Abstract
Background and Purpose
Because of gastrointestinal irritation and kidney toxicity associated with non-steroidal anti-inflammatory drugs and the cardiovascular problems of Coxibs use, developing novel anti-inflammatory agents with reduced toxicity and improved selectivity remains a major challenge. Depending on our previous work, a novel series of pyridopyrimidinones IIIa-i has been synthesized via reaction of 6-amino-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one (I) and phenyldiazenyl aromatic aldehydes (IIa-i). All the new constructed compounds were fully characterized by elemental and spectral analysis.
Methods
The target compounds IIIa–i were investigated for their potential towards COX inhibition, anti-inflammatory properties using carrageenan induced edema model in rat paw, and the ulcer indices of the most active members.
Results
The ethyl pyridopyrmidinone-benzoates IIIf, IIIg and IIIh showed superior inhibitory activity of carrageenan induced edema to celecoxib. Furthermore, the pyridopyrimidinones IIId, IIIf, IIIg, and IIIi exerted improved COX-2 inhibitory activity (IC50 = 0.67–1.02 µM) comparing to celecoxib (IC50 = 1.11 µM). Moreover, the gastric ulcerogenic potential assay of compounds IIIf–h revealed their lower ulcerogenic liability than indomethacin with comparable effect to celecoxib.
Conclusion
Virtual docking investigation of the most active candidates IIId, IIIf, IIIg and IIIi in the active site of COX-2 enzyme showed that these compounds implied interaction and binding motif similar to the cocrystallized ligand bromocelecoxib.
Keywords: cyclooxygenase inhibitors, anti-inflammatory activity, ulcerogenic effects, tricyclic pyridopyrimidines
Introduction
Inflammation is a cellular reaction to any harmful stimuli and conditions like tissue damage and infection.1–3 This physiological response includes the delivery of blood components to the local infection site or injury triggering vasodilation and increased vascular permeability.4–7 NSAIDs (Non-steroidal anti-inflammatory drugs) are clinically indicated for relieving fever, pain, and inflammation by suppressing cyclooxygenase (COX) enzymes.8,9 Cyclooxygenases (COXs) are key enzymes responsible for transforming arachidonic acid, which is released on affected tissues by the effect of phospholipase A2 to various prostaglandins.10,11 The major two isoforms of cyclooxygenase are COX-1 and 2.12 The constitutive COX-1 mediates the formation of diverse cytoprotective prostaglandins, which are responsible for lining the gastric mucosa, inducing platelet aggregation and preserving homeostasis.13,14 However, the inducible COX-2 is accountable for synthesizing of pain and inflammatory mediating prostaglandins.15,16
Conventional NSAIDs non-selectively inhibit COX-1 and 2 isozymes, therefore their administration is associated with gastrointestinal side effects.17–19 Therefore, selective COX-2 inhibitors were developed to achieve enhanced safety profile on gastric mucosa. Nonetheless, certain members such as rofecoxib and valdecoxib have been associated with increased probability of myocardial infarction incidences as well as hypertensive actions.20 In this regard, discovery of selective COX-2 blockers for management of pain and inflammation with diminished side actions emerged as an urgent medical need.
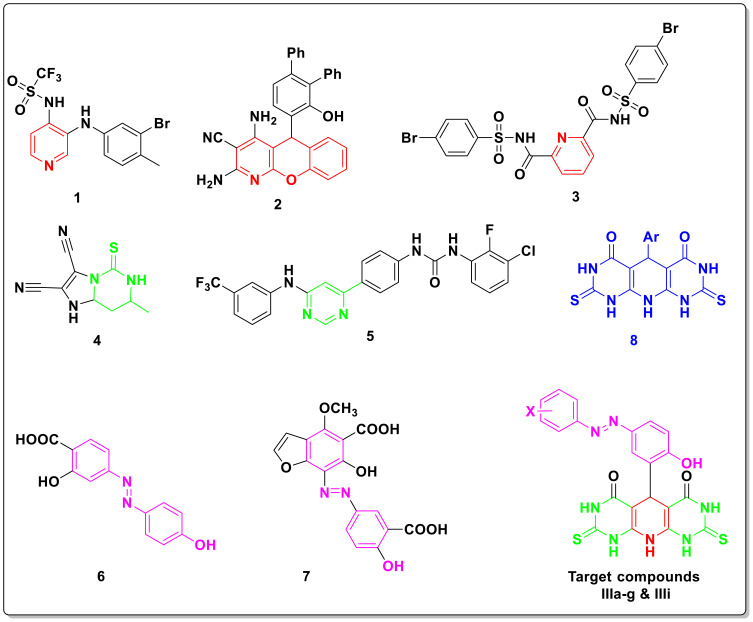
The pyridine nucleus has been incorporated in many core structures of several anti-inflammatory agents.21–23 For example, the trifluoromethanesulfonamide pyridine (1) (Figure 1) was reported as COX-2 inhibitor with higher COX-2 selectivity index (SI = 15.35) than celecoxib (SI =7.46).24 Moreover, Chung et al reported a set of different tricyclic chromeno-pyridines as promising anti-inflammatory agents.25 As a representative example, compound 2 exerted potent anti-inflammatory effect through reducing the formation of PGE2 at 10–20 mg/kg dose. Furthermore, the pyridoacylsulfonamide derivative 3 was described as COX-2 inhibitor with IC50 equal to 5.6 µM,26 and it strongly inhibited PGE2 (IC50 = 0.15 µM) in a comparable potency to celecoxib (IC50 = 0.10 µM).
Figure 1.
Representative examples of previously identified anti-inflammatory pyridines (1–3), pyrimidines (4, 5), azo containing derivatives (6,7), tricyclic pyridopyrimidine (8), and target compounds IIIa–i.
On the contrary, a number of pyrimidine-based small molecules have been reported as potent anti-inflammatory candidates.27 As an example, the hexahydroimidazo[1,2-c]pyrimidine 4 was documented to possess 34.3% anti-inflammatory activity at 50 mg/kg dose.28 Furthermore, Keche et al reported a series of pyrimidine-diarylurea conjugates such as compound 5, which demonstrated higher inhibitory properties against interleukin IL-6 (96%) and proactive kinase TNF-ὰ (78%) than that exhibited by dexamethasone against interleukin IL-6 (86%) and TNF-ὰ (72%).29 Several 4-(phenyldiazenyl)phenol containing compounds 6 and 7 exhibited anti-inflammatory activity in case of inflammatory bowel disease and ulcerative colitis.30–33 A variety of tricyclic pyridodipyrimidinones 8 was identified and elicited promising anti-inflammatory activities with low incidence of gastric ulcer.34,35
According to reported mentioned studies and in continuation to our former studies for identification of selective COX-2 blockers,12,19,36–44 further derivatives of pyridodipyrimidinone scaffold IIIa–i have been prepared and biologically investigated for their potential anti-inflammatory properties. The target molecules have been designed via conjugation of the privileged tricyclic pyridodipyrimidinone with 4-(phenyldiazenyl) phenol structural feature in a single chemical entity in an attempt to achieve selective COX-2 blocking activity along with favorable anti-inflammatory activity and minimized gastric side effects (Figure 1).
