | “I had a lot of difficulty dealing with solitude. The uncertainty and anxiety disturbed by sleep.” |
| “I was bored and felt imprisoned, which worsened my anxiety.” |
DISCUSSION
Our participants reported increased night sleep time and decreased daytime sleepiness during lockdown, an effect more marked in teleworkers than in in-person workers. A plurality of participants reported delaying their bedtime and wakeup time, which was driven by participants with IH. Half of participants with NT1 reported a decrease in or disappearance of cataplexy. Stimulant dosage was decreased by nearly one-third of participants. In contrast, half of all participants reported insomnia during lockdown. Neuropsychological wellness was associated with decreasing sleepiness scores. A minority of participants disliked the lockdown, while 3 times as many enjoyed it. Many participants reported that the lockdown allowed the reallocation of time usually spent commuting toward sleep, hobbies, and family time. Many also reported benefitting from a longer sleep time, a freer napping schedule, and improved daytime vigilance. By contrast, some expressed feelings of isolation and psychological distress during lockdown.
Daytime sleepiness decreased in all disorders (although less in NT1 than in NT2 and IH), on average by 1 point of ESS. This modest but significant change was obtained despite unchanged or decreased dosage of stimulants in most participants. Decreased fatigue and better concentration were reported in one-third of participants. The mechanisms of these improvements may be different across diseases, including increased nighttime sleep, sleep phase delay, free napping schedules, and a general psychological well-being (which correlated with improved sleepiness). People with narcolepsy generally have normal 24-hour total sleep time, but experience an ultradian rhythm, characterized by multiple sleep periods (including refreshing naps) rather than a single, consolidated sleep period.10 In their open-ended statements, many participants reported that, during lockdown, they were freer to nap as needed, improving their somnolence, although there was no quantitative assessment of changes in nap frequency. In addition, cataplexy mostly improved in our sample, possibly as a consequence of improved sleepiness and decreased social interaction (a primer of cataplexy attacks). Contrary to people with narcolepsy, people with IH often report long sleep times, evening chronotypy,11,12 and long, unrefreshing naps.13 Participants slept 1 hour longer during than before lockdown, and this was more marked in IH, probably decreasing sleepiness and fatigue, as observed during vacation. A frequent sleep phase delay was found in our population (as in the general working population during the COVID-19 pandemic2–4,14), possibly reflecting a shift toward their innate chronotype absent the usual social constraints. A similar phase delay has been reported among patients with narcolepsy in Brazil during the COVID-19 pandemic (although in our sample, phase delay was driven by IH more than narcolepsy).15 It is possible that, under lockdown conditions, many people with narcolepsy and IH more easily adapted their sleep schedule in accordance with their biological needs.
Half of participants, however, experienced insomnia (more in NT1) at some point during the lockdown (although the survey did not assess baseline insomnia). In their open-ended statements, many participants who reported worsened sleep quality attributed this to increased anxiety and loss of their usual zeitgebers. In comparison, a large survey study among the general population in France during the same time period as our study demonstrated a prevalence of 74% of troubled sleep during the pandemic (increased from 49% in a previous survey in 2017).16 Similar findings in the general population have been reported in a Canadian survey study, which further found that only 6% of the general population reported improved sleep during the pandemic.17 The striking differences between our observations in people with hypersomnia and the reported data in the general population are likely a reflection of the fundamental neurobiological differences underlying different sleep needs between these populations. People with hypersomnia have been subject to the same factors posited to cause sleep disturbances during the pandemic as the general population (stress, increased screen time, etc) and many participants did report disturbed sleep. However, it appears that, for most participants, the factors negatively impacting sleep were outweighed by the advantages associated with a less-constrained sleeping schedule.
The COVID-19 pandemic has normalized telework globally on a large scale, which is likely to have a lasting impact beyond the pandemic. In our study, a decrease in sleepiness and fatigue as well as an increase in sleep time (reallocated from commuting time) were observed in teleworkers but not in-person workers. Many of our participants also specifically evoked teleworking and its more adaptable schedule as a key factor in improving sleep-wake quality and work performance and related easing in social stigma and anxiety related to their hypersomnia symptoms. These findings support the data reported by Postiglione et al18 showing that, during the first COVID-19–related lockdown in Italy, 22 participants with NT1 who worked from home experienced increased sleep time and decreased ESS scores. Our results extend these findings to participants with NT2 and IH. Rodrigues Aguilar et al15 did not report an association between changes in employment and narcolepsy symptoms, although they did not perform any direct comparisons between teleworkers and in-person workers. Narcolepsy and IH impair work, resulting in decreased health-related quality of life6,7,19–22 and in loss of or change in employment in 30.3–42.7%.7,20,23 Patients report significant social stigma associated with sleepiness and cataplexy. Fear of being perceived as lazy is common, and people frequently adopt avoidance behaviors out of fear of precipitating cataplectic attacks in front of others.24,25 Ozaki and colleagues23 have reported that the autonomy to control one’s job schedule (including nap opportunities) was associated with increased quality of life in people with NT1, NT2, or IH.23 Despite these compelling data, workplace and schedule adaptations may be underprovided, although the reasons are not clear.26 Taken together, our results, combined with this existing literature, suggest that offering increased access to telework, or perhaps simply allowing work environment and scheduling adjustments to better accommodate the needs of people with hypersomnia, has the potential to improve sleepiness, work performance, and quality of life.
