Abstract
Study Objectives:
Although cognitive impairment in obstructive sleep apnea (OSA) is primarily attributed to intermittent hypoxemia and sleep fragmentation, hypercapnia may also play a role in patients whose OSA is complicated by hypoventilation. This study investigated the impact of hypercapnia on cognitive function in severe sleep-disordered breathing (OSA accompanied by hypoventilation).
Methods:
Patients with severe OSA (apnea-hypopnea index >30 events/h; n = 246) underwent evaluation for accompanying hypoventilation with polysomnography that included continuous transcutaneous carbon dioxide (TcCO2) monitoring and awake arterial blood gas analysis. Patients were categorized as having no hypoventilation (n = 84), isolated sleep hypoventilation (n = 40), or awake hypoventilation (n = 122). Global cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA), memory with the Rey Auditory Verbal Learning Test (RAVLT), and processing speed with the Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV), Digit Symbol Coding subtest (DSC).
Results:
Apnea-hypopnea index was similar across groups (P = .15), but the sleep and awake hypoventilation groups had greater nocturnal hypoxemia compared with the no-hypoventilation group (P < .01). Within all groups, mean MoCA scores were < 26, which is the validated threshold to indicate mild cognitive impairment; RAVLT scores were lower than age-matched norms only in the awake-hypoventilation group (P ≤ .01); and DSC scores were lower than age-matched norms within all groups (P < .01). In multivariable regression analyses, higher arterial partial pressure of carbon dioxide (PaCO2) and TcCO2 during wakefulness were associated with lower MoCA and DSC scores (P ≤ .03), independent of confounders including overlap syndrome (OSA + chronic obstructive pulmonary disease).
Conclusions:
Awake hypoventilation is associated with greater deficits in cognitive function in patients with severe sleep-disordered breathing.
Citation:
Beaudin AE, Raneri JK, Ayas NT, Skomro RP, Smith EE, Hanly PJ; on behalf of Canadian Sleep and Circadian Network. Contribution of hypercapnia to cognitive impairment in severe sleep-disordered breathing. J Clin Sleep Med. 2022;18(1):245–254.
Keywords: sleep-disordered breathing, hypoventilation syndrome, hypercapnia, cognitive function
BRIEF SUMMARY
Current Knowledge/Study Rationale: Cognitive impairment in patients with obstructive sleep apnea is primarily attributed to intermittent hypoxia and sleep fragmentation. However, hypercapnia may also play a role in patients whose obstructive sleep apnea is complicated by hypoventilation.
Study Impact: This study found that hypercapnia during wakefulness, but not during sleep, was associated with worse cognitive function in patients with severe sleep-disordered breathing (ie, obstructive sleep apnea accompanied by hypoventilation) independent of confounders including hypoxemia, sleep fragmentation, and overlap syndrome (obstructive sleep apnea + chronic obstructive pulmonary disease).
INTRODUCTION
Obstructive sleep apnea (OSA) is associated with impaired cognitive function and is an independent risk factor for mild cognitive impairment (MCI) and dementia.1–4 Impaired cognitive function in OSA is primarily attributed to chronic nocturnal intermittent hypoxemia and sleep fragmentation.5,6 Although there is strong evidence linking greater sleep fragmentation and impaired cognitive function in OSA,1 the relationship between nocturnal hypoxemia and reduced cognitive function is modest and inconsistent.7,8 Consequently, additional pathophysiologic mechanisms, such as awake and nocturnal hypercapnia, have been considered.9–12
Patients with OSA experience varying degrees of hypercapnia depending upon the severity of their OSA and comorbidities such as obesity and chronic obstructive pulmonary disease (COPD) that predispose them to hypoventilation. In uncomplicated OSA, hypercapnia may be transient during apneas or hypopneas.13 Alternatively, sustained hypercapnia during wakefulness and/or sleep may occur in patients with OSA with accompanying obesity hypoventilation syndrome (OHS)14 or COPD.15 Experimental studies in animals and healthy humans exposed to hypercapnia report electroencephalogram (EEG) slowing,10,16–18 while reduced amplitude of neural oscillations19 and altered mental and psychomotor function have been reported in humans.20,21 Moreover, Wang et al22 reported a positive correlation between awake hypercapnia and EEG slowing in OHS and overlap syndrome (OSA + COPD) patients and decreased awake hypercapnia following positive airway pressure therapy was associated with faster sleep EEG.9
While these studies demonstrate that hypercapnia can cause meaningful, and reversible, changes in brain electrical activity, it is unclear whether hypercapnia contributes to impaired cognitive function in patients whose OSA is accompanied by hypoventilation. One small study in obese patients with OSA found negative associations between awake hypercapnia and memory, processing speed and reaction time.23 However, transcutaneous carbon dioxide (TcCO2) was not monitored during sleep, which precluded the ability to determine whether patients with isolated sleep hypoventilation (ie, prodromal OHS14) also have impaired cognitive function. Another small study of patients with OHS with severe OSA found a negative correlation between EEG slowing during non–rapid eye movement (NREM) sleep and psychomotor vigilance but also did not investigate the relationship between TcCO2 and cognitive function.24 Accordingly, the objective of this study was to investigate the impact of hypercapnia during wakefulness and sleep on cognitive performance in a large group of patients with severe sleep-disordered breathing (SDB).
METHODS
This study included a subset of English-speaking patients (n = 246) enrolled in the multicenter Canadian Sleep and Circadian Network OSA observational cohort study of adults referred to participating academic sleep centers for suspected OSA.7 Patients were recruited from a single center (Sleep Centre, Foothills Medical Centre, Calgary, AB, Canada) who underwent split-night polysomnography (PSG) for suspected sleep hypoventilation based upon clinical assessment and review of preliminary home sleep apnea testing. Typical home sleep apnea testing indicators of potential hypoventilation included arterial oxyhemoglobin saturation (pulse oximetry [SpO2]) continually < 90% and episodes of sustained hypoxemia, defined as an SpO2 < 85% for at least 5 consecutive minutes.25 Additional tests included arterial blood gas (ABG) analysis immediately prior to the PSG, continuous TcCO2 measurement during sleep, pulmonary function tests (PFTs), a sleep questionnaire, and cognitive testing during a single daytime visit. Patients from other Canadian Sleep and Circadian Network centers were excluded because ABG analyses and TcCO2 monitoring were not performed during PSG.
This study was performed according to the Declaration of Helsinki and approved by the Conjoint Health Research Ethics Board of the University of Calgary (REB16-0211). Patients were informed of study requirements prior to providing written informed consent.
