Abstract
Study Objectives:
References for the evaluation of obstructive sleep apnea often exceed the sleep clinic’s capacity. We aimed to assess the noninferiority of a nurse-communicated model compared with a traditional physician-led model for the initial management of uncomplicated obstructive sleep apnea in the sleep clinic.
Methods:
In this noninferiority, open-label randomized controlled trial, patients referred for the evaluation of uncomplicated obstructive sleep apnea (home sleep apnea test with respiratory event index ≥ 20 events/h) were randomized to a nurse-communicated or a physician-led management. The primary endpoint was noninferiority in the mean change from baseline of the Epworth Sleepiness Scale score at 3 and 6 months, assuming a noninferiority margin of −2.0 points. Secondary outcomes included quality of life (Quebec Sleep Questionnaire) and positive airway pressure adherence.
Results:
Two hundred participants were randomized to a nurse-communicated (n = 101) or physician-led management (n = 99). Overall, 48 participants were lost at follow-up (27.7% and 20.4% in the nurse-communicated and physician-led groups, respectively). Most participants were treated with positive airway pressure (78.2% and 80.6% in the nurse-communicated and physician-led management groups, respectively). There was substantial missing data for the Epworth Sleepiness Scale (33% and 58% at 3 and 6 months in the nurse-communicated group and 29% and 55% in the physician-led group) and Quebec Sleep Questionnaire (86% and 91% at 3 and 6 months and 79.6% and 85.7% in the physician-led group). The difference in mean change between groups for the Epworth Sleepiness Scale was −0.71 (95% confidence interval −2.25 to 0.83) at 3 months and −0.21 (95% confidence interval −1.85 to 1.45) at 6 months. For each domain of the Quebec Sleep Questionnaire at 3 and 6 months, the lower bound of the 95% confidence interval was greater than the prespecified noninferiority margin. Positive airway pressure adherence was similar between groups.
Conclusions:
Among patients with uncomplicated obstructive sleep apnea, nurse-communicated management was noninferior to physician-led management in terms of sleepiness, quality of life, and positive airway pressure adherence at 6 months.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Management of Sleep Apnea Patients by a Clinical Nurse (Supernurse), URL: https://clinicaltrials.gov/ct2/show/NCT03455920; Identifier: NCT03455920.
Citation:
Lajoie AC, Privé A, Roy-Hallé A, Pagé D, Simard S, Séries F. Diagnosis and management of sleep apnea by a clinical nurse: a noninferiority randomized clinical trial. J Clin Sleep Med. 2022;18(1):89–97.
Keywords: sleep-disordered breathing, obstructive sleep apnea, positive airway pressure, nurse-communicated management
BRIEF SUMMARY
Current Knowledge/Study Rationale: Referrals for the evaluation of obstructive sleep apnea often exceed the sleep clinic capacity, thus generating long waiting lists and substantial delays in treatment initiation.
Study Impact: This study proposes that integrating a clinical nurse experienced in the care of patients with sleep disorders in the initial evaluation of uncomplicated obstructive sleep apnea compares with specialized sleep-physician directed evaluation and care in terms of symptomatic response, quality of life, and positive airway pressure therapy adherence. Including appropriately trained registered nurses to the sleep clinic workforce could accelerate obstructive sleep apnea treatment initiation; however, studies assessing reduction in wait lists specifically are warranted.
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep breathing disorder characterized by recurrent upper airway collapse during sleep leading to intermittent hypoxemia and sleep fragmentation.1 It is a highly prevalent disease, affecting 9–38% of the general population depending on the patient population and diagnostic criteria.2 OSA has been linked with numerous cardiovascular and metabolic consequences, namely ischemic heart disease,3,4 arrythmias,5,6 hypertension,7 cerebrovascular disease,8 and diabetes,9 as well as impaired cognitive function10 and health-related quality of life.11 Screening for and treating symptomatic OSA has beneficial impacts on sleep-related quality of life, excessive daytime sleepiness, systemic hypertension, and mood, as well as being cost-effective.12,13
Nevertheless, OSA remains largely underdiagnosed and undertreated.14,15 The diagnosis of OSA requires the expertise of a sleep physician, often respirologists, and access to specialized sleep testing equipment.16 References for the evaluation of OSA currently exceeds the sleep clinic’s evaluation capacity, thus creating important delays.17 In a study from Ontario, mean time to OSA treatment initiation was nearly a year, with accessibility to the sleep laboratory being the major obstacle.17 With the ageing population and the obesity epidemic, referrals to the sleep clinic are projected to increase, mirroring the rise in OSA’s prevalence.18
The integration of specialized nurse in the sleep clinic has been proposed to optimize the sleep clinic’s capacity and reduce treatment initiation delays. A previous study looking at a nurse-communicated management of snoring and OSA clinics suggested beneficial effects on patient-related outcomes, such as daytime sleepiness and quality of life.19 In 2009, a randomized trial assessed the noninferiority of 2 models of care for positive airway pressure (PAP) treatment initiation in patients diagnosed with moderate-to-severe OSA according to results of ambulatory oximetry.19 In the simplified model of care, an experienced nurse supervised ambulatory PAP titration using an auto-adjusting device, whereas in the physician-directed model, patients underwent a diagnostic and a titration in-laboratory polysomnography to determine optimal therapeutic PAP pressure. They concluded that the simplified model of care was noninferior to the physician-directed interventions, as well as being less costly.