Appreciation of the lockdown was clustered with measures of psychological well-being (including stress, mood, irritability, concentration, and fatigue). Participants who enjoyed the lockdown commonly evoked that they had more time to spend with their loved ones, pursuing hobbies, introspection, and physical activities. Many specifically mentioned gratitude at no longer having to squander time commuting to and from work. Furthermore, improved psychological wellness and decreased sleepiness were correlated, although the direction of causality may be bidirectional. By contrast, many participants related decreased psychological well-being, related not only to anxiety concerning COVID-19 but also due to boredom, isolation, and loss of freedom. Approximately one-third of participants reported worsened mood, stress, and irritability during the lockdown. This proportion is similar to those reported in the general population in systematic reviews and meta-analyses reporting prevalence between 30% and 40% for stress and mood and anxiety disorders.27,28 One might have expected a higher proportion of our participants to experience psychological disturbances than what we observed, as people with hypersomnia are known to be at increased risk of anxiety and mood disorders.29 This may be partly related to pandemic-related social assistance programs in France, which guaranteed salaries in the case of work leave caused by the pandemic, protecting citizens from loss of employment and mitigating financial burden compared with many other countries.
We acknowledge some limitations to our study. First, our survey had a modest response rate (26.5%). Despite this, the sample was substantial and included participants with well-characterized disorders, diagnosed in a reference center, and included participants with IH, a disorder less studied than narcolepsy. The response rate is likely attributable to the length of the survey (mean completion time: 24 minutes), a high volume of spam mails, and a single presentation of the study without reminders. Participants being asked to recall their pre-lockdown somnolence introduces the possibility of recall bias. This was necessary, however, as ESS scores shortly prior to lockdown were not available in most participants’ charts. The questionnaire was designed “in house” as a means to assess the real-world experience of our patients with hypersomnias. However, the inclusion of validated scales assessing the severity of narcolepsy and IH would have been informative and would have added to the robustness of our data. Some perspectives expressed by participants during this first lockdown in the first few months of the COVID-19 pandemic may not be reflective of their experience during subsequent lockdowns, as the pandemic has endured. Notably, use of online video communications technology (such as Zoom; Zoom Video Communications Inc., San Jose, California, US) may have been less widespread during initial lockdown. Thus, participants may not yet have experienced so-called “Zoom-fatigue.”30 Although our population was large, the group of in-person workers was relatively small, which may have limited statistical power for between-group comparisons.
In conclusion, we found that participants with IH, NT1, and NT2 experienced increased sleep time and decreased daytime somnolence during the first COVID-19–related lockdown. This may have been driven by decreased social and occupational constraints on sleep-wake habits, allowing an economy of commuting time, increased nighttime sleep, physiological circadian realignment, and increased nap opportunities. We also noted that teleworking was associated with decreased fatigue and somnolence. These findings provide preliminary evidence suggesting that workplace adaptations for people with hypersomnia that ease social sleep constraints (including partial teleworking and protected nap time) may be beneficial. Because of its potential importance for patient advocacy efforts, this hypothesis warrants confirmation in future prospective studies.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Sleep Disorders Unit, Pitié-Salpêtrière University Hospital, Paris, France. Financial support was provided by PNMR3 (National Program for Rare Diseases) to I.A. M.N. has received an educational grant from Paladin Labs Pharmaceuticals and from the Department of Neurosciences at the University of Montreal. S.L.-S. has speaking engagements with UCB Pharma. I.A. has received consultant’s fees from Idorsia Pharma and Ono Pharma, and speaker honoraria from UCB Pharma. The other authors report no conflicts of interest.
ABBREVIATIONS
- COVID-19
coronavirus disease 2019
- ESS
Epworth Sleepiness Scale
- IH
idiopathic hypersomnia
- NT1
narcolepsy type 1
- NT2
narcolepsy type 2
REFERENCES
- 1.Sommaire analytique du 17 mars 2020. Journal Officiel des Lois et Decrets 66 edition. https://www.legifrance.gouv.fr/eli/jo/2020/3/17/0066. Published date: 03/17/2020. Date accessed 01/15/2021.
