Abstract
As the most prevalent type of RNA modification in eukaryotes, N6-methyladenosine (m6A) can modulate RNA fates such as processing, splicing, maturation, export, stability, translation, and degradation. Circular RNAs (circRNAs), a novel type of non-coding RNA (ncRNAs) characterized by a covalently closed loop structure, play an essential role in various physiological and pathological processes. Extensive studies have revealed that m6A modification is widespread in circRNAs and influences their biogenesis and functions. Intriguingly, circRNAs can affect m6A modification by regulating m6A regulatory proteins. In this review, we summarize the characteristics and biological functions of m6A and circRNAs and focus on recent advances in the interaction of m6A modification and circRNAs. In addition, the potential clinical applications of m6A modification and circRNAs in diagnosis and therapeutic targets are discussed.
Keywords: N6-methyladenosine, circular RNAs, m6A-modified circRNAs, biological functions, clinical applications
Graphical abstract
In this review, Xu et al. comprehensively summarize the characteristics and biological functions of m6A and circRNAs, respectively, and focus on recent advances in the interactive effects of m6A modification and circRNAs. In addition, the potential clinical applications of m6A modification and circRNAs in diagnosis and therapeutic targets are discussed.
Introduction
Due to far-reaching advances in science and technology, many chemical modifications of DNA, RNA, and proteins have been identified, and chemical modification of RNA is the most abundant.1 As a result of the widespread presence of >100 RNA modifications in different RNA types, including eukaryotic messenger RNAs (mRNAs), non-coding RNAs (ncRNAs), and viral RNA genomes, a new field of epitranscriptomics has arisen.2,3 RNA methylation is one of the predominant forms of epigenetic modification, accounting for >60% of RNA modifications.4 The common sites of RNA methylation mainly include N6-methyladenosine (m6A), 5-methylcytosine (m5C), N1-methyladenosine (m1A) and 7-methylguanosine (m7G). As the most prevalent type among these methylations, m6A, which describes a methylation at the N6 position of adenosine and enriched in the consensus sequence RRACH (R: A or G; A: m6A; and H: A, C, or U), has become a hotspot in the research community.5 The biological function of m6A modification is regulated by three types of core proteins: methyltransferases (writers), demethylases (erasers), and RNA-binding proteins (RBPs; readers), indicating a dynamic and reversible process. Abnormalities in m6A regulators resulting in aberrant m6A levels are associated with various diseases, such as cancer, neurological disease, and embryo retardation.6 Moreover, accumulating evidence has shown that m6A possesses potential clinical applications as a biomarker in the prevention, diagnosis, treatment, and prognosis of diseases.7,8
High-throughput sequencing for RNA methylation has revealed that m6A is usually located in the 3′ untranslated region (3′ UTR) near the termination codon and within internal long exons,9 which can modulate RNA fate such as processing, splicing, maturation, export, stability, translation, and degradation.10 Also, the modification occurring near the 5′ cap can facilitate translation initiation in a cap-independent manner.11 In addition, there is a crosslink between m6A peaks with the 3′ UTR and binding sites of ncRNAs, suggesting a mechanism by which m6A modification can regulate RNA transcripts through interaction with ncRNAs such as microRNAs (miRNAs).12,13 Specifically, circular RNAs (circRNAs), a novel type of ncRNA characterized by a covalently closed loop structure, have demonstrated that they play an important role in the occurrence and development of diseases by sponging miRNAs, regulating alternative splicing, modulating parental gene expression, acting as scaffolds in protein complexes, and encoding peptides.14,15 Recently, thousands of circRNAs derived from human tissues have attracted much attention due to their conservation, stability, and abundance in tissues and body fluids, which make them a new star in the field of liquid biopsies.16,17 Since the specific m6A modification of circRNAs was first reported in 2017,18 research on m6A circRNAs is in full swing. Zhou et al. confirmed that m6A is widespread in circRNAs and expressed in cell-type-specific patterns, suggesting the important regulatory role of m6A in the biological function of circRNAs.19 Nevertheless, the interaction network between m6A modifications and circRNAs remains unclear. In this review, we summarize the characteristics and biological functions of m6A and circRNAs and focus on recent research progress on the interaction of m6A modification and circRNAs. In addition, the potential clinical applications of m6A modification and circRNAs in diagnosis and therapeutic targets are discussed.