Results and Discussion
Chemistry
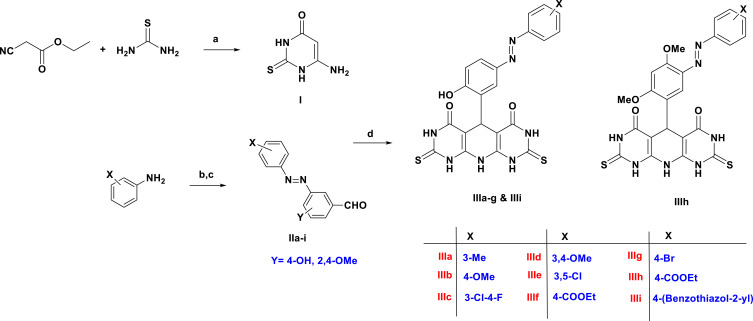
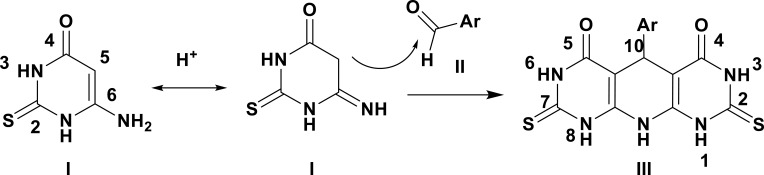
The key building block, pyrimidine-4-one (I) and phenyldiazenyl aromatic aldehydes IIa–i, were chemically synthesized as shown in Scheme 1. Condensation of the ethyl cyanoacetate ester with thiourea in presence of sodium ethoxide as a strong base afforded compound I in quantitative yield.45 On the other hand, phenyldiazenyl aldehydes IIa–i were achieved through diazotization of various anilines followed by treatment with aromatic aldehydes under basic conditions.46–48 The synthesis of the target pyridopyrimidinones IIIa–i was accomplished in 66–93% yield by treatment of compound I with various phenyldiazenyl aromatic aldehydes in CH3OH containing a catalytic amount of HCl adopting the reported method.34 In this reaction, the acidic polar solvent favors the reaction progress by the creation of a 6-imino that directs to higher nucleophilic character of carbon five, causing reaction to occur on the aromatic aldehydic carbonyl (Scheme 2). The chemical structures of newly prepared compounds IIIa–i have been elucidated via different spectroscopic and elemental analysis. 1HNMR data for the novel compounds IIIa-i is shown in Table 1.
Scheme 1.
The synthetic pathway of molecules IIIa–i. Reagents and conditions: a) NaOC2H5, C2H5OH, reflux, 6h, 99%; b) NaNO2, HCl, 0 oC, 2 h; c) Aromatic aldehyde, NaOH, stirring, 0 oC, 12 h; d) conc. HCl, methanol, rt, 7h, 66–93%.
Scheme 2.
The reasonable mechanism for compound III formation.
Table 1.
NMR Data of the Novel Compounds IIIa-I
| Compound No. | 1HNMR | 13CNMR |
|---|---|---|
| IIIa | δ 2.41 (s, 3H, CH3), 4.71 (s, 1H, pyridine), 6.63–6.70 (m, 3H, 2NH, H-3\), 7.00–7.20 (m, 2H, H-4\, 5\), 7.30–7.63 (m, 2H, H-2\, 6\), 7.67–7.75 (m, 2H, H-4\, 2\), 11.56–11.75 (s, 3H, 3NH), 12.40 (s, 1H, OH). | δ 21.4, 29.7, 78.6, 115.6, 120.2, 120.9, 122.0, 124.2, 125.2, 129.6, 131.4, 139.2, 145.4, 152.7, 153.3, 160.7, 163.1, 175.0. |
| IIIb | δ 3.77 (s, 3H, OCH3), 5.29 (s, 1H, pyridine), 6.82–6.91 (m, 5H, H-3\, 3\, 5\, 2NH), 7.54–7.57 (m, 2H, H-6\, 4\), 7.80 (d, J = 8.4 Hz, 2H, H-2\, 6\), 11.58–11.62 (m, 3H, 3NH), 12.14 (s, 1H, OH). | δ 29.4, 56.5, 90.5, 113.9, 115.8, 121.9, 123.9, 125.9, 125.8, 144.7, 145.5, 153.9, 161.8, 164.3, 166.9, 174.5. |
| IIIc | δ 5.31 (s, 1H, pyridine), 6.68–6.89 (m, 4H, H-3\, 5\, 2NH), 7.11–7.21 (m, 2H, H-4\, 6\), 7.59 (s, 1H, H-6\), 7.83 (s,1H, H-2\), 11.49–11.90 (s, 3H, 3NH), 12.01 (s, 1H, OH). | δ 29.7, 91.0, 115.8, 117.9, 121.2, 121.2, 122.3, 123.4, 125.4, 126.9, 145.1, 149.3, 153.36, 160.0, 162.8, 168.0, 173.0. |
| IIId | δ 3.84 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 5.29 (s, 1H, pyridine), 6.82–6.85 (m, 4H, H-3\, 5\, 2NH), 7.11–7.16 (m, 2H, H-2\, 6\), 7.54–7.57 (m, 2H, H-6\, H-4\), 11.92–11.97 (m, 3H, 3NH), 12.03 (s, 1H, OH). | δ 31.2, 55.9, 56.2, 90.5, 111.6, 115.6, 115.8, 115.9, 121.5, 123.9, 125.7, 145.2, 145.7, 146.7, 151.3, 154.0, 162.8, 166.5, 173.7. |
| IIIe | δ 5.31 (s, 1H, pyridine CH), 6.61–6.67 (m, 3H, H-3\, 2NH), 6.87 (d, J = 8.4 Hz, 1H, H-5\), 7.65–7.71 (m, 2H, H-4\, H-6), 7.77 (s, 1H, H-6\), 7.96 (s,1H, H-2\), 11.52–11.88 (s, 3H, 3NH), 12.02 (s, 1H, OH). | δ 29.7, 89.0, 115.8, 121.1, 121.8, 122.4, 125.5, 129.3, 135.3, 145.1, 153.3, 154.3, 160.6, 163.1, 173.0 |
| IIIf | δ 1.35 (t, J = 6.8 Hz, 3H, CH3), 3.36 (q, J = 6.8 Hz, 2H, CH2), 5.31 (s, 1H, pyridine), 6.63–6.89 (m, 3H, H-3\, 2NH), 7.65–7.68 (m, 2H, H-4\, 6\), 7.88 (d, J = 8.4 Hz, 2H, H-3\, 5\), 8.11 (d, J = 8.4 Hz, 2H, H-2\, 6\), 11.90–11.91 (s, 3H, 3NH), 12.06 (s, 1H, OH). | δ, 14.6, 29.7, 61.4, 90.7, 115.8, 121.6, 122.6, 123.2, 125.7, 130.8, 131.0, 145.5, 153.3, 155.3, 160.5, 163.1, 165.7, 172.9. |
| IIIg | δ, 5.30 (s, 1H, pyridine), 6.63–6.68 (m, 4H, H-3\, 4\, 2NH), 7.62–7.65 (m, 3H, H-6\, 3\, 5\), 7.73 (d, 1H, J = 8 Hz, H-2\, H-6), 11.92–12.01 (m, 3H, 3NH), 12.06 (s, 1H, OH). | δ 29.7, 78.6, 115.7, 121.2, 123.8, 124.4, 125.6, 126.8, 132.8, 145.2, 151.5, 153.3, 159.8, 163.1, 175.0. |
| IIIh | δ 1.33 (t, J = 7.2 Hz, 3H, CH3), 3.73 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 4.33 (q, J = 7.2 Hz, 2H, CH2), 4.75 (s, 1H, pyridine CH), 6.42–6.56 (m, 3H, H-3\, 2NH), 7.53 (s, 1H, H-6\), 8.12 (d, J = 8.4 Hz, 2H, H-3\, 5\), 8.13 (d, J = 8.4 Hz, 2H, H-2\, 6\), 10.37 (s, 1H, NH), 11.68 (s, 2H, 2NH). | δ, 14.6, 19.0, 56.4, 56.5, 61.3, 85.7, 101.2, 115.6, 122.4, 125.2, 130.6, 130.9, 153.2, 156.6, 165.1, 167.3, 168.7, 174.9. |
| IIIi | δ 5.35 (s, 1H, pyridine CH), 6.89–7.48 (m, 6H, H-3\,4\,3\, 4\, 2NH), 7.53–7.77 (m, 3H, H-6\, 3\,5\), 7.69 (d, 1H, J = 8 Hz, H-2\, 6\), 8.13–8.23 (m, 2H, H-2,6\), 11.92–12.01 (m, 3H, 3NH), 12.06 (s, 1H, OH). | δ 29.7, 90.8, 115.8, 121.3, 122.8, 123.4, 123.8, 125.8, 126.21, 127.2, 134.3, 135.1, 145.5, 153.3, 154.0, 154.0, 160.1, 166.6, 166.8, 173.0. |
Pharmacological Activity
Assay of COX Inhibition
The newly prepared azo molecules IIIa–i were investigated for their COX blocking action – in terms of IC50 – by enzyme immunoassay (EIA) utilizing ovine COX-1/2 assay kit. Furthermore, SI (selectivity index) was assessed as IC50 against COX-1/IC50 against COX-2 applying positive standard celecoxib. As shown in Table 2, the obtained data pointed out that the pyridopyrimidinones IIIa–i exhibited modest to moderate blocking activity to COX-1 (IC50 = 3.25–11.23 µM), and favorable suppressing activity for COX-2 (IC50 = 0.67–4.78 µM). While the pyridopyrimidinone IIId, possessing 3,4-dimethoxyphenyl, was the highest COX-2 blocker (IC50 = 0.67 µM), IIIf emerged as the best selective member to COX-2 (SI = 11.82) being superior to celecoxib.