Considering the increasing number of references for evaluation of new patients with clinical suspicion of sleep apnea, other strategies must be implemented to preserve quality of care. Therefore, this study aims to assess the noninferiority of a nurse-communicated initial sleep clinic consultation compared with a traditional physician-led consultation on patient-related outcomes.
METHODS
Trial design
We conducted a randomized open-label noninferiority study in a tertiary care university hospital. The research protocol was designed a priori and approved by the Institut Universitaire de Cardiologie et de Pneumologie de Québec research ethics committee. The study protocol is available on clinicaltrials.gov (NCT03455920). Financial support was provided by Sleep Apnea Research and Teaching Fund of the Institut Universitaire de Cardiologie et de Pneumologie-UL Foundation and the Alphonse L’Espérance Funds.
Participants
Patients were recruited from the Institut Universitaire de Cardiologie et de Pneumologie sleep clinic following the completion of a home sleep apnea test (HSAT; level 3 sleep study). In our center, references to the sleep clinic are reviewed by an experienced respirologist who determines if the patient, based on clinical information provided by the referee, fulfills the criteria to have an HSAT prior to their initial sleep clinic consultation.16 As per American Academy of Sleep Medicine guidelines, patients with a suspicion of complicated OSA (significant cardiorespiratory disease or history of recent stroke, chronic opioid medication use, potential respiratory muscle weakness, awake hypoventilation, or suspected sleep-related hypoventilation or suspected comorbid parasomnia/sleep disorders) should be investigated with an upfront polysomnography rather than HSAT and were excluded from this study, as were those requiring clinical assessment before sleep testing (eg, insufficient information).16 Thus, patients who were included in this study were those who had a HSAT prior to their initial sleep clinic visit showing a respiratory events index of ≥ 20 events/h, a central apnea index ≤ 5 events/h, and less than 15% of total sleep time with a saturation (SpO2) below 90%. Respiratory events were scored according to American Academy of Sleep Medicine criteria and using a 3% oxygen desaturation threshold.20 Patients were excluded if they had a body mass index of ≥ 35 kg/m2, had a polysomnography prior to their first visit at the sleep clinic or required an urgent sleep clinic evaluation (eg, occupational risk, severe excessive daytime sleepiness (Epworth Sleepiness Scale [ESS] score > 20/24), recurrent hospitalization for respiratory insufficiency or pregnancy).
Randomization
Patients were randomized in a 1:1 ratio to a nurse-communicated or physician-led management using a randomization list generated by the study statistician using SAS software v9.4. (Cary, NC USA) Randomization was stratified by blocks of 20 patients to ensure an equal number of patients in each group in case of premature termination of study.
Interventions
Patients randomized to the nurse-communicated management group had their initial sleep clinic evaluation conducted by a trained clinical nurse (A.R.H and D.P). The clinical nurse completed the entire sleep questionnaire, physical examination, reviewed the diagnostic testing (HSAT), and discussed therapeutic options with the patient. Before patient discharge, the respirologist appointed to the sleep clinic reviewed and discussed each case with the clinical nurse to confirm or adjust the final treatment plan and determine the need for further investigations or referral, if appropriate.
Before the participation in the study, the nurses (A.R.H and D.P) received a specific training pertaining to the diagnosis and management of OSA. The training included documentation on diagnostic and therapeutic options for OSA, observation periods in the sleep laboratory to gain expertise in the scoring of HSAT and an observation period with a head, eyes, ear, nose, and throat (HEENT) physician and with a sleep-specialized respirologist to gain expertise on the clinical evaluation of OSA and the different therapeutic options. The minimal observation period was 1 month and could be extended if deemed necessary. On average, the nurses had 115 hours of training. This training, which was similar between both nurses, was complementary to our clinical nurses’ prior years of experience in the follow-up of patients with sleep breathing disorders. Before their involvement in the study, the clinical nurses worked full-time at the sleep clinic for at least 2 years. In Quebec (Canada), a clinical nurse obtains a bachelor’s degree. Furthermore, the evaluation of the physical and mental condition of a symptomatic individual falls within the scope of practice of a clinical nurse, as per the Order or Nurses of Quebec (Ordre des infirmières et infirmier du Québec or OIIQ) deontology code. We consulted a representative of the OIIQ and the nursing services director of our hospital to ensure that our procedure would not infringe current provincial regulations.
Patients randomized to the physician-led evaluation had their first sleep clinical evaluations performed by the respirologist appointed to the sleep clinic. Both groups were evaluated using the same consultation sheet to standardize each first-visit evaluation and data collection.
Treatment options were standard and consisted of PAP (continuous or automatic), mandibular advancement device, HEENT surgery, weight loss, positional therapy, and observation.