- 2. Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID-19 outbreak: practical recommendations from a task force of the European CBT-I Academy. J Sleep Res. 2020; 29( 4): e13052. [DOI] [PubMed] [Google Scholar]
- 3. Cellini N, Canale N, Mioni G, Costa S. Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J Sleep Res. 2020; 29( 4): e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R, Pandi-Perumal SR. COVID-Somnia: How the Pandemic Affects Sleep/Wake Regulation and How to Deal with it? Sleep Vigil 2020 42. 2020;4(2):51–53. [DOI] [PMC free article] [PubMed]
- 5. Vignatelli L, D’Alessandro R, Mosconi P, et al. ; GINSEN (Gruppo Italiano Narcolessia-Studio Epidemiologico Nazionale). Health-related quality of life in Italian patients with narcolepsy: the SF-36 Health Survey. Sleep Med. 2004; 5( 5): 467– 475. [DOI] [PubMed] [Google Scholar]
- 6. Ervik S, Abdelnoor M, Heier MS, Ramberg M, Strand G. Health-related quality of life in narcolepsy. Acta Neurol Scand. 2006; 114( 3): 198– 204. [DOI] [PubMed] [Google Scholar]
- 7. Dodel R, Peter H, Spottke A, et al. Health-related quality of life in patients with narcolepsy. Sleep Med. 2007; 8( 7–8): 733– 741. [DOI] [PubMed] [Google Scholar]
- 8. Sateia MJ. International Classification Of Sleep Disorders-third edition: highlights and modifications. Chest. 2014; 146( 5): 1387– 1394. [DOI] [PubMed] [Google Scholar]
- 9. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006; 3( 2): 77– 101. [Google Scholar]
- 10. Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy—clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019; 15( 9): 519– 539. [DOI] [PubMed] [Google Scholar]
- 11. Landzberg D, Trotti LM. Is idiopathic hypersomnia a circadian rhythm disorder? Curr Sleep Med Rep. 2019; 5( 4): 201– 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009; 32( 6): 753– 759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trotti LM. Central disorders of hypersomnolence. Continuum (Minneap Minn). 2020; 26( 4): 890– 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franceschini C, Musetti A, Zenesini C, et al. Poor sleep quality and its consequences on mental health during the COVID-19 lockdown in Italy. Front Psychol. 2020; 11: 574475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodrigues Aguilar AC, Frange C, et al. The effects of the COVID-19 pandemic on patients with narcolepsy. J Clin Sleep Med. 2021; 17( 4): 621– 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beck F, Léger D, Fressard L, Peretti-Watel P, Verger P; Coconel Group. Covid-19 health crisis and lockdown associated with high level of sleep complaints and hypnotic uptake at the population level. J Sleep Res. 2021; 30( 1): e13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robillard R, Dion K, Pennestri MH, et al. Profiles of sleep changes during the COVID-19 pandemic: demographic, behavioural and psychological factors. J Sleep Res. 2021; 30( 1): e13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Postiglione E, Pizza F, Ingravallo F, et al. Impact of COVID-19 pandemic lockdown on narcolepsy type 1 management. Brain Behav. 2021; 11( 1): e01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Broughton RJ, Guberman A, Roberts J. Comparison of the psychosocial effects of epilepsy and narcolepsy/cataplexy: a controlled study. Epilepsia. 1984; 25( 4): 423– 433. [DOI] [PubMed] [Google Scholar]
- 20. Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res. 2001; 10( 1): 75– 81. [DOI] [PubMed] [Google Scholar]
- 21. Dodel R, Peter H, Walbert T, et al. The socioeconomic impact of narcolepsy. Sleep. 2004; 27( 6): 1123– 1128. [DOI] [PubMed] [Google Scholar]
- 22. Teixeira VG, Faccenda JF, Douglas NJ. Functional status in patients with narcolepsy. Sleep Med. 2004; 5( 5): 477– 483. [DOI] [PubMed] [Google Scholar]
- 23. Ozaki A, Inoue Y, Hayashida K, et al. Quality of life in patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time: comparison between patients on psychostimulants, drug-naïve patients and the general Japanese population. Sleep Med. 2012; 13( 2): 200– 206. [DOI] [PubMed] [Google Scholar]
- 24. Kapella MC, Berger BE, Vern BA, Vispute S, Prasad B, Carley DW. Health-related stigma as a determinant of functioning in young adults with narcolepsy. PLoS One. 2015; 10( 4): e0122478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raggi A, Plazzi G, Ferri R. Health-related quality of life in patients with narcolepsy: a review of the literature. J Nerv Ment Dis. 2019; 207( 2): 84– 99. [DOI] [PubMed] [Google Scholar]
- 26. White M, Charbotel B, Fort E, et al. Academic and professional paths of narcoleptic patients: the Narcowork study. Sleep Med. 2020; 65: 96– 104. [DOI] [PubMed] [Google Scholar]
- 27. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020; 16( 1): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu T, Jia X, Shi H, et al. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord. 2021; 281: 91– 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dauvilliers Y, Paquereau J, Bastuji H, Drouot X, Weil JS, Viot-Blanc V. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry. 2009; 80( 6): 636– 641. [DOI] [PubMed] [Google Scholar]
- 30. Wiederhold BK. Connecting through technology during the coronavirus disease 2019 pandemic: avoiding “Zoom fatigue”. Cyberpsychol Behav Soc Netw. 2020; 23( 7): 437– 438. [DOI] [PubMed] [Google Scholar]