Polysomnography
All patients underwent split-night PSG that included EEG (C3, C4, M1, M2, O1, O2), left and right electrooculograms, submental electromyograms, airflow using nasal pressure (Braebon, Ottawa, ON, Canada) and oral thermistor (Protech; Philips Respironics, Murrysville, PA), thoracic and abdomen respiratory excursions (Respitrace; Ambulatory Monitoring, Ardsley, NY), finger pulse oximetry (NATUS Embla Systems, Tonawanda, NY), and TcCO2 (TCM4; Radiometer Medical, Copenhagen, Denmark). Calibration and placement details for TcCO2 monitoring are provided in the supplemental material. All data were continuously recorded and stored electronically for subsequent scoring (Sandman; Tyco Healthcare, Kanata, ON, Canada). Polysomnograms were manually scored by experienced registered PSG technologists according to the American Academy of Sleep Medicine criteria.26 An obstructive apnea was defined as a decrease in respiratory airflow amplitude by ≥ 90% for at least 10 seconds with continued respiratory efforts. An obstructive hypopnea was defined as a decrease in respiratory airflow amplitude of ≥ 30% lasting for at least 10 seconds followed by a decrease in SpO2 of ≥ 3% or an arousal from sleep.
Arterial blood sampling
Arterial blood samples were collected immediately prior to the PSG by a respiratory therapist with the patient supine, awake, and breathing room air. Samples were analyzed for the partial pressures of carbon dioxide (PaCO2) and oxygen (PaO2), pH (pHa), and bicarbonate (GEM Premier 5000; Instrumentation Laboratories, Bedford, MA).
Sleep questionnaire
The sleep questionnaire included demographics (age, height, weight, sex), medical history, comorbidities, medications, sleep schedule, symptoms of restless legs syndrome, and insomnia. The Epworth Sleepiness Scale (ESS)27 and the Pittsburgh Sleep Quality Index (PSQI)28 were used to assess daytime sleepiness and sleep quality. Additional details are provided in the supplemental material and a recent publication.7
Cognitive testing
Administration and scoring of cognitive tests were performed by qualified personnel. Tests included the Montreal Cognitive Assessment (MoCA) of global cognition,29 the Rey Auditory Verbal Learning Test (RAVLT) of episodic memory,30 and the Wechsler Adult Intelligence Scale, Fourth Edition, Digit Symbol Coding (DSC) subtest of information processing speed.31 Test details are provided in the supplemental material and a recent publication.7
Data analyses
Patients were categorized as having no hypoventilation, isolated sleep hypoventilation, or awake hypoventilation based upon ABG and TcCO2. Since Calgary is located at ∼1100 m above sea level where normal PaCO2 is 30–40 mmHg during wakefulness, PaCO2 >40 mmHg was used to define awake hypoventilation.32 Table 1 outlines categorization criteria. For completeness, analyses were repeated using the standard, sea-level criteria of PaCO2 >45 mmHg to define awake hypoventilation26 (Table S1 in the supplemental material). For patients without a PaCO2 measurement (10 with no hypoventilation, 3 with sleep hypoventilation), the TcCO2 value recorded at the beginning of the PSG with the patient supine, awake, and breathing room air was used. PSG data were obtained from the diagnostic portion of study. Overlap syndrome was defined as OSA accompanied by either PFT evidence of COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] class 1–433) or, if PFT data were not available, self-reported history of COPD or asthma, or a smoking history > 10 pack-years.34
Table 1.
Hypoventilation categorization criteria.
| Category | Criteria |
|---|---|
| No hypoventilation | PaCO2 ≤ 40 mmHg; TcCO2 increase during sleep <10 mmHg; and peak TcCO2 during sleep ≤ 55 mmHg |
| or | |
| PaCO2 ≤ 40 mmHg; TcCO2 increase during sleep ≥ 10 mmHg; and peak TcCO2 during sleep ≤ 50 mmHg | |
| Sleep hypoventilation | PaCO2 ≤ 40 mmHg; TcCO2 increase during sleep ≥ 10 mmHg; and peak TcCO2 during sleep > 50 mmHg |
| or | |
| PaCO2 ≤ 40 mmHg and peak TcCO2 during sleep > 55 mmHg | |
| Awake hypoventilation | PaCO2 > 40 mmHg |
PaCO2 = arterial partial pressure of carbon dioxide, TcCO2 = transcutaneous partial pressure of CO2.
Sleep duration was quantified from the PSQI question 4 (“How many hours of actual sleep do you get at night?”). Patients were categorized as having short sleep duration if they reported less than 6 hours of sleep per night.35
Self-reported medications were categorized according to their drug classification.36 For clinical relevance, we have reported only medications known to impact cognition37 (antidepressants, atypical antipsychotics, benzodiazepines, cannabinoids, dopaminergics, gabapentinoids, nonbenzodiazepines, opioids, and statins).
A validated MoCA score <26 (range: 0–3029) was operationally defined to indicate the presence of MCI because it has acceptable discriminatory ability to identify patients with OSA and MCI.38 However, since a MoCA <26 may be too liberal to detect MCI39 we also calculated the prevalence of a MoCA < 25 and <24. RAVLT delayed free recall and recognition, and DSC scores were expressed as z-scores relative to age-matched norms.40–42
Statistical analyses
Continuous descriptive variables were compared across groups using 1-way analyses of variance incorporating a Tukey-Kramer correction for post hoc comparisons; categorical variables were compared using a chi-square goodness-of-fit test. Group mean RAVLT delayed recall and recognition and DSC z-scores were compared to zero (hypothesized no difference from age-adjusted norms) using 1-sample t tests. Cognitive scores were compared across groups using analyses of covariance. Covariates included age, sex, low education (≤12 years of formal education, except for the MoCA, which includes a correction for low education), apnea-hypopnea index (AHI), hypoxemia (percentage of total sleep time with SpO2 <90% [T90]), sleep fragmentation (arousal/awakening index), and the presence (or absence) of excessive daytime sleepiness (ESS >10), poor sleep quality (PSQI > 5), short sleep duration (<6 hours/night), comorbidities (including overlap syndrome), and medication use. Relationships between cognitive scores, PaCO2, and TcCO2 were assessed using multivariable linear regression adjusting for the same confounders included in our analyses of covariance. Analyses were performed with Statistical Analysis Software (version 9.4; SAS Institute, Cary, NC) and an α ≤ 0.05 was considered significant.
RESULTS
The sleep- and awake-hypoventilation groups had a lower mean SpO2 and greater T90 compared with the no-hypoventilation group (Table 2), COPD was more prevalent in the awake-hypoventilation group, and the sleep-hypoventilation group had fewer patients using antidepressants (Table 3). By design, PaCO2 was highest in the awake-hypoventilation group. Compared with the no-hypoventilation group, baseline TcCO2, peak TcCO2 during sleep, and the increase in TcCO2 during the transition from wakefulness to sleep were higher in the sleep- and awake-hypoventilation groups. Furthermore, smoking history and PFT evidence of airflow obstruction were similar across groups, although forced vital capacity was lower in patients with awake hypoventilation (Table 4). The prevalence of overlap syndrome in the no-hypoventilation, sleep-hypoventilation, and awake-hypoventilation groups was 33%, 43%, and 38% (χ2, P = .58). Group differences were comparable when patients were categorized using PaCO2 >45 mmHg, except the sleep-hypoventilation group had the lowest AHI and a lower arousal/awakening index compared with the no-hypoventilation group, hypertension and opioid use were more prevalent in the awake-hypoventilation group, and PFT evidence of airflow obstruction was greater in the sleep- and awake-hypoventilation groups (Table S2, Table S3, and Table S4 in the supplemental material), resulting in 45% of patients in these groups having overlap syndrome compared with 31% of patients with no hypoventilation (χ2, P = .09).
Table 2.
Patient demographics, measures of OSA severity and hypercapnia, daytime sleepiness, and sleep characteristics when categorizedusing PaCO2 > 40 mmHg to indicate awake hypoventilation.
| No Hypoventilation | Sleep Hypoventilation | Awake Hypoventilation | P | |
|---|---|---|---|---|
| n | 84 | 40 | 122 | |
| Female, n (%) | 31 (36.9) | 21 (52.5) | 58 (47.5) | .18 |
| Age, y | 53.0 ± 12.9 | 55.7 ± 11.1 | 55.0 ± 12.1 | .41 |
| Education ≤ 12 years, n (%) | 19 (22.6) | 12 (30.0) | 43 (35.2) | .15 |
| BMI, kg/m2 | 41.0 ± 8.7 | 39.5 ± 8.4 | 43.0 ± 9.1 | .07 |
| White, n (%) | 73 (86.9) | 37 (92.5) | 104 (85.2) | .87 |
| OSA severity | ||||
| AHI, events/h | 75.9 ± 48.9 | 59.9 ± 37.0 | 75.9 ± 49.4 | .15 |
| ODI (3%), events/h | 73.4 ± 35.6 | 64.8 ± 28.5 | 76.8 ± 37.8 | .18 |
| ODI (4%), events/h | 63.3 ± 40.4 | 55.1 ± 30.5 | 66.2 ± 41.3 | .30 |
| Mean SpO2, (%) | 87.5 ± 3.9 | 85.4 ± 4.0* | 84.3 ± 4.9* | <.01 |
| T90, % TST | 67.8 ± 26.9 | 81.3 ± 20.7* | 86.2 ± 19.1* | <.01 |
| Arousal/awakening index, /h | 51.3 ± 29.2 (n = 81) | 42.2 ± 25.9 (n = 38) | 48.9 ± 28.5 (n = 121) | .27 |
| Arterial blood gases and TcCO2 | ||||
| PaCO2, mmHg | 36.9 ± 2.7a | 38.2 ± 2.5b | 44.3 ± 3.0*,† | <.01 |
| PaO2, mmHg | 70.1 ± 9.9 (n = 75) | 66.9 ± 11.4 (n = 37) | 60.8 ± 7.9*,† | <.01 |
| pHa | 7.44 ± 0.03 (n = 75) | 7.44 ± 0.02 (n = 37) | 7.42 ± 0.03*,† (n = 120) | <.01 |
| Bicarbonate, mEq/L | 24.7 ± 2.1 (n = 75) | 25.9 ± 1.4* (n = 37) | 28.7 ± 2.2*,† (n = 121) | <.01 |
| Baseline TcCO2, mmHg | 39.6 ± 3.1 | 42.3 ± 3.4* | 46.3 ± 3.9*,† (n = 119) | <.01 |
| Peak TcCO2 during sleep, mmHg | 46.7 ± 3.4 | 55.8 ± 4.2* | 55.3 ± 6.0* (n = 119) | <.01 |
| ΔTcCO2, mmHg | 7.2 ± 2.4 | 13.5 ± 2.9* | 9.0 ± 4.1*,† (n = 119) | <.01 |
| Daytime sleepiness | ||||
| ESS score | 11.7 ± 5.0 | 10.3 ± 5.5 | 10.7 ± 4.9 (n = 121) | .27 |
| ESS > 10, n (%) | 55 (65.5) | 20 (50.0) | 71 (58.7) | .25 |
| Sleep characteristics | ||||
| PSQI global score | 9.7 ± 4.4 | 8.6 ± 2.9 | 9.6 ± 3.9 | .28 |
| PSQI > 5, n (%) | 74 (88.1) | 37 (92.5) | 107 (88.4) | .74 |
| Sleep duration (h) | 6.4 ± 1.9 | 6.4 ± 1.2 | 6.4 ± 1.8 | .99 |
| Sleep <6 hours, n (%) | 43 (51.2) | 21 (52.5) | 67 (54.9) | .87 |
| ISI (total score) | 14.7 ± 6.4 (n = 83) | 12.9 ± 5.4 | 13.5 ± 6.2 | .20 |
| No insomnia, n (%) | 14 (16.9) | 7 (17.5) | 26 (21.3) | .56 |
| Subthreshold insomnia, n (%) | 23 (27.7) | 16 (40.0) | 36 (29.5) | |
| Moderate insomnia, n (%) | 36 (43.4) | 16 (40.0) | 49 (40.2) | |
| Severe insomnia, n (%) | 10 (12.0) | 1 (2.5) | 11 (9.0) | |
| RLS, n (%) | 19 (22.6) | 7 (17.5) | 34 (27.9) | .37 |
| RLS severity (range: 1–7) | 5.2 ± 1.3 | 4.1 ± 1.8 | 5.1 ± 1.7 | .30 |
Values are n (%) for categorical variables (P values = χ2) and means ± SDs for continuous variables (P values= ANOVA). aTcCO2 baseline values used for 10 patients who did not have an arterial blood gas. bTcCO2 baseline values used for 3 patients who did not have an arterial blood gas. *P <.05 vs no hypoventilation; †P <.05 vs sleep hypoventilation. AHI = apnea-hypopnea index, ANOVA = analysis of variance, BMI = body mass index, ESS = Epworth Sleepiness Scale, ISI = Insomnia Severity Index, mean SpO2 = mean arterial oxyhemoglobin saturation during sleep, ODI = oxygen desaturation index based upon either 3% or 4% desaturations, OSA = obstructive sleep apnea, PaCO2 = arterial partial pressure of carbon dioxide, PaO2 = arterial partial pressure of oxygen, pHa = arterial pH, PSQI = Pittsburgh Sleep Quality Index, RLS = restless legs syndrome, T90 = percentage of total sleep time with SpO2 <90%; TcCO2 = transcutaneous partial pressure of carbon dioxide, ΔTcCO2 = increase in TcCO2 during transition from wakefulness to sleep, TST = total sleep time.
Table 3.
Patient comorbidities and medication use when categorized using PaCO2 > 40 mmHg to indicate awake hypoventilation.
| No Hypoventilation | Sleep Hypoventilation | Awake Hypoventilation | P | |
|---|---|---|---|---|
| n | 84 | 40 | 122 | |
| Comorbidities, n (%) | ||||
| Hypertension | 48 (57.1) | 20 (50.0) | 74 (60.7) | .49 |
| Diabetes | 26 (31.0) | 9 (22.5) | 43 (35.2) | .32 |
| High cholesterol | 40 (47.6) | 16 (40.0) | 62 (50.8) | .49 |
| Kidney disease | 7 (8.3) | 1 (2.5) | 11 (9.0) | .39 |
| Coronary artery disease | 11 (13.1) | 3 (7.5) | 17 (13.9) | .56 |
| Heart failure | 4 (4.8) | 1 (2.5) | 3 (2.5) | .63 |
| Atrial fibrillation | 3 (3.6) | 3 (7.5) | 8 (6.6) | .57 |
| Prior stroke | 2 (2.4) | 1 (2.5) | 6 (4.9) | .58 |
| Chronic obstructive pulmonary disease | 4 (4.8) | 4 (10.0) | 19 (15.6) | .05 |
| Asthma | 20 (23.8) | 9 (22.5) | 38 (31.1) | .39 |
| Medications, n (%) | ||||
| Statins | 33 (39.3) | 9 (22.5) | 53 (43.4) | .06 |
| Antidepressant | 26 (31.0) | 3 (7.5) | 41 (33.6) | .01 |
| Gabapentinoid | 11 (13.1) | 5 (12.5) | 19 (15.6) | .83 |
| Opioid | 13 (15.5) | 2 (5.0) | 9 (7.4) | .09 |
| Nonbenzodiazepine | 5 (6.0) | 1 (2.5) | 8 (6.6) | .63 |
| Atypical antipsychotic | 2 (2.4) | 2 (5.0) | 9 (7.4) | .29 |
| Benzodiazepine | 3 (3.6) | 0 (0) | 3 (2.5) | .48 |
| Cannabinoid | 4 (4.8) | 1 (2.5) | 3 (2.5) | .63 |
| Dopaminergic | 0 (0) | 0 (0) | 5 (4.1) | .08 |
Values are n (%) for categorical variables (P value = χ2). PaCO2 = arterial partial pressure of carbon dioxide.
Table 4.
Smoking status and pulmonary function data.
| No Hypoventilation | Sleep Hypoventilation | Awake Hypoventilation | P | |
|---|---|---|---|---|
| Smoking status | ||||
| n | 84 | 40 | 122 | |
| Never, n (%) | 40 (47.6) | 17 (42.5) | 45 (36.9) | .27 |
| Current, n (%) | 7 (8.3) | 6 (15.0) | 23 (18.9) | |
| Past, n (%) | 37 (44.1) | 17 (42.5) | 54 (44.3) | |
| Smoking history,a pack-years | 23.9 ± 18.3 | 27.6 ± 22.5 | 30.4 ± 29.4 | .41 |
| Pulmonary function | ||||
| n | 49 | 24 | 94 | |
| FEV1 (% predicted) | 85.2 ± 14.8 | 76.5 ± 15.4 | 74.3 ± 18.4* | < .01 |
| FVC (% predicted) | 87.0 ± 11.4 | 79.7 ± 20.0 | 80.1 ± 15.5* | .03 |
| FEV1:FVC | 76.1 ± 10.8 | 71.7 ± 9.6 | 72.2 ± 13.4 | .16 |
| Airflow limitation classification, n (%) | ||||
| None | 39 (79.6) | 15 (62.5) | 60 (64.5) | .19 |
| Mild: GOLD 1 | 2 (4.1) | 1 (4.2) | 3 (3.2) | |
| Moderate: GOLD 2 | 7 (14.3) | 8 (33.3) | 24 (25.8) | |
| Severe: GOLD 3 | 0 (0.0) | 0 (0.0) | 6 (6.5) | |
| Very severe: GOLD 4 | 1 (2.0) | 0 (0.0) | 0 (0.0) |
Values are n (%) for categorical variables (P values = χ2) and means ± SDs for continuous variables (P values = ANOVA). aCurrent and past smokers only. *P < .05 vs no hypoventilation. ANOVA = analysis of variance, FEV1 = forced expiratory volume in 1 second, FEV1:FVC = ratio between FEV1 and FVC, FVC = forced vital capacity, GOLD = Global Initiative for Chronic Obstructive Lung Disease classification of airflow limitation.
PSG measures of sleep from the diagnostic portion of the split-night studies were similar across groups, except patients with awake hypoventilation spent less time in rapid eye movement (REM) sleep compared with patients with sleep hypoventilation (Table S5 in the supplemental material). When categorized using PaCO2 >45 mmHg, patients with sleep hypoventilation spent less time in stage 1 NREM sleep and had higher sleep efficiency compared with patients with no hypoventilation.
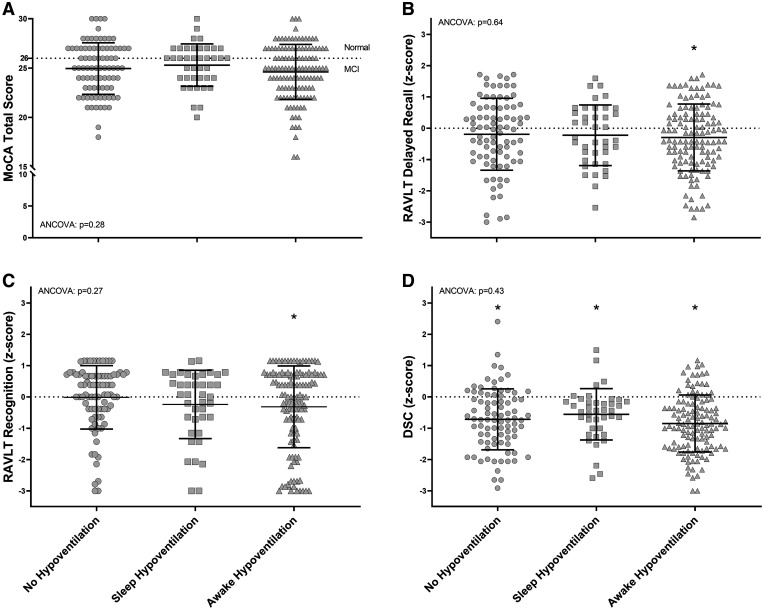
Mean MoCA scores were <26 in all groups (P = .22; Figure 1A). Within the no-hypoventilation, sleep-hypoventilation, and awake-hypoventilation groups the prevalence of a MoCA <26 was 56%, 45%, and 60%, respectively (χ2, P = .26), while 42%, 35%, and 44% had a MoCA <25 (χ2, P = .59) and 30%, 20%, and 31% had a MoCA <24 (χ2, P = .39) (Table S6 in the supplemental material). Mean RAVLT delayed recall and recognition scores were lower than age-adjusted norms in the awake-hypoventilation group (P <.01), whereas the mean DSC score was lower than age-adjusted norms in all groups (P <.01; Figure 1B–D). Results were similar when PaCO2 > 45 mmHg defined awake hypoventilation, except the mean RAVLT delayed recall within the no-hypoventilation group was lower than age-adjusted norms (P = .02) (Table S6 and Figure S1 in the supplemental material).
Figure 1. Cognitive testing across groups.
MoCA (A), RAVLT delayed recall (B), RAVLT delayed recognition (C), and DSC scores (D) across groups categorized using PaCO2 > 40 mmHg to indicate awake hypoventilation. Asterisks (*) indicate where the group mean is significantly different from zero (ie, hypothesized no difference from age-adjusted norms; dotted lines), with P ≤ .05; ANCOVA: group comparisons adjusted for age, sex, low education, AHI, T90, sleep fragmentation (arousal/awakening index), daytime sleepiness (ESS), sleep quality (PSQI), sleep duration, comorbidities (including overlap syndrome), and medication use. AHI = apnea-hypopnea index, ANCOVA = analysis of covariance, DSC = Wechsler Adult Intelligence Scale, 4th Edition, Digit Symbol Coding subtest, ESS = Epworth Sleepiness Scale, MoCA = Montreal Cognitive Assessment, PSQI = Pittsburgh Sleep Quality Index, RAVLT = Rey Auditory Verbal Learning Test, T90 = percentage of total sleep time with SpO2 <90%.
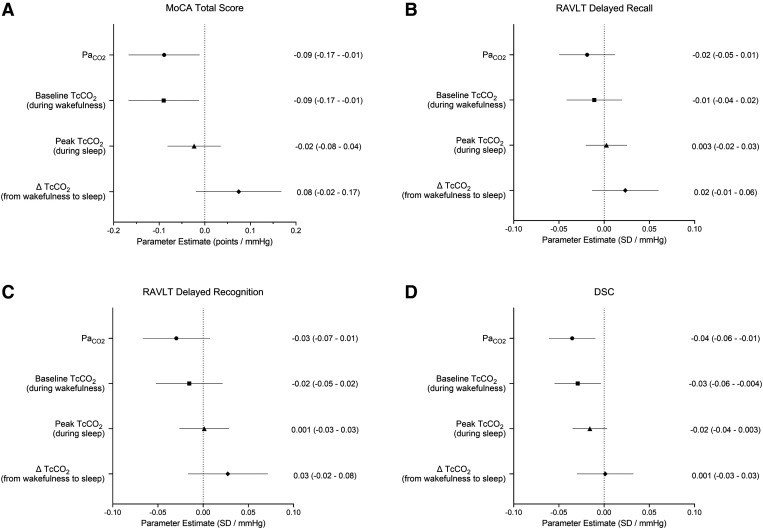
Lower MoCA and DSC, but not RAVLT delayed recall or recognition, were associated with higher PaCO2 (P ≤ .03) and baseline TcCO2 (P ≤ .03) values independent of age, sex, low education (DSC and RAVLT), AHI, T90, sleep fragmentation, daytime sleepiness, sleep quality, sleep duration, comorbidities (including overlap syndrome), and medication use (Figure 2). Cognitive scores were not associated with the peak TcCO2 during sleep or the change in TcCO2 during the transition from wakefulness to sleep with adjustments for the same confounders (P ≥ .10 for all regression models).
Figure 2. Relationships between cognitive testing and PaCO2, and TcCO2 measurements.
Parameter estimates (95% confidence interval) from multivariable regression analyses assessing the individual relationships between MoCA (A) (n = 238), RAVLT delayed recall (B) (n = 237), RAVLT delayed recognition (C) (n = 237), and the DSC scores (D) (n = 238) with PaCO2, TcCO2 recorded at the beginning of the PSG (baseline TcCO2), peak TcCO2 during sleep (peak TcCO2), and the change in TcCO2 during the transition from wakefulness to sleep (ΔTcCO2). Parameter estimates are independent of age, sex, low education (RAVLT and DSC scores only), AHI, hypoxemia (T90), sleep fragmentation (arousal/awakening index), daytime sleepiness (ESS), sleep quality (PSQI), sleep duration, comorbidities (including overlap syndrome), and medication use. AHI = apnea-hypopnea index, DSC = Wechsler Adult Intelligence Scale, 4th Edition, Digit Symbol Coding subtest, ESS = Epworth Sleepiness Scale, MoCA = Montreal Cognitive Assessment, PaCO2 = arterial partial pressure of CO2, PSG = polysomnography, PSQI = Pittsburgh Sleep Quality Index, RAVLT = Rey Auditory Verbal Learning Test, T90 = percentage of total sleep time with SpO2 < 90%, TcCO2 = transcutaneous CO2.
Results were unaltered by removing the 13 patients from the analyses for whom baseline TcCO2 was substituted for PaCO2.
DISCUSSION
This study investigated the impact of hypercapnia during wakefulness and sleep on cognitive function in patients with severe SDB. We found that (1) cognitive impairment was common, with 45–60% of patients within each group having a MoCA <26; (2) only patients with hypercapnia during wakefulness had worse memory performance compared with age-adjusted norms, while all groups had lower information processing speed; and (3) higher PaCO2 was associated with lower MoCA and DSC scores after adjusting for confounders, including hypoxemia, sleep fragmentation, and overlap syndrome.
OSA is associated with reduced measures of global cognitive function,38 memory, executive function, attention, and visuospatial cognitive abilities.1,2 The finding that the mean MoCA was <26 in all groups aligns with our prior study in a larger sleep clinic population of patients with untreated OSA.7 Specifically, we found that the mean MoCA for participants with severe OSA was 24.8 ± 2.9 and that 55% (177 out of 320) of participants had a MoCA <26. Furthermore, in a previous study of 394 sleep clinic patients, 41% with severe OSA (AHI >40 events/h) had a MoCA <26, with a mean MoCA score of 25.6 ± 3.0.43 Consequently, we believe that the participants in the current study are representative of sleep clinic cohorts with severe OSA. The memory deficits observed in delayed verbal recall and recognition in the awake-hypoventilation group are also consistent with previous studies in patients with OSA.44 Moreover, the deficits observed in information processing speed across all groups are also comparable to prior studies in patients with OSA.45 Of note, the greater deficits in information processing speed compared with memory within all groups are likely due to the DSC test requiring multiple cognitive, and noncognitive, domains such as short-term memory, attention, concentration, learning, psychomotor speed, visuo-perception, and motor speed.31
Traditionally, chronic intermittent hypoxemia and sleep fragmentation are considered the primary mechanisms leading to cognitive impairment in untreated OSA.5,6 Based upon a positive relationship between hypercapnia during wakefulness and EEG slowing (higher Delta:Alpha ratio) during sleep in patients with respiratory failure, OHS, and overlap syndrome, hypercapnia was proposed to contribute to this complication.9–12,22 These same investigators found a negative correlation between EEG slowing during NREM sleep and psychomotor vigilance in 15 patients with OHS (PaCO2 >45 mmHg) with severe OSA (AHI: 78 ± 47 events/h).24 Our observation that patients with awake hypercapnia performed worse on the RAVLT and DSC compared with age-adjusted norms supports the hypothesis that awake hypercapnia contributes, at least in part, to impaired daytime function, including diminished cognition in patients with severe SDB, and specifically in those with OHS.
A previous study of 39 obese patients (24 normocapnic; 15 with awake hypercapnia [PaCO2 >45 mmHg]) investigated the relationship between hypercapnia and cognitive function in OSA.23 Compared with normocapnic patients, those with awake hypercapnia had worse information processing speed and logical memory performance and increased reaction time within psychomotor vigilance tasks (Stroop and Eriksen flanker tasks). These observations are similar to Sivam et al24 who found that patients with OHS with severe OSA had slower reaction time on the Stroop test, but in contrast with our findings of no difference in global cognitive function (MoCA), memory (RAVLT), or information processing speed (DSC) between patients with awake and no hypoventilation using either PaCO2 > 40 mmHg or PaCO2 > 45 mmHg to indicate awake hypercapnia. There are several possible explanations for these differences. First, awake hypercapnia was more severe in these previous studies with PaCO2 = 52.8 ± 8.4 mmHg23 and 51.4 ± 5.1 mmHg24 compared with our 44.3 ± 3.0 mmHg (PaCO2 > 40 mmHg criteria) and 48.1 ± 2.2 mmHg (PaCO2 > 45 mmHg criteria). Second, in contrast to our study, neither of the previous studies adjusted their group comparisons for differences in age, sex, and education. Finally, since Kung et al23 did not report medication use, it is possible that hypercapnic patients in their study were taking more medications that can impact cognition.
In addition to group comparisons, to further explore the relationship between hypercapnia during wakefulness and sleep and cognitive function we quantified the relationships between measures of hypercapnia while awake (PaCO2, baseline TcCO2) and during sleep (peak TcCO2 during sleep and the increase in TcCO2 during the transition from wakefulness to sleep) using multivariable linear regression analyses of data pooled across all patients. In these analyses, MoCA and DSC scores had negative associations with PaCO2 and baseline TcCO2, independent of multiple confounders, including nocturnal hypoxemia, sleep fragmentation, and overlap syndrome, while RAVLT delayed recall and recognition were not associated with either measure of awake hypercapnia (Figure 2). In contrast, there were no relationships between cognitive scores and indices of hypercapnia during sleep (Figure 2). Although a negative association between awake PaCO2 and MoCA score has been reported in patients with COPD,46 this is the first study to report a similar relationship in patients with severe SDB adjusting for the presence of overlap syndrome. The negative association between PaCO2 and DSC scores is comparable to that observed by Kung et al,23 but the absence of an association between PaCO2 and memory in our study contrasts to those by Kung et al, who reported negative associations between PaCO2 and indices of logical memory. These contrasting findings may be related to differences in hypercapnia severity and medication use between studies discussed earlier.
This is the first study to examine the impact of hypercapnia isolated to sleep (quantified with TcCO2 monitoring) on cognitive function in patients with severe SDB. The lack of any association between peak TcCO2 during sleep and the increase in TcCO2 during the transition from wakefulness to sleep and cognitive scores suggest that, in contrast to awake hypercapnia, hypercapnia isolated to sleep does not contribute to cognitive deficits observed in this patient population. It may be that the “hypercapnic load” that develops during sleep is insufficient to impair cognition, or that any impairment that develops during sleep is corrected by restoration of normocapnia during wakefulness.
In this study, we used PaCO2 >40 mmHg to identify awake hypercapnia for our primary patient categorization. This threshold was chosen because Calgary is ∼1100 m above sea level where the normal range of PaCO2 is 30–40 mmHg.32 However, we repeated our analyses using sea-level criteria of PaCO2 >45 mmHg to indicate awake hypercapnia.26 Although our revised awake-hypoventilation group was substantially smaller (38 vs 122), our results and conclusions did not change. Additionally, we used the validated MoCA score <26 to indicate the presence of MCI,29 but the MoCA’s sensitivity and specificity for detecting MCI varies according to clinical settings.38 Although a MoCA <26 was found to have acceptable discriminatory ability to identify patients with OSA and MCI, a MoCA <27 has been reported to be the optimal threshold to detect MCI in patients with moderate-to-severe OSA.38 Notwithstanding that by using a MoCA <26, we may have underestimated the prevalence of MCI in our patient cohort, we believe that this more conservative threshold strengthened our statistical analysis.
This study has limitations. First, all patients underwent split-night PSGs, which have the potential to underestimate the prevalence of sleep hypoventilation if the diagnostic portion does not include REM sleep. To address this, we determined the change in peak TcCO2 during transition from NREM to REM sleep in the sleep-hypoventilation group participants who had >10 minutes of REM sleep. The mean ± SD increase was 2 ± 2 mmHg. Next, we added 4 mmHg to the peak TcCO2 during sleep of participants in the no-hypoventilation group who did not have REM sleep and recategorized those who met our criteria for sleep hypoventilation and repeated our statistical analyses. Although 20 participants moved from the no-hypoventilation group into the sleep-hypoventilation group, this did not change the results and conclusions. Second, participants had very severe OSA, which could limit the translation of our findings to patients with milder OSA. However, most patients who have hypercapnia from SDB have severe OSA. Furthermore, patients with mild and moderate OSA do not usually have hypercapnia, unless they have a significant comorbidity such as severe COPD. Consequently, we believe that the impact of hypoventilation due to SDB is most relevant to the patient population we investigated. Third, ABGs were not measured the morning after the sleep study as the results would have been confounded by continuous positive airway pressure and/or bilevel positive airway pressure titration during the second part of the night. Consequently, we were unable to evaluate TcCO2 signal drift across the night, which raises the potential for some participants to be miscategorized regarding hypoventilation. However, the TcCO2 monitors we used, when properly calibrated prior to PSG (details in the Supplemental Methods), are designed for up to 8 hours of continuous recording with minimal drift and without a need for recalibration for measurements to show good agreement with PaCO2 values.47,48 Moreover, we only used TcCO2 data from the diagnostic portion of the split-night PSGs, which had mean recording times of ∼160 to 180 minutes across the 3 participant groups (Table S5). Therefore, any slow drift in the TcCO2 signal likely had minimal impact on the categorization of participants. Fourth, we did not perform quantitative EEG analyses of the PSG, which precluded our ability to investigate the association between hypercapnia and EEG parameters. Consequently, we are unable to determine if the cognitive deficits observed in patients with awake hypoventilation were related to previously described hypercapnia-induced EEG slowing.11,12 Fifth, we included patients with comorbidities (including overlap syndrome) and those taking medications known to impact cognition since these are commonly seen in sleep clinic populations. However, we controlled for these potential confounders in our statistical analyses and found independent associations between our indices of hypercapnia and cognitive scores. Sixth, the predominance of White patients may limit the generalizability of results to other ethnic populations. Finally, since this is a cross-sectional study, causal inferences between hypercapnia and MCI cannot be made from our results. Noteworthy strengths of our study include the large sample size compared with previous studies,23,24 standardized ABG analyses performed the same night as patients’ PSG, and continuous TcCO2 monitoring during the PSG.
Clinical implications
Our results support the hypothesis that awake hypercapnia contributes to the development of impaired cognitive function in patients with severe SDB.11,12 Further research is needed to determine whether this clinical phenotype, if untreated, leads to progressive cognitive impairment and whether correction of awake hypercapnia, over and above correction of nocturnal hypoxemia and sleep fragmentation, improves these outcomes.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. A.E.B was supported by postdoctoral fellowships from the Canadian Alzheimer Association & Canadian Consortium on Neurodegeneration in Aging (CCNA), Campus Alberta Neuroscience, and the Canadian Institutes of Health Research (CIHR); and funds from a Brain Canada operating grant. J.K.R. was supported by funds from the Cumming School of Medicine, Sleep Research Program (University of Calgary), and the Canadian Sleep and Circadian Network (CSCN). E.E.S. holds the Katthy Taylor Chair in Vascular Dementia from the University of Calgary. This study was funded by the CSCN, which is funded through a CIHR Community Development Program grant. R.P.S. reports that they have provided consulting work to ResMed, GSK, and AstraZeneca outside the submitted work. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank their patients for the donation of their valuable time and interest in this study. They also thank the Sleep Centre staff at Foothills Medical Centre in Calgary for their assistance. Author contributions: A.E.B., N.T.A, R.P.S., E.E.S., and P.J.H. conceived the experimental design; J.K.R. helped with patient recruitment and data acquisition; A.E.B., E.E.S., and P.J.H. performed the statistical analyses. All authors contributed to the interpretation of the data. A.E.B. wrote the first draft of the manuscript. A.E.B., J.K.R., N.T.A., R.P.S., E.E.S., and P.J.H. critically reviewed the manuscript for important intellectual property. A.E.B., J.K.R., N.T.A., R.P.S., E.E.S., and P.J.H. approved the final manuscript. A.E.B., J.K.R., N.T.A., R.P.S., E.E.S., and P.J.H. agree to be accountable for all aspects of the work.
ABBREVIATIONS
- ABG
arterial blood gas
- AHI
apnea-hypopnea index
- COPD
chronic obstructive pulmonary disease
- DSC
Wechsler Adult Intelligence Scale, 4th Edition, Digit Symbol Coding subtest
- EEG
electroencephalogram
- ESS
Epworth Sleepiness Scale
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- OHS
obesity-hypoventilation syndrome
- OSA
obstructive sleep apnea
- MCI
mild cognitive impairment
- MoCA
Montreal Cognitive Assessment
- NREM
non–rapid eye movement
- PaCO2
partial pressure of arterial carbon dioxide
- PFT
pulmonary function test
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- RAVLT
Rey Auditory Verbal Learning Test
- REM
rapid eye movement
- SDB
sleep-disordered breathing
- SpO2
arterial oxyhemoglobin saturation measured by pulse oximetry
- T90
percentage of total sleep time with SpO2<90%
- TcCO2
transcutaneous carbon dioxide
REFERENCES
- 1. Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013; 18( 1): 61– 70. [DOI] [PubMed] [Google Scholar]
- 2. Gagnon K, Baril AA, Gagnon JF, et al. Cognitive impairment in obstructive sleep apnea. Pathol Biol (Paris). 2014; 62( 5): 233– 240. [DOI] [PubMed] [Google Scholar]
- 3. Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011; 306 ( 6): 613– 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma RA, Varga AW, Bubu OM, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly: a longitudinal study. Am J Respir Crit Care Med. 2018; 197( 7): 933– 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Champod AS, Eskes GA, Foster GE, et al. Effects of acute intermittent hypoxia on working memory in young healthy adults. Am J Respir Crit Care Med. 2013; 187 ( 10): 1148– 1150. [DOI] [PubMed] [Google Scholar]
- 6. Sforza E, Roche F. Sleep apnea syndrome and cognition. Front Neurol. 2012; 3: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beaudin AE, Raneri JK, Ayas NT, et al. Cognitive function in a sleep clinic cohort of patients with obstructive sleep apnea. Ann Am Thorac Soc. 2021; 18( 5): 865– 875. [DOI] [PubMed] [Google Scholar]
- 8. Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance—the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep. 2011; 34( 3): 303– 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Piper AJ, Yee BJ, et al. Hypercapnia is a key correlate of EEG activation and daytime sleepiness in hypercapnic sleep disordered breathing patients. J Clin Sleep Med. 2014; 10( 5): 517– 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Yee BJ, Wong KK, et al. Comparing the effect of hypercapnia and hypoxia on the electroencephalogram during wakefulness. Clin Neurophysiol. 2015; 126( 1): 103– 109. [DOI] [PubMed] [Google Scholar]
- 11. Wang D, Yee BJ, Rowsell L. Sleep-disordered breathing-related neurocognitive impairment, time to think beyond hypoxia and sleep fragmentation? Sleep Breath. 2015; 19( 1): 23– 24. [DOI] [PubMed] [Google Scholar]
- 12. Wang D, Thomas RJ, Yee BJ, Grunstein RR. Hypercapnia is more important than hypoxia in the neuro-outcomes of sleep-disordered breathing. J Appl Physiol 1985. 2016; 120( 12): 1484– 1486. [DOI] [PubMed] [Google Scholar]
- 13. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010; 90( 1): 47– 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masa JF, Pépin JL, Borel JC, Mokhlesi B, Murphy PB, Sánchez-Quiroga MA. Obesity hypoventilation syndrome. Eur Respir Rev. 2019; 28( 151): 180097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Owens RL, Malhotra A. Sleep-disordered breathing and COPD: the overlap syndrome. Respir Care. 2010; 55( 10): 1333– 1344, discussion 1344–1346. [PMC free article] [PubMed] [Google Scholar]
- 16. Schindler U, Betz E. Influence of severe hypercapnia upon cerebral cortical metabolism, CSF electrolyte concentrations and EEG in the cat. Bull Eur Physiopathol Respir. 1976; 12( 2): 277– 284. [PubMed] [Google Scholar]
- 17. Smith LJ, Greene SA, Moore MP, Keegan RD. Effects of altered arterial carbon dioxide tension on quantitative electroencephalography in halothane-anesthetized dogs. Am J Vet Res. 1994; 55( 4): 467– 471. [PubMed] [Google Scholar]
- 18. Forslid A, Ingvar M, Rosén I, Ingvar DH. Carbon dioxide narcosis: influence of short-term high concentration carbon dioxide inhalation on EEG and cortical evoked responses in the rat. Acta Physiol Scand. 1986; 127( 3): 281– 287. [DOI] [PubMed] [Google Scholar]
- 19. Hall EL, Driver ID, Croal PL, et al. The effect of hypercapnia on resting and stimulus induced MEG signals. Neuroimage. 2011; 58( 4): 1034– 1043. [DOI] [PubMed] [Google Scholar]
- 20. Hesser CM, Fagraeus L, Adolfson J. Roles of nitrogen, oxygen, and carbon dioxide in compressed-air narcosis. Undersea Biomed Res. 1978; 5( 4): 391– 400. [PubMed] [Google Scholar]
- 21. Fothergill DM, Hedges D, Morrison JB. Effects of CO2 and N2 partial pressures on cognitive and psychomotor performance. Undersea Biomed Res. 1991; 18( 1): 1– 19. [PubMed] [Google Scholar]
- 22. Wang D, Piper AJ, Wong KK, et al. Slow wave sleep in patients with respiratory failure. Sleep Med. 2011; 12( 4): 378– 383. [DOI] [PubMed] [Google Scholar]
- 23. Kung S-C, Shen Y-C, Chang E-T, Hong Y-L, Wang L-Y. Hypercapnia impaired cognitive and memory functions in obese patients with obstructive sleep apnoea. Sci Rep. 2018; 8( 1): 17551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sivam S, Poon J, Wong KKH, et al. Slow-frequency electroencephalography activity during wake and sleep in obesity hypoventilation syndrome. Sleep. 2020; 43( 2): zsz214. [DOI] [PubMed] [Google Scholar]
- 25. Braganza MV, Hanly PJ, Fraser KL, Tsai WH, Pendharkar SR. Predicting CPAP failure in patients with suspected sleep hypoventilation identified on ambulatory testing. J Clin Sleep Med. 2020; 16( 9): 1555– 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rule, Terminology and Technical Specifications. Version 2.2. Darien, IL: American Academy of Sleep Medicine; 2015. [Google Scholar]
- 27. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991; 14( 6): 540– 545. [DOI] [PubMed] [Google Scholar]
- 28. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28( 2): 193– 213. [DOI] [PubMed] [Google Scholar]
- 29. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53( 4): 695– 699. [DOI] [PubMed] [Google Scholar]
- 30. Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique (Les problems). [The psychological examination in cases of traumatic encepholopathy. Problems.] Arch Psychol. 1941; 28: 215– 285. [Google Scholar]
- 31. Wechsler D. Wechsler Adult Intelligence Scale. 4th ed. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 32. Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med. 1999; 160( 5 Pt 1): 1525– 1531. [DOI] [PubMed] [Google Scholar]
- 33. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017; 195( 5): 557– 582. [DOI] [PubMed] [Google Scholar]
- 34. Han MK, Dransfield MT, Martinez FJ. Chronic Obstructive Pulmonary Disease: Definition, Clinical Manifestations, Diagnosis, and Staging. In: Stoller JK, Hollingsworth H, eds. Waltham, MA; 2020. [Google Scholar]
- 35. Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010; 14( 4): 239– 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kane SP. Top 250 Drugs: Drug List by Therapeutic Category. n.d. Available from: https://clincalc.com/Downloads/Top250Drugs-DrugList.pdf. Accessed August 16, 2021.
- 37. Lusic Kalcina L, Pavlinac Dodig I, Pecotic R, Valic M, Dogas Z. Psychomotor performance in patients with obstructive sleep apnea syndrome. Nat Sci Sleep. 2020; 12: 183– 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gagnon K, Baril A-A, Montplaisir J, et al. Detection of mild cognitive impairment in middle-aged and older adults with obstructive sleep apnoea. Eur Respir J. 2018; 52( 5): 1801137. [DOI] [PubMed] [Google Scholar]
- 39. Ihle-Hansen H, Vigen T, Berge T, et al. Montreal Cognitive Assessment in a 63- to 65-year-old Norwegian cohort from the general population: data from the Akershus Cardiac Examination 1950 Study. Dement Geriatr Cogn Disord Extra. 2017; 7( 3): 318– 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 41. Gale SD, Baxter L, Connor DJ, Herring A, Comer J. Sex differences on the Rey Auditory Verbal Learning Test and the Brief Visuospatial Memory Test-Revised in the elderly: normative data in 172 participants. J Clin Exp Neuropsychol. 2007; 29 ( 5): 561– 567. [DOI] [PubMed] [Google Scholar]
- 42. Smith EE, O’Donnell M, Dagenais G, et al. ; PURE Investigators. Early cerebral small vessel disease and brain volume, cognition, and gait. Ann Neurol. 2015; 77( 2): 251– 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen R, Xiong KP, Huang JY, et al. Neurocognitive impairment in Chinese patients with obstructive sleep apnoea hypopnoea syndrome. Respirology. 2011; 16( 5): 842– 848. [DOI] [PubMed] [Google Scholar]
- 44. Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013; 36( 2): 203– 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kilpinen R, Saunamäki T, Jehkonen M. Information processing speed in obstructive sleep apnea syndrome: a review. Acta Neurol Scand. 2014; 129( 4): 209– 218. [DOI] [PubMed] [Google Scholar]
- 46. Qian H, Lin H, Li Y. [Assessment of cognition and associated factors in patients with stable chronic obstructive pulmonary disease.] Zhonghua Jie He He Hu Xi Za Zhi. 2014; 37( 10): 769– 773. [PubMed] [Google Scholar]
- 47. Storre JH, Magnet FS, Dreher M, Windisch W. Transcutaneous monitoring as a replacement for arterial PCO(2) monitoring during nocturnal non-invasive ventilation. Respir Med. 2011; 105( 1): 143– 150. [DOI] [PubMed] [Google Scholar]
- 48. Janssens JP, Borel JC, Pépin JL; SomnoNIV Group. Nocturnal monitoring of home non-invasive ventilation: the contribution of simple tools such as pulse oximetry, capnography, built-in ventilator software and autonomic markers of sleep fragmentation. Thorax. 2011; 66( 5): 438– 445. [DOI] [PubMed] [Google Scholar]