Follow-up after the initial sleep clinic visit was the same for both groups and was conducted according to our sleep clinic’s standard of care. For patients who were prescribed PAP therapy, the sleep clinic nurse (A.R.H or D.P.) contacted the participants over the phone 4–6 weeks after treatment initiation to review any issues with the device and reviewed the therapeutic and adherence reports obtained from the device download (communicated by the PAP provider or obtained through the cloud database). Device downloads were also reviewed at 3 and 6 months to monitor and collect PAP adherence data. Any adherence or therapeutic issues that could not be immediately resolved (eg, high PAP reported apnea-hypopnea index on the device report, residual excessive daytime sleepiness, suspicion of treatment emergent central sleep apnea, refractory intolerance to therapy despite usual mitigation measures) were discussed with the attending sleep clinic respirologist and a follow-up or additional testing visit was organized, if needed. For both groups, the PAP device provider was involved in PAP therapy initiation, initial mask fitting, and troubleshooting pertaining to any mask/device issue. They also shared therapeutic and adherence data from the PAP device download for patients whose information was unavailable through the cloud database. A follow-up visit with the sleep clinic respirologist was planned for the patients who were prescribed mandibular advancement device, HEENT surgery, weight loss, positional therapy, or observation.
Outcomes
The primary outcome was noninferiority in the mean change from baseline in ESS score at 3 and 6 months. The ESS is a self-administered questionnaire with 8 items rated on a 4-point scale (ranging from 0 to 3, with a maximum of 24 points) assessing the propensity of an individual to fall asleep in certain situations. Secondary outcomes included noninferiority in the mean change from baseline in Quebec Sleep Questionnaire (QSQ) and difference between groups in treatment adherence. The QSQ was developed specifically to assess health-related quality of life in patients with OSA.21 It has 32 items scored on a 7-point Likert scale, providing a quality of life score for five different domains: 1) excessive sleepiness, 2) diurnal symptoms, 3) nocturnal symptoms, 4) emotions, and 5) social interactions. A lower score is associated with higher impact on the affected domain. Minimal clinically important difference differs for each domain and has been reported previously as follows: daytime sleepiness: 1.8, diurnal symptoms: 2.0, nocturnal symptoms: 1.5, emotions: 1.1, social interactions: 2.5.21
PAP adherence and efficacy (% nights used and % nights with use > 4 hours) was assessed at 3 and 6 months by obtaining PAP device downloads from the PAP provider or by accessing cloud-based data. Unfortunately, no self-reported adherence or therapeutic efficacy data were available at 3 or 6 months for non-PAP therapies (such as mandibular advancement devices, positional therapy, or HEENT surgery) due to delays in initiating therapy or obtaining a repeat sleep study or follow-up visit. All participants were analyzed for ESS and QSQ, regardless of treatment type or adherence.
Data collection
Data were collected by consulting the patient’s electronic file. Baseline demographic data and OSA variables (respiratory event index [apneas + hypopneas/total recording time], respiratory disturbance index [apneas + hypopneas + respiratory event-related arousals/total recording time]), oxygen desaturation index, percentage of the total recording time with an SpO2 < 90% were collected at baseline. ESS and QSQ were administered on the first visit, and participants were instructed to mail an ESS and QSQ questionnaire at 3-month and 6-month time. Baseline questionnaires were completed upon first visit and participants would be sent home with the 3-month and 6-month questionnaires, which they were to return to the sleep clinic nurse by email or regular mail. Reminders were issued by the sleep clinic nurse in case there were delays in returning the questionnaires. However, due to the low response rate, participants were called at 6 months to complete ESS over the phone and an additional copy of the QSQ was sent by email or mail to be completed. Missing ESS scores were also retrieved retrospectively from the PAP provider’s patient data, if available, within +/−2 weeks from the projected 3-month and 6-month timepoints. Despite these measures, the rate of response for the QSQ at 6 months remained low. Patients were considered lost at follow-up if no ESS and no QSQ data were available at 3 months and 6 months.
PAP parameters from the devices’ reports (PAP mode, median or mean PAP pressure, PAP machine reported apnea-hypopnea index, percentage of nights with greater than 4 hours of use, and overall percentage of use) were collected at 3 months and 6 months.
Statistical analysis
Baseline categorical variables are expressed in frequencies (%) and were analyzed using Fisher’s exact test, while continuous variables were reported as means ± standard deviation and analyzed using a Student’s t-test. Statistical analysis for the primary and secondary endpoints comparing the mean change from baseline in ESS and QSQ scores at 3 months and 6 months between groups were analyzed using a linear mixed-model with an unrestricted covariance structure and 2 fixed factors: 1 linked to the comparison between physician and nurse and the other associated with the 3 visits, with an interaction term between factors. The normality assumption was verified using the Shapiro-Wilks tests using residuals from the statistical model and transformed by the Cholesky's metric for repeated measurements. The Brown and Forsythe's variation of Levene's test statistic will be used to verify the homogeneity of variances. The lower bound of the 95% confidence interval (CI) was used to determine noninferiority. For each domain of the QSQ, a change greater or equal to predefined minimal clinically important difference (defined above) was used to determine noninferiority. PAP adherence (% nights with device usage, % nights with device usage > 4 hours and mean use [hours/night]) at 3 months and 6 months in each group was analyzed using independent t-test. Data were analyzed using an intention to treat principle, and all tests were performed at a 5% significance level.
Sample size
Using the change in ESS as the outcome of interest and assuming a noninferiority margin of 2.0 points (standard deviation of 2.0 points) and a drop-out rate of 10% in each arm, we estimated a sample size of 200 patients (100 patients per group) would be necessary (β = 0.90). The noninferiority margin was selected based on the minimal clinically important difference, as previously reported.22 The 1-sided significance level (alpha) of the test was set at 0.025.
RESULTS
Participants
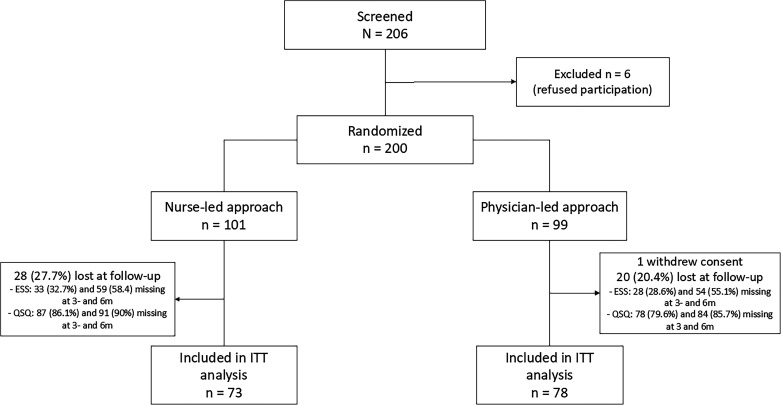
The enrolment process is summarized in Figure 1. A total of 206 eligible patients were approached, of which 200 consented to participate in the study and were randomized to nurse-communicated (n = 101) or physician-led (n = 99) management. One patient in the physician-led group withdrew consent before inclusion in the study. Overall, 48 patients were lost at follow-up (27.7% and 20.0% in the nurse-communicated and physician-led group, respectively). Key baseline characteristics, OSA metrics or prescribed treatment for participants lost to follow-up were comparable between intervention groups (Table S1 in the supplemental material). At 3 months, 68 and 70 participants had an ESS score and 14 and 20 a QSQ in the nurse-communicated and physician-led groups, respectively. At 6 months, these numbers were 42 and 44 for the ESS and 10 and 14 for the QSQ. At 3 months and 6 months, ESS data were available for 79% and 67% of participants who were prescribed PAP and for 34% and 22% of participants who were offered non-PAP therapies (Figure S1 in the supplemental material). For the QSQ, these numbers were much lower (18% and 12% at 3 months and 6 months for PAP and 12% at both timepoints for non-PAP therapies).
Figure 1. Study flow chart.
Lost at follow-up indicates no follow-up data for ESS and QSQ (at 3 and 6 months) was available. ESS = Epworth Sleepiness Scale, ITT = intention-to-treat, m = months, QSQ = Quebec Sleep Questionnaire.
Baseline characteristics are presented in Table 1. Apart from higher neck circumference and a higher proportion of patients with dyslipidemia in the nurse-communicated management group, the baseline characteristics, including OSA severity, were otherwise well-balanced between groups. There was no significant difference in ESS or QSQ scores between groups at baseline. Recommended OSA treatments were similar, with PAP being the most prescribed treatment modality in both groups (78.2% and 80.6% in the nurse-communicated and physician-led groups, respectively).
Table 1.
Baseline characteristics of patients.
| Nurse-Communicated (n = 101); Age: 55.6 ± 13.1 (range: 26.0–87.0) | Physician-Led (n = 98); Age: 54.6 ± 12.5 (range: 29.0–88.0) | |
|---|---|---|
| Men, n (%) | 75.0 (74.3) | 66.0 (67.4) |
| Body mass index, kg/m2 | 30.2 ± 4.2 | 30.3 ± 4.4 |
| Neck circumference, cma | 41.5 ± 4.1a | 38.8 ± 4.1a,** |
| Prior ENT surgery, n (%)b | 31.0 (30.7) | 19 (19.4) |
| Comorbidities, n (%) | ||
| Arterial hypertension | 43.0 (42.5) | 30.0 (30.6) |
| Cardiovascular disease | 9.0 (8.9) | 11 (11.2) |
| Diabetes mellitus | 11.0 (10.9) | 5.0 (5.1) |
| Dyslipidemia | 39.0 (38.6) | 24.0 (24.5)* |
| Atrial fibrillation | 4.0 (4.0) | 3.0 (3.1) |
| Obstructive sleep apnea variables (HSAT) | ||
| REI, events/h | 32.1 ± 12.2 | 34.6 ± 15.3 |
| Supine index, events/h | 46.1 ± 18.7 | 52.4 ± 20.9* |
| ODI 3%, events/h | 32.9 ± 12.4 | 33.9 ± 14.9 |
| T90, % of TRT | 6.2 ± 5.6 | 7.6 ± 9.0 |
| Treatment recommended (n, %) | ||
| Positive airway pressure | 79.0 (78.2) | 79.0 (80.6) |
| Mandibular advancement device | 2.0 (2.0) | 1.0 (1.0) |
| Upper airway surgery | 6.0 (5.9) | 6.0 (6.1) |
| Weight loss | 2.0 (2.0) | 0.0 (0.0) |
| Positional therapy | 2.0 (2.0) | 2.0 (2.0) |
| No treatment | 10.0 (9.9) | 10.0 (10.2) |
| Epworth Sleepiness Scale score | 10.7 ± 4.6 (range: 2.0–21.0) | 9.5 ± 5.2 (range: 0.0–21.0) |
| Epworth Sleepiness Scale score > 10, n (%) | 53.0 (52.5) | 42.0 (42.9) |
| Quebec Sleep Questionnaire score | ||
| Daytime sleepiness | 4.7 ± 1.5 | 4.9 ± 1.6 |
| Diurnal symptoms | 4.3 ± 1.7 | 4.3 ± 1.7 |
| Nocturnal symptoms | 4.5 ± 1.3 | 4.5 ± 1.4 |
| Emotions | 5.0 ± 1.3 | 5.0 ± 1.4 |
| Social | 5.0 ± 1.5 | 5.0 ± 1.5 |
Values are expressed as means ± standard deviation unless otherwise specified. *P < .05, **P < .001. an = 93 in nurse-communicated management group and n = 32 in physician-led management group. bPrior ENT surgery refers to any nasal (turbinate reduction, septoplasty, rhinoseptoplasy, or rhinoplasty) or throat (tonsillectomy or uvulopalatopharyngoplasty) surgery. ENT = ear, nose, and throat, HSAT = home sleep apnea test, ODI = oxygen desaturation index (3%), RDI = respiratory disturbance index (apneas + hypopneas + respiratory event related arousals/total recording time), REI = respiratory events index (apneas + hypopneas/total recording time), T90 = percentage of total recording time (TRT) spent with a saturation < 90%.
Primary outcomes: change in ESS at 3 months and 6 months
The mean ESS at 3 months was 6.19 (standard deviation 4.89) in the nurse-communicated group and 5.79 (3.97) in the physician-led group. The difference in mean change between groups at 3 months was −0.71 (−2.25 to 0.83) (Table 2). The mean ESS at 6 months was 5.88 (3.89) in the nurse-communicated group and 5.05 (3.54) in the physician-led group. The difference in mean change between groups at 6 months was −0.21 (95% CI: −1.85 to 1.45). The lower bound of the 95% CI of the difference in mean change in ESS was used to determine noninferiority. Thus, because the lower bound of the 95% CI for the difference in mean change at 6 months is greater than the prespecified noninferiority margin of −2.0, the results these results support the noninferiority of management by the nurse-communicated group at 6 months (Figure 2). We could not conclude to noninferiority of the nurse-communicated management group at 3 months because the lower bound of the 95% CI (−2.25) was lower than the prespecified noninferiority margin (−2.0).
Table 2.
Change in Epworth Sleepiness Scale Score at 3 and 6 months.
| Nurse-Communicated Group | Physician-Led Group | Difference in Mean Change (SEM) | 95% CI | |
|---|---|---|---|---|
| 3 Months | −4.44 (−5.54 to −3.36) (n = 68) | −3.74 (−4.82 to −2.66) (n = 70) | −0.71 (0.78) | −2.25 to 0.83 |
| 6 Months | −4.85 (−6.03 to −3.67) (n = 42) | −4.64 (−5.80 to −3.48) (n = 44) | −0.21 (0.84) | −1.86 to 1.45 |
Values are presented as mean change from baseline (95% CI). CI = confidence interval, SEM = standard error of the mean.
Figure 2. Difference in mean change for ESS between nurse-communicated and physician-led management.
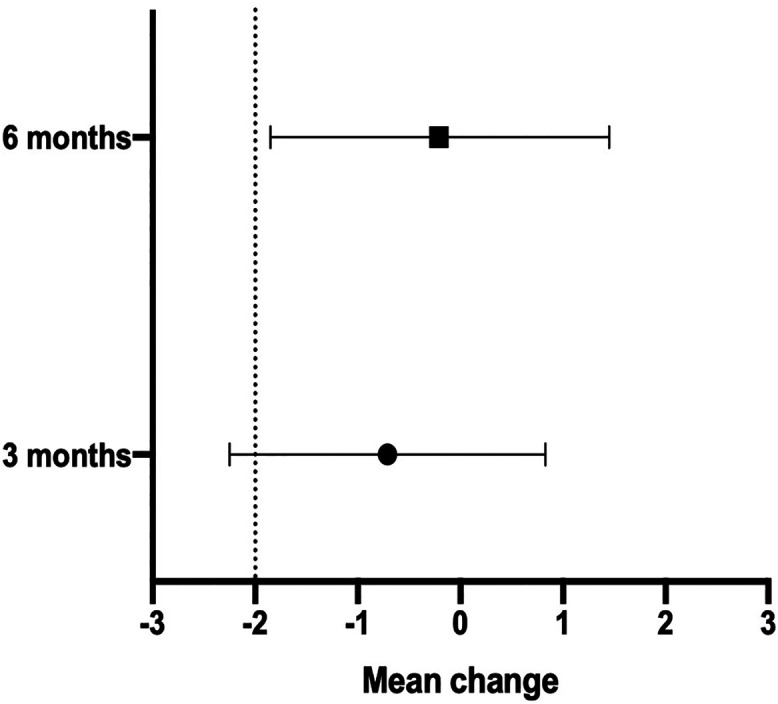
ESS = Epworth Sleepiness Scale.
Secondary outcomes: change in QSQ at 3 months and 6 months
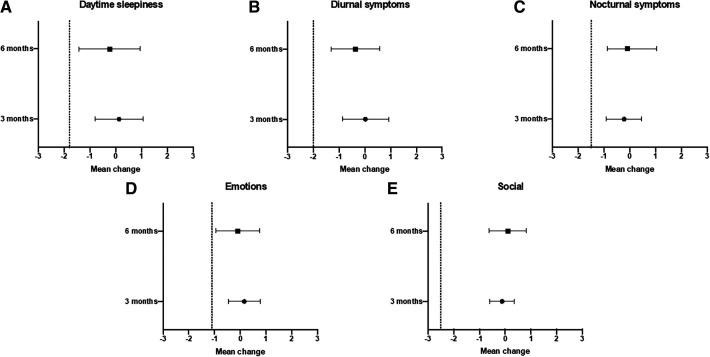
The differences in QSQ scores for each domain are detailed in Table 3. We used the lower bound of the 95% CI of the between groups difference in mean change in each domain of the QSQ at 3 months and 6 months to determine noninferiority according to prespecified thresholds (see Methods section). For each domain at 3 months and 6 months, the lower bound of the 95% CI was greater than the prespecified noninferiority margin, indicating noninferiority of the nurse-communicated management with respect to the QSQ (Figure 3).
Table 3.
Change in QSQ score at 3 and 6 months.
| QSQ Domain | Nurse-Communicated Group | Physician-Led Group | Difference in Mean Change (SEM) | 95% CI |
|---|---|---|---|---|
| Daytime sleepiness | ||||
| 3 months | 0.90 (0.18 to 1.61) (n = 14) | 0.77 (0.16 to 1.37) (n = 20) | 0.13 (0.46) | −0.81 to 1.07 |
| 6 months | 0.90 (0.00 to 1.79) (n = 10) | 1.13 (0.35 to 1.90) (n = 14) | −0.23 (0.56) | −1.41 to 0.95 |
| Diurnal symptoms | ||||
| 3 months | 0.97 (0.28 to 1.65) (n = 14) | 0.95 (0.37 to 1.52) (n = 20) | 0.02 (0.44) | −0.87 to 0.92 |
| 6 months | 0.54 (−0.18 to 1.26) (n = 10) | 0.90 (0.29 to 1.51) (n = 14) | −0.36 (0.46) | −1.30 to 0.58 |
| Nocturnal symptoms | ||||
| 3 months | 1.31 (0.80 to 1.82) (n = 14) | 1.53 (1.08 to 1.98) (n = 20) | −0.22 (0.34) | −0.91 to 0.46 |
| 6 months | 1.27 (0.55 to 1.99) (n = 10) | 1.18 (0.56 to 1.81) (n = 14) | 0.08 (0.46) | −0.87 to 1.04 |
| Emotions | ||||
| 3 months | 0.58 (0.11 to 1.05) (n = 14) | 0.43 (0.03 to 0.82) (n = 20) | 0.15 (0.31) | −0.46 to 0.77 |
| 6 months | 0.33 (−0.31 to 0.98) (n = 10) | 0.43 (−0.11 to 0.98) (n = 14) | −0.10 (0.40) | −0.95 to 0.75 |
| Social | ||||
| 3 months | 1.04 (0.68 to 1.39) (n = 14) | 1.16 (0.84 to 1.48) (n = 20) | −0.12 (0.24) | −0.60 to 0.36 |
| 6 months | 1.43 (0.89 to 1.97) (n = 10) | 1.33 (0.85 to 1.81) (n = 14) | 0.10 (0.36) | −0.63 to 0.82 |
Values are presented as mean change from baseline (95% CI). Minimal clinically important difference (MCID) differs for each domain and has been reported previously as follows: daytime sleepiness: 1.8; diurnal symptoms: 2.0; nocturnal symptoms: 1.5; emotions: 1.1; social interactions: 2.5.21 CI = confidence interval, QSQ = Quebec Sleep Questionnaire, SEM = standard error of the mean.
Figure 3. Difference in mean change for QSQ between nurse-communicated and physician-led management.
QSQ = Quebec Sleep Questionnaire.
Secondary outcomes: treatment adherence
Adherence data were only available for those on PAP therapy, which was recommended for 78% and 81% of patients in the nurse-communicated and physician-led management groups, respectively. PAP adherence data were available for 86.1% and 93.7% of participants in the nurse-communicated and physician-led groups at 3 months, respectively, and 65.8% and 76.0% at 6 months. There was no significant difference between groups in terms of mean nightly use, % of nights of use, % nights with usage ≥ 4 hours. Treatment efficacy was similar in both groups, with a PAP machine reported apnea-hypopnea index < 5 events/h in both groups at 3 months and 6 months (Table 4).
Table 4.
PAP therapy adherence at 3 and 6 months.
| 3 Months | 6 Months | |||||
|---|---|---|---|---|---|---|
| Nurse- Communicated Group (n = 68) | Physician-Led Group (n = 74) | P | Nurse-Communicated Group (n = 52) | Physician-Led Group (n = 60) | P | |
| Percentage of nights used | 87.4 ± 23.0 | 93.3 ± 11.0 | .05 | 86.9 ± 18.4 | 88.8 ± 18.0 | .58 |
| Percentage nights ≥ 4 hours | 74.6 ± 29.3 | 81.7 ± 23.7 | .11 | 73.7 ± 26.2 | 71.8 ± 28.5 | .72 |
| Mean use (hours/night) | 6.13 ± 1.9 | 6.4 ± 1.7 | .38 | 6.3 ± 1.6 | 6.2 ± 1.7 | .59 |
| PAP machine–reported AHI, events/h | 4.5 ± 4.1 | 4.1 ± 5.3 | .62 | 3.1 ± 2.7 | 3.8 ± 5.5 | .41 |
Values are presented as mean ± standard deviation. AHI = apnea-hypopnea index, PAP = positive airway pressure.
DISCUSSION
In this open-label randomized controlled trial we found that nurse-communicated management of uncomplicated OSA in the sleep clinic was noninferior to standard of care physician-directed management, with respect to improvement in self-reported sleepiness at 6 months, quality of life, and PAP adherence at 3 months and 6 months.
The diagnosis and management of chronic diseases by practician or specialized, registered nurses have been proven successful in numerous pathologies, namely diabetes, hypertension, and heart failure.23–26 Until recently, the role of nurses in the sleep clinic has been centered on the management of OSA after treatment initiation, often with PAP, and promoting its adherence.19 A prior open-label noninferiority randomized controlled trial found that a simplified model of care, consisting of ambulatory overnight oximetry for the diagnosis of OSA and nurse-communicated management, was similar to a standard management trajectory where OSA was diagnosed by polysomnography and managed by a physician.19 In this study, participants had to be willing to try PAP to be included, with those refusing or failing PAP being referred to a sleep physician. This differs from our nurse-communicated management model, where after receiving extensive training on the different modalities available for OSA treatment, the nurses would discuss the appropriate therapy with the participants based on their OSA characteristics and preferences. The recommended treatments were similar between nurse-led and physician-led management groups, with PAP being the preferred modality, but also included upfront mandibular advancement device or positional therapy in adequate candidates. Furthermore, PAP adherence was similar in both groups. However, most participants on PAP benefited from early follow-up of adherence by the sleep clinic nurse, as it is a standard procedure in our center and has been proven beneficial to promote PAP adherence in previous studies.27
Increasing the workforce in the sleep clinic is of particular importance in an era where the demand for the initial management of OSA often exceeds the sleep clinic’s capacity and produces significant delays in treatment initiation.28 Untreated OSA has been associated with numerous adverse consequences, such as cardiovascular and metabolic diseases as well as motor vehicle and workplace accidents related to excessive daytime sleepiness.27 Involving advanced practice providers, such as nurse practioners or trained clinical nurses, could reduce delays in treatment initiation and was recently supported by a report from the American Academy of Sleep Medicine Sleep-Disordered Breathing Collaboration Summit.28 Other studies also suggest including primary care physicians in ambulatory management of uncomplicated OSA is comparable to specialized care.27 However, in some countries such as Canada, access to a primary care physicians is a challenge and adding additional tasks to their workload is counterintuitive.
Our results must be interpreted in the context of uncomplicated OSA, as all participants included in the study had a level 3 HSAT showing OSA without significant central sleep apnea or sustained hypoxemia suggestive of hypoventilation. In our center, referrals for a suspicion of OSA are triaged by the respirologist appointed to the sleep clinic, ensuring that participants with suspicion of an alternate diagnosis or potential complicated OSA, such as referrals with significant cardiopulmonary, neuromuscular or cerebrovascular disease, chronic opioid medication or suspicion of hypoventilation, hypoxemia or comorbid sleep disorder, will receive the appropriate investigation by polysomnography and specialized management.16 With this strategy, in our clinic, sleep apnea diagnosis, treatment discussion, and prescription can be completed within 1 visit in approximately 75% of patients. Furthermore, our registered nurses received extensive training in HEENT and regular oversight from the sleep physician, which may be difficult to reproduce in certain settings where these resources are unavailable (eg, nontertiary or regional care centers). Our model may also be difficult to implement in countries where regulations preclude nurses, outside of advanced practice providers, from being involved in the evaluation and management of chronic diseases.
The most important limitation of this study was the important number of missing ESS and QSQ questionnaires at 3-month and 6-month follow-up, despite attempts to reach out to the participants and mitigate data loss. Indeed, 27.7% and 20.4% of the participants in the nurse-communicated and physician-led groups failed to complete the follow-up ESS and QSQ, the latter being available for less than 20% of participants in both groups at 3 months and 6 months. There were no significant differences in the baseline or treatment characteristics of participants lost to follow-up between groups. However, such as high number of missing data yields imprecision and may have introduced bias and impaired the capacity to determine noninferiority of the nurse-communicated management approach at 3 months for the ESS. However, it is unlikely that a bigger sample size would have modified our conclusions, apart from yielding a tighter CI. Other studies have reported a fairly high percentage of missing data with self-reported outcomes, such as quality of life.29 The extent of the missing data in our study may, in part, reflect the loss to follow-up we experience clinically within the sleep center. However, relying on the participants to remember when to complete and return the questionnaires instead of mailing them at the appropriate time or using an electronic database was perhaps the biggest driving factor explaining the importance of data missingness in each group.
Lastly, we should mention the possibility of selection bias, especially in light of the excellent compliance to PAP therapy (> 85% of nights at 3 months and 6 months in both groups) in our study. This exceeds what is reported in the literature and may reflect inclusion of a population more motivated to engage with therapy and more likely to experience to symptomatic improvement with PAP.30
In conclusion, among patients with uncomplicated OSA, nurse-led management was noninferior to physician-led management in terms of sleepiness or quality of life at 6 months. There was no difference in treatment allocation nor PAP adherence among patient treated between groups.
DISCLOSURE STATEMENT
All authors have read and approved this manuscript. This study was funded by the Sleep Apnea Research and Teaching Fund of the Institut Universitaire de Cardiologie et de Pneumologie-UL Foundation and the Alphonse L’Espérance Fund. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Mr. Simon Gakwaya for his participation in patient follow-up.
ABBREVIATIONS
- CI
confidence interval
- ESS
Epworth Sleepiness Scale
- HEENT
head, ear, eyes, nose, and throat
- HSAT
home sleep apnea test
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- QSQ
Quebec Sleep Questionnaire
REFERENCES
- 1. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017; 34: 70– 81. [DOI] [PubMed] [Google Scholar]
- 3. Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005; 365( 9464): 1046– 1053. [DOI] [PubMed] [Google Scholar]
- 4. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009; 373( 9657): 82– 93. [DOI] [PubMed] [Google Scholar]
- 5. Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004; 110( 4): 364– 367. [DOI] [PubMed] [Google Scholar]
- 6. Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003; 107( 20): 2589– 2594. [DOI] [PubMed] [Google Scholar]
- 7. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342( 19): 1378– 1384. [DOI] [PubMed] [Google Scholar]
- 8. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005; 353( 19): 2034– 2041. [DOI] [PubMed] [Google Scholar]
- 9. Pamidi S, Wroblewski K, Broussard J, et al. Obstructive sleep apnea in young lean men: impact on insulin sensitivity and secretion. Diabetes Care. 2012; 35( 11): 2384– 2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013; 18( 1): 61– 70. [DOI] [PubMed] [Google Scholar]
- 11. Silva GE, Goodwin JL, Vana KD, Quan SF. Obstructive sleep apnea and quality of life: comparison of the SAQLI, FOSQ, and SF-36 questionnaires. Southwest J Pulm Crit Care. 2016; 13( 3): 137– 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Streatfeild J, Hillman D, Adams R, Mitchell S, Pezzullo L. Cost-effectiveness of continuous positive airway pressure therapy for obstructive sleep apnea: health care system and societal perspectives. Sleep. 2019; 42( 12): zsz181. [DOI] [PubMed] [Google Scholar]
- 13. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: An American academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019; 15( 2): 301– 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simpson L, Hillman DR, Cooper MN, et al. High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep Breath. 2013; 17( 3): 967– 973. [DOI] [PubMed] [Google Scholar]
- 15. Costa LE, Uchôa CHG, Harmon RR, Bortolotto LA, Lorenzi-Filho G, Drager LF. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart. 2015; 101( 16): 1288– 1292. [DOI] [PubMed] [Google Scholar]
- 16. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017; 13( 3): 479– 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rotenberg B, George C, Sullivan K, Wong E. Wait times for sleep apnea care in Ontario: a multidisciplinary assessment. Can Respir J. 2010; 17( 4): 170– 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Twells LK, Gregory DM, Reddigan J, Midodzi WK. Current and predicted prevalence of obesity in Canada: a trend analysis. CMAJ Open. 2014; 2( 1): E18– E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antic NA, Buchan C, Esterman A, et al. A randomized controlled trial of nurse-led care for symptomatic moderate-severe obstructive sleep apnea. Am J Respir Crit Care Med. 2009; 179( 6): 501– 508. [DOI] [PubMed] [Google Scholar]
- 20. Berry RB, Budhiraja R, Gottlieb DJ, et al. ; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012; 8( 5): 597– 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lacasse Y, Bureau MP, Sériès F. A new standardised and self-administered quality of life questionnaire specific to obstructive sleep apnoea. Thorax. 2004; 59( 6): 494– 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel S, Kon SS, Nolan CM, et al. Minimum clinically important difference of the Epworth Sleepiness Scale. Eur Resp J . 2017; 50: PA330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Litaker D, Mion L, Planavsky L, Kippes C, Mehta N, Frolkis J. Physician - nurse practitioner teams in chronic disease management: the impact on costs, clinical effectiveness, and patients’ perception of care. J Interprof Care. 2003; 17( 3): 223– 237. [DOI] [PubMed] [Google Scholar]
- 24. Health Quality Ontario. Specialized nursing practice for chronic disease management in the primary care setting: an evidence-basedanalysis. Ont Health Technol Assess Ser. 2013; 13( 10): 1– 66. [PMC free article] [PubMed] [Google Scholar]
- 25. Khunti K, Stone M, Paul S, et al. Disease management programme for secondary prevention of coronary heart disease and heart failure in primary care: a cluster randomised controlled trial. Heart. 2007; 93( 11): 1398– 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Q, Shen Y, Chen Y, Li X, Zhan Y. Impacts of nurse-led clinic and nurse-led prescription on hemoglobin A1c control in type 2 diabetes: A meta-analysis. Medicine (Baltimore). 2019; 98( 23): e15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. World J Otorhinolaryngol Head Neck Surg. 2015; 1( 1): 17– 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosen IM, Rowley JA, Malhotra RK, Kristo DA, Carden KA, Kirsch DB; American Academy of Sleep Medicine Board of Directors. Strategies to improve patient care for obstructive sleep apnea: a report from the American Academy of Sleep Medicine Sleep-Disordered Breathing Collaboration Summit. J Clin Sleep Med. 2020; 16( 11): 1933– 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fielding S, Maclennan G, Cook JA, Ramsay CR. A review of RCTs in four medical journals to assess the use of imputation to overcome missing data in quality of life outcomes. Trials. 2008; 9( 1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008; 5( 2): 173– 178. [DOI] [PMC free article] [PubMed] [Google Scholar]