Abstract
Study Objectives:
Children with Down syndrome (DS) are at risk of obstructive sleep apnea (OSA), but the access to sleep lab polysomnography (PSG) is limited. Simplified techniques are needed, such as polygraphy coupled with pulse transit time (PTT-PG) that detects respiratory events and the total autonomic arousals index (PTTAI). Our objective was to assess the ability of PTT-PG compared with PSG to diagnose OSA in children with DS.
Methods:
In this prospective multicenter study, patients with DS underwent a full-night PSG coupled with PTT. Sleep questionnaires (Sleep Disturbance Scale for Children and Pediatric Sleep Questionnaire) were filled by parents. PSG and PTT-PG results were compared to test their sensibility and specificity to diagnose OSA.
Results:
A total of 53 patients with DS were included; their median age was 9.3 years. An obstructive apnea-hypopnea index (OAHI) by PSG > 1 event/h was found in 36 (68%) patients, OAHI was > 1 and < 5 events/h in 18 patients (34%), ≥ 5 and < 10 events/h in 11 patients (21%), and ≥ 10 events/h in 7 patients (13%). OAHI was larger on PSG than on PTT-PG (P = .0005). For OSA diagnosis, the sensitivity was excellent for OAHI by PTT-PG if the added total PTTAI was > 1 event/h (1.0) and the specificity was high for the Pediatric Sleep Questionnaire (0.88) and OAHI > 1 event/h on PTT-PG (1.0).
Conclusions:
More than two-thirds of children with DS referred for screening by a genetics specialist had OSA diagnosed by PSG. With its excellent sensitivity and specificity, PTT-PG could be a good and simplified alternative to PSG to diagnose OSA in children with DS.
Citation:
Ioan I, Weick D, Sevin F, et al. Pulse transit time as a diagnostic test for OSA in children with Down syndrome. J Clin Sleep Med. 2022;18(1):119–128.
Keywords: Down syndrome, sleep, apnea, pulse transit time, children
BRIEF SUMMARY
Current Knowledge/Study Rationale: The American Academy of Pediatrics has recommended that a 1-night polysomnography be performed in children with Down syndrome before 4 years of age due to the elevated incidence of obstructive sleep apnea in this population. However, the access to polysomnography is limited and polygraphy coupled with pulse transit time measurement to detect autonomic arousals related to respiratory events could be a good alternative.
Study Impact: In our study, more than two-thirds of children with Down syndrome referred for screening by a genetics specialist had obstructive sleep apnea diagnosed by polysomnography. With its excellent sensitivity and specificity, polygraphy coupled with pulse transit time measurement could be a good and simplified alternative to polysomnography to diagnose obstructive sleep apnea in children with Down syndrome.
INTRODUCTION
The annual incidence of Down syndrome (DS), ranging from 1 in 650 to 1 in 1,000 births worldwide and 1 in 2,000 births in France, has substantially decreased over the last years due to prenatal screening.1 The prevalence of obstructive sleep apnea (OSA) is estimated to be 30%–97% in children with DS,1–3 which is higher compared with the general population for whom the prevalence is up to 4%.4 Morphological characteristics of DS, such as midfacial and mandibular hypoplasia, micrognathia, narrower upper airways, glossoptosis, macroglossia, global muscular and pharyngeal hypotonia, predispose these patients to OSA.1,5,6 Upper airway infections are chronic and persistent in children with DS and lead to hypertrophy of adenoids and tonsils, thus aggravating OSA.5,6 In order to prevent cardiovascular morbidity, neurocognitive impairment, attention deficit, and learning disability,7,8 early diagnosis and treatment of OSA are necessary. The diagnosis and severity assessment of OSA involve a full-night polysomnography (PSG) in a sleep laboratory consisting of electroencephalogram, electro-oculogram, and chin electromyogram recordings to score sleep stages and nasal cannula, thoraco-abdominal belts, and oximetry to obtain respiratory parameters. Despite the recommendations of the American Academy of Pediatrics to evaluate OSA in children with DS,9 the access to pediatric sleep laboratories is limited due to an increasing demand, their nonavailability in most regions, the expensive cost, and the time constraints for registration and interpretation. Moreover, the results of PSG depend on the equipment used and the environment in which it is performed as sleep can be altered in these settings, especially in children due to hospital anxiety, noise, and interventions for sensors repositioning. Simplified diagnostic methods, such as ventilatory polygraphy (PG), can be useful in this context. Indeed, PG measures only the respiratory parameters during sleep, and the recording can be performed at home; it therefore represents an alternative method that is more accessible, less expensive, and potentially associated with better sleep quality as the child sleeps in his/her own bed during recording. However, the main disadvantage of PG is the underestimation of respiratory events related to arousals, leading to a falsely lower obstructive apnea-hypopnea index (OAHI). This limitation may be in part overcome by coupling PG to pulse transit time (PTT) recording (PTT-PG). PTT is a technique that records the time necessary for the arterial pressure wave to travel from the aortic valve to the fingertip, which corresponds to the time interval between R waves from the electrocardiogram and pulse wave. PTT reflects the esophageal pressure10 and it decreases when the blood pressure increases (ie, during an arousal). PTT therefore detects the subcortical autonomic arousals and it has previously been shown that these arousals are more sensitive than respiratory arousals detected using cortical electroencephalography (EEG) for OSA diagnosis in children.10–14 In addition, the PTT arousal index (PTTAI) was found to have an excellent diagnostic utility for assessing moderate and severe OSA in children.11,12
The first objective of the study was to evaluate the ability of PTT-PG to diagnose OSA in children with DS compared with PSG. The hypothesis was that the use of PTT would improve the detection of respiratory events associated with arousals in PG. The second objective was to assess whether validated sleep apnea questionnaires were able to detect the presence of OSA diagnosed by PSG.
METHODS
Patients
A prospective, multicenter, regional clinical study including children with DS followed up and recruited from 2 university hospitals (Saint-Etienne, Lyon) was conducted between 2010 and 2013 (Programme Hospitalier de Recherche Clinique (PHRC) no. 0808017). All the sleep studies were performed in the pediatric sleep unit in Lyon (Hôpital Femme Mère Enfant, Hospices Civils de Lyon). The participation was proposed by genetics specialists (D.S., B.D.F.) to pediatric patients with DS aged 2 to 18 years for whom the diagnosis was confirmed by karyotype analysis. Exclusion criteria were as follows: age < 2 years, presence of cardiomyopathy with ejection fraction of < 55%, and severe behavioral disorders impeding on sleep recording as severe autism disorder or severe attention-deficit/hyperactivity disorder. The protocol was explained to the child and his/her parents and a written consent was obtained from the parents. The study was approved by the ethics committee of the Hospices Civils de Lyon and the Commission National de l’Informatique et des Libertés (CNIL; French national data protection agency; protocol no. 0808017, version no. 9.1 from November 8, 2020).
Study design and questionnaires
Children were hospitalized 1 night for a full video PSG in the sleep laboratory. The clinical history of patients was reviewed and physical examination was performed by the sleep physicians (D.W., P.F.). Parents filled in sleep questionnaires, such as the Sleep Disturbance Scale for Children (SDSC)15 and Pediatric Sleep Questionnaire (PSQ).16 The SDSC has 27 items, each scored from 1 to 5 according to severity, and investigates the presence of disorders for initiating and maintaining sleep (abnormal if score ≥ 17), sleep-disordered breathing (SDB; abnormal if score ≥ 7), disorders of arousal (abnormal if score ≥ 5), sleep-wake transition disorders (abnormal if score ≥ 13), disorders of excessive somnolence (abnormal if score ≥ 12), and sleep hyperhidrosis. The total SDSC score is considered pathological if ≥ 55.15 The PSQ is focused on SDB; a total score > 8 and a diurnal sleepiness score > 1 are considered pathological.16
Clinical and ear, nose, and throat exams
Obesity was defined as a body mass index (BMI) z-score > 2.17 An ear, nose, and throat (ENT) exam was made before PSG to evaluate the presence of nasopharyngeal obstruction, rhinitis, septal deviation, adenoids, and palatine and lingual tonsil hypertrophy. The ENT exam was considered abnormal (positive) if tonsil volume was ≥ 3 on the Friedman score or if the Mallampati score (evaluating the buccal aperture) was ≥ 3, or if a macroglossia was found.
PSG and PG with PTT
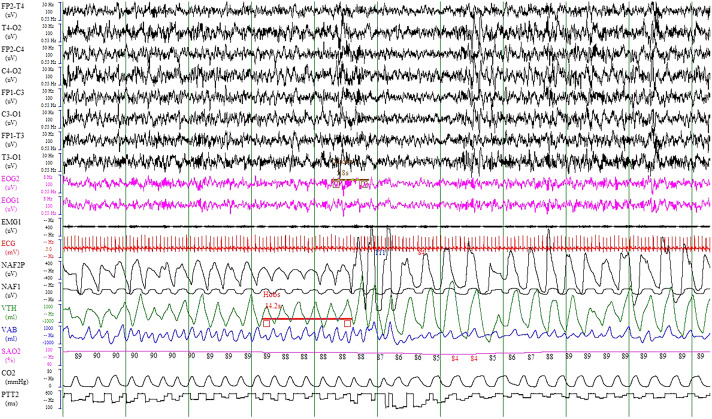
One-night video PSG in the sleep laboratory under nurse supervision was performed using the polysomnograph Morpheus (Medatec-Medical Data, Brussels, Belgium). Eight EEG bilateral channels were recorded and then referenced to the mastoids according to the 10–20 system (FP1-A2, C3-A2, O1-A2, T3-A2, FP2-A1, C4-A1, O2-A1, T4-A1). PSG measurements also included bilateral electro-oculography (right and left); chin, tibia, and diaphragm electromyography; nasal cannula; oro-nasal thermistor; thoraco-abdominal belts; oximetry for pulse oxygen saturation (SpO2); plethysmographic wave recording, 1 electrocardiogram, microphone, and CO2 transcutaneous pressure (PtcCO2) recording by the SenTec system (SenTec Digital Monitor, Therwil, Switzerland). PTT was recorded by an Eden Trace III(Bennett) device (California), connected to the polysomnograph and the synchronized software (Figure 1). This system was previously validated in children by a French center.10
Figure 1. Polysomnographic trace showing obstructive hypopnea with EEG arousal and PTT decrease.
Electroencephalogram (EEG) channels (FP2, C4, T4, O2, FP1, C3, T3, O1), electro-oculography (EOG1-2), electromyography (EMG1), nasal cannula (NAF2P), oro-nasal thermistor (NAF1), thoraco-abdominal belts (VTH-VAB), oximetry for pulse oxygen saturation (SaO2), electrocardiogram (ECG), CO2, and pulse transit time (PTT2). This picture represents 2 minutes of recording.
Sleep and respiratory analysis
PSG without PTT was first read and interpreted by a sleep physician (D.W.), and several months later PTT-PGs were read together by 2 physicians (D.W. and F.S.) with consensus agreement about the interpretation of events. PTT-PG was scored after eliminating sleep data from the original PSG and consisted of 5 channels: nasal cannula, thoraco-abdominal belts, oximetry, electrocardiogram, and PTT.
For PSG, the sleep was coded following the American Academy of Sleep Medicine recommendations.18 EEG arousals were defined as an abrupt shift of EEG frequency including alpha, theta, and/or frequencies > 16 Hz, but not spindles, lasting > 3 seconds and < 15 seconds and preceded by > 10 seconds of stable sleep. For PTT-PG, autonomic arousals corresponded to a decrease in the baseline of at least 15 milliseconds and lasting at least 5 seconds.10 For both PSG and PTT-PG, the arousal was considered respiratory related if it occurred within a 5-second delay of apnea or hypopnea. The arousal index was defined as the number of arousals per hour of total sleep time (TST). Total and respiratory EEG arousal indexes from PSG and total and respiratory PTTAI from PTT-PG were retained for analysis. By PTT-PG, the TST necessary to evaluate respiratory indexes was coded according to the notes from the nurse.
The respiratory events were scored in conformity with the pediatric American Academy of Sleep Medicine guidelines19 for both PSG and PTT-PG. The obstructive apnea index, obstructive hypopnea index (OHI), OAHI, central apnea index (CAI), mean SpO2, percentage of TST with an SpO2 < 90%, and 3% desaturation index were reported. By PTT-PG, the OAHI included the obstructive respiratory events associated with autonomic arousals. The mean and maximal PtcCO2 values were retained for analysis and a hypoventilation was defined as PtcCO2 > 50 mm Hg for > 25% of TST.
An OAHI > 1 event/h of sleep and a CAI > 5 events/h were considered pathological.20,21 OSA was considered mild if the OAHI was > 1 and < 5 events/h, moderate if the OAHI was ≥ 5 and < 10 events/h, and severe if the OAHI was ≥ 10 events/h.
Statistical analysis
The statistical analysis was performed using the software SAS University Edition (SAS Institute, Cary, NC). Quantitative variables were expressed as medians (minimum–maximum; 95% confidence interval) and qualitative variables as counts (percentage).
Children were divided in 2 groups: OSA group for OAHI on PSG > 1 event/h and no-OSA group for OAHI on PSG ≤ 1 events/h. Wilcoxon nonparametric test or chi-square or Fisher’s exact test were used as needed to compare demographic and sleep characteristics of child patients from with OSA vs no-OSA groups. PSG and PTT-PG results were compared using paired-sample Wilcoxon test. Correlations between the demographic, sleep, and respiratory parameters were estimated using the Spearman’s correlation test. The P values were adjusted for multiple comparisons using Bonferroni correction; P < .001 was considered statistically significant for comparisons performed between the OSA and no-OSA groups and P < .003 was considered statistically significant for comparisons performed between PSG and PTT-PG.
The sensitivity, specificity, and positive and negative predictive values and their 95% confidence interval were computed to test the ability of questionnaires, ENT exam, and PTT-PG parameters to diagnose an OAHI > 1 event/h on PSG.
RESULTS
Patient characteristics
A total of 56 children with DS underwent 1-night full video PSG; 3 children were excluded, 2 because they did not sleep during the night and 1 because oxygen therapy was administered during the recording due to his underlying pathology that might have underestimated the desaturating hypopneas. A total of 53 patients were included in the study. There were 30 (57%) males; their median (minimum–maximum) age was 9.3 (2.7–17) years (Table 1), and 11 (21%) were younger than 5 years old at the time of the evaluation. A total of 29 (55%) children had a cardiac history—that is, ventricular septal defects in 10 (19%), atrial septal defects in 4 (8%), an association of the 2 defects in 4 (7%), atrioventricular canal in 6 (11%), patent arterial duct in 1 (2%), patent arterial duct associated with ventricular septal defect in 1 (2%), atrial septal defect in 1 (2%), mitral insufficiency in 1 (2%), and bicuspid aortic valve in 1 (2%). A total of 38 (72%) children had an ENT history—that is, recurrent otitis in 27 (51%), deafness in 4 (8%), and chronic rhinitis in 7 (13%). Twenty-six (49%) children had an ENT surgery: adeno-tonsillectomy for 8 (15%), tonsillectomy for 7 (13%), adenoidectomy for 6 (11%), and tympanostomy tube insertion for 5 (9%). The medical history revealed hypothyroidism in 8 (15%) children, asthma in 5 (9%), gastroesophageal reflux in 5 (9%), mastocytosis in 1 (2%), type 1 diabetes in 1 (2%), humoral immunodeficiency in 1 (2%), recurrent urinary infections in 2 (4%), scoliosis in 1 (2%), autism in 1 (2%), and other behavioral disorder in 1 (2%). Overall, 20 (38%) children had a medical treatment (l-thyroxine, anti-asthma treatment, proton pump inhibitors, antihistamines, and/or Risperidone (Risperdal, Janssen-Cilag NV, Beerse, Belgium)), and no children had cardiac disease at the time of recording; 5 (9%) patients had a BMI z-score > 2 (Table 1).
Table 1.
Clinical characteristics and questionnaire results of children with Down syndrome with and without OSA.
| All Children | No-OSA Group | OSA Group | |
|---|---|---|---|
| Number of children (%) | 53 (100) | 17 (32) | 36 (68) |
| Age, y | 9.3 (2.7, 17) | 9 (3, 17) | 9 (2.7, 17) |
| Boys | 30 (57) | 10 (59) | 20 (56) |
| BMI, kg/m2 | 18 (14, 27) | 19 (14, 23) | 18 (14, 27) |
| BMI z-score | 0.58 (−1.54, 3.14) | 0.84 (−1.13, 1.89) | 0.55 (−1.54, 3.14) |
| BMI z-score > 2 | 5 (9) | 0 (0) | 5 (14) |
| Tonsil volume | 2 (0, 3) | 2 (0, 3) | 2 (0, 3) |
| Tonsil volume ≥ 3 | 10 (19) | 1 (6) | 8 (23) |
| Mallampati score | 2 (1, 4) | 3 (1, 4) | 2 (1, 4) |
| Score ≥ 3 | 15 (28) | 4 (24) | 10 (29) |
| SDSC SDB score | 7 (2, 15) | 5 (3, 9) | 8 (2, 15)* |
| Score ≥ 7 | 27 (51) | 6 (35) | 21 (58) |
| SDSC disorders of initiation and maintaining sleep score | 13 (7, 25) | 11 (7, 25) | 14 (7, 24) |
| Score ≥ 17 | 11 (21) | 3 (18) | 8 (23) |
| SDSC disorders of arousal score | 4 (2, 11) | 5 (2, 11) | 4 (3, 10) |
| Score ≥ 5 | 22 (42) | 8 (47) | 14 (38) |
| SDSC sleep-wake transition disorders score | 13 (3, 22) | 12 (5, 22) | 14 (3, 19) |
| Score ≥ 13 | 26 (49) | 6 (35) | 20 (56) |
| SDSC disorders of excessive somnolence score | 8 (5, 47) | 7 (5, 14) | 10 (5, 47) |
| Score ≥ 12 | 18 (34) | 5 (29) | 13 (36) |
| SDSC total score | 47 (28, 80) | 41 (29, 62) | 52 (28, 80) |
| Score ≥ 55 | 15 (28) | 2 (12) | 13 (36) |
| PSQ excessive diurnal sleepiness score | 0 (0, 3) | 0 (0, 1) | 0 (0, 3) |
| Score > 1 | 7 (13) | 0 | 7 (19) |
| PSQ total score | 6 (0, 12) | 4 (0, 7) | 7 (1, 12)† |
| Score > 8 | 7 (13) | 0 | 7 (19) |
Data are expressed as median (minimum, maximum) or n (%). *P = .008; †P = .004. BMI = body mass index, OSA = obstructive sleep apnea, PSQ = Pediatric Sleep Questionnaire, SDB = sleep-disordered breathing, SDSC = Sleep Disturbance Scale for Children.
Questionnaires
The results from the sleep questionnaires showed that 15 (28%) children had an abnormal total SDSC score with pathological SDB scores for 27 (51%), and the PSQ total score was abnormal for 7 (13%) patients (Table 1).
PSG results
The median (minimum–maximum) sleep efficiency of children with DS was 85% (40%–95%), OAHI of 2.6 (0–31) events/h, and SpO2 of 95% (86%–97%) (Table 2). Seven (13%) patients had an SpO2 < 90% for > 10% of TST, 5 (9%) of them with cardiac pathologies, and 8 (15%) patients had a PtcCO2 > 50 mm Hg for > 25% of TST. An OAHI > 1 event/h was found in 36 (68%) children; the OAHI was > 1 and < 5 events/h in 18 (34%), ≥ 5 and < 10 events/h in 11 (21%), and ≥ 10 events/h in 7 (13%). No patient had a CAI > 5 events/h.
Table 2.
Polysomnographic results of children with Down syndrome with and without OSA.
| All Children | No-OSA Group | OSA Group | |
|---|---|---|---|
| Number of children (%) | 53 (100) | 17 (32) | 36 (68) |
| Total recording time, min | 617 (479, 720) [586, 617] | 603 (479, 720) [562, 634] | 618 (485, 691) [586, 621] |
| TST, min | 484 (154, 588) [425, 481] | 495 (154, 588) [348, 504] | 480 (319, 567) [443, 488] |
| Sleep efficiency, % | 85 (30, 96) [77, 85] | 85 (30, 95) [69, 89] | 85 (60, 96) [79, 95] |
| N1 sleep, min | 70 (0, 154) [61, 81] | 61 (0, 120) [43, 79] | 79 (0, 154) [64, 88] |
| N1 sleep, %TST | 16 (0, 35) [14, 18] | 14 (0, 31) [10, 17] | 18 (0, 35) [14, 20] |
| N2 sleep, min | 213 (32, 355) [181, 221] | 227 (32, 305) [158, 244] | 199 (60, 355) [179, 224] |
| N2 sleep, %TST | 45 (15, 63) [40, 46] | 44 (20, 61) [38, 48] | 45 (15, 63) [39, 47] |
| N3 sleep, min | 105 (30, 889) [91, 153] | 108 (30, 889) [59, 257] | 105 (43, 186) [96, 115] |
| N3 sleep, %TST | 22 (7, 60) [22, 27] | 24 (7, 60) [21, 35] | 22 (11, 34) [21, 25] |
| REM sleep, min | 75 (0, 131) [66, 86] | 80 (0, 125) [49, 92] | 75 (0, 131) [68, 90] |
| REM sleep, %TST | 18 (0, 28) [14, 18] | 18 (0, 26) [11, 19] | 18 (0, 28) [14, 19] |
| Sleep latency, min | 21 (3, 208) [25, 49] | 16 (4, 208) [0, 95] | 21 (3, 85) [24, 43] |
| REM sleep latency, min | 182 (65, 397) [182, 235] | 197 (65, 397) [126, 279] | 176 (100, 374) [182, 238] |
| Obstructive apnea index, events/h | 1.3 (0, 19) [1.6, 4.1] | 0.2 (0, 0.8) [0.1, 0.3] | 2.5 (0, 19) [2.2, 5.3]* |
| Obstructive hypopnea index, events/h | 0.5 (0, 13) [0.9, 2.9] | 0.1 (0, 0.8) [0.03, 0.3] | 0.9 (0, 13) [1.2, 3.7] |
| Obstructive apnea-hypopnea index, events/h | 2.3 (0, 31) [3.0, 6.7] | 0.3 (0, 1.0) [0.2, 0.5] | 4.9 (1.1, 31) [4.5, 9.4]† |
| Central apnea index, events/h | 0 (0, 1.3) [0.02, 0.2] | 0 (0, 0.5) [–0.1, 0.2] | 0 (0, 1.3) [0.02, 0.2] |
| Total EEG arousal index, events/h | 12 (0, 33) [11, 15] | 11.3 (0, 32) [8.2, 15] | 12 (0, 33) [11, 16] |
| Respiratory EEG arousal index, events/h | 1.8 (0, 24) [2.2, 5.1] | 0.5 (0, 3.9) [0.2, 12] | 2.5 (0, 24) [3.0, 7.0]† |
| Mean SpO2, % | 95 (86, 97) [94, 95] | 95 (93, 97) [94, 96] | 95 (86, 97) [93, 95] |
| TST with SpO2 < 90%, % | 0 (0, 97) [1.5, 13] | 0 (0, 23) [−1.6, 4.6] | 0.3 (0, 97) [1.6, 18] |
| 3% Desaturation index, events/h | 0.2 (0, 151) [−0.7, 12] | 0.1 (0, 9.9) [−0.5, 2.1] | 0.3 (0, 151) [−1.5, 17] |
| TST with CO2 transcutaneous pressure > 50 mm Hg, % | 0 (0, 92) [5.6, 26] | 0 (0, 92) [−10, 49] | 0, 9 (0, 69) [4, 24] |
| n (%) | 9 (17) | 2 (22)‡ | 6 (24)§ |
Data are expressed as median (minimum, maximum) [95% confidence interval] unless otherwise indicated. *P = .0002; †P ≤ .0001; ‡n = 9; §n = 25. EEG = electroencephalography, OSA = obstructive sleep apnea, REM = rapid eye movement, SpO2 = pulse oxygen saturation, TST = total sleep time.
Comparison between PSG and PTT-PG
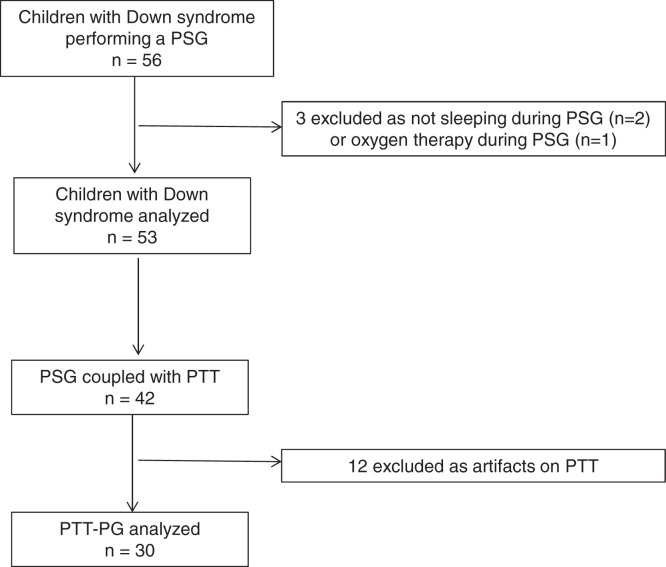
PTT recording was performed on 42 (78%) children with DS, including 26 (62%) boys; their median (minimum–maximum) age was 8.3 (2.7–17) years. A total of 12 (29%) recordings were noninterpretable due to a technical problem with the PTT sensor, defined by artifacts > 75% of the recording time. The flow diagram of children undergoing a PSG and PTT-PG is represented in Figure 2. The comparison between PSG and PTT-PG parameters was made on the remaining 30 (71%) recordings belonging to 18 (60%) boys; the median (minimum–maximum) age of these 30 patients was 8.7 (2.7–17) years. The obstructive apnea index (P = .0008) and OAHI (P = .0005) were significantly larger on PSG than PTT-PG; there was no significant difference for the obstructive hypopnea index, obstructive apnea and obstructive hypopnea maximal duration, CAI or oximetry indexes, SpO2, percentage of TST with SpO2 < 90%, or desaturation index. No significant difference was found between total EEG arousal index and total PTTAI (P = .001), whereas the respiratory EEG arousal index was significantly larger than respiratory PTTAI (P = .0005; Table 3).
Figure 2. Flow diagram of children with Down syndrome undergoing PG and PTT-PG measurement.
PG = polygraphy, PSG = polysomnography, PTT = pulse transit time, PTT-PG = polygraphy coupled with pulse transit time.
Table 3.
Comparison between PSG and PTT-PG parameters.
| PSG | PTT-PG | |
|---|---|---|
| Number of children | 30 | 30 |
| Boys, n (%) | 18 (60) | 18 (60) |
| Total recording time, min | 620 (511, 720) [597, 632] | 617 (510, 720) [596, 631] |
| Obstructive apnea index, events/h | 1.5 (0, 19) [1.6, 5.0] | 0.4 (0, 6.2) [0.4, 1.5]* |
| Obstructive hypopnea index, events/h | 0.5 (0, 13) [0.7, 3.3] | 0 (0, 6.4) [0, 1.0] |
| Obstructive apnea maximal duration, s | 15 (6, 48) [14, 22] | 16 (6, 33) [14, 22] |
| Obstructive hypopnea maximal duration, s | 14 (7, 32) [9, 24] | 15 (10, 25) [9, 24] |
| OAHI, events/h | 2.5 (0, 31) [2.7, 8.1] | 0.7 (0, 16) [0.7, 3.3]* |
| Number of patients with OAHI, n (%) | ||
| > 1 | 22 (73) | 11 (37) |
| < 1 | 8 (27) | 19 (63) |
| > 1 to < 5 | 10 (33) | 7 (23) |
| > 5 to < 10 | 7 (23) | 3 (10) |
| > 10 | 5 (17) | 1 (3) |
| Central apnea index, events/h | 0 (0, 1.3) [0, 0.2] | 0 (0, 0.5) [0, 0.2] |
| Total EEG arousal index (events/h) on PSG vs total autonomic arousal index (events/h) on PTT-PG | 11 (4.1, 27) [9.7, 14] | 5.4 (0.1, 29) [4.2, 8.5] |
| Respiratory EEG arousal index (events/h) on PSG vs respiratory autonomic arousal index (events/h) on PTT-PG | 2.0 (0, 21) [2.2, 6.1] | 0.4 (0, 12) [0.4, 2.3]* |
| Mean SpO2, % | 95 (90, 96) [94, 95] | 95 (92, 97) [94, 95] |
| Percentage of total sleep time spent with SpO2 < 90% | 0 (0, 57) [−0.3, 82] | 0.1 (0, 57) [−0.1, 8.3] |
| 3% Desaturation index, events/h | 0.2 (0, 6.1) [0.2, 1.1] | 0 (0, 4.3) [−0.1, 0.5] |
Data are expressed as median (minimum, maximum) [95% confidence interval] unless otherwise indicated. *P ≤ .0005. EEG = electroencephalography, OAHI = obstructive apnea-hypopnea index, PSG = polysomnography, PTT-PG = polygraphy coupled with pulse transit time, SpO2 = pulse oxygen saturation.
No significant difference was found between the number of patients with an OAHI > 1 event/h on PSG compared with PTT-PG, regardless of the severity of OSA. No patient had a CAI > 5 events/h on PSG or PTT-PG.
Correlations
No significant correlation was found between SDSC or PSQ scores and OAHI by PSG, total and respiratory EEG arousal indexes, or total and respiratory PTTAI or desaturation indexes. The OAHI by PSG did not correlate with age, BMI, or BMI z-score. The OAHI by PSG correlated positively with total (r = .47, P = .009) and respiratory (r = .81, P < .0001) EEG arousal indexes and with total PTTAI (r = .48, P = .007). Total PTTAI correlated positively with OAHI by PTT-PG (r = .51, P = .004) and with respiratory PTTAI (r = .83, P < .0001).
Comparison between the OSA (n = 36) and no-OSA (n = 17) groups
There was no significant difference in age, BMI, and BMI z-score between the 2 groups. There were more children in the OSA group who had a BMI z-score > 2, a tonsil volume ≥ 3, and a Mallampati score ≥ 3 compared with the no-OSA group, but those differences did not reach statistical significance.
Patients with OSA had higher SDSC-SDB scores (P = .008) compared with children without OSA. There was no other significant difference between children regarding the SDSC subscales. The PSQ total score was significantly higher in the OSA group compared with the no-OSA group (P = .004; Table 1).
PSG results showed no difference for TST, sleep efficiency, sleep and rapid eye movement sleep latency, and durations for the sleep stages between the OSA and no-OSA groups. The obstructive apnea index (P = .0002) and OAHI (P < .0001) were significantly higher in children with OSA compared with children without OSA, and there was no significant difference for the obstructive hypopnea index and CAI between the 2 groups. The respiratory EEG arousal index was significantly larger in patients with OSA compared with those without OSA (P < .0001) and there was no significant difference regarding the total EEG arousal index. The percentage of TST with SpO2 < 90% was higher in the OSA group compared with the no-OSA group, but this difference was not statistically significant (P = .008); there was no difference regarding the mean SpO2 and the desaturation index between both groups. More patients with OSA had a PtcCO2 > 50 mm Hg for > 25% of TST, but this difference was not statistically significant (Table 2).
Sensitivity, specificity, and positive and negative predictive values
The SDSC SDB score exhibited poor sensitivity and specificity for diagnosing an OAHI > 1 event/h on PSG while the PSQ had a high specificity and positive predictive value with low sensitivity and negative predictive value. ENT exam showed low sensitivity and specificity.
Specificity and positive predictive value were excellent for OAHI > 1 event/h on PTT-PG, followed by OAHI > 1 event/h and respiratory PTTAI for diagnosing an OAHI > 1 event/h on PSG. On the contrary, sensitivity and negative predictive value were maximal for OAHI > 1 event/h and total PTTAI, followed by OAHI > 1 event/h and respiratory PTTAI or OAHI > 1 event/h on PTT-PG (Table 4).
Table 4.
Sensitivity, specificity, positive and negative predictive values, and their 95% confidence interval for OAHI > 1 event/h and > 3 events/h on polysomnography.
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | |
|---|---|---|---|---|
| OAHI > 1 | ||||
| SDSC SDB | 0.50 (0.28–0.72) | 0.63 (0.24–0.91) | 0.69 (0.41–0.89) | 0.31 (0.11–0.59) |
| PSQ total score | 0.27 (0.09–0.46) | 0.88 (0.65–1.00) | 0.86 (0.60–1.00) | 0.30 (0.12–0.49) |
| Abnormal ENT exam | 0.59 (0.39–0.80) | 0.38 (0.04–0.71) | 0.72 (0.52–0.93) | 0.25 (0.01–0.50) |
| OAHI > 1 on PTT-PG | 0.50 (0.29–0.71) | 1.00 (1.00–1.00) | 0.58 (0.36–0.80) | 0.42 (0.20–0.64) |
| OAHI > 1 + respiratory PTTAI > 1 on PTT-PG | 0.59 (0.39–0.80) | 0.88 (0.65–1.00) | 0.93 (0.79–1.00) | 0.44 (0.19–0.68) |
| OAHI > 1 + total PTTAI > 1 on PTT-PG | 1.00 (1.00–1.00) | 0.13 (0.00–0.35) | 0 | 1.00 (1.00–1.00) |
| OAHI > 3 | ||||
| PSQ total score | 0.33 (0.10–0.65) | 0.83 (0.59–1.00) | 0.35 (0.16–0.57) | 0.65 (0.43–0.84) |
| OAHI > 3 on PTT-PG | 0.42 (0.15–0.72) | 0.94 (0.73–1.00) | 0.29 (0.13–0.51) | 0.71 (0.49–0.87) |
| OAHI > 3 + respiratory PTTAI > 3 on PTT-PG | 0.42 (0.15–0.72) | 0.94 (0.73–1.00) | 0.29 (0.13–0.51) | 0.71 (0.49–0.87) |
| OAHI > 3 + total PTTAI > 3 on PTT-PG | 0.75 (0.43–0.95) | 0.28 (0.10–0.53) | 0.38 (0.09–0.76) | 0.63 (0.24–0.91) |
Values in bold correspond to a significant P value. ENT = ear, nose, and throat, OAHI = obstructive apnea-hypopnea index, PSQ = Pediatric Sleep Questionnaire, PTTAI = autonomic arousal index on PTT-PG, PTT-PG = polygraphy coupled with pulse transit time, SDB = sleep-disordered breathing, SDSC = Sleep Disturbance Scale for Children.
A combination of abnormal PSQ score and OAHI by PTT-PG showed a very large sensitivity 1.0 (0.85–1), a specificity of 0.88 (0.65–1.0), and an excellent negative predictive value 1.0 (1.0–1.0).
When considering a threshold of 3 events/h for OAHI on PSG (Table 4), the specificity of PSQ total score of OAHI > 3 events/h on PTT-PG combined or not with respiratory PTTAI > 3 events/h did not change. The specificity of the combination of OAHI > 3 events/h and respiratory PTTAI > 3 events/h on PTT-PG increased; nonetheless, the sensitivity of the association between OAHI > 3 events/h and total PTTAI > 3 events/h on PTT-PG decreased.
DISCUSSION
The present study conducted in children with DS showed that more than two-thirds of patients referred by a genetics specialist for screening purposes, irrespective of the presence or absence of sleep problems, presented an OSA diagnosed by PSG and had a fragmented sleep with high total and respiratory arousal indexes. SDSC SDB had poor sensitivity and specificity while the PSQ was very specific for diagnosing OSA on PSG. PTT-PG exhibited an excellent specificity, and a maximal sensitivity was obtained by adding the total autonomic arousal index by PTT.
The prevalence of OSA in children with DS is high, mostly due to their morphological features. Nevertheless, some differences have been reported in terms of prevalence between studies, mainly dependent on the inclusion criteria. The prevalence observed herein was similar to previous studies for which participants were included for a screening purpose,6 while it has been found to be even higher (∼97%) in studies for which only snoring children with DS were included.3,22 Another factor influencing OSA prevalence is the OAHI threshold used to define OSA, which varies between studies; for example, with a threshold set at > 2 events/h, the prevalence has been found to be 75%.23,24 Half of the participants with OSA included in the present study had a moderate to severe OAHI and some had an obstructive alveolar hypoventilation, while few had a PtcCO2 > 50 mm Hg. Previous studies have reported an increased PtcCO2 in children with DS, regardless of the presence of OSA and its severity, due to muscular hypotonia and/or ventilatory control alteration.25,26
As the questionnaires are parent-reported, parents commonly underestimate the severity of the sleep abnormalities of their child.22 Nevertheless, the SDSC found that half of the population with DS had pathological scores for items describing SDB, insomnia, sleep-wake transition disorders, and disorder of arousal. Additionally, children with OSA had a significantly higher SDSC SDB score. Children were referred for PSG by a genetics specialist not because of their symptoms but for screening, and the large number of sleep disorders identified can be explained by the late age of PSG diagnosis as sleep issues often worsen with time. This highlights the importance of systematic detection of OSA and sleep disturbance in patients with DS at an early age. In the population studied herein, the PSQ total score showed a good specificity in diagnosing an OAHI > 1 event/h on PSG, while, in contrast to other studies, SDSC SDB had a poor diagnostic value.25 The low predictive value of parental reporting is in line with a recent study in which even a well-designed questionnaire with powerful psychometric properties demonstrated limited usefulness in young children with DS for screening for moderate to severe OSA.27 Moreover, the ENT exam showed a low sensitivity and low specificity and no significant difference was seen for the Mallampati score or tonsil volume between children with and without OSA. There was no correlation between the presence of OSA and tonsillar hyperplasia, as previously reported,28,29 demonstrating the importance of sleep studies in this population.