m6A modification machinery
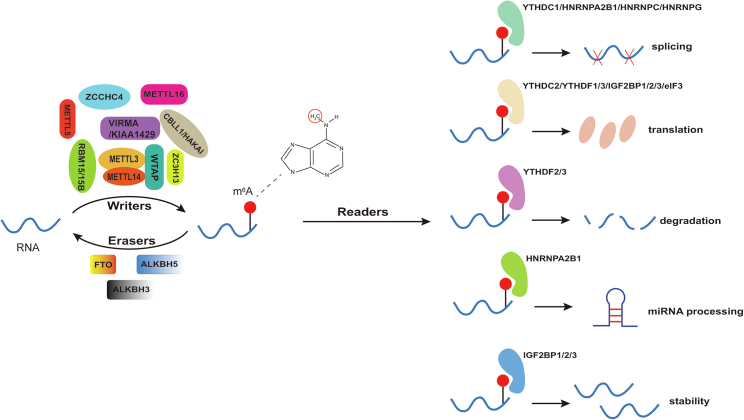
As a dynamic and reversible process in eukaryotic cells, m6A modification is catalyzed by methyltransferase and demethylase, which act as writers and erasers to add and remove, respectively, m6A. RBPs called readers recognize m6A sites to interact with RNA and perform corresponding biological functions (Figure 1; Table 1). The dysregulation of writer, eraser, and reader proteins is involved in the pathogenesis of multiple diseases.
Figure 1.
m6A modification machinery
As a dynamic and reversible process in eukaryotic cells, m6A modification is catalyzed by methyltransferase and demethylase acting as writers and erasers to add and remove m6A, respectively. RNA-binding proteins called readers recognize m6A sites to combinate with RNA and perform corresponding biological functions.
Table 1.
The core regulatory proteins in m6A modification
| Type | Regulator | Function | Mechanism | References |
|---|---|---|---|---|
| Methyltransferase | METTL3 | writer | transfer methyl from S-adenosyl methionine (SAM) to adenine bases | Wang et al.20,21 |
| METTL14 | writer | maintain the stability of METTL3-14 heterodimer and recognize the m6A-specific sequence (RRACH) | Wang et al.20,21 | |
| WTAP | writer | facilitate m6A modification by guiding METTL3-14 heterodimer localization to nuclear spots | Ping et al.22 | |
| VIRMA/KIAA1429 | writer | recruit METTL3/METTL14/WTAP complex in 3′ UTR and near the termination codon and regulate alternative polyadenylation | Yue et al.23 | |
| RBM15/RBM15B | writer | bind to U-rich region and guide METTL3/METTL14/WTAP complex to m6A-specific motifs | Knuckles et al.,24 Patil et al.25 | |
| ZC3H13 | writer | combine with WTAP to promote MTC deposition in nuclear spots and enhance m6A modification | Knuckles et al.24 | |
| CBLL1/HAKAI | writer | support efficient methylation through the nuclear location of ZC3H13-WTAP-VIRMA-CBLL1 | Wen et al.26 | |
| ZCCHC4 | writer | new m6A methyltransferase for human 28S rRNA; involved in translation biology | Ma et al.27 | |
| METTL16 | writer | target U6 small nuclear RNA (snRNA) at position 43 and recognize the 5′ splice site during pre-mRNA splicing | Warda et al.28 | |
| METTL5 | writer | catalyze m6A installation for 18S rRNA by forming METTL5-TRMT112 heterodimeric complex to increase metabolic stability | van Tran et al.29 | |
| Demethylases | FTO | eraser | mediate demethylation of m6A in RNA and m1A in tRNA | Jia et al.,30 Wei et al.31 |
| ALKBH5 | eraser | preferentially recognize the m6A for demethylation | Zheng et al.32 | |
| ALKBH3 | eraser | mediate the demethylation of m6A, m1A, and m3C on tRNA | Ueda et al.,33 Chen et al.34 | |
| RNA-binding proteins (RBPs) | YTHDC1 | reader | regulate mRNA splicing and mediate export of m6A-containing mRNA from the nucleus to the cytoplasm | Xiao et al.,35 Roundtree et al.36 |
| YTHDC2 | reader | essential for the enhancement of translation efficiency and regulation of mammalian spermatogenesis | Tanabe et al.,37 Mao et al.,38 Hsu et al.39 | |
| YTHDF1 | reader | mediate translation promotion via the interaction with translation initiation complex | Wang et al.40 | |
| YTHDF2 | reader | induce the instability and accelerate the degradation of m6A-methylated mRNA through the interaction with P bodies | Wang et al.,41 Batista et al.,42 Geula et al.43 | |
| YTHDF3 | reader | facilitates translation and affects the decay of m6A-containing mRNA in synergy with YTHDF1 and YTHDF2, respectively | Shi et al.44 | |
| HNRNPA2B1 | reader | bind m6A-bearing RNAs to elicit alternative splicing effects and interact with the microRNA (miRNA) microprocessor complex protein DGCR8 to promote primary miRNA processing | Alarcon et al.45 | |
| HNRNPC | reader | affect the abundance and alternative splicing of target RNAs | Liu et al.46,47 | |
| HNRNPG | reader | affect the abundance and alternative splicing of target RNAs | Liu et al.46,47 | |
| IGF2BP1/2/3 | reader | promote mRNA stability and translation in an m6A-dependent manner | Huang et al.48 | |
| eIF3 | reader | bind to the 5′ UTR of m6A-bearing mRNA and recruit the 43S ribosomal complex to initiate translation in a cap-independent manner | