Abstract
Study Objectives:
To carry out an analysis of leg movement activity during sleep in a polysomnography dataset of patients with multiple sclerosis (MS) in comparison to idiopathic restless legs syndrome (iRLS) and healthy controls.
Methods:
In this cross-sectional, observational, instrumental study, 57 patients (males/females: 11/46; mean age 46.2 ± 10.2 years) with a diagnosis of MS underwent a telephone interview assessing the 5 standard diagnostic criteria for RLS and polysomnography. Sleep architecture and leg movement activity during sleep were subsequently compared: 1) 40 patients with MS without RLS (MS–RLS) vs 28 healthy controls; 2) 17 patients with MS with RLS (MS+RLS) vs 35 patients with iRLS; 3) MS+RLS vs MS–RLS.
Results:
MS–RLS and MS+RLS presented increased sleep latency, percentage of sleep stage N1, and reduced total sleep time compared to healthy controls and iRLS, respectively. The periodic limb movements during sleep (PLMS) index was higher in MS–RLS than in healthy controls (P = .035) and lower in MS+RLS compared to iRLS (P = .024). PLMS in MS+RLS were less periodic, less often bilateral, and with shorter single movements compared to the typical PLMS in iRLS.
Conclusions:
MS is a risk factor for RLS, PLMS, and for a lower sleep quality in comparison to healthy patients. PLMS in MS+RLS are fewer and shorter if compared to iRLS. Our results suggest a dissociation between motor (PLMS) and sensory symptoms (RLS sensory component) in RLS secondary to MS, with possible treatment implications.
Citation:
Ferri R, Sparasci D, Castelnovo A, et al. Leg movement activity during sleep in multiple sclerosis with and without restless legs syndrome. J Clin Sleep Med. 2022;18(1):11–20.
Keywords: periodic limb movements, restless legs syndrome, multiple sclerosis, polysomnography
BRIEF SUMMARY
Current Knowledge/Study Rationale: Restless legs syndrome (RLS) is 4-fold more frequent in multiple sclerosis (MS). Sleep-related motor component of symptomatic RLS in MS has not been investigated so far. It is still unclear if MS is a risk factor also for periodic limb movements during sleep and if the leg movement activity during sleep in MS-related RLS is similar of that observed in idiopathic RLS.
Study Impact: Besides RLS, MS is a risk factor also for periodic limb movements during sleep. In MS, periodic limb movements during sleep are less periodic, less bilateral, and inversely correlated to RLS severity, rather than positively as in idiopathic RLS. Periodic limb movements during sleep alone are associated to sleep disruption in MS. MS seems to be a candidate pathological model of dissociation between the sensory and motor component of RLS. This dissociative clinical feature can help in tailoring the best therapeutic approach to this secondary form of RLS.
INTRODUCTION
Multiple sclerosis (MS) is a chronic disease of the central nervous system characterized by demyelination and axon injury associated with inflammatory activity. MS represents the major cause of nontraumatic permanent disability in young adults.1 The clinically isolated syndrome2 is the first clinical episode that is consistent with a demyelinating etiology and suggestive of MS. The conversion of clinically isolated syndrome to definite MS occurs in 20–75% of the cases,3 when the magnetic resonance imaging shows dissemination of lesions in space and, as evidence for dissemination in time, the simultaneous presence of gadolinium-enhancing and nonenhancing lesions or the presence of cerebrospinal fluid oligoclonal bands.4
Sleep disorders are very common in patients affected by MS. In particular, several studies have identified MS as clearly associated with restless legs syndrome (RLS). The prevalence of RLS in MS ranges between 13.3% and 41%, with MS patients having a 4-fold to 5-fold higher risk than the general population to develop RLS.5,6 Among patients with MS, RLS has been found to be associated with several factors, such as longer disease duration, more severe disability, lower sleep quality, depression and use of antidepressants, fatigue, sleepiness, cervical spinal damage, and worse quality of life.7–11 When RLS occurs in patients with MS, it is considered to be a symptomatic form, implying a causal role for MS in inducing RLS. However, the causal link between these 2 diseases has not yet been definitely proven, and it is still unclear if the MS-related RLS differs from idiopathic RLS (iRLS) under the clinical viewpoint. Indeed, in MS, RLS seems to be more severe and is not clearly associated with iron deficiency or an evident family history.5 Although the role of genetic factors cannot be definitely excluded, the latter finding might suggest that strategic inflammatory lesions might trigger RLS in MS, which would support RLS being considered mediated by MS lesions, contributing to identify the neuronal networks implicated in the pathogenesis of RLS.
Up to over 90% of iRLS patients present a periodic limb movements during sleep index (PLMSI) higher than 5/h.12 Despite being sensitive, the presence of periodic limb movements during sleep (PLMS) is a supportive criterion for the diagnosis of RLS since it is poorly specific.13 A recent polysomnography (PSG) study inferred a relatively low prevalence of PLMS in patients with MS-related RLS (70.8% with PLMS ≥ 5/h).14 PLMSI in MS has been ascertained by 6 previous controlled studies in which, however, the overlap between RLS and PLMS was not investigated. In 2 of these, the PLMSI in patients with MS was higher than in healthy controls,15,16 with the global average PLMSI being slightly above the threshold of 15/h, usually indicated as the upper normative value in adults.17 The other 4 studies found no difference in PLMSI between patients and controls,18–21 suggesting that MS is a risk factor for RLS but not for PLMS. However, all these studies limited their analysis to the PLMSI computation and did not investigate the association with RLS. When used alone, PLMSI is largely insufficient and unspecific in depicting the nature and the structure of leg movement activity (LMA) during sleep.22 A deeper analysis of the time structure of LMA during sleep is able to show crucial information in other symptomatic forms of RLS/PLMS, such as those associated with sleep apnea, narcolepsy, or rapid eye movement sleep behavior disorders, in which the PLMS index alone can hardly find significant differences with iRLS.23–26
The aim of the current study was to investigate LMA during sleep in patients with MS-related RLS; in particular, this study searched for differences in the time structure of LMA during sleep between MS-related RLS and iRLS and, separately, between patients with MS without RLS and a representative sample of healthy non-RLS controls. Secondary aim was to compare the same parameters in patients with MS with and without RLS, also taking into account the confounding factor of psychotropic drug therapy.