Table 2.
In vitro COXs Inhibitory Action of Molecules IIIa–i
| Compound No. | IC50 (µM)a | SIb | |
|---|---|---|---|
| COX-1 | COX-2 | ||
| IIIa | 7.88 ± 0.14 | 4.78 ± 0.08 | 1.65 |
| IIIb | 6.86 ± 0.12 | 2.51 ± 0.09 | 2.73 |
| IIIc | 10.52 ± 0.24 | 3.87 ± 0.07 | 2.72 |
| IIId | 4.00 ± 0.09 | 0.67 ± 0.02 | 5.97 |
| IIIe | 7.52 ± 0.11 | 2.11 ± 0.07 | 3.56 |
| IIIf | 11.23 ± 0.27 | 0.95 ± 0.01 | 11.82 |
| IIIg | 9.20 ± 0.22 | 1.02 ± 0.03 | 9.02 |
| IIIh | 7.84 ± 0.16 | 2.43 ± 0.07 | 3.23 |
| IIIi | 3.25 ± 0.07 | 0.69 ± 0.02 | 4.71 |
| Celecoxib | 7.34 ± 0.18 | 1.11 ± 0.04 | 6.61 |
Notes: aIC50: compound concentration required to produce 50% inhibition of COX-1 or COX-2 for means of three determinations, bold figures refer to submicromolar; bSI = IC50 (COX-1)/ IC50 (COX-2).
The nature of substitution pattern on both proximal and distal phenyl rings of pyridopyrimidinone had a substantial role in modulating compound’s selectivity and activity against COX-2. The hydroxybenzaldehyde derived pyridopyrimidinones IIId (COX-2, IC50 = 0.67 µM), IIIi (COX-2, IC50 = 0.69 µM), IIIf (COX-2, IC50 = 0.95 µM), and IIIg (COX-2, IC50 = 1.02 µM), elicited distinct inhibition for COX-2 in comparable pattern to celecoxib (IC50 = 1.11 µM). Upon comparing the activity of 4-ethyl carboxylate derivatives IIIf and IIIh, it was evident that appendage of hydroxyl group (IIIf; COX-2, IC50 = 0.95 µM, SI = 11.82) on the proximal phenyl is advantageous than 2,4-dimethoxy substitution (IIIh; COX-2, IC50 = 2.43 µM, SI = 3.23) for achieving better COX-2 suppressive activity and selectivity. Moreover, it was found that replacing the 3-chloro-4-fluorophenyl of IIIc (COX-2, IC50 = 3.87 µM) with 3,4-dimethoxyphenyl led to 5.8-fold improvement in activity (IIId; COX-2, IC50 = 0.67 µM). Of special significance, pyridopyrimidinones IIIf (SI = 11.82) and IIIg (SI = 9.02) displayed remarkable selectivity for COX-2 outperforming that observed for celecoxib (SI = 6.61).
In vivo Anti-Inflammatory Action
Animal and ethics: we used in this study adult male Wister albino rats weighing 150–180g. Before any experimental study, rats are given 14 days to acclimate. The rats were kept in a controlled environment with access to water and food. All assays and practical animal studies had been done in Nahda university and adapting rules for care of animals in lab in accordance with NIH Guidelines for the Care and Use of Laboratory Animals. The design of the current work was authorized from Nahda University ethical committee, Beni-Suef, Egypt (NUB-059-019).
Carrageenan induced edema model in rat paw was used to evaluate the anti-inflammatory activity of the target candidates IIIa–i and celecoxib was chosen as the positive control.
The pyridopyrimidinones were orally administrated in a dose of 50 mg/kg nearly earlier persuading inflammation through SC (subcutaneous injection) of carrageenan. Inhibition of carrageenan induced inflammation was estimated by measuring the changes of paw dimensions after 1, 3 and 5 h (Table 3). The obtained findings showed that the pyridopyrimidinones IIIf and IIIh had superior anti-inflammatory properties than celecoxib at the three examined time intervals. In addition, the target compound IIIg exhibited higher anti-inflammatory potential than displayed by celecoxib after 3 and 5 hours.
Table 3.
In vivo Anti-Inflammatory Activities of Compounds IIIa–i
| Compound No. | Anti-Inflammatory Activity % (AI)a | ||
|---|---|---|---|
| 1 h | 3 h | 5 h | |
| IIIa | 0.95 ± 0.10* (34%) | 0.83 ± 0.12* (41%) | 0.85 ± 0.06*(39%) |
| IIIb | 1.00 ± 0.11* (31%) | 0.95 ± 0.12 (32%) | 0.90 ± 0.14 (36%) |
| IIIc | 1.10 ± 0.08 (24%) | 1.00 ± 0.10 (29%) | 0.65 ± 0.05*** (54%) |
| IIId | 1.05 ± 0.10 (28%) | 0.95 ± 0.12 (32%) | 1.05 ± 0.13 (55%) |
| IIIe | 1.05 ± 0.12 (28%) | 0.95 ± 0.09 (32%) | 0.78 ± 0.08** (45%) |
| IIIf | 0.60 ± 0.12***(52%) | 0.58 ± 0.11***(59%) | 0.48 ± 0.14*** (66%) |
| IIIg | 1.08 ± 0.09 (26%) | 0.65 ± 0.13***(54%) | 0.60 ± 0.15*** (57%) |
| IIIh | 0.49 ± 0.05***(67%) | 0.58 ± 0.11***(63%) | 0.58 ± 0.17***(57%) |
| IIIi | 1.10 ± 0.14 (24%) | 0.80 ± 0.15**(43%) | 0.68 ± 0.13** (52%) |
| Control | 1.45 ± 0.15 (0%) | 1.58 ± 0.15 (0%) | 1.33 ± 0.10 (0%) |
| Celecoxib | 0.83 ± 0.09**(43%) | 0.80 ± 0.11** (43%) | 0.65 ± 0.10*** (54%) |
Notes: aThe presented values are the average of triplicate experiments ± SEM, Significance levels *p ˂ 0.05, **p ˂ 0.01 and ***p ˂ 0.001 as compared to the control group.
