Abstract
Study Objectives:
Both obesity and airways disease can lead to chronic hypercapnic respiratory failure, which can be managed with positive airway pressure (PAP) therapy. The efficacy of PAP has been studied in obesity hypoventilation syndrome as well as in chronic hypercapnic chronic obstructive pulmonary disease patients, but not in patients where both obesity and airway obstruction coexist. This pilot study aims to compare the efficacy of continuous positive airway pressure vs bilevel positive airway pressure spontaneous mode in the treatment of hypoventilation disorder with obesity and obstructive airways disease.
Methods:
We sequentially screened PAP-naïve patients with stable chronic hypercapnic respiratory failure (PaCO2 > 45 mm Hg), obesity (body mass index > 30 kg/m2), and obstructive airways disease. Participants were randomized to continuous positive airway pressure or bilevel positive airway pressure spontaneous mode treatment for 3 months. Participants were blinded to their PAP allocation. Change in awake PaCO2 was the primary endpoint. Secondary endpoints included change in lung function, daytime sleepiness, sleep quality, quality of life, PAP adherence, and neurocognitive function.
Results:
A total of 32 individuals were randomized (mean ± SD: age 61 ± 11 years, body mass index 43 ± 7 kg/m2, PaCO2 54 ± 7 mm Hg, forced expiratory volume in 1 second 1.4 ± 0.6L, apnea-hypopnea index 59 ± 35 events/h). Sixteen participants in each PAP group were analyzed. Bilevel positive airway pressure yielded a greater improvement in PaCO2 compared to continuous positive airway pressure (9.4 mm Hg, 95% confidence interval, 4.3–15 mm Hg). There were no significant differences in PAP adherence, sleepiness, sleep quality, or neurocognitive function between the two therapies.
Conclusions:
Although both PAP modalities improved hypercapnic respiratory failure in this group of individuals, bilevel positive airway pressure spontaneous mode showed greater efficacy in reducing PaCO2.
Clinical Trial Registration: Registry: Australian New Zealand Clinical Trials Registry; Name: Nocturnal ventilatory support in obesity hypoventilation syndrome; URL: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12605000096651; Identifier: ACTRN12605000096651.
Citation:
Zheng Y, Yee BJ, Wong K, Grunstein R, Piper A. A pilot randomized trial comparing CPAP vs bilevel PAP spontaneous mode in the treatment of hypoventilation disorder in patients with obesity and obstructive airway disease. J Clin Sleep Med. 2022;18(1):99–107.
Keywords: chronic obstructive pulmonary disease, obstructive sleep apnea, overlap syndrome, obesity, hypercapnic respiratory failure, positive airway pressure therapy
BRIEF SUMMARY
Current Knowledge/Study Rationale: The efficacy of positive airway pressure therapy has not been explored in patients with hypercapnic respiratory failure due to contributing factors of both obesity and chronic obstructive pulmonary disease. This study compares the effectiveness of continuous positive airway pressure vs bilevel positive airway pressure (spontaneous mode) in improving respiratory failure.
Study Impact: The current study suggests bilevel positive airway pressure therapy (spontaneous mode) is superior to continuous positive airway pressure therapy in reversing hypercapnic respiratory failure among patients with both obesity and airways disease. As these patients are often excluded from clinical trials in obesity hypoventilation syndrome or hypercapnic chronic obstructive pulmonary disease, this is the first randomized clinical trial comparing different modes of positive airway pressure therapy studying this population.
INTRODUCTION
Obesity and chronic obstructive pulmonary disease (COPD) are both common conditions with increasing prevalence worldwide. The reported proportion of patients with coexisting COPD and obesity varies between different cohorts and is likely influenced by COPD phenotype and the severity of the airflow limitation.1 Available data suggest that obesity is more prevalent in patients with COPD than the general population.1
Both COPD and obesity place significant strain on the respiratory system. Altered lung mechanics, increased burden of respiration, and reduced respiratory muscle efficiency are some of the shared physiological derangements.2–4 Although most patients with COPD or obesity can compensate, maintain ventilation, and remain eucapnic, a minority will develop chronic hypoventilation (hypercapnic COPD or obesity hypoventilation syndrome). Sleep can further exacerbate the dysfunction as it leads to reduced respiratory motor neuron output, increased upper airway resistance (ie, obstructive sleep apnea, OSA), and diminished chemoreceptor sensitivity.5 The pathophysiological effects of both COPD and obesity are not well studied, but their coexistence likely increases the risk of developing chronic hypercapnia.
The treatment of chronic respiratory failure has been examined in randomized clinical trials for both obesity hypoventilation syndrome (OHS) and chronic hypercapnic COPD. Continuous positive airway pressure (CPAP) therapy is equivalent to bilevel positive airway pressure (BPAP) therapy in stable OHS with concurrent severe OSA,6–9 while BPAP is the preferred option in OHS without severe OSA.10 In COPD with concurrent OSA (overlap syndrome), CPAP is the therapy of choice, including those with awake hypercapnia, with studies showing improvements in PaCO2.11–13 CPAP has also been associated with reduced hospitalization and mortality14 but has not been directly compared to BPAP in this cohort. Patients who have both obesity and obstructive airways disease contributing to their hypoventilation are often excluded from clinical trials—patients with a forced expiratory ratio < 0.7 are excluded from OHS trials,7–9 while the presence of obesity or OSA often results in exclusion in long-term BPAP trials in pure COPD.15 A randomized clinical trial has not been previously conducted to directly compare different positive airway pressure (PAP) modalities in this particular cohort of patients.
In this pilot clinical trial the primary goal was to compare the efficacy of BPAP and CPAP spontaneous (S) mode in reversing ventilatory failure (reduction in PaCO2) over a 3-month treatment period among obese patients with hypoventilation disorder and concurrent obstructive airways disease. Secondary outcomes included changes in spirometry indices, weight, health-related quality of life, quality of sleep, and sleepiness.
METHODS
Participants
Patients with obesity (body mass index [BMI] > 30 kg/m2) and stable daytime hypercapnia (PaCO2 > 45 mm Hg) presenting to the Sleep Disorders Centre, Royal Prince Alfred Hospital, were screened for suitability for trial enrollment. Those without significant respiratory or neuromuscular disorders were diagnosed as OHS and recruited for other studies. Participants in whom an obstructive ventilatory defect on spirometry was found (ratio of forced expiratory volume in 1 second/forced vital capacity or forced expiratory ratio < 0.7) or clinician-diagnosed COPD were invited to participate in the current study. Other inclusion criteria included (1) no neuromuscular or chest wall skeletal disorders, (2) not currently being treated with PAP therapy, and (3) no major psychiatric illness or unstable medical conditions that would affect the patient’s ability to participate in the trial. Individuals did not need to have symptoms of sleep-disordered breathing to be included in this trial.
Study design
This study was designed as a single-blinded randomized control trial with two parallel groups, comparing Bilevel PAP (S mode) with CPAP over 3 months. The Bilevel PAP group received noninvasive ventilation using an S mode of ventilatory support. The CPAP group used a fixed-pressure CPAP mode. The protocol included a planned change to Bilevel PAP in the event of treatment failure in the CPAP group. Treatment failure was defined by (1) oxygen saturation remaining below 80% continuously for 10 minutes, (2) a rise in transcutaneous CO2 > 10 mm Hg during rapid eye movement sleep, or (3) an increase in awake CO2 of 10 mm Hg despite PAP use. Polysomnography was used to titrate PAP settings at randomization.
The Human Research Ethics Committees at the Royal Prince Alfred Hospital approved the project, and all participants provided written informed consent. This study was registered at anzctr.org as part of ACTRN12605000096651.
Randomization and masking
Demographic information, anthropomorphic data, medical history, and medications were collected at recruitment. Spirometry, baseline diagnostic sleep study, and daytime seated arterial blood gases were measured to assess eligibility criteria. Following baseline data collection, participants were randomly assigned (1:1) using block randomization and computer-generated sequence. Allocation concealment was maintained using sequentially numbered opaque, sealed envelopes. Participants were blinded to their allocated treatment arm.
Interventions
All patients had laboratory-based (type 1) diagnostic and PAP titration studies using commercially available digital sleep systems following recognized guidelines and scored according to Rechtschaffen and Kales scoring classification by experienced sleep scientists unaware of the patient’s involvement in the trial. Additional monitoring of transcutaneous carbon dioxide was performed. Further details are included in the supplemental material.
Following randomization and on a separate night from the diagnostic study all participants underwent a conventional in-laboratory titration study using the PAP mode corresponding to their allocated arm (either fixed CPAP or Bilevel S mode). For CPAP titration, pressure was manually increased in 1 cm H2O increments with the aim of preventing obstruction, flow limitation, desaturation, and arousal. For BPAP titration, expiratory PAP was increased in 1 cm H2O increments with the aim of abolishing obstructive events and if inspiratory efforts did not consistently trigger inspiratory PAP, while the inspiratory PAP was initially set 4 cm H2O higher than expiratory PAP and then increased to eliminate hypopneas and improve saturation. Supplemental oxygen was added at 1–2 L/min to maintain SpO2 > 90% if SpO2 remained below 88% in nonrapid eye movement sleep during patients’ allocated treatment study despite optimization of ventilation or at maximum pressure that eliminated obstructive apneic or hypopneic events.
Participants were prescribed the titrated settings as determined by their PAP titration studies. In addition to the usual standard of care (management of their airways disease, instructions on lifestyle modification), patients were instructed to use their allocated PAP device nightly and received education as per usual clinical care. Patients were contacted at 2 weeks following initiation of therapy and encouraged to call the clinical service at any time if problems or queries arose.
Instrumentation and measurements
Participants were evaluated on two occasions, at baseline and after 3 months from initiating PAP treatment. The primary outcome PaCO2 was assessed by daytime arterial blood gas analysis. Secondary outcomes assessed included other arterial blood gas parameters, anthropometric data, spirometry indices, compliance, Epworth Sleepiness Scale,16 sleep quality using the Pittsburgh Sleep Quality Index,17 and health-related quality of life using the Medical Outcomes Survey Short Form-36 (SF-36).18 Neurocognitive evaluations included a psychomotor vigilance test (sustained attention, reaction time), digit span task (working memory), trail-making test (executive functioning), and digit symbol substitution test (cognitive dysfunction). Procedural details are included in the supplemental material.
Statistical analysis
An a priori power calculation suggested that a sample size of 13 in each group would be needed to detect a difference in the mean change in arterial CO2 of 7 mm Hg with a power of 80% and a P < .05. Additional patients were recruited to allow for dropouts.
Baseline characteristics were expressed as mean and SD or percentages with 95% confidence intervals and compared using Student t test and χ2 analysis, respectively. Intention-to-treat analysis was performed. Analysis of primary and secondary endpoints and exploratory neurocognitive tests were done using a linear mixed model for repeated measures. The model included fixed categorical effects for treatment arm (BPAP or CPAP), time (baseline and 3 months), and their interaction and a random intercept, adjusted for covariates (baseline values of age, BMI, absolute forced expiratory volume in 1 second [FEV1], and apnea-hypopnea index). Posthoc analysis using linear mixed models was also performed with additional covariates (baseline PaCO2 and PAP adherence) for the primary outcome. These covariates were selected as they were known or anticipated important prognostic variables that can affect outcome comparisons. There was no imputation for missing data as a mixed-effects model was used for analysis. The differences in mean, 95% confidence interval, and P value were reported with the significance test based on a two-sided α of 0.05. An additional analysis of covariance was performed for the primary outcome with adjustment for PAP adherence. PAP adherence was compared using an unpaired t test. Data management was performed using SPSS software IBM Corp. released 2017. IBM SPSS Statistics for Macintosh, Version 25.0. Armonk, NY: IBM Corp.
RESULTS
Participant characteristics
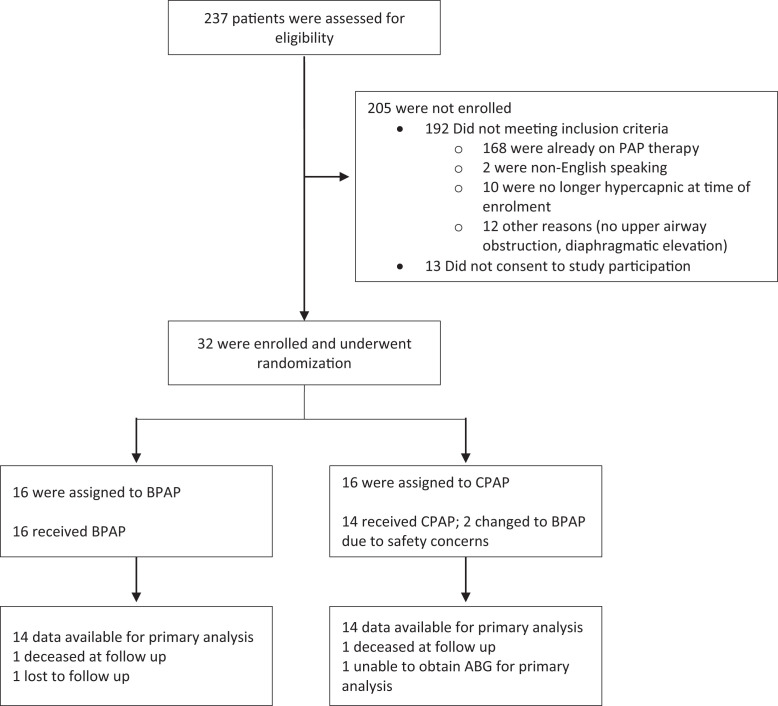
Between December 2003 and February 2012, a total of 237 patients were assessed for inclusion criteria. A total of 32 participants met eligibility criteria and enrolled to the study (Figure 1).
Figure 1. Enrollment and randomization.
ABG = arterial blood gas, BPAP = bilevel positive airway pressure, CPAP = continuous positive airway pressure, PAP = positive airway pressure.
Table 1 summarizes the baseline demographic data. The mean age across both groups was 61 (± 11) years. All but 1 patient had moderate or severe OSA, with a mean apnea-hypopnea index of 59 (± 35) events/h. The mean BMI was 43 (± 7) kg/m2, with the majority of patients having class III obesity (20/32 had BMI > 40 kg/m2). The mean FEV1 was 1.4 (± 0.6) L. Baseline age and forced vital capacity were lower in the BPAP arm. There were more males in the CPAP arm, whereas sex distribution was even in the BPAP arm. There were no significant differences in baseline PaCO2, FEV1, and BMI between groups. The mean titrated settings were inspiratory PAP 15.8 cm H2O and expiratory PAP 9.7 cm H2O for the BPAP arm and 12.7 cm H2O for the CPAP arm. Table 2 compares the polysomnography data from the diagnostic study with titration studies (at recruitment and trial exit). During the initial titration study, 5 participants of the CPAP arm (mean = 1.4 L/min) and 6 participants of the BPAP arm (mean = 1.7 L/min) required and were prescribed supplemental oxygen in addition to their PAP therapy.
Table 1.
Baseline characteristics.
| BPAP (n = 16) | CPAP (n = 16) | All (n = 32) | |
|---|---|---|---|
| Age (y) | 57 (9.7) | 65 (10.7) | 61 (10.9) |
| Sex (% female) | 50 | 13 | 31 |
| BMI (kg/m2) | 45 (7.8) | 40 (5.8) | 43 (7.2) |
| Neck circumference (cm) | 46 (4.9) | 49 (3.5) | 47 (4.4) |
| Waist circumference (cm) | 132 (10.2) | 133 (10.7) | 133 (10.3) |
| Hip circumference (cm) | 137 (14.1) | 131 (14.8) | 134 (14.5) |
| Spirometry | |||
| FEV1 (L) | 1.3 (0.6) | 1.5 (0.5) | 1.4 (0.6) |
| FEV1 (% predicted) | 45 (19.8) | 51 (17.5) | 48 (18) |
| FVC (L) | 2.0 (0.9) | 2.6 (0.7) | 2.3 (0.8) |
| FVC (% predicted) | 58 (23) | 68 (20) | 64 (22) |
| FER (%) | 62 (8) | 59 (11) | 60 (10) |
| ABG | |||
| PaCO2 (mm Hg) | 57 (8) | 52 (5.6) | 54 (7.4) |
| PaO2 (mm Hg) | 60 (11) | 57 (9) | 59 (10) |
| Bicarbonate (mmol) | 33 (5) | 31 (3.8) | 32 (4.6) |
| Base excess (mmol) | 6.5 (4.5) | 5.5 (3.4) | 6 (4.0) |
| pH | 7.39 (0.03) | 7.39 (0.03) | 7.39 (0.03) |
| PSG | |||
| AHI (events/h) | 57 (34) | 61 (37) | 59 (35) |
| % TST < 90% | 93 (70–99) | 84 (58–96) | 85 (68–98) |
| % TST < 80% | 28 (17–47) | 17 (5–37) | 23 (7–43) |
| % NREM sleep | 90 (9) | 87 (9) | 89 (9) |
| % REM sleep | 10 (9) | 13 (9) | 11 (9) |
| TcCO2 mean (mm Hg) | 57 (13) | 50 (6) | 54 (11) |
| TcCO2 peak (mm Hg) | 69 (15) | 65 (9) | 67 (12) |
Values displayed as mean (standard deviation), except for % TST < 90% and % TST < 80%, which are displayed as median (interquartile range). Please note TcCO2 data were only available for 7 patients in the BPAP arm and 5 patients in the CPAP arm. ABG = arterial blood gas, AHI = apnea-hypopnea index, BMI = body mass index, BPAP = bilevel positive airway pressure, CPAP = continuous positive airway pressure, FER = forced expiratory ratio, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, NREM = nonrapid eye movement, PSG = polysomnography, REM = rapid eye movement, TcCO2, transcutaneous CO2, TST = total sleep time.