METHODS
Participants
A cross-sectional, observational, instrumental, single-center study in a population of patients affected with MS or clinically isolated syndrome, according to McDonald criteria,27 was carried out at the Neurocenter of Southern Switzerland. Patients older than 18 years, with an Expanded Disability Status Scale score < 7 (range 0–10), and a magnetic resonance imaging performed within the 12 months preceding the evaluation were consecutively recruited from the local MS center. Exclusion criteria were Mini Mental Status Examination score lower than 24; recent (within the last 3 months) clinical MS relapse; history of drug and/or alcohol abuse; any serious general medical condition such as decompensated cardiopulmonary disease, cancer, or decompensated renal failure; any major neurological condition other than MS that could interfere with the correct execution of the study design; respiratory disturbance index > 10/h at PSG. No specific limitations were used regarding disease modifying therapies for MS. On the basis of its clinical course, MS was classified as primary progressive, secondary progressive, or relapsing remitting.
At the screening visit, patients were interviewed concerning their medical history and received a complete clinical and neurological examination. Within 1 week from the screening visit, the patients underwent a full-night PSG. Additionally, all patients underwent a structured interview evaluating the presence of RLS, conducted by one PSG-blinded neurologist expert in sleep medicine (MM). A patient was considered to be affected by RLS if she/he met the 5 standard diagnostic criteria13: 1) an urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs; where the urge to move or unpleasant sensations 2) begin or worsen during periods of rest or inactivity such as lying or sitting; 3) are partially or totally relieved by movement, such as walking or stretching; and 4) are worse in the evening or night than during the day or only occur in the evening or night; and where 5) the occurrence of the above features is not solely accounted for as symptoms primary to another medical or a behavioral condition. On this basis, patients were classified into 2 groups: 1) patients affected with MS without RLS symptoms (MS–RLS) and 2) patients affected with MS and RLS (MS+RLS). All patients affected with RLS underwent the validated self-administered International RLS Rating Scale (10 items, score range between 0 and 40) to measure the severity of their RLS symptoms.28
For this study we also retrospectively identified and included patients with iRLS, age-matched and sex-matched with MS+RLS, whose recordings had been utilized in previous studies published by our groups and had been recorded in a standard way. Routine blood tests and neurophysiological investigation (electromyogram and electroneurography of the lower limbs) were normal in these patients. The sleep respiratory pattern of each patient was polygraphically assessed in a previous recording (within 1 week) or during the same recording, and patients with an apnea-hypopnea index > 10 events/h were not included. Neurological examination results were unremarkable in all patients. None of the patients was under any drug treatment for RLS or other neurologic diseases at the time of the recording.
Also, a group of healthy controls, age-matched and sex-matched with MS-RLS, was retrospectively identified and included in this study and screened to exclude those with any current or prior symptoms suggestive of RLS, according to the same minimal criteria set by the International RLS Study Group for the diagnosis of RLS.13
Healthy controls and patients with iRLS had to be in general good health and were excluded if any of the following was present: a diagnosis of any (other) significant sleep disorder(s), major mental illness including any indications of cognitive problems as determined by history, any history of neuroleptic-induced akathisia, or use of any neuroleptic in the past year.
Standard protocol approvals, registrations, and patient consents
According to the regulatory requirements of Switzerland, the protocol of the study was approved by the local Independent Ethics Committee. Eligible patients signed a written informed consent to participate to the study.
Polysomnographic recording and scoring
A full-night PSG recording was obtained for each participant, which included electroencephalogram (at least 3 channels, 1 frontal, 1 central, and 1 occipital, referred to the contralateral earlobe); electrooculogram (2 channels), electromyogram of the submentalis muscle and of both tibialis anterior muscles, and electrocardiogram (1 derivation). Electromyogram signals were band-pass filtered at 10 − 100 Hz, with a notch filter at 50 Hz. At the beginning of each recording session, the amplitude of the electromyogram signal from the 2 tibialis anterior muscles was assured to be below 2 µV at rest.
Sleep stages were visually scored on 30-second epochs29 and all leg movements during sleep were identified following standard criteria30 (see references for a more detailed description of each parameter reported below), with the subsequent calculation of a series of parameters including:
a) Total LMS index, n/h;
b) PLMS index, n/h, LMS included in regular and noninterrupted sequences of at least 4 with onset-to-onset intermovement interval (IMI) 10–90 s;
c) Short-interval LMS (SILMS) index, n/h, LMS with preceding IMI < 10 s;
d) Isolated LMS (ISOLMS) index, n/h, LMS with IMI > 90 s and LMS with IMI 10–90 s not meeting all the criteria for PLMS;
e) Percentage of bilateral PLMS, PLMS formed by two to four monolateral LMS from the two legs overlapping each other within 0.5-s windows with a combined total duration < 15 s;
f) Periodicity Index, PLMS/Total LMS ratio;
g) PLMS duration, s;
h) SILMS duration, s;
i) ISOLMS duration, s;
j) PLMS index in rapid eye movement sleep, n/h;
k) PLMS index in NREM sleep, n/h.
All sleep leg movement onset-to-onset IMI from each recording were counted in each participant for 2-second classes (0.5 < IMI ≤ 2, 2 < IMI ≤ 4, 4 < IMI ≤ 6, …, 98 < IMI ≤100), and group grand averages were obtained, which were used for statistical analysis. Finally, hourly night distribution histograms of the number of PLMS during the first 8 recording hours were also obtained for each group of patients.
Statistical analysis
Descriptive statistics were used, followed by between-group comparisons by means of the Student’s t-test. Also, Bonferroni correction for multiple comparisons was used and the Cohen’s d effect size was computed, when appropriate. Frequency data were analyzed by means of the chi-square or Fisher’s exact test, as appropriate. Correlations were assessed by means of the Pearson’s correlation coefficient; following the Cohen’s indications, we considered correlations 0.10, 0.30, and 0.50 as corresponding to small, medium, and large sizes, respectively. The level for statistical significance was set at P < .05.
Subsequently, we checked for possible simultaneous associations of PLMS index and RLS status (yes/no) used as independent factors/predictors on selected sleep architecture parameters, considered as dependent variables, by means of the General Regression Models module offered by the commercially available software STATISTICA v.6, StatSoft Inc. (this software was also used for all other statistical tests carried out in this study). This module allows to build models for designs with categorical predictor variables, as well as with continuous predictor variables. For each dependent variable, the statistical significance of the association of PLMS index was obtained by considering the effect of the other independent factor (RLS status) and vice versa.