Since clinical exam and history are nonspecific and undiagnosed and untreated OSA is associated with serious cognitive, behavioral, and somatic consequences, the American Academy of Pediatrics has recommended that a 1-night PSG be performed in children with DS before 4 years of age due to the elevated incidence of OSA in this population.9 Because pediatric sleep laboratories are rare and in-laboratory PSG is poorly tolerated by children due to the need for hospitalization and the high number of leads and sensors, alternative methods have been used to facilitate the recording of these patients, such as PTT-PG. When compared with PSG, PTT-PG demonstrated several benefits and drawbacks. PTT-PG has fewer sensors to place on the body, but the PTT signal was uninterpretable in one-quarter of child patients due to poor quality. PTT-PG showed a significantly smaller obstructive apnea index and OAHI than PSG. This was expected because, without EEG data, respiratory indexes in PG are underestimated, as previously reported,30,31 due to the events not causing desaturation but just arousal and not counted in PG. The TST was difficult to assess on PTT-PG and the parts with artifacts or when the PTT sensor was not working were eliminated, thus reducing the sensitivity of this exam. Consequently, one-third of patients were misdiagnosed as no-OSA on PTT-PG. Nevertheless, the use of PTT was likely to improve the detection of some of the nondesaturating respiratory events; yet, the total and respiratory arousal indexes remained statistically reduced on PTT-PG compared with PSG. However, PTT associated with classical PG allows appreciation of the sleep fragmentation10 as total PTTAI was positively correlated with OAHI on PSG and on PTT-PG. These subcortical autonomic responses are considered as a primary form of cortical arousal, and appear more frequently in children at the end of a respiratory event and are not visualized on classical EEG.10,32 Altogether, the number of patients with OAHI > 1 event/h was not different on PSG compared with PTT-PG, and when considering an OAHI > 1 event/h on PTT-PG, the specificity to diagnose an OAHI > 1 event/h on PSG was maximal, yet the sensitivity was poor. When adding respiratory PTTAI > 1 event/h to OAHI > 1 event/h on PTT-PG, the sensitivity did not improve much, but when total PTTAI > 1 event/h was considered, the sensitivity became maximal.
The correlation between OAHI obtained by PSG and total PTTAI has already been assessed in a population of symptomatic children suspected of OSA and showed that a threshold of total PTTAI > 5.4 events/h had a very good sensitivity and specificity and was significantly correlated with OAHI11,12; the correlation was stronger when OAHI was > 3 events/h during sleep.11 The authors concluded that PTT seems to be an excellent screening tool for moderate and severe OSA associated with an OAHI > 3 events/h during sleep, yet it is not adequate for mild OSA.11,12 Part of our results confirm these previous studies; the specificity of OAHI > 3 events/h on PTT-PG combined with respiratory PTTAI > 3 events/h to diagnose an OAHI > 3 events/h on PSG was high, but the sensitivity of the combination of OAHI > 3 events/h and total PTTAI > 3 events/h on PTT-PG decreased when the OAHI threshold increased.
Other studies using simpler techniques for the diagnosis of OSA in children with DS used at-home PG25,28,32 and have shown that more than three-quarters of PG were technically acceptable after 1 night and almost all failed recordings when repeated were acceptable. At-home PG exhibited maximal sensitivity and very good specificity when an OAHI ≥ 3 events/h was considered as a cutoff for OSA diagnosis. Scoring sleep-wake states was based on available signals and improved by using actigraphy; thus, at-home PG proved to be enthusiastically accepted by parents and children, as many cited prior experiences of sleeping poorly in a hospital setting.28 More than 80% of parents expressed willingness to repeat home cardiorespiratory PG in the future and more than 60% of parents found the equipment “easy or OK” to use.33
The limitations of the present study were the problem with the PTT device, which led to approximately one-quarter of noninterpretable traces due, in part, to the use of an old device and, in part, to the many motion artifacts, as children with DS are reported to be more restless during sleep compared with children with normal development. Another limitation of the study is its small sample size due to the difficulty to recruit participants. Indeed, parents are resistant to include their child in a study due to their belief in the inevitable progression of their child’s DS, even though OSA therapy could lead to improved quality of life and better cognitive and cardiovascular evolution.34 Another limitation is the lack of a real control group, but the aim of our study was to determine the incidence of OSA in a population of children with DS addressed by the genetics specialist for a screening purpose, irrespective of the child’s symptoms.
To conclude, patients with DS are prone to SDB mostly due to their anatomical airway structure; OSA was found in two-thirds of children referred by a genetics specialist for screening, and half of them had moderate to severe OSA. The PSQ had high specificity and PTT-PG with total autonomic arousals had high sensitivity; yet, it was highly dependent on the quality of the signal. The combination of PSQ questionnaire and PG-PTT exhibited excellent sensitivity with a strong negative predictive value and could improve the screening of these patients. Similar to prior reports, the present study supports screening for sleep disorders at an early age as treatment may improve the prognosis of children with DS.
DISCLOSURE STATEMENT
Each author listed on the manuscript has seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript. This study was funded by Programme Hospitalier de Recherche Clinique (PHRC). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Hélène Boyer for providing language help. Author contributions: Conception and study design: Bénédicte De Fréminville, Damien Sanlaville, Patricia Franco. Patient data collection: Diane Weick, Mohamed Akkari. Polysomnographic analysis: Diane Weick. PTT-PG analysis: Diane Weick, François Sevin. Statistical analysis: Iulia Ioan, Cyril Schweitzer. Interpretation of results: Iulia Ioan, Cyril Schweitzer, Mohamed Akkari, Laurianne Coutier, Marine Thieux, Benjamin Putois, Cyril Schweitzer, Patricia Franco. Preparation of the manuscript: Iulia Ioan, Diane Weick, Marine Thieux, Patricia Franco.
ABBREVIATIONS
- BMI
body mass index
- CAI
central apnea index
- DS
Down syndrome
- EEG
electroencephalography
- ENT
ear, nose, and throat
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PG
ventilatory polygraphy
- PSG
polysomnography
- PSQ
Pediatric Sleep Questionnaire
- PtcCO2
CO2 transcutaneous pressure
- PTT
pulse transit time
- PTTAI
pulse transit time arousal index
- SDB
sleep-disordered breathing
- SDSC
Sleep Disturbance Scale for Children
- SpO2
pulse oxygen saturation
- TST
total sleep time
REFERENCES
- 1. Churchill SS, Kieckhefer GM, Landis CA, Ward TM. Sleep measurement and monitoring in children with Down syndrome: a review of the literature, 1960-2010. Sleep Med Rev. 2012; 16( 5): 477– 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dyken ME, Lin-Dyken DC, Poulton S, Zimmerman MB, Sedars E. Prospective polysomnographic analysis of obstructive sleep apnea in Down syndrome. Arch Pediatr Adolesc Med. 2003; 157( 7): 655– 660. [DOI] [PubMed] [Google Scholar]
- 3. Fitzgerald DA, Paul A, Richmond C. Severity of obstructive apnoea in children with Down syndrome who snore. Arch Dis Child. 2007; 92( 5): 423– 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brockmann PE, Perez JL, Moya A. Feasibility of unattended home polysomnography in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2013; 77( 12): 1960– 1964. [DOI] [PubMed] [Google Scholar]
- 5. Ng DK, Hui HN, Chan CH, et al. Obstructive sleep apnoea in children with Down syndrome. Singapore Med J. 2006; 47( 9): 774– 779. [PubMed] [Google Scholar]
- 6. Shott SR. Down syndrome: common otolaryngologic manifestations. Am J Med Genet C Semin Med Genet. 2006; 142C( 3): 131– 140. [DOI] [PubMed] [Google Scholar]
- 7. Andreou G, Galanopoulou C, Gourgoulianis K, Karapetsas A, Molyvdas P. Cognitive status in Down syndrome individuals with sleep disordered breathing deficits (SDB). Brain Cogn. 2002; 50( 1): 145– 149. [DOI] [PubMed] [Google Scholar]
- 8. O’Driscoll DM, Horne RS, Davey MJ, et al. Cardiac and sympathetic activation are reduced in children with Down syndrome and sleep disordered breathing. Sleep. 2012; 35( 9): 1269– 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bull MJ; Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011; 128( 2): 393– 406. [DOI] [PubMed] [Google Scholar]
- 10. Pépin JL, Delavie N, Pin I, et al. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest. 2005; 127( 3): 722– 730. [DOI] [PubMed] [Google Scholar]
- 11. Bradley J, Galland BC, Bakker JP, et al. Pulse transit time and assessment of childhood sleep disordered breathing. Arch Otolaryngol Head Neck Surg. 2012; 138( 4): 398– 403. [DOI] [PubMed] [Google Scholar]
- 12. Brietzke SE, Katz ES, Roberson DW. Pulse transit time as a screening test for pediatric sleep-related breathing disorders. Arch Otolaryngol Head Neck Surg. 2007; 133( 10): 980– 984. [DOI] [PubMed] [Google Scholar]
- 13. Katz ES, Lutz J, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res. 2003; 53( 4): 580– 588. [DOI] [PubMed] [Google Scholar]
- 14. Yanney MP, Prayle AP, Rowbotham NJ, Kurc M, Tilbrook S, Ali N. Observational study of pulse transit time in children with sleep disordered breathing. Front Neurol. 2020; 11: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996; 5( 4): 251– 261. [DOI] [PubMed] [Google Scholar]
- 16. Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000; 1( 1): 21– 32. [DOI] [PubMed] [Google Scholar]
- 17. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000; 320( 7244): 1240– 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 19. Berry RB, Budhiraja R, Gottlieb DJ, et al. ; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012; 8( 5): 597– 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaditis AG, Alonso Alvarez ML, Boudewyns A, et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J. 2016; 47( 1): 69– 94. [DOI] [PubMed] [Google Scholar]
- 21. Kritzinger FE, Al-Saleh S, Narang I. Descriptive analysis of central sleep apnea in childhood at a single center. Pediatr Pulmonol. 2011; 46( 10): 1023– 1030. [DOI] [PubMed] [Google Scholar]
- 22. Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1991; 88( 1): 132– 139. [PubMed] [Google Scholar]
- 23. Lee CF, Lee CH, Hsueh WY, Lin MT, Kang KT. Prevalence of obstructive sleep apnea in children with Down syndrome: a meta-analysis. J Clin Sleep Med. 2018; 14( 5): 867– 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nerfeldt P, Sundelin A. Obstructive sleep apnea in children with Down syndrome: prevalence and evaluation of surgical treatment. Int J Pediatr Otorhinolaryngol. 2020; 133: 109968. [DOI] [PubMed] [Google Scholar]
- 25. Ikizoglu NB, Kiyan E, Polat B, Ay P, Karadag B, Ersu R. Are home sleep studies useful in diagnosing obstructive sleep apnea in children with Down syndrome? Pediatr Pulmonol. 2019; 54( 10): 1541– 1546. [DOI] [PubMed] [Google Scholar]
- 26. Richard N, Beydon N, Berdah L, Corvol H, Aubertin G, Taytard J. Nocturnal hypoventilation in Down syndrome children with or without sleep apnea. Pediatr Pulmonol. 2020; 55( 5): 1246– 1253. [DOI] [PubMed] [Google Scholar]
- 27. Grantham-Hill S, Evans HJ, Tuffrey C, et al. Psychometric properties and predictive value of a screening questionnaire for obstructive sleep apnea in young children with Down syndrome. Front Psychiatry. 2020; 11: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hill CM, Evans HJ, Elphick H, et al. Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome. Sleep Med. 2016; 27-28: 99– 106. [DOI] [PubMed] [Google Scholar]
- 29. Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. Sleep. 2016; 39( 3): 699– 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abdelghani A, Roisman G, Escourrou P. Evaluation of a home respiratory polygraphy system in the diagnosis of the obstructive sleep apnea syndrome. Article in French. Rev Mal Respir. 2007; 24( 3 Pt 1): 331– 338. [DOI] [PubMed] [Google Scholar]
- 31. Coutier L, Bierme P, Thieux M, et al. The role of sleep laboratory polygraphy in the evaluation of obstructive sleep apnea syndrome in Robin infants. Sleep Med. 2020; 72: 59– 64. [DOI] [PubMed] [Google Scholar]
- 32. McNamara F, Issa FG, Sullivan CE. Arousal pattern following central and obstructive breathing abnormalities in infants and children. J Appl Physiol (1985). 1996; 81( 6): 2651– 2657. [DOI] [PubMed] [Google Scholar]
- 33. Kingshott RN, Gahleitner F, Elphick HE, et al. Cardiorespiratory sleep studies at home: experience in research and clinical cohorts. Arch Dis Child. 2019; 104( 5): 476– 481. [DOI] [PubMed] [Google Scholar]
- 34. Horne RS, Wijayaratne P, Nixon GM, Walter LM. Sleep and sleep disordered breathing in children with Down syndrome: effects on behaviour, neurocognition and the cardiovascular system. Sleep Med Rev. 2019; 44: 1– 11. [DOI] [PubMed] [Google Scholar]