Table 2.
PSG summary comparison (baseline vs during titration studies).
| BPAP (n = 16) | CPAP (n = 16) | |
|---|---|---|
| Age (y) | 57 (9.7) | 65 (10.7) |
| Sex (% female) | 50 | 13 |
| BMI (kg/m2) | 45 (7.8) | 40 (5.8) |
| Baseline PSG | ||
| AHI (events/h) | 57 (34) | 61 (37) |
| % TST < 90% | 93 (70–99) | 84 (58–96) |
| % TST < 80% | 28 (12–47) | 17 (5–37) |
| Nadir SpO2 (%) | 47 (40–64) | 52 (45–64) |
| Titration study 1 | ||
| % TST < 90% | 44 (4–85) | 67 (37) |
| % TST < 80% | 0 (0–0) | 19 (27) |
| Nadir SpO2 (%) | 82 (78–86) | 66 (16) |
| Titration study 2 | ||
| AHI (events/h) | 7 (9) | |
| % TST < 90% | 15 (2–42) | |
| % TST < 80% | 0 (0–0) | |
| Nadir SpO2 (%) | 84 (81–86) | |
Baseline diagnostic PSG and titration study 1 (16 patients in each arm) were done at time of recruitment, and titration study 2 was done after exit from trial (data from 17 patients available). The majority of titration study 2 were CPAP studies. The age, BMI, and AHI are displayed as mean (standard deviation). The % TST < 90%, % TST < 80%, and nadir SpO2 are displayed as median (interquartile range). AHI = apnea-hypopnea index, BMI = body mass index, BPAP = bilevel positive airway pressure, CPAP = continuous positive airway pressure, PSG = polysomnography, TST = total sleep time.
Primary outcome
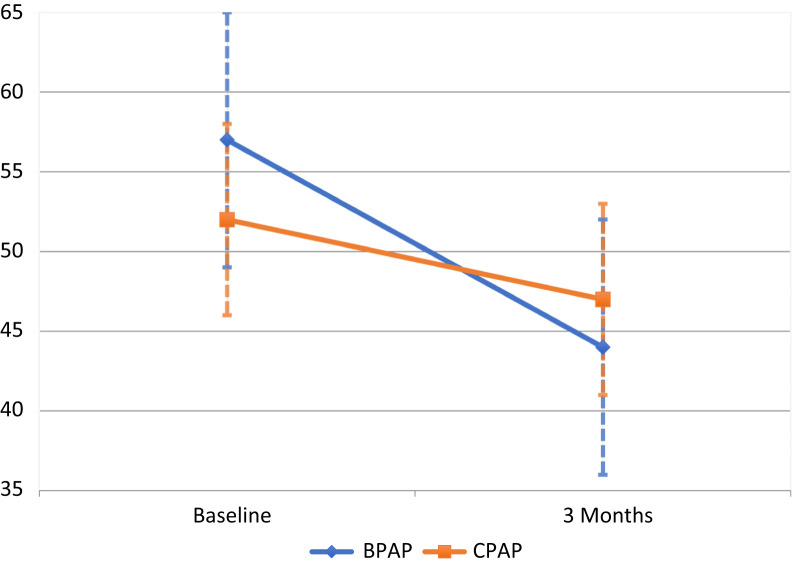
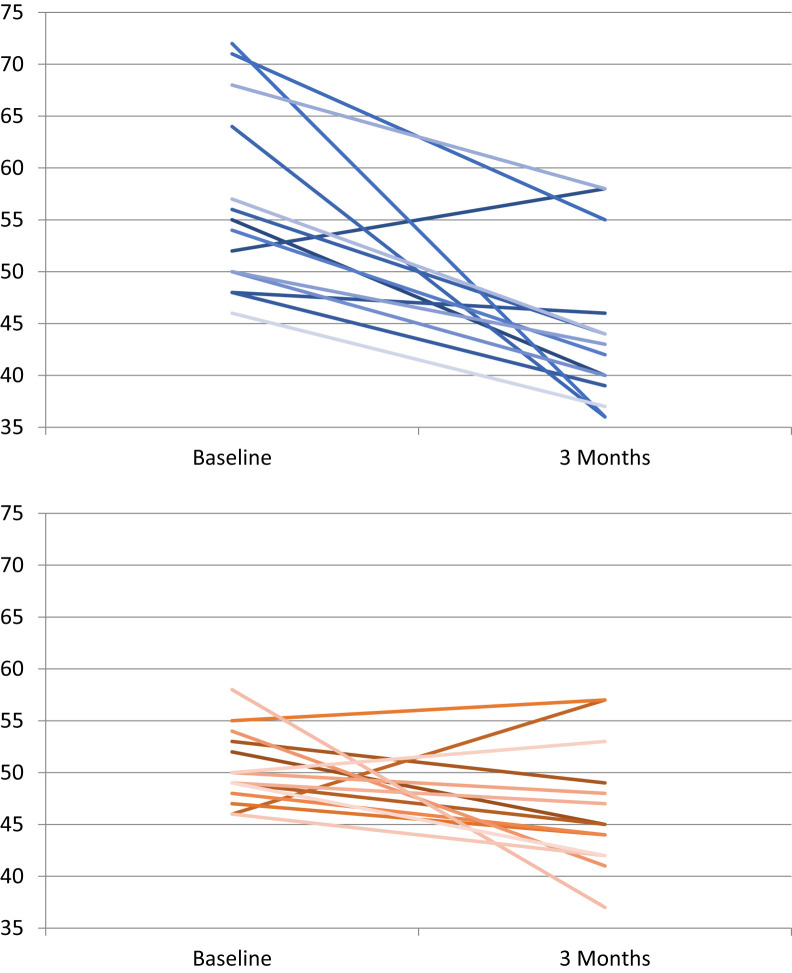
The intergroup analysis indicated that BPAP S mode was superior to CPAP in reducing PaCO2 (9.4 mm Hg, (95% confidence interval, 4.3–15 mm Hg, P = .001; see Table 3). The BPAP advantage persisted with additional adjustment for baseline PaCO2 and PAP adherence (9.6 mm Hg, 95% confidence interval, 2.1–17 mm Hg, P = .01). There was a significant improvement in PaCO2 in the BPAP arm (P < .01) and CPAP arm (P < .05) (Figure 2 and Figure 3). Two patients in the CPAP arm were switched to BPAP due to safety concerns based on their CPAP titration study (details in supplemental material) but were analyzed as per their allocated arm during randomization. Inclusion of these two patients within the BPAP arm during analysis did not alter the intergroup comparison. Ten participants in the BPAP arm and eight participants in the CPAP arm had a PaCO2 within the normal range at the end of 3 months.
Table 3.
Baseline measurements and changes with treatment related to outcomes of pulmonary function and weight.
| BPAP, Mean (SD) | CPAP, Mean (SD) | Intergroup Differences† | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 Months | Baseline | 3 Months | Mean (CI) | P Value | |
| ABG | ||||||
| PaCO2 (mm Hg) | 57 (8) | 44 (8)** | 52 (6) | 47 (6)* | 9.4 (4.3 to 15) | .001 |
| Bicarbonate (mmol/L) | 33 (5) | 27 (3)** | 31 (3) | 28 (3)* | 4.8 (1.8 to 7.8) | .004 |
| Spirometry | ||||||
| FEV1 (L) | 1.3 (0.6) | 1.6 (0.7) | 1.5 (0.5) | 1.6 (0.5) | −0.3 (−0.5 to −0.0) | .04 |
| FVC (L) | 2.0 (0.9) | 2.3 (0.9) | 2.6 (0.7) | 2.5 (0.6) | −0.5 (−0.9 to −0.0) | .04 |
| Weight (kg) | 125 (28) | 124 (28) | 120 (19) | 120 (19) | 2.8 (−2.0 to 7.7) | NS |
Adjusted for baseline values of the variables analyzed and age, sex, BMI, AHI, and FEV1. *P < .05 intragroup difference (3 months–baseline). **P < .01 intragroup difference (3 months–baseline). ABG = arterial blood gas, AHI = apnea-hypopnea index, BMI = body mass index, BPAP = bilevel positive airway pressure, CI = confidence interval, CPAP = continuous positive airway pressure, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, NS = not significant, SD = standard deviation.
Figure 2. Change in mean PaCO2 (mm Hg) after 3 months of positive airway pressure therapy compared to baseline.
Dashed whiskers represent standard deviation. BPAP = bilevel positive airway pressure, CPAP = continuous positive airway pressure.
Figure 3. Change in individual PaCO2 (mm Hg) values before (baseline) and after (3 months) positive airway pressure therapy.
Blue: bilevel positive airway pressure therapy. Red: continuous positive airway pressure therapy.
Secondary outcomes
There was a greater improvement in both FEV1 and forced vital capacity in the BPAP arm compared to the CPAP arm (0.3 L and 0.5 L, respectively). BPAP was also associated with a significant improvement in the mental component of SF-36 compared to CPAP. There were no significant intergroup differences in other secondary outcomes summarized in Table 3, Table 4, and Table 5).
Table 4.
Baseline measurements and changes with treatment related to quality of life, quality of sleep, and sleepiness scores.
| BPAP, Mean (SD) | CPAP, Mean (SD) | Intergroup Differences† | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 Months | Baseline | 3 Months | Mean (CI) | P Value | |
| ESS | 12 (6) | 4 (3)** | 13 (5) | 8 (6)* | 2.8 (−1.4 to 7.9) | NS |
| Global PSQI | 12 (4) | 6 (3)** | 10 (5) | 8 (4) | 3.8 (−0.5 to 8.2) | NS |
| SF-36 | ||||||
| Physical component | 32 (12) | 35 (12) | 32 (11) | 32 (11) | −2.5 (−11 to 6.5) | NS |
| Mental component | 19 (13) | 39 (17)** | 34 (17) | 41 (15) | −15 (−29 to −1.4) | .03 |
Adjusted for baseline values of the variables analyzed and age, sex, BMI, AHI, and FEV1. *P < .05 intragroup difference (3 months–baseline). **P < .01 intragroup difference (3 months–baseline). AHI = apnea-hypopnea index, BMI = body mass index, BPAP = bilevel positive airway pressure, CI = confidence interval, CPAP = continuous positive airway pressure, ESS = Epworth Sleepiness Scale, FEV1 = forced expiratory volume in 1 second, NS = not significant, PSQI = Pittsburgh Sleep Quality Index, SD = standard deviation, SF-36 = Medical Outcome Survey Short Form-36.
Table 5.
Baseline measurements and changes with treatment related to outcomes of neurocognitive tests.
| BPAP, Mean (SD) | CPAP, Mean (SD) | Intergroup Differences† | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 Months | Baseline | 3 Months | Mean (CI) | P Value | |
| PVT | ||||||
| Lapses | 13 (13) | 8 (13) | 5 (7) | 5 (9) | 5.5 (−2.0 to 13) | NS |
| Median: RT (ms) | 361 (97) | 315 (80) | 316 (60) | 297 (54) | 35 (−28 to 98) | NS |
| Mean slowest 10% RT (s) | 1.6 (0.7) | 2.1 (0.6)* | 2.1 (0.5) | 2.3 (0.6) | −0.4 (−0.8 to 0.0) | NS |
| Digit span forward | 7 (2) | 8 (2) | 7 (2) | 8 (2) | 0.5 (−0.8 to 1.9) | NS |
| Digit span backward | 5 (2) | 5 (2) | 5 (1) | 6 (2) | 1 (−0.8 to 2.5) | NS |
| Trail-making test | 129 (39) | 116 (42) | 134 (61) | 116 (45) | −13 (−51 to 24) | NS |
| Digit symbol substitution | 36 (12) | 39 (16) | 35 (10) | 40 (11) | −0.4 (−8.0 to 7.2) | NS |
Adjusted for baseline values of the variables analyzed and age, sex, BMI, AHI, and FEV1. *P < .05 intragroup difference (3 months–baseline). AHI = apnea-hypopnea index, BMI = body mass index, BPAP = bilevel positive airway pressure, CI = confidence interval, CPAP = continuous positive airway pressure, FEV1 = forced expiratory volume in 1 second, NS = not significant, PVT = psychomotor vigilance test, RT = reaction time, SD = standard deviation.
The mean weight did not change in either group with therapy. Both BPAP and CPAP reduced daytime sleepiness measured by the Epworth Sleepiness Scale (P < .01) at 3 months compared to baseline. The BPAP arm also showed improvements in sleep quality using the Pittsburgh Sleep Quality Index (P < .05) and mental component of SF-36 in the BPAP arm (P < .01) at 3 months compared to baseline.
Exploratory neurocognitive testing did not identify significant intergroup differences in performance. BPAP improved the mean of the slowest 10% reaction times on the psychomotor vigilance test.
The mean adherence calculated at the end of the 3-month therapy period was 4.1 (± 2.5) hours (BPAP) and 5.6 (± 2.3) hours (CPAP) (P = .10). Eight out of 14 patients in the BPAP arm and 10 out of 15 patients in the CPAP arm had average use ≥ 4 hours at the end of trial download. No major therapy-related adverse effect was reported.
DISCUSSION
To our knowledge, this is the first randomized controlled trial that compares PAP therapies in patients with chronic hypercapnic respiratory failure in the setting of concurrent obesity and COPD. Compared to baseline, 3 months of PAP therapy resulted in significant decreases in PaCO2 in both BPAP and CPAP arms, with BPAP S mode superior to CPAP in reducing PaCO2 in our study population. BPAP allocation was also associated with a greater improvement in FEV1, forced vital capacity, and the mental component of SF-36 when compared to CPAP. There were no significant differences between the two arms in other secondary outcomes and PAP adherence.
In clinical trials involving OHS patients with severe OSA and without airways disease (forced expiratory ratio > 0.7), BPAP and CPAP had similar efficacy in improving ventilatory failure.7–9 As sleep apnea plays an important role in the development of hypercapnia in this OHS phenotype, CPAP is effective in resolving upper airway obstruction. CPAP also has a volume-inflation effect, which improves V/Q mismatch due to small airway closure2 and over time improves central ventilatory drive.19 In contrast, the addition of nocturnal BPAP in addition to usual care is recommended in chronic stable hypercapnic COPD patients,15,20 where BPAP is thought to provide improved minute ventilation, resting of fatigued respiratory muscle, and better V/Q matching.3,21 Studies of OHS patients exclude those with concurrent lung disease,7–9 while studies of hypercapnic COPD do not include patients with OSA.15 As a consequence, few studies have reported outcomes of PAP therapy in patients with hypercapnic OSA and moderate to severe lung disease. In OHS and in overlap syndrome, predictors of CPAP failure include less severe OSA, reduced lung function, and more sleep time spent in SpO2 < 90%.2,21 Although the majority of our study participants (26/32) had severe OSA (a mean baseline apnea-hypopnea index 59 events/h), they also had severe impairment in their lung function and a large portion of their total sleep time was spent with SpO2 < 90% in their diagnostic study. It appears that while CPAP is an effective alternative to BPAP in the majority of obese hypercapnic patients the added burden of airways disease favors treatment with BPAP in reducing awake PaCO2 in our participants, even though compliance with therapy was lower.
When comparing study population characteristics of this study to that of OHS or hypercapnic COPD the additive effects of obesity-driven sleep apnea and lung disease are apparent. At a similar baseline PaCO2, our study population had a lower baseline BMI compared to OHS clinical trials8,9 but better lung function (FEV1) compared to hypercapnic COPD trials.22 As expected, nearly all our participants (31/32) would fit the definition of overlap syndrome (presence of both COPD and OSA).
PAP therapy, in particular BPAP, has been associated with improved lung function7,11,12,20,23 and reduction in weight7–9,12 in some of the COPD and OHS trials, but no significant change in spirometry indices or weight were observed in our study. Participants in the BPAP arm did have a more significant change in spirometry indices than the CPAP arm, however it is not clear if this is related to having lower baseline values and whether their airways disease was optimally controlled at the time of enrollment.
Our data suggest that this cohort of patients is subject to a high burden of symptoms. They have increased daytime sleepiness, poor quality of sleep, and low health-related quality of life. Baseline daytime sleepiness, as assessed by the Epworth Sleepiness Scale, was comparable to OHS populations7–9 as well as nonhypercapnic overlap syndrome24 with similar improvements with treatment. Our cohort had worse mental health status (mental component of SF-36 Health Survey) than that reported in nonhypercapnic overlap syndrome and pure COPD.25 BPAP but not CPAP therapy was associated with improvement in mental health, although it is not clear if this was due to lower baseline mental component SF-36 scores in the BPAP arm.
Prevalence of cognitive impairment is higher in patients with COPD than in healthy controls and appears to correlate with the severity of disease.26 Although less explored, neurocognitive impairment is also seen in OHS and is more common than in healthy and obese controls.27 The underlying mechanism is not well understood, but contributing factors include hypoxia, hypercapnia, and sleep fragmentation.28 Results from our cohort did not differ significantly from previous reports of neuropsychological function in OSAS and COPD groups.29,30 PAP therapy over 3 months did not appear to improve neurocognitive test performances significantly, a finding similar to that with an OHS cohort over the same treatment period.31
Benefits from PAP therapy are dependent on good adherence. PAP adherence is an independent predictor of mortality in both overlap syndrome and OHS.32,33 The average adherence to PAP was similar to other clinical trials in hypoventilation disorders.7–9,22 Although the expectation is that BPAP would be better tolerated given a lower exhalation pressure, adherence was not significantly different between the two arms, with participants on CPAP showing a slightly higher average hours of use. This is despite patients in the BPAP arm reporting better within-group improvements in sleep quality compared to the CPAP group.
There are several limitations to this trial. This study specifically recruited a population with hypercapnia, and thus the results of the data apply only to this subset of obese patients with concurrent COPD. Although nearly our entire cohort had by definition overlap syndrome, the study conclusion cannot be applied to the general overlap syndrome population, where the majority of patients are not hypercapnic or obese and CPAP is likely to be sufficient. The severity of airway obstruction and therefore contribution of coexisting airways disease to hypoventilation would have varied between recruited individuals. A subgroup of patients with mild airways disease and severe OSA may do equally well on either PAP therapy. The recruited participants are from a single site and may lack applicability to other population centers. While patients were blinded to their allocated therapy, investigators were not, and this could have introduced biases.
Many of the treatment effects found were from within-group analysis. These findings are not conclusive and will need to be replicated in larger studies. The study population was small and not powered for secondary endpoints. As the studied population has a high burden of disease and other medical comorbidities, they are challenging to recruit and retain—2 participants were lost to follow-up due to death unrelated to respiratory failure/PAP treatment. A control (non-PAP) arm was not included in the study design as the majority of patients likely to be recruited were expected to be symptomatic from their sleep apnea/ventilatory failure.
Recruitment for the study was slower than anticipated, with many patients presenting with acute respiratory decompensation or referred for review after already being established on either CPAP or BPAP therapy by other centers. There has been a delay in data analysis and reporting of results after the completion of recruitment.
A spontaneous mode of BPAP was used in this study, and other modes using a backup rate or volume-assured pressure support may have been even more effective. During the time when the trial was conducted the PAP devices were not able to provide data on residual apnea-hypopnea index on treatment.
Despite randomization, there were intergroup differences in baseline measurements (age, sex, and BMI). Statistical analysis was able to adjust for differences in these covariates. Although not statistically significant, the relatively higher baseline PaCO2 would favor BPAP’s overall treatment effect. Although the participants were not informed of their PAP treatment allocation the PAP devices are not masked, and in theory participants still had the ability to unblind themselves as the PAP machines in the two treatment arms were not physically identical.
Future studies will need a larger sample size through multicenter trials and be powered to measure clinically meaningful outcomes such as hospitalizations, cardiovascular outcomes, and mortality over more extended periods. Further phenotyping studies are required to help determine biomarkers that will predict optimal initial PAP modality. The optimal pressure targets are unclear in this group of patients. Recent COPD trials suggest a benefit of larger driving pressure, while a higher expiratory PAP is required to offset the OSA, both of which need to be balanced with patient acceptance and adherence to therapy along with the possible impact of higher leak on the effectiveness of therapy.
CONCLUSIONS
In the single-center pilot randomized controlled trial, BPAP was more effective in improving PaCO2 than CPAP in patients with hypoventilation disorder in the setting of obesity and concurrent obstructive airways disease. BPAP also resulted in a greater change in lung function (FEV1 and forced vital capacity) and quality of life (mental component of SF-36) when compared to CPAP. There were no significant differences between groups in weight loss, daytime sleepiness, sleep quality, neurocognitive testing, or adherence over 3 months.
ABBREVIATIONS
- BMI,
body mass index
- BPAP,
bilevel positive airway pressure
- COPD,
chronic obstructive pulmonary disease
- CPAP,
continuous positive airway pressure
- FEV1,
forced expiratory volume in 1 second
- OHS,
obesity hypoventilation syndrome
- OSA,
obstructive sleep apnea
- PAP,
positive airway pressure
- S mode,
spontaneous mode
- SF-36,
Medical Outcomes Survey Short Form 36
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Royal Prince Alfred Hospital. Ronald Grunstein is an NHMRC research fellow. The authors report no conflicts of interest.
REFERENCES
- 1. Franssen FM, O’Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. obesity and COPD. Thorax. 2008; 63( 12): 1110– 1117. [DOI] [PubMed] [Google Scholar]
- 2. Piper A. Obesity hypoventilation syndrome: weighing in on therapy options. Chest. 2016; 149( 3): 856– 868. [DOI] [PubMed] [Google Scholar]
- 3. Budweiser S, Jörres RA, Pfeifer M. Treatment of respiratory failure in COPD. Int J Chron Obstruct Pulmon Dis. 2008; 3( 4): 605– 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calverley PM. Respiratory failure in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003; 47: 26s– 30s. [DOI] [PubMed] [Google Scholar]
- 5. McNicholas WT, Hansson D, Schiza S, Grote L. Sleep in chronic respiratory disease: COPD and hypoventilation disorders. Eur Respir Rev. 2019; 28( 153): 190064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masa JF, Mokhlesi B, Benítez I, et al.; Spanish Sleep Network. Long-term clinical effectiveness of continuous positive airway pressure therapy versus non-invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open-label, randomised controlled trial. Lancet. 2019; 393( 10182): 1721– 1732. [DOI] [PubMed] [Google Scholar]
- 7. Masa JF, Corral J, Alonso ML, et al.; Spanish Sleep Network. Efficacy of different treatment alternatives for obesity hypoventilation syndrome. Pickwick study. Am J Respir Crit Care Med. 2015; 192( 1): 86– 95. [DOI] [PubMed] [Google Scholar]
- 8. Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008; 63( 5): 395– 401. [DOI] [PubMed] [Google Scholar]
- 9. Howard ME, Piper AJ, Stevens B, et al. A randomised controlled trial of CPAP versus non-invasive ventilation for initial treatment of obesity hypoventilation syndrome. Thorax. 2017; 72( 5): 437– 444. [DOI] [PubMed] [Google Scholar]
- 10. Masa JF, Corral J, Caballero C, et al.; Spanish Sleep Network. Non-invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnoea. Thorax. 2016; 71( 10): 899– 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mansfield D, Naughton MT. Effects of continuous positive airway pressure on lung function in patients with chronic obstructive pulmonary disease and sleep disordered breathing. Respirology. 1999; 4( 4): 365– 370. [DOI] [PubMed] [Google Scholar]
- 12. de Miguel J, Cabello J, Sánchez-Alarcos JM, Alvarez-Sala R, Espinós D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath. 2002; 6( 1): 3– 10. [DOI] [PubMed] [Google Scholar]
- 13. Toraldo DM, De Nuccio F, Nicolardi G. Fixed-pressure nCPAP in patients with obstructive sleep apnea (OSA) syndrome and chronic obstructive pulmonary disease (COPD): a 24-month follow-up study. Sleep Breath. 2010; 14( 2): 115– 123. [DOI] [PubMed] [Google Scholar]
- 14. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010; 182( 3): 325– 331. [DOI] [PubMed] [Google Scholar]
- 15. Ergan B, Oczkowski S, Rochwerg B, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J. 2019; 54( 3): 1901003. [DOI] [PubMed] [Google Scholar]
- 16. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991; 14( 6): 540– 545. [DOI] [PubMed] [Google Scholar]
- 17. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28( 2): 193– 213. [DOI] [PubMed] [Google Scholar]
- 18. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992; 30( 6): 473– 483. [PubMed] [Google Scholar]
- 19. Chau EH, Lam D, Wong J, Mokhlesi B, Chung F, Warner DS. Obesity hypoventilation syndrome: a review of epidemiology, pathophysiology, and perioperative considerations. Anesthesiology. 2012; 117( 1): 188– 205. [DOI] [PubMed] [Google Scholar]
- 20. Macrea M, Oczkowski S, Rochwerg B, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020; 202( 4): e74– e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajian B, De Backer J, Sneyers C, et al. Pathophysiological mechanism of long-term noninvasive ventilation in stable hypercapnic patients with COPD using functional respiratory imaging. Int J Chron Obstruct Pulmon Dis. 2017; 12: 2197– 2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA. 2017; 317( 21): 2177– 2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014; 2( 9): 698– 705. [DOI] [PubMed] [Google Scholar]
- 24. Economou N-T, Ilias I, Velentza L, et al. Sleepiness, fatigue, anxiety and depression in chronic obstructive pulmonary disease and obstructive sleep apnea - overlap - syndrome, before and after continuous positive airway pressure therapy. PLoS One. 2018; 13( 6): e0197342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soler X, Gaio E, Powell FL, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015; 12( 8): 1219– 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torres-Sánchez I, Rodríguez-Alzueta E, Cabrera-Martos I, López-Torres I, Moreno-Ramírez MP, Valenza MC. Cognitive impairment in COPD: a systematic review. J Bras Pneumol. 2015; 41( 2): 182– 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Argun Baris S, Tuncel D, Ozerdem C, et al. The effect of positive airway pressure therapy on neurocognitive functions, depression and anxiety in obesity hypoventilation syndrome. Multidiscip Respir Med. 2016; 11( 1): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andreou G, Vlachos F, Makanikas K. Effects of chronic obstructive pulmonary disease and obstructive sleep apnea on cognitive functions: evidence for a common nature. Sleep Disord. 2014;2014: 768210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roehrs T, Merrion M, Pedrosi B, Stepanski E, Zorick F, Roth T. Neuropsychological function in obstructive sleep apnea syndrome (OSAS) compared to chronic obstructive pulmonary disease (COPD). Sleep. 1995; 18( 5): 382– 388. [DOI] [PubMed] [Google Scholar]
- 30. Lv Z, Hu P, Jiang Y, et al. Changes in spatial working memory in stable chronic obstructive pulmonary disease: a retrospective study. BioMed Res Int. 2020;2020: 7363712. [DOI] [PMC free article] [PubMed]
- 31. Sivam S, Poon J, Wong KKH, et al. Slow-frequency electroencephalography activity during wake and sleep in obesity hypoventilation syndrome. Sleep. 2020; 43( 2): zsz214. [DOI] [PubMed] [Google Scholar]
- 32. Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013; 9( 8): 767– 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castro-Añón O, Pérez de Llano LA, De la Fuente Sánchez S, et al. Obesity-hypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS One. 2015; 10( 2): e0117808. [DOI] [PMC free article] [PubMed] [Google Scholar]