RESULTS
In total, 57 MS patients met the inclusion and exclusion criteria set for this study. Patients with relapsing-remitting MS were the vast majority (n = 47), there were 5 patients with clinically isolated syndrome, only 2 and 3 patients, respectively, with primary-progressive MS and secondary-progressive MS. MS–RLS were 40 (70.2%; 35 women and 5 men, mean age 43.9 years ± 10.60 standard deviation [SD]), while MS+RLS were 17 (29.8%; 11 women and 6 men, mean age 51.5 years ± 6.83 SD). These 2 subgroups were significantly different for both age (t value = 2.707, P = .009) and sex (chi-square = 3.98, P = .046); for this reason, 2 different control groups were arranged, following the inclusion/exclusion criteria described above. The healthy participant group comprised 28 individuals (19 women and 9 men; mean age 42.5 years ± 14.19 SD, t value = 0.454, not significant), while the iRLS group included 35 patients (25 women and 10 men; mean age 50.4 years ± 6.69 SD, t value = 0.523, not significant). The mean International RLS score was significantly lower in MS+RLS than in patients with iRLS (19.7 ± 7.52 SD vs 24.9 ± 4.79 SD, respectively, t value = 2.556, P = .015).
MS patients without RLS vs normal controls
Sleep architecture was abnormal in MS–RLS and characterized by reduced sleep period time, total sleep time, and percentage of sleep stage N2; conversely, sleep latency, number of stage shifts, and percentage of sleep stage N1 were increased in the same patients who also presented a marginally significant increased percentage of sleep stage N3 (Table 1).
Table 1.
Sleep architecture and leg movement activity during sleep parameters in controls and MS patients without RLS.
| Controls (n = 28) |
MS Patients (n = 40) |
Student’s t-Test | Effect Size | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t-Value | P ≤ | Cohen’s d | |
| Sleep architecture | |||||||
| Time in bed, min | 490.3 | 82.21 | 459.5 | 47.33 | 1.953 | NS | 0.459 |
| Sleep period time, min | 474.4 | 79.82 | 421.3 | 62.75 | 3.070 | .003* | 0.740 |
| Total sleep time, min | 417.7 | 66.14 | 376.6 | 66.71 | 2.508 | .015 | 0.619 |
| Sleep latency, min | 11.4 | 12.21 | 28.5 | 30.01 | −2.844 | .006 | −0.745 |
| REM latency, min | 123.3 | 87.34 | 94.9 | 64.43 | 1.545 | NS | 0.370 |
| Stage shifts/h | 13.4 | 4.51 | 19.4 | 6.16 | −4.374 | .000044* | −1.107a |
| Awakenings/h | 6.2 | 2.93 | 5.6 | 2.63 | 0.899 | NS | 0.219 |
| Sleep efficiency, % | 85.6 | 7.43 | 82.0 | 11.85 | 1.447 | NS | 0.370 |
| Stage W, % | 11.5 | 7.26 | 10.5 | 8.64 | 0.499 | NS | 0.125 |
| Stage N1, % | 6.2 | 3.85 | 9.3 | 4.32 | −3.030 | .0035* | −0.754 |
| Stage N2, % | 49.8 | 9.27 | 43.7 | 8.21 | 2.849 | .0058 | 0.694 |
| Stage N3, % | 14.7 | 9.16 | 18.7 | 6.65 | −2.067 | .043 | −0.495 |
| Stage R, % | 17.8 | 5.27 | 17.8 | 6.56 | −0.011 | NS | −0.003 |
| Leg movement activity | |||||||
| PLMS index | 2.3 | 5.46 | 8.5 | 14.54 | −2.158 | .035 | −0.567 |
| SILMS index | 2.3 | 4.31 | 3.1 | 2.64 | −1.009 | NS | −0.238 |
| ISOLMS index | 7.5 | 5.50 | 9.3 | 4.59 | −1.459 | NS | −0.354 |
| Total LMS index | 12.0 | 14.29 | 20.9 | 17.51 | −2.212 | .03 | −0.555 |
| Bilateral PLMS, % | 32.3 | 25.68 | 38.8 | 58.55 | −0.371 | NS | −0.145 |
| Periodicity index | 0.092 | 0.152 | 0.225 | 0.255 | −2.473 | .016 | −0.635 |
| PLMS duration, s | 2.1 | 0.64 | 2.1 | 0.83 | 0.143 | NS | 0.054 |
| SILMS duration, s | 2.5 | 1.46 | 2.2 | 1.09 | 0.904 | NS | 0.217 |
| ISOS duration, s | 2.5 | 0.78 | 2.4 | 0.75 | 0.700 | NS | 0.172 |
| PLMS index in REM sleep | 1.9 | 4.13 | 7.3 | 19.26 | −1.472 | NS | −0.392 |
| PLMS index in NREM sleep | 2.5 | 6.54 | 8.9 | 14.96 | −2.141 | .036 | −0.559 |
Significant after Bonferroni correction. aEffect size value > 0.8 (large effect). ISOLMS = isolated leg movements during sleep, LMS = leg movements during sleep, MS = multiple sclerosis, NREM = non-rapid eye movement, NS = not significant, PLMS = periodic leg movements during sleep, REM = rapid eye movement, SILMS = short-interval leg movements during sleep.
Moreover, some differences in LMA during sleep were also observed between MS–RLS and healthy controls, involving the PLMS index, during total and non-rapid eye movement sleep, total LMS index, and the periodicity index, which were increased (Table 1). The remaining parameters were not significantly different.
The proportion of MS–RLS with a PLMSI > 15/h was 8 out of 32 patients (25.0%), while this figure was 1 out of 27 (3.7%) in healthy controls (Fisher’s exact test: P = .049).