Close inspection of the results listed in Table 3 underscored that the pyridopyrimidinone derivatives substituted with ethyl ester IIIf (Anti-inflammatory (AI) % = 52–66) and IIIh (AI % = 57–67) displayed higher activity for inhibition of edema than those congeners containing either electron donating (IIId; AI % = 28–55) or electron withdrawing (IIIc; AI % = 24–54) groups, particularly after 1 and 3 hours. In harmony with the COX-2 inhibitory assessment, the 4-benzothiazol-2-yl containing pyridopyrimidinone IIIi exerted superior in vivo anti-inflammatory activity than its corresponding methoxy derivative IIIb. Moreover, introducing bromine on compound IIIg augmented the anti-inflammatory activity (3 h; AI % = 54, 5 h; AI % = 57).
The ethyl acetoxy group in compounds IIIf and IIIh has important role in vivo activity anti-inflammatory activity also bromine at for position 4 in compound IIIg. Also, the ethyl acetoxy group in IIIf and IIIh increases absorption so fast onset of action consequently high anti-inflammatory activities 52% and 67% respectively but bromine in IIIg delay onset of action firstly in compared with IIIf and IIIh.
Ulcerogenic Liability
The top three active pyridopyrimidinones IIIf–h were further evaluated for their gastric ulcerogenic liability in rats (Table 4). The ulcerogenicity liability of the investigated pyridopyrimidinones was compared with both COX-1 inhibitor (indomethacin) and COX-2 inhibitor (celecoxib). Interestingly, compound IIIf had the lowest ulcerogenic effect, which might be attributed to its potential selectivity for COX-2 (SI = 11.82). Additionally, all of the tested candidates showed lower ulcerogenic action than the standard indomethacin.
Table 4.
Gastric Ulcerogenic Effect of Compounds IIIf–h
| Comp. No. | Ulcer Number | Ulcer Index | Relative Ulcerogenicity to Celecoxib |
|---|---|---|---|
| IIIf | 3.75 ± 0.15 | 3.75 ± 0.11 | 1.25 |
| IIIg | 6.75 ± 0.07 | 4.75 ± 0.14 | 1.58 |
| IIIh | 6.50 ± 0.11 | 5.25 ± 0.13 | 1.75 |
| Celecoxib | 3.25 ± 0.17 | 3.00 ± 0.09 | 1 |
| Indomethacin | 14.25 ± 0.31 | 22.5 ± 0.21 | 7.5 |
Molecular Docking Studies
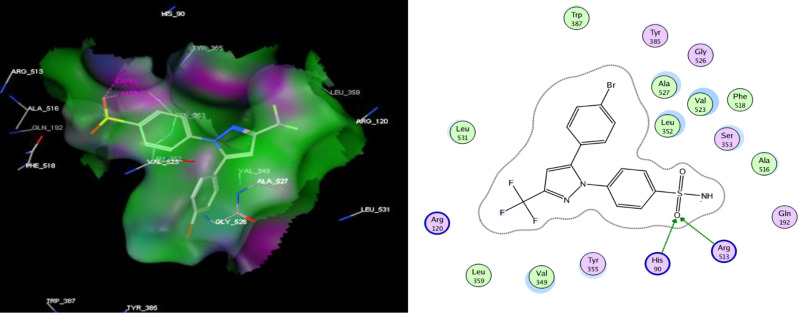
To acquire insights about the underlying mechanism of action of the newly investigated pyridopyrimidinones, virtual docking of the highly selective COX-2 inhibitors IIId, IIIf, IIIg and IIIi within the active binding site of COX-2 enzyme was studied. The co-crystal structure of COX-2 complex with SC-558, a selective COX-2 blocker, was acquired from PDB (protein data bank: 1CX2),49 and the virtual docking was conducted using MOE (Molecular Operating Environment; 2010). Validation of docking protocol had been performed by redocking the ligand bromocelecoxib (SC-558) into COX-2 active site with root mean standard deviation (RMSD) of 1.1524, and showed an energy score (S) of −11.93 kcal/mol. The ligand sulphonyl group was engaged in two hydrogen bonds with the receptor amino acids His90 and Arg513 (Figure 2, Table 5).
Figure 2.
The 2D (right panel) and 3D (left panel) putative binding mode of SC-558.
Table 5.
The Virtual Docking Data of Compounds IIId, IIIf, IIIg, IIIi and SC-558
| Compound No. | Affinity Kcal/mol | No. of HBs | Distance (Ao) from Main Residue | Functional Group | |
|---|---|---|---|---|---|
| IIId | −13.95 | 5 | 2.97 | Tyr355 | C=O |
| 3.20 | His90 | OH | |||
| 2.99 | Ser353 | OH | |||
| 3.28 | Ser530 | OCH3 | |||
| 2.97 | Tyr385 | OCH3 | |||
| IIIf | −13.89 | 2 | 2.65 | Arg120 | C=O |
| 3.25 | His90 | N=N | |||
| IIIg | −11.20 | 3 | 2.68 | Arg120 | C=O |
| 2.15 | His90 | N=N | |||
| 2.98 | Tyr355 | C=O | |||
| IIIi | −14.52 | 3 | 3.11 | Arg120 | C=O |
| 2.87 | His90 | N=N | |||
| 3.09 | Tyr355 | C=O | |||
| SC-558 | −11.93 | 2 | 2.41 | Arg513 | -SO2 |
| 2.30 | His90 | -SO2 | |||
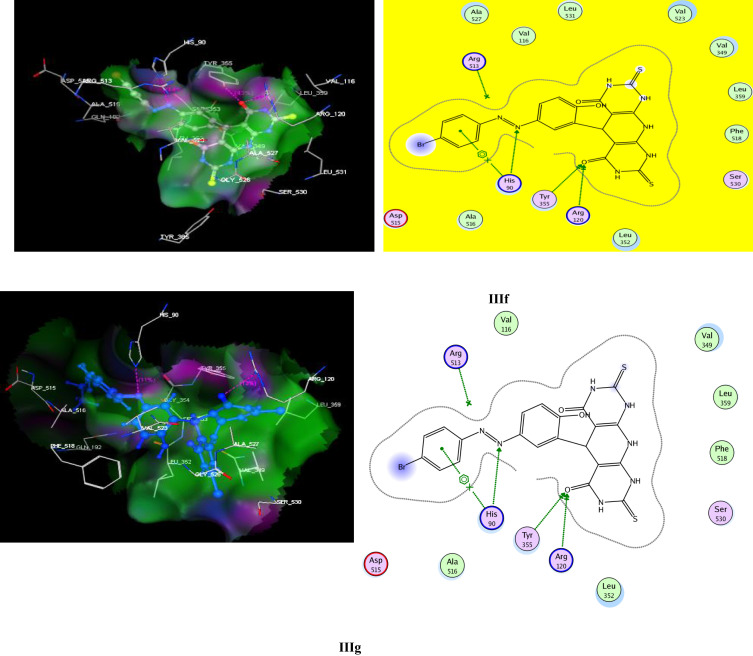
Interestingly, compound IIIf showed profitable fitting with COX-2 with superior docking score (S = −13.89 kcal/mol) to bromocelecoxib (S = −11.93 kcal/mol). Furthermore, IIIf displayed two HB interactions through its carbonyl oxygen with Arg120 (2.65 A°) as well as azo moiety with His90 (3.25 A°) (Figure 3; Table 5). Also, the pyridopyrimidinone IIIg exhibited score energy (S) of −11.20 kcal/mol, and was able to form three HB interactions with Arg120, His90 and Tyr355 (Figure 3).
Figure 3.
The 2D (right panel) and 3D (left panel) putative binding mode of compounds IIIf and IIIg.
Conclusion
Novel derivatives of pyridopyrimidinones IIIa–i were prepared and assessed in vitro and in vivo for their COXs and carrageenan induced edema anti-inflammatory activities, respectively. Preliminary screening of the target compounds disclosed that the pyridopyrimidinone IIIf possessing ethyl acetate had the best activity with potent edema inhibition in percent = 52% after one hour, 59% after three hours and 66% after five hours. Moreover, certain members among this focused library were identified to be selective COX-2 inhibitors. Particularly, IIId, IIIf, IIIg and IIIi, exerted superior inhibition for COX-2 (IC50 = 0.67–1.02 µM) than celecoxib (IC50 = 1.11 µM). Ulcerogenic accountability of compounds IIIf–h exhibited their comparable activity to celecoxib along with less ulcerogenic effect than indomethacin. It was noted that the lipophilic group (ethyl ester or benzothiazol-2-yl) containing compounds IIIf, IIIh and IIIi elicited superior anti-inflammatory effect and better selectivity for COX-2 than other derivatives. Overall, combining the privileged pyridodipyrimidinone scaffold with diphenylazo structural feature in single molecule with appropriate hydrophobic substitution pattern may represent a promising core structure for further design of potent anti-inflammatory agents with minimized gastric side effects.
Experimental
Chemistry
Melting points were determined with Thomas-Hoover capillary apparatus and uncorrected. Infrared (IR) spectra of the new compounds were detected utilizing FT-IR spectrometer (Nicolet 550 Series II Magna) as films on NaCl plates, and expressed in wave number (cm−1). 1H NMR and 13C NMR spectra were recorded with Bruker Avance III 400 MHz in deuterated dimethyl sulfoxide (DMSO‑d6). Chemical shifts were measured in ppm (δ scale), and the coupling constant (J) values were expressed in Hertz (Hz). Mass spectra were recorded using Hewlett Packard 5988 spectrometer. Elemental microanalyses for N, C, and H were measured utilizing Perkin-Elmer 2400 analyzer (Perkin-Elmer, Norwalk, CT, USA), at Cairo University (Micro analytical unit), Egypt, and all analyzed compounds were within ± 0.4% of the assessed values. Thin layer chromatography (TLC) was carried out utilizing silica gel plates (Germany, MERCK 60F 254, 0.25 mm), a mixture of chloroform /methanol (9.5:0.5 mL) as eluent and visualized with UV lamp. All chemicals and reagents were commercially purchased and used directly without purification. 6-Aminopyrimidinone derivative I and the aldehyde derivatives IIa–i were prepared adopting the reported methods.45,50,51
Synthesis of Pyridopyrimidinones IIIa–i
6-Amino-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one (I) (2.86 g, 20 mmol), appropriate aromatic aldehyde (IIa–i) (10 mmol), and conc. hydrochloric acid (5 mL) in methanol (30 mL) were stirred at room temperature (rt) for 7 h. The precipitated product was collected, washed with cold ethyl alcohol, dried and crystallized from DMF to afford the target molecules IIIa–i in pure forms.
5-(2-Hydroxy- 5-(m-Tolyldiazenyl) Phenyl)−2,8- Dithioxo-2, 3, 5, 8, 9, 10-Hexahydropyrido[2,3-D:6,5d′]Dipyrimidine-4,6–(1H,7H)-Dione (IIIa)
Yield (85%), yellowish white crystals, m.p. > 300 °C; IR (cm−1): 1649 (C=O), 3223(NH), 3415(OH); 1H NMR δ 2.41 (s, 3H, CH3), 4.71 (s, 1H, pyridine), 6.63–6.70 (m, 3H, 2NH, H-3\), 7.00–7.20 (m, 2H, H-4\, 5\), 7.30–7.63 (m, 2H, H-2\, 6\), 7.67–7.75 (m, 2H, H-4\, 2\), 11.56–11.75 (s, 3H, 3NH), 12.40 (s, 1H, OH); 13C NMR δ 21.4, 29.7, 78.6, 115.6, 120.2, 120.9, 122.0, 124.2, 125.2, 129.6, 131.4, 139.2, 145.4, 152.7, 153.3, 160.7, 163.1, 175.0; EIMS (m/z) 491 (M+, 19.44%). Elemental analysis of C22H17N7O3S2: C, 53.76; H, 3.49; N, 19.95. Found: C, 53.40; H, 3.56; N, 20.03.
5-(2-Hydroxy-5-(4-Methoxyphenyl)Diazenyl) Phenyl)−2,8-Dithioxo-2, 3, 5, 8, 9, 10-Hexahydropyrido[2,3-D:6,5d′]dipyrimidine-4,6–(1H,7H)-Dione (IIIb)
Yield (80%), greyish white crystals;; m.p. > 300°C; IR (cm−1): 1669 (C=O), 3257(NH), 3419 (OH); 1H NMR δ 3.77 (s, 3H, OCH3), 5.29 (s, 1H, pyridine), 6.82–6.91 (m, 5H, H-3\, 3\, 5\, 2NH), 7.54–7.57 (m, 2H, H-6\, 4\), 7.80 (d, J = 8.4 Hz, 2H, H-2\, 6\), 11.58–11.62 (m, 3H, 3NH), 12.14 (s, 1H, OH); 13C NMR δ 29.4, 56.5, 90.5, 113.9, 115.8, 121.9, 123.9, 125.9, 125.8, 144.7, 145.5, 153.9, 161.8, 164.3, 166.9, 174.5; EIMS (m/z) 507 (M+, 24.52%). Elemental analysis of C22H17N7O4S2: C, 52.06; H, 3.38; N, 19.32. Found: C, 52.00; H, 3.50; N, 19.53.
5(5-((3-Chloro-4-Fluorophenyl) Diazenyl)-2-Hydroxyphenyl)−2, 8- Dithioxo-2, 3, 5, 8, 9, 10-Hexahydropyrido[2,3-D:6,5d′]Dipyrimidine-4,6–(1H,7H)-Dione (IIIc)
Yield (66%), whitish grey crystals, m.p. > 300°C; IR (cm−1): 1641 (C=O), 3040(ArH), 3150(NH), 3422(OH); 1H NMR δ 5.31 (s, 1H, pyridine), 6.68–6.89 (m, 4H, H-3\, 5\, 2NH), 7.11–7.21 (m, 2H, H-4\, 6\), 7.59 (s, 1H, H-6\), 7.83 (s,1H, H-2\), 11.49–11.90 (s, 3H, 3NH), 12.01 (s, 1H, OH); 13C NMR δ 29.7, 91.0, 115.8, 117.9, 121.2, 121.2, 122.3, 123.4, 125.4, 126.9, 145.1, 149.3, 153.36, 160.0, 162.8, 168.0, 173.0; EIMS (m/z) 529 (M+, 29.39%), 67 (100%). Elemental analysis of C21H13ClFN7O3S2: C, 47.59; H, 2.47; N, 18.50. Found: C, 47.50; H, 2.45; N, 18.54.
5-(5-((3,4-Dimethoxyphenyl-2-Diazenyl)-2-Hydroxyphenyl)−2, 8- Dithioxo-2, 3, 5, 8, 9, 10-Hexahydropyrido[2,3-D:6,5d′]Dipyrimidine-4,6-(1H,7H)-Dione (IIId)
Yield (80%), yellow crystals, m.p. > 300°C; IR (cm−1): 3419 (OH), 3215 (NH), 1667 (C=O); 1H NMR δ 3.84 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 5.29 (s, 1H, pyridine), 6.82–6.85 (m, 4H, H-3\, 5\, 2NH), 7.11–7.16 (m, 2H, H-2\, 6\), 7.54–7.57 (m, 2H, H-6\, H-4\), 11.92–11.97 (m, 3H, 3NH), 12.03 (s, 1H, OH); 13C NMR δ 31.2, 55.9, 56.2, 90.5, 111.6, 115.6, 115.8, 115.9, 121.5, 123.9, 125.7, 145.2, 145.7, 146.7, 151.3, 154.0, 162.8, 166.5, 173.7; EIMS (m/z) 537 (M+, 32.07%). Elemental analysis of C23H19N7O5S2: C, 51.39; H, 3.56; N, 18.24. Found: C, 51.55; H, 3.45; N, 18.03.
5-(5-((3,4-Dichlorophenyl-2-Diazenyl)-2-Hydroxyphenyl)−2, 8- Dithioxo-2, 3, 5, 8, 9, 10-Hexahydropyrido[2,3-D:6,5d′]Dipyrimidine-4,6-(1H,7H)-Dione (IIIe)
Whitish yellow crystals (yield 75%), m.p. > 300°C; IR (cm−1); 3416 (OH), 3180 (NH), 1652 (C=O); 1H NMR δ 5.31 (s, 1H, pyridine CH), 6.61–6.67 (m, 3H, H-3\, 2NH), 6.87 (d, J = 8.4 Hz, 1H, H-5\), 7.65–7.71 (m, 2H, H-4\, H-6\), 7.77 (s, 1H, H-6\), 7.96 (s,1H, H-2\), 11.52–11.88 (s, 3H, 3NH), 12.02 (s, 1H, OH); 13C NMR δ 29.7, 89.0, 115.8, 121.1, 121.8, 122.4, 125.5, 129.3, 135.3, 145.1, 153.3, 154.3, 160.6, 163.1, 173.0; EIMS (m/z) 545 (M+, 20.55%). Elemental analysis of C21H13Cl2N7O3S2: C, 46.16; H, 2.40; N, 17.94. Found: C, 46.00; H, 2.56; N, 18.23.
Ethyl (E)-4-((3-(4,6-Dioxo-2,8-Dithioxo-1,2,3,4,5,6,7,8,9,10-Decahydropyrido[2,3-D:6,5-D’]Dipyrimidin-5-Yl)-4-Hydroxyphenyl)Diazenyl)Benzoate (IIIf)
Yield (82%), yellowish white crystals, m.p. > 300°C; IR (cm−1): 1686 (C=O), 3229(NH), 3451(OH); 1H NMR δ, 1.35 (t, J = 6.8 Hz, 3H, CH3), 3.36 (q, J = 6.8 Hz, 2H, CH2), 5.31 (s, 1H, pyridine), 6.63–6.89 (m, 3H, H-3\, 2NH), 7.65–7.68 (m, 2H, H-4\, 6\), 7.88 (d, J = 8.4 Hz, 2H, H-3\, 5\), 8.11 (d, J = 8.4 Hz, 2H, H-2\, 6\), 11.90–11.91 (s, 3H, 3NH), 12.06 (s, 1H, OH); 13C NMR δ, 14.6, 29.7, 61.4, 90.7, 115.8, 121.6, 122.6, 123.2, 125.7, 130.8, 131.0, 145.5, 153.3, 155.3, 160.5, 163.1, 165.7, 172.9; EIMS (m/z) 549 (M+, 18.52%). Elemental analysis of C24H19N7O5S2: C, 52.45; H, 3.48; N, 17.84. Found: C, 52.55; H, 3.52; N, 18.01.
(E)-5-(5-((4-Bromophenyl) Diazenyl)-2-Hydroxyphenyl)-2.8-Dithioxo-2, 3, 5,8,9,10-Hexahydropyrido [2,3-D:6,5-D’]Dipyrimidine-4,6(1H,7H)-Dione (IIIg)
Yield (75%), yellowish white crystals, m.p. > 300°C; IR (cm−1): 1657 (C=O), 3185(NH), 3413(OH); 1H NMR δ, 5.30 (s, 1H, pyridine), 6.63–6.68 (m, 4H, H-3\, 4\, 2NH), 7.62–7.65 (m, 3H, H-6\, 3\, 5\), 7.73 (d, 1H, J = 8 Hz, H-2\, H-6), 11.92–12.01 (m, 3H, 3NH), 12.06 (s, 1H, OH); 13C NMR δ 29.7, 78.6, 115.7, 121.2, 123.8, 124.4, 125.6, 126.8, 132.8, 145.2, 151.5, 153.3, 159.8, 163.1, 175.0; EIMS (m/z) 556 (M+, 15.28%). Elemental analysis of C21H14BrN7O3S2: C, 45.53; H, 2.54; N, 17.62. Found: C, 45.33; H, 2.50; N, 17.60.
Ethyl (E)-4-((5-(4,6-Dioxo-2,8-Dithioxo-1,2,3,4,5,6,7,8,9,10-Decahydropyrido[2,3-D:6,5-D’]Dipyrimidin-5-Yl)-2,4-Dimethoxyphenyl)Diazenyl)Benzoate (IIIh)
Yield (70%), yellowish white crystals, m.p. > 300°C; IR (cm−1): 1645 (C=O), 3425(NH),; 1H NMR δ 1.33 (t, J = 7.2 Hz, 3H, CH3), 3.73 (s, 3H, OCH3), 3.74 (s, 3H, OCH3), 4.33 (q, J = 7.2 Hz, 2H, CH2), 4.75 (s, 1H, pyridine CH), 6.42–6.56 (m, 3H, H-3\, 2NH), 7.53 (s, 1H, H-6\), 8.12 (d, J = 8.4 Hz, 2H, H-3\, 5\), 8.13 (d, J = 8.4 Hz, 2H, H-2\, 6\), 10.37 (s, 1H, NH), 11.68 (s, 2H, 2NH); 13C NMR δ, 14.6, 19.0, 56.4, 56.5, 61.3, 85.7, 101.2, 115.6, 122.4, 125.2, 130.6, 130.9, 153.2, 156.6, 165.1, 167.3, 168.7, 174.9; EIMS (m/z) 593 (M+, 8.69%). Elemental analysis of C26H23N7O6S2: C, 52.61; H, 3.91; N, 16.52. Found: C, 52.51; H, 4.03; N, 16.43.
(E)-5-(5-((4-(Benzo[D]thiazol-2-Yl)Phenyl)Diazenyl)-2-Hydroxyphenyl)-2,8-Dithioxo-2,3,5,8,9,10-Hexahydropyrido[2,3-D:6,5-D’]Dipyrimidine-4,6(1H,7H)-Dione (IIIi)
Yield (93%), whitish yellow crystals, m.p. > 300°C; IR (cm−1): 1172(C=S), 1651 (C=O), 3181(NH), 3419 (OH); 1H NMR, δ 5.35 (s, 1H, pyridine CH), 6.89–7.48 (m, 6H, H-3\,4\,3\, 4\, 2NH), 7.53–7.77 (m, 3H, H-6\, 3\,5\), 7.69 (d, 1H, J = 8 Hz, H-2\, 6\), 8.13–8.23 (m, 2H, H-2,6\), 11.92–12.01 (m, 3H, 3NH), 12.06 (s, 1H, OH); 13C NMR δ 29.7, 90.8, 115.8, 121.3, 122.8, 123.4, 123.8, 125.8, 126.21, 127.2, 134.3, 135.1, 145.5, 153.3, 154.0, 154.0, 160.1, 166.6, 166.8, 173.0; EIMS (m/z) 610 (M+, 6.46%). Elemental analysis of C28H18N8O3S3: C, 55.07; H, 2.97; N, 18.35. Found: C, 55.38; H, 3.12; N, 18.74.
Pharmacological Activity Studies
All utilized procedures in the pharmacological evaluation were carried out as described earlier. Colorimetric assay of COXs,20 anti-inflammatory activity (in-vivo),40 ulcerogenic liability52 were cited in the Supplementary Materials.
Molecular Docking
The virtual docking study was performed by utilizing the x-ray crystal structure of COX-2 enzyme (pdb code: 1CX2).28 Ligand and protein preparation (3D protonation for the amino acid side chain of enzyme, addition of hydrogen atoms, and deletion of all water of crystallization away from the active site) was performed using MOE software (version 2010, Chemical Computing Group Inc., QC, Canada). The pyridopyrimidinones compounds were sketched in their three-dimensional (3D) structures by Chemo-Draw, protonated, and subjected to energy minimization. Molecular docking of these compounds has been applied, amino acid interactions were examined, and the hydrogen bond lengths were recorded.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Jouf University under grant No (DSR-2021-01-0303)”.
Also, authors thanks Prof. Hossam M. Hassan, Vice Dean of the Faculty of Pharmacy, Nahda University Beni-Suef Egypt. For supporting this work.
Statistical Analysis
The significant difference for groups was measured utilizing one-way ANOVA followed by Dunnett’s test. Significant differences are at *P >0.05, **P > 0.01 and ***P >0.001, and GraphPad Prism software (version 5) was used for statistical tests (version 5).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428. doi: 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846. doi: 10.1038/nature01320 [DOI] [PubMed] [Google Scholar]
- 3.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 4.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323. doi: 10.1038/nature09782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72(1):219–246. doi: 10.1146/annurev-physiol-021909-135846 [DOI] [PubMed] [Google Scholar]
- 7.Abdellatif KR, Abdelgawad MA, Labib MB, Zidan TH. Synthesis and biological evaluation of new diarylpyrazole and triarylimidazoline derivatives as selective cox‐2 inhibitors. Arch Pharm. 2017;350(8):1600386. doi: 10.1002/ardp.201600386 [DOI] [PubMed] [Google Scholar]
- 8.Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104(3):2S–8S. doi: 10.1016/S0002-9343(97)00203-9 [DOI] [PubMed] [Google Scholar]
- 9.Brune K, Patrignani P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J Pain Res. 2015;8:105. doi: 10.2147/JPR.S75160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Nat Acad Sci. 1992;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdellatif K, Abdelall E, Bakr R. Nitric oxide-NSAIDs donor prodrugs as hybrid safe anti-inflammatory agents. Curr Top Med Chem. 2017;17(8):941–955. doi: 10.2174/1568026616666160927153435 [DOI] [PubMed] [Google Scholar]
- 12.Abdelgawad MA, Bakr RB, Omar HA. Design, synthesis and biological evaluation of some novel benzothiazole/benzoxazole and/or benzimidazole derivatives incorporating a pyrazole scaffold as antiproliferative agents. Bioorg Chem. 2017;74:82–90. doi: 10.1016/j.bioorg.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 13.Bakr RB, Azouz AA, Abdellatif KR. Synthesis, cyclooxygenase inhibition, anti-inflammatory evaluation and ulcerogenic liability of new 1-phenylpyrazolo [3, 4-d] pyrimidine derivatives. J Enzyme Inhib Med Chem. 2016;31:6–12. doi: 10.1080/14756366.2016.1186018 [DOI] [PubMed] [Google Scholar]
- 14.Abdellatif KR, Abdelgawad MA, Elshemy HA, Alsayed SS, Kamel G. Synthesis and anti-inflammatory evaluation of new 1, 3, 5-triaryl-4, 5-dihydro-1h-pyrazole derivatives possessing an aminosulphonyl pharmacophore. Arch Pharm Res. 2015;38(11):1932–1942. doi: 10.1007/s12272-015-0606-7 [DOI] [PubMed] [Google Scholar]
- 15.Wang D, DuBois RN. The role of cox-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29(6):781. doi: 10.1038/onc.2009.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griswold DE, Adams JL. Constitutive cyclooxygenase (cox‐1) and inducible cyclooxygenase (cox‐2): rationale for selective inhibition and progress to date. Med Res Rev. 1996;16(2):181–206. doi: [DOI] [PubMed] [Google Scholar]
- 17.Lazzaroni M, Bianchi Porro G. Gastrointestinal side‐effects of traditional non‐steroidal anti‐inflammatory drugs and new formulations. Aliment Pharmacol Ther. 2004;20:48–58. doi: 10.1111/j.1365-2036.2004.02037.x [DOI] [PubMed] [Google Scholar]
- 18.Vane J, Bakhle Y, Botting R. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38(1):97–120. doi: 10.1146/annurev.pharmtox.38.1.97 [DOI] [PubMed] [Google Scholar]
- 19.Abdelrahman MH, Youssif BG, Abdelazeem AH, et al. Synthesis, biological evaluation, docking study and ulcerogenicity profiling of some novel quinoline-2-carboxamides as dual coxs/lox inhibitors endowed with anti-inflammatory activity. Eur J Med Chem. 2017;127:972–985. doi: 10.1016/j.ejmech.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 20.Abdelgawad MA, Bakr RB, El-Gendy AO, Kamel GM, Azouz AA, Bukhari SNA. Discovery of a cox-2 selective inhibitor hit with anti-inflammatory activity and gastric ulcer protective effect. Future Med Chem. 2017;9(16):1899–1912. doi: 10.4155/fmc-2017-0115 [DOI] [PubMed] [Google Scholar]
- 21.Lacerda RB, de Lima CK, da Silva LL, et al. Discovery of novel analgesic and anti-inflammatory 3-arylamine-imidazo [1, 2-a] pyridine symbiotic prototypes. Bioorg Med Chem. 2009;17(1):74–84. doi: 10.1016/j.bmc.2008.11.018 [DOI] [PubMed] [Google Scholar]
- 22.Abdelgawad MA, Bakr RB, Azouz AA. Novel pyrimidine-pyridine hybrids: synthesis, cyclooxygenase inhibition, anti-inflammatory activity and ulcerogenic liability. Bioorg Chem. 2018;77:339–348. doi: 10.1016/j.bioorg.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 23.Girgis AS, Mishriky N, Ellithey M, Hosni HM, Farag H. Novel synthesis of [1]-benzothiepino [5, 4-b] pyridine-3-carbonitriles and their anti-inflammatory properties. Bioorg Med Chem. 2007;15(6):2403–2413. doi: 10.1016/j.bmc.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 24.Renard J-F, Lecomte F, Hubert P, de Leval X, Pirotte B. N-(3-arylaminopyridin-4-yl) alkanesulfonamides as pyridine analogs of nimesulide: cyclooxygenases inhibition, anti-inflammatory studies and insight on metabolism. Eur J Med Chem. 2014;74:12–22. doi: 10.1016/j.ejmech.2013.12.033 [DOI] [PubMed] [Google Scholar]
- 25.Chung S-T, Huang W-H, Huang C-K, et al. Synthesis and anti-inflammatory activities of 4h-chromene and chromeno [2, 3-b] pyridine derivatives. Res Chem Intermed. 2016;42(2):1195–1215. doi: 10.1007/s11164-015-2081-7 [DOI] [Google Scholar]
- 26.Lu X, Zhang H, Li X, et al. Design, synthesis and biological evaluation of pyridine acyl sulfonamide derivatives as novel cox-2 inhibitors. Bioorg Med Chem. 2011;19(22):6827–6832. doi: 10.1016/j.bmc.2011.09.034 [DOI] [PubMed] [Google Scholar]
- 27.Bekhit AA, Fahmy HT, Rostom SA, Baraka AM. Design and synthesis of some substituted 1h-pyrazolyl-thiazolo [4, 5-d] pyrimidines as anti-inflammatory–antimicrobial agents. Eur J Med Chem. 2003;38(1):27–36. doi: 10.1016/S0223-5234(02)00009-0 [DOI] [PubMed] [Google Scholar]
- 28.Sondhi SM, Singh N, Johar M, Kumar A. Synthesis, anti-inflammatory and analgesic activities evaluation of some mono, bi and tricyclic pyrimidine derivatives. Bioorg Med Chem. 2005;13(22):6158–6166. doi: 10.1016/j.bmc.2005.06.063 [DOI] [PubMed] [Google Scholar]
- 29.Keche AP, Hatnapure GD, Tale RH, Rodge AH, Birajdar SS, Kamble VM. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg Med Chem Lett. 2012;22(10):3445–3448. doi: 10.1016/j.bmcl.2012.03.092 [DOI] [PubMed] [Google Scholar]
- 30.Garjani A, Davaran S, Rashidi M, Malek N. Protective effects of some azo derivatives of 5-aminosalicylic acid and their pegylated prodrugs on acetic acid-induced rat colitis. DARU J Pharmaceut Sci. 2004;12:24–30. [Google Scholar]
- 31.Abdu-Allah HH, El-Shorbagi A-NA, Abdel-Moty SG, El-Awady R, Abdel-Alim A. 5-aminosalyclic acid (5-asa): a unique anti-inflammatory salicylate. Med Chem. 2016;6(05):306–315. doi: 10.4172/2161-0444.1000361 [DOI] [Google Scholar]
- 32.Sheng SF, Zheng HX, Liu J, Zhao ZB. Synthesis of phenol-class azo derivatives of 4-aminosalicylic acid. Chin Chem Lett. 2008;19(4):419–422. doi: 10.1016/j.cclet.2008.01.042 [DOI] [Google Scholar]
- 33.Hassan GS, Soliman GA. Design, synthesis and anti-ulcerogenic effect of some of furo-salicylic acid derivatives on acetic acid-induced ulcerative colitis. Eur J Med Chem. 2010;45(9):4104–4112. doi: 10.1016/j.ejmech.2010.05.071 [DOI] [PubMed] [Google Scholar]
- 34.Mohamed MS, Awad SM, Sayed AI. Synthesis of certain pyrimidine derivatives as antimicrobial agents and anti-inflammatory agents. Molecules. 2010;15(3):1882–1890. doi: 10.3390/molecules15031882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdelgawad MA, Labib MB, Ali WA, Kamel G, Azouz AA, EL-Shaymaa E-N. Design, synthesis, analgesic, anti-inflammatory activity of novel pyrazolones possessing aminosulfonyl pharmacophore as inhibitors of cox-2/5-lox enzymes: histopathological and docking studies. Bioorg Chem. 2018;78:103–114. doi: 10.1016/j.bioorg.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 36.Abdelgawad MA, Labib MB, Abdel-Latif M. Pyrazole-hydrazone derivatives as anti-inflammatory agents: design, synthesis, biological evaluation, cox-1, 2/5-lox inhibition and docking study. Bioorg Chem. 2017;74:212–220. doi: 10.1016/j.bioorg.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 37.Abdelgawad MA, Mohamed AM, Musa A, Mostafa EM, Awad HM. Synthesis, chromatographic separation and antimicrobial evolution of new azoquinoline-8-ol. J Pharmaceut Sci Res. 2018;10:1314–1318. [Google Scholar]
- 38.Bakr RB, Elkanzi NA, Ghoneim AA, Moustafa S. Synthesis, molecular docking studies and in vitro antimicrobial evaluation of novel pyrimido [1, 2-a] quinoxaline and triazino [4, 3-a]-quinoxaline derivatives. Heterocycles. 2018;96(11):1941–1957. doi: 10.3987/COM-18-13955 [DOI] [Google Scholar]
- 39.Elkanzi NA, Bakr RB, Ghoneim AA. Design, synthesis, molecular modeling study, and antimicrobial activity of some novel pyrano [2, 3‐b] pyridine and pyrrolo [2, 3‐b] pyrano [2.3‐d] pyridine derivatives. J Heterocycl Chem. 2019;56:406–416. [Google Scholar]
- 40.Bakr RB, Ghoneim AA, Azouz AA. Selective cyclooxygenase inhibition and ulcerogenic liability of some newly prepared anti-inflammatory agents having thiazolo [4, 5-d] pyrimidine scaffold. Bioorg Chem. 2019;88:102964. doi: 10.1016/j.bioorg.2019.102964 [DOI] [PubMed] [Google Scholar]
- 41.Belal A, Abdelgawad MA. New benzothiazole/benzoxazole-pyrazole hybrids with potential as cox inhibitors: design, synthesis and anticancer activity evaluation. Res Chem Intermed. 2017;43(7):3859–3872. doi: 10.1007/s11164-016-2851-x [DOI] [Google Scholar]
- 42.Oraby AK, Abdellatif KR, Abdelgawad MA, Attia KM, Dawe LN, Georghiou PE. 2, 4‐disubstituted phenylhydrazonopyrazolone and isoxazolone derivatives as antibacterial agents: synthesis, preliminary biological evaluation and docking studies. ChemistrySelect. 2018;3(11):3295–3301. doi: 10.1002/slct.201800174 [DOI] [Google Scholar]
- 43.Abdellatif RA, Abdelgawad M, Elshemy. AH, Kahk M, El Amir M. Design, synthesis, antioxidant and anticancer activity of new coumarin derivatives linked with thiazole, isoxazole or pyrazole moiety. Lett Drug Des Discov. 2017;14(7):773–781. doi: 10.2174/1570180813666161026153743 [DOI] [Google Scholar]
- 44.Bakr B, Mehany. BM, Abdellatif RA. Synthesis, egfr inhibition and anti-cancer activity of new 3, 6-dimethyl-1-phenyl-4-(substituted-methoxy) pyrazolo [3, 4-d] pyrimidine derivatives. Curr Med Chem Anticancer Agents. 2017;17:1389–1400. [DOI] [PubMed] [Google Scholar]
- 45.Taddei D, Slawin AM, Woollins JD. 2‐(benzylsulfanyl)‐6‐chloro‐9‐isopropylpurine, a valuable intermediate in the synthesis of diaminopurine cyclin dependent kinase inhibitors. European J Org Chem. 2005;2005(5):939–947. doi: 10.1002/ejoc.200400748 [DOI] [Google Scholar]
- 46.Bhuvaneswari K, Nagasundaram N, Lalitha A. Synthesis, anti‐inflammatory activity, and molecular docking study of novel azo bis antipyrine derivatives against cyclooxygenase‐2 enzyme. J Chin Chem Soc. 2021;68:27–33. [Google Scholar]
- 47.Korade SN, Patil JD, Gaikwad DS, et al. Synthesis and biological activities of novel aryldiazo substituted heterocycles. Org Prep Proced Int. 2020;52(2):147–165. doi: 10.1080/00304948.2020.1716625 [DOI] [Google Scholar]
- 48.Korade SN, Pore DM. Basic ionic liquid [DPPA] cl− catalyzed synthesis of fluorescent 3‐acetoacetyl− 6‐aryldiazenyl‐coumarins. ChemistrySelect. 2019;4:4804–4808. doi: 10.1002/slct.201900332 [DOI] [Google Scholar]
- 49.Kurumbail RG, Stevens AM, Gierse JK, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384(6610):644–648. doi: 10.1038/384644a0 [DOI] [PubMed] [Google Scholar]
- 50.Khanmohammadi H, Erfantalab M, Bayat A, Babaei A, Sohrabi M. New 1, 2, 4-triazole-based azo–azomethine dyes. Part ii: synthesis, characterization, electrochemical properties and computational studies. Spectrochim Acta a Mol Biomol Spectrosc. 2012;97:876–884. doi: 10.1016/j.saa.2012.07.041 [DOI] [PubMed] [Google Scholar]
- 51.Arbabi HA, Soltani SS, Salehi H, Rezazadeh S, Zonouzi A, Toosibashi M. Convenient synthesis of heterocyclic azo dyes in the class of pyranopyrazoles and chromenes. J Chem Res. 2018;42(2):68–72. doi: 10.3184/174751918X15177611816526 [DOI] [Google Scholar]
- 52.Cho CH, Ogle CW. Cholinergic-mediated gastric mast cell degranulation with subsequent histamine h1-and h2-receptor activation in stress ulceration in rats. Eur J Pharmacol. 1979;55(1):23–33. doi: 10.1016/0014-2999(79)90144-4 [DOI] [PubMed] [Google Scholar]