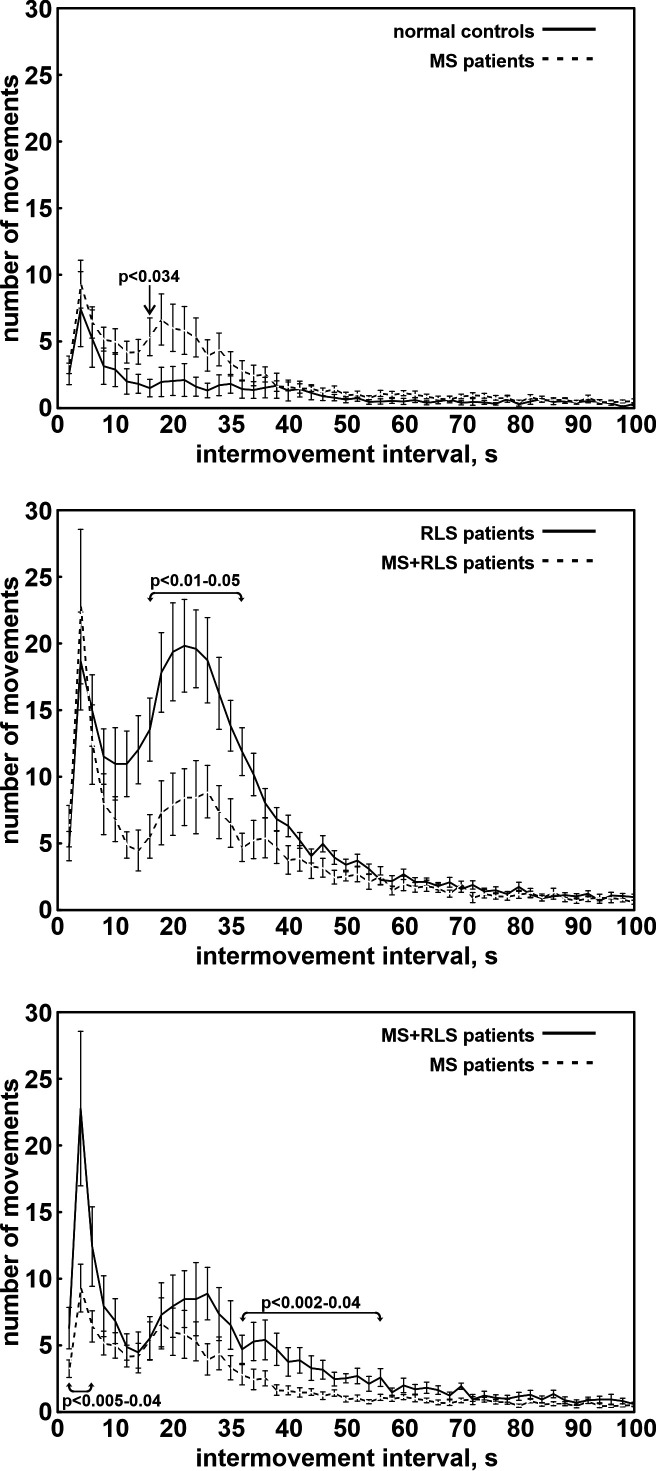
The increase in PLMS in MS–RLS was also evident in the distribution of IMI of the total LMA during sleep (Figure 1, top), with a peak ranging approximately between 10 and 40 s. However, no significant difference was obtained in the comparison with the same distribution in healthy controls.
Figure 1. Distribution of the number of PLMS/h during sleep recorded in the participants.
(Top) Comparison between controls and patients with MS without RLS. (Middle) Comparison between patients with idiopathic RLS and patients with MS with RLS. (Bottom) Comparison between patients with MS without and with RLS. Whiskers indicate standard error. MS = multiple sclerosis, PLMS = periodic limb movements during sleep, RLS = restless legs syndrome.
On the contrary, the analysis of the distribution of the number of PLMS/h during sleep showed that MS–RLS tended to have higher values than healthy controls during the first 7 hours of sleep, reaching statistical significance during the third and sixth hours (Figure 1, top). Moreover, a decline of the PLMS/h could be observed during sleep in MS–RLS, but not in healthy controls.
MS patients with RLS vs patients with idiopathic RLS
Sleep architecture in MS+RLS, compared to that of patients with iRLS, was characterized by reduced time in bed, sleep period time, and total sleep time, as well as increased number of stage shifts and percentage of sleep stage N1 (Table 2).
Table 2.
Sleep architecture and leg movement activity during sleep parameters in patients with idiopathic RLS and MS patients with RLS.
| Idiopathic RLS (n = 35) | MS with RLS (n = 17) | Student’s t-Test | Effect Size | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t-Value | P ≤ | Cohen’s d | |
| Sleep architecture | |||||||
| Time in bed, min | 526.9 | 91.24 | 446.4 | 48.92 | 3.398 | .0013* | 1.100 |
| Sleep period time, min | 497.9 | 90.04 | 413.0 | 55.63 | 3.562 | .00082* | 1.135 |
| Total sleep time, min | 415.1 | 97.17 | 362.8 | 56.93 | 2.049 | .046 | 0.657 |
| Sleep latency, min | 20.0 | 21.09 | 26.4 | 21.92 | −1.004 | NS | −0.295 |
| REM latency, min | 123.6 | 65.99 | 108.4 | 77.73 | 0.738 | NS | 0.212 |
| Stage shifts/h | 12.2 | 3.40 | 20.1 | 6.32 | −5.878 | .000001* | −1.556 |
| Awakenings/h | 4.7 | 2.63 | 5.8 | 2.18 | −1.531 | NS | −0.468 |
| Sleep efficiency, % | 78.1 | 12.44 | 81.2 | 8.63 | −0.909 | NS | −0.285 |
| Stage W, % | 17.2 | 13.16 | 12.0 | 8.82 | 1.471 | NS | 0.464 |
| Stage N1, % | 7.2 | 5.07 | 10.7 | 4.44 | −2.429 | .019 | −0.735 |
| Stage N2, % | 44.8 | 11.45 | 40.0 | 10.61 | 1.458 | NS | 0.437 |
| Stage N3, % | 15.5 | 8.15 | 20.1 | 9.85 | −1.784 | NS | −0.509 |
| Stage R, % | 15.3 | 5.67 | 17.2 | 6.55 | −1.084 | NS | −0.312 |
| Leg movement activity | |||||||
| PLMS index | 27.5 | 18.94 | 15.2 | 15.17 | 2.327 | .024 | 0.715 |
| SILMS index | 4.5 | 4.15 | 7.2 | 6.16 | −1.860 | NS | −0.511 |
| ISOLMS index | 11.0 | 5.42 | 12.7 | 4.12 | −1.175 | NS | −0.364 |
| Total LMS index | 43.0 | 22.43 | 35.1 | 19.44 | 1.230 | NS | 0.373 |
| Bilateral PLMS, % | 33.8 | 24.67 | 18.0 | 17.76 | 2.288 | .027 | 0.734 |
| Periodicity index | 0.601 | 0.189 | 0.362 | 0.228 | 3.999 | .00021* | 1.143 a |
| PLMS duration, s | 2.8 | 0.66 | 2.2 | 0.92 | 2.436 | .019 | 0.693 |
| SILMS duration, s | 2.5 | 0.79 | 2.0 | 0.51 | 2.431 | .019 | 0.770 |
| ISOS duration, s | 3.3 | 0.99 | 2.1 | 0.65 | 4.663 | .000023* | 1.474 a |
| PLMS index in REM sleep | 8.6 | 16.07 | 9.8 | 11.85 | −0.272 | NS | −0.085 |
| PLMS index in NREM sleep | 31.6 | 21.34 | 16.9 | 17.35 | 2.464 | .017 | 0.755 |
Significant after Bonferroni correction. aEffect size value > 0.8 (large effect). ISOLMS = isolated leg movements during sleep, LMS = leg movements during sleep, MS = multiple sclerosis, NS = not significant, PLMS = periodic leg movements during sleep, RLS = restless legs syndrome, SILMS = short-interval leg movements during sleep.
Regarding LMA during sleep, MS+RLS had a lower PLMS index, during total and non-rapid eye movement sleep, percentage of bilateral PLMS, periodicity index, and duration of PLMS, SILMS, and ISOLMS, than patients with iRLS (Table 2).
A PLMSI > 15/h was found in 25 out of 35 patients (71.4%) with iRLS, and 6 out of 17 patients (35.3%) in MS with RLS group (chi-square test: 6.21, P = .013).
Even if the distribution of IMI of the total LMA during sleep (Figure 1, middle) was characterized in both groups by 2 main peaks at around 4 s and in the range 10–50 s, the main peak of patients with iRLS was significantly higher in the range 16–32 s.
The distribution of the number of PLMS/h during sleep showed that both MS+RLS and patients with iRLS had a similar decline throughout the night. However, patients with iRLS tended to have higher values, with a significant difference for the third hour (Figure S1, middle).
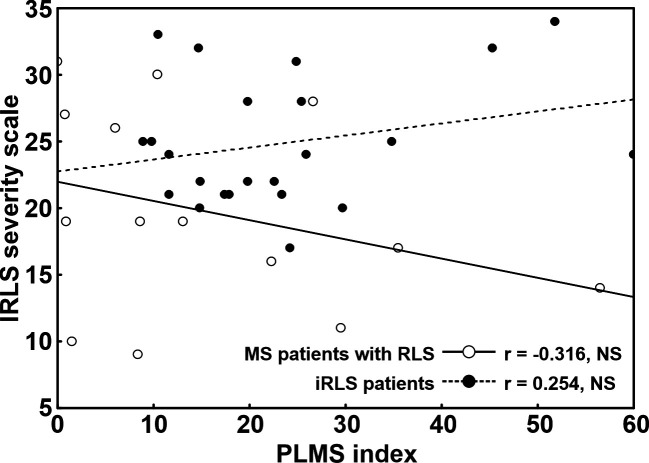
We also assessed the correlation between PLMSI and International RLS severity score separately in MS+RLS and patients with iRLS (Figure 2). In MS+RLS this correlation was negative, with a decreasing number of PLMS in patients with higher RLS severity, and characterized by a moderate correlation coefficient size (although statistically not significant, probably due to the relatively small sample size). On the contrary, in patients with iRLS, the same correlation was positive, with a tendency of PLMSI to be higher in patients with more severe RLS, with a low-to-moderate correlation coefficient size.
Figure 2. Correlation between PLMSI and IRLS severity score, separately, in MS+RLS (empty circles) and iRLS (black-filled circles) patients.
The difference between the correlation coefficients found in MS+RLS and in iRLS, however, was statistically significant (z = 1.68, P < .046).
Leg movement activity during sleep in MS patients with vs without RLS
While MS+RLS tended to show a general increase in LMA during sleep compared to MS–RLS, a significant difference was obtained only for SILMS, ISOLMS, and, consequently, total LMS (Table 3). A PLMSI > 15/h was found in 6 out of 17 (35.3%) MS+RLS patients and 8 out of 40 patients (20%) in the MS–RLS group (Fisher exact test: P = .185).
Table 3.
Leg movement activity during sleep parameters in MS patients without and with RLS.
| MS patients (n = 40) |
MS with RLS (n = 17) |
Student’s t-Test | Effect Size | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t-Value | P ≤ | Cohen’s d | |
| PLMS index | 8.5 | 14.54 | 15.2 | 15.17 | −1.577 | NS | −0.453 |
| SILMS index | 3.1 | 2.64 | 7.2 | 6.16 | −3.541 | .00082* | −0.865a |
| ISOLMS index | 9.3 | 4.59 | 12.7 | 4.12 | −2.668 | .01 | −0.790 |
| Total LMS index | 20.9 | 17.51 | 35.1 | 19.44 | −2.723 | .0086 | −0.771 |
| Bilateral PLMS, % | 38.8 | 58.55 | 18.0 | 17.76 | 1.383 | NS | 0.482 |
| Periodicity index | 0.225 | 0.255 | 0.362 | 0.228 | −1.909 | NS | −0.566 |
| PLMS duration, s | 2.1 | 0.83 | 2.2 | 0.92 | −0.434 | NS | −0.132 |
| SILMS duration, s | 2.2 | 1.09 | 2.0 | 0.51 | 0.784 | NS | 0.255 |
| ISOS duration, s | 2.4 | 0.75 | 2.1 | 0.65 | 1.483 | NS | 0.441 |
| PLMS index in REM sleep | 7.3 | 19.26 | 9.8 | 11.85 | −0.493 | NS | −0.155 |
| PLMS index in NREM sleep | 8.9 | 14.96 | 16.9 | 17.35 | −1.757 | NS | −0.493 |
Significant after Bonferroni correction. aEffect size value > 0.8 (large effect). MS = multiple sclerosis, NS = not significant, RLS = restless legs syndrome, PLMS = periodic leg movements during sleep, SILMS = short-interval leg movements during sleep, ISOLMS = isolated leg movements during sleep, LMS = leg movements during sleep.
Also, the distribution of IMI of the total LMA during sleep (Figure 1, bottom) was characterized by 2 main peaks at around 4 s and in the approximate range 10–60 in both groups; however, the main peak of MS+RLS tended to be higher and reached a statistically significantly difference in the rage 36–56 s.
The distribution of the number of PLMS/h during sleep showed that both MS+RLS and MS–RLS had a decline throughout the night more evident for MS+RLS; however, MS+RLS tended to have higher values, with a statistically significant difference for the second and fifth hour (Figure 1, bottom).
Leg movement activity during sleep in patients with MS treated with antidepressants
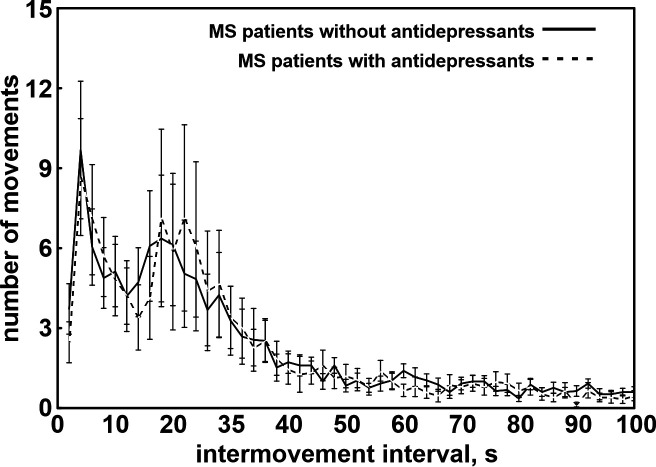
Fifteen out of 40 (37.5%) MS–RLS were under antidepressants, as well as 4 out of 17 (23.5%) MS+RLS (chi-square = 1.05, NS). Because of the low number of patients with antidepressants in the group of MS+RLS, we performed a further comparison between patients under antidepressants and patients without antidepressants only in the MS–RLS group (Figure 3). This also allowed us to avoid the eventual confounding effect of the presence of RLS.
Figure 3. Distribution of intermovement intervals of the total leg movement activity during sleep recorded in the participants.
Linear regression lines are also shown (continuous line for MS+RLS and dashed line for iRLS). iRLS = idiopathic restless legs syndrome, MS = multiple sclerosis, NS = not significant, r = Pearson’s correlation coefficient, RLS = restless legs syndrome.
Notwithstanding that all MS–RLS and under antidepressants were women (5 of 25 without antidepressants were men) and that patients under antidepressant treatment were significantly older than those without (48.5 years ± 9.05 SD vs 41.2 years ± 10.68 SD, t value = 2.213; P = .033), none of the parameters of LMA during sleep was statistically different between these 2 groups. The distribution of IMI of the total LMA during sleep was also strikingly similar between these 2 subgroups (see Figure S1 in the supplemental material). Only 1 patient was taking amitriptyline, the others were all under selective serotonin reuptake inhibitors or serotonin–norepinephrine reuptake inhibitors.
Association between PLMS index or RLS sleep disruption/fragmentation
In addition, in the whole MS group, we assessed possible simultaneous associations of PLMS index and RLS status with selected sleep architecture parameters, indicative of sleep disruption/fragmentation. PLMS index was significantly associated with decreased sleep efficiency and with increased percentage of wakefulness during the sleep period (Table 4). Both correlations showed values that can be considered of “medium size”, following the Cohen’s indications.31 RLS status was not associated with any of the sleep architecture parameters considered.
Table 4.
Correlation between PLMS index or presence/absence of RLS and selected sleep architecture parameters in the whole group of 57 MS patients.
| PLMS Index | RLS/No RLS | |||
|---|---|---|---|---|
| Partial Correlation | P < | Partial Correlation | P < | |
| Total sleep time, min | −0.184 | NS | 0.061 | NS |
| Stage shifts/h | 0.168 | NS | −0.022 | NS |
| Awakenings/h | −0.015 | NS | −0.034 | NS |
| Sleep efficiency, % | −0.271 | .044 | −0.023 | NS |
| Stage W, % | 0.369 | .0052 | −0.002 | NS |
| Stage N1, % | −0.182 | NS | 0.152 | NS |
| Stage N2, % | 0.102 | NS | −0.128 | NS |
| Stage N3, % | −0.180 | NS | −0.121 | NS |
| Stage R, % | −0.096 | NS | 0.023 | NS |
NS = not significant.
DISCUSSION
The present study shows the first analysis of LMA during sleep performed in a relatively large PSG dataset of patients with MS in comparison with iRLS and healthy controls.
As expected, our findings confirm that MS is a risk factor for RLS and for a lower sleep quality in comparison to healthy patients and regardless of the associated LMA. Patients with MS sleep worse than healthy controls even when they have no RLS. At the same time, patients with MS and RLS sleep worse than iRLS. Both of these results support the idea that MS per se is a determinant for low sleep quality and that RLS is an additional contributor to sleep disruption.
Sleep-related polysomnographic parameters in MS were considered in 6 case-control studies, of which only 1 found a reduced of total sleep time in the MS group compared to the control group.15,16,19–21,32,33 We did not find a worse sleep efficiency in MS than in controls, although it emerged as the most affected parameter in the other PSG studies involving MS patients.9 The reasons of the impact of MS on sleep are still unclear. Some authors hypothesized the role of neuroinflammation, while others suggested a direct involvement of demyelinating lesions or even the role of the disease-related psychological burden.34–36 Common symptoms of MS such as pain, paresthesia, spasticity, and MS drug side effects also contribute to worsen the sleep quality and to generate insomnia.
Concerning the LMA, the main aim of our study, we provided 2 important observations.
First, we showed that PLMSI was increased in MS–RLS with respect to normal controls, either considering the mean value in terms of percentage of participants with PLMSI exceeding the clinical threshold value of 15/h. This finding was also confirmed by the time-of-night distribution analysis and suggests that MS per se represents a risk factor for PLMS independently from the presence of RLS. LMA in MS–RLS is similar to the typical PLMS with the typical isolated and short interval movements. This result is reinforced by the higher periodicity of LMA in MS and by the fact that only non-rapid eye movement sleep-related PLMS are more represented in the MS group. None of the previously published case-control PSG studies on MS analyzed the data after excluding the RLS component.
Second, when comparing LMA in MS+RLS and iRLS, the PLMS index in the MS+RLS group was significantly lower. Also, the total LMA differed because of differences in PLMS and not in ISOLMS or SILMS. Thus, the nonperiodic components of LMA, another expression of sleep disruption, were similar in iRLS and MS+ RLS. On the contrary, PLMS in MS+RLS had some different features compared to the typical PLMS seen in patients with iRLS, ie, a less frequent bilateral expression, lower periodicity index, and shorter duration of single motor events. Of note, none of the parameters of LM activity during sleep was influenced by the intake of antidepressants, which therefore does not seem to represent a confounding factor in these patients.
In summary, on the one hand patients with MS had more PLMS than controls even in the absence of RLS, suggesting the role of MS as a risk factor for PLMS; on the other hand, RLS in MS had a less important motor component (fewer and shorter PLMS) than in iRLS. These findings suggest a dissociation between the motor (PLMS) and sensory (uncomfortable and unpleasant sensations in the legs) components being more important than PLMS in MS+RLS than iRLS. The finding of an opposite direction of the correlation between PLMSI and iRLS, negative in MS+RLS and positive in iRLS, further reinforce the idea of a dissociation between motor and sensory RLS components in MS. This dissociation may result from the inflammatory and neurodegenerative neural damage typical of MS, suggesting that MS somewhat represents a model of dissociation of RLS, similarly to that induced by specific drugs in the experimental setting.37 The results from this study confirm the existence of a specific, predominantly sensory RLS phenotype in MS. A neurological lesion load mainly involving descending motor pathways might favor the appearance of PLMS without triggering a whole RLS disorder. On the other hand, lesions in the ascending sensory pathways or affecting thalamo-cortical circuits might elicit the typical “urge to move”, not necessarily accompanied by PLMS. However, it cannot be excluded that at least a portion of patients with MS with RLS might be affected by a typical and genetically determined “primary” form of RLS, due to the epidemiology of this condition. In the age range of the participants in this study, based on epidemiological data, a prevalence of primary RLS around 3–5% can be expected.38
This study has some limitations. First, the small sample size particularly of the MS+RLS group requires further confirmation in larger cohorts. Spine and brain magnetic resonance images were not evaluated, and 1 study found that the presence of spinal cord lesions increased the risk of RLS in patients with MS.39 Future studies including imaging data should evaluate if, in MS patients, RLS with PLMS is associated with a different topographic pattern of neural damage, compared to the pure sensory RLS form. Due to sample size limits, we compared patients with and without intake of antidepressants only in MS–RLS group. Equally, we could not make a comparison taking into consideration the assumption of α2δ ligands and benzodiazepines, 2 drug classes that are known to affect PLMS, because of the low number of patients with MS under this treatment. The lack of data on ferritin levels limits the possibility to speculate on the possible causal links between RLS and MS. In a previous study, iron-storage indicators did not differ between patients with MS with and without RLS.6 Since inflammation and neurodegeneration in MS have been documented to be affected by aberrant iron metabolism and deposition,40 iron involvement in MS-related RLS pathogenesis should be further investigated. The exclusion of patients with an Expanded Disability Status Scale ≥ 7 and the relatively low median Expanded Disability Status Scale in our group of patients with MS limits the generalizability of our results. The lack of a structured assessment of the response of both RLS and PLMS to dopamine agonists is a further limitation, which would require a future interventional trial.
In conclusion, our findings support the importance to always search for RLS in MS, but the role of PLMS as supportive diagnostic criteria is limited in these patients. Other factors, such as the positive response to medications, should be taken into consideration when diagnosing difficult cases. PLMS should also be investigated in patients without RLS but with unexplained sleep disruption. A confirmation and a further characterization of the present findings might also improve our knowledge on the pathways implicated in both the sensory and motor components of RLS and, overall, might guide future treatment options. The preponderance of sensory symptoms without PLMS, or vice versa, sleep disruption with PLMS and without RLS, might suggest a different therapeutic approach, which can be based on the choice of α2δ ligands or opioids, in the first case, and dopamine agonists, in the second.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by a grant ABREOC from Ente Ospedaliero Cantonale (EOC); Swiss MS Society (SMSS). A grant from Ente Ospedaliero Cantonale (EOC) for senior researchers supported the participation of PD Dr. Zecca and Prof. Manconi. The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: M.M. and R.F. contributed to study concept and design, revised the manuscript for intellectual content. D.S. and R.F. drafted the text for intellectual content and contributed to data acquisition and analysis. A.C., S.M., K.T., N.T., C.C., G.R., G.D., C.Z. contributed to data acquisition and analysis and revised the manuscript for intellectual content. M.M., R.F., and D.S. contributed to figure and table preparation, and interpretation of data.
ABBREVIATIONS
- IMI
inter-movement interval
- iRLS
idiopathic restless legs syndrome
- ISOLMS
isolated LMS
- LMA
leg movement activity
- LMS
limb movements in sleep
- MS
multiple sclerosis
- PLMS
periodic limb movements during sleep
- PLMSI
periodic limb movements during sleep index
- PSG
polysomnography
- RLS
restless legs syndrome
- SD
standard deviation
- SILMS
short-interval LMS
REFERENCES
- 1. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclereosis. N Engl J Med. 2000; 343: 938– 952. [DOI] [PubMed] [Google Scholar]
- 2. Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012; 11( 2): 157– 169. [DOI] [PubMed] [Google Scholar]
- 3. Förster M, Graf J, Mares J, Aktas O, Hartung HP, Kremer D. Drug treatment of clinically isolated syndrome. CNS Drugs. 2019; 33( 7): 659– 676. [DOI] [PubMed] [Google Scholar]
- 4. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018; 17( 2): 162– 173. [DOI] [PubMed] [Google Scholar]
- 5. Zecca C, Manconi M, Fulda S, Gobbi C. Restless legs syndrome in multiple sclerosis. CNS Neurol Disord Drug Targets. 2013; 11( 8): 1061– 1069. [DOI] [PubMed] [Google Scholar]
- 6. Manconi M, Ferini-Strambi L, Filippi M, et al. Italian REMS Study Group. Multicenter case-control study on restless legs syndrome in multiple sclerosis: the REMS study. Sleep. 2008; 31( 7): 944– 952. [PMC free article] [PubMed] [Google Scholar]
- 7. Giannaki CD, Aristotelous P, Stefanakis M, et al. Restless legs syndrome in Multiple Sclerosis patients: a contributing factor for fatigue, impaired functional capacity, and diminished health-related quality of life. Neurol Res. 2018; 40( 7): 586– 592. [DOI] [PubMed] [Google Scholar]
- 8. Contentti EC, Lopez PA, Balbuena ME, Finkelstein AM, Tkachuk V. Poster Session 1. Prevalence of restless legs syndrome/Willis-Ekbom disease in multiple sclerosis: A case-control study in Argentina. Mult Scler J. 2017; 23( 3_suppl): 85– 426. [Google Scholar]
- 9. Tanioka K, Castelnovo A, Tachibana N, et al. Framing multiple sclerosis under a polysomnographic perspective. Sleep. 2020; 43( 3): zsz232. [DOI] [PubMed] [Google Scholar]
- 10. Lebrato Hernández L, Prieto León M, Cerdá Fuentes NA, Uclés Sánchez AJ, Casado Chocán JL, Díaz Sánchez M. Síndrome de piernas inquietas en esclerosis múltiple: evaluación de factores de riesgo y repercusión clínica [available online 26 February 2021]. Neurología (English Edition) . 2019;S0213-4853(19)30030-1. [DOI] [PubMed] [Google Scholar]
- 11. Kotterba S, Neusser T, Norenberg C, et al. Sleep quality, daytime sleepiness, fatigue, and quality of life in patients with multiple sclerosis treated with interferon beta-1b: results from a prospective observational cohort study. BMC Neurol. 2018; 18( 1): 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J; Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of HealthInternational Restless Legs Syndrome Study Group. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003; 4( 2): 101– 119. [DOI] [PubMed] [Google Scholar]
- 13. Allen RP, Picchietti DL, Garcia-Borreguero D, et al. International Restless Legs Syndrome Study Group. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014; 15( 8): 860– 873. [DOI] [PubMed] [Google Scholar]
- 14. Sparasci D, Ferri R, Castelnovo A, et al. Restless legs syndrome and periodic limb movements in 86 patients with multiple sclerosis. Sleep. 2021: zsab066. [DOI] [PubMed] [Google Scholar]
- 15. Chen JH, Liu XQ, Sun HY, Huang Y. Sleep disorders in multiple sclerosis in China: clinical, polysomnography study, and review of the literature. J Clin Neurophysiol. 2014; 31( 4): 375– 381. [DOI] [PubMed] [Google Scholar]
- 16. Kaynak H, Altintaş A, Kaynak D, et al. Fatigue and sleep disturbance in multiple sclerosis. Eur J Neurol. 2006; 13( 12): 1333– 1339. [DOI] [PubMed] [Google Scholar]
- 17. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 18. Braley TJ, Chervin RD, Segal BM. Fatigue, tiredness, lack of energy, and sleepiness in multiple sclerosis patients referred for clinical polysomnography. Mult Scler Int. 2012; 2012: 673936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaminska M, Kimoff RJ, Benedetti A, et al. Obstructive sleep apnea is associated with fatigue in multiple sclerosis. Mult Scler. 2012; 18( 8): 1159– 1169. [DOI] [PubMed] [Google Scholar]
- 20. Beran RG, Ainley LAE, Holland G. Sleepiness in multiple sclerosis: A pilot study. Sleep Biol Rhythms. 2008; 6( 4): 194– 200. [Google Scholar]
- 21. Trojan DA, Kaminska M, Bar-Or A, et al. Polysomnographic measures of disturbed sleep are associated with reduced quality of life in multiple sclerosis. J Neurol Sci. 2012; 316( 1-2): 158– 163. [DOI] [PubMed] [Google Scholar]
- 22. Ferri R. The time structure of leg movement activity during sleep: the theory behind the practice. Sleep Med. 2012; 13( 4): 433– 441. [DOI] [PubMed] [Google Scholar]
- 23. Ferri R, Zucconi M, Manconi M, et al. Different periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndrome. Sleep. 2006; 29( 12): 1587– 1594. [DOI] [PubMed] [Google Scholar]
- 24. Manconi M, Ferri R, Zucconi M, Fantini ML, Plazzi G, Ferini-Strambi L. Time structure analysis of leg movements during sleep in REM sleep behavior disorder. Sleep. 2007; 30( 12): 1779– 1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferri R, Franceschini C, Zucconi M, et al. Sleep polygraphic study of children and adolescents with narcolepsy/cataplexy. Dev Neuropsychol. 2009; 34( 5): 523– 538. [DOI] [PubMed] [Google Scholar]
- 26. Manconi M, Zavalko I, Bassetti CL, Colamartino E, Pons M, Ferri R. Respiratory-related leg movements and their relationship with periodic leg movements during sleep. Sleep. 2014; 37( 3): 497– 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69( 2): 292– 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharon D, Allen RP, Martinez-Martin P, et al. International RLS Study Group. Validation of the self-administered version of the international Restless Legs Syndrome study group severity rating scale - The sIRLS. Sleep Med. 2019; 54: 94– 100. [DOI] [PubMed] [Google Scholar]
- 29. Berry RB, Albertario CL, Harding SM, et al. ; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5. Darien, IL: American Academy of Sleep Medicine; 2018. [Google Scholar]
- 30. Ferri R, Fulda S, Allen RP, et al. International and European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG). World Association of Sleep Medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the International and the European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG). Sleep Med. 2016; 26: 86– 95. [DOI] [PubMed] [Google Scholar]
- 31. Cohen J. Statistical Power Analysis for the Behavioural Science. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 32. Elkattan MM, El-Serafy OA, Helmy SM, et al. Exploring the relationship between fatigue and sleep disturbances in multiple sclerosis. Egypt J Neurol Psychiatry Neurosurg; 2009. [Google Scholar]
- 33. Zhang W, Chen X, Su S, et al. Exogenous melatonin for sleep disorders in neurodegenerative diseases: a meta-analysis of randomized clinical trials. Neurol Sci. 2016; 37: 57– 65. [DOI] [PubMed] [Google Scholar]
- 34. Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004; 89( 5): 2119– 2126. [DOI] [PubMed] [Google Scholar]
- 35. Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun . 2002; 16( 5): 503– 512. [DOI] [PubMed] [Google Scholar]
- 36. Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009; 10( 3): 199– 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012; 71( 6): 834– 844. [DOI] [PubMed] [Google Scholar]
- 38. Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012; 16( 4): 283– 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manconi M, Rocca MA, Ferini-Strambi L, et al. Restless legs syndrome is a common finding in multiple sclerosis and correlates with cervical cord damage. Mult Scler. 2008; 14( 1): 86– 93. [DOI] [PubMed] [Google Scholar]
- 40. Stankiewicz JM, Neema M, Ceccarelli A. Iron and multiple sclerosis. Neurobiol Aging. 2014; 35( Suppl 2): S51– S58. [DOI] [PubMed] [Google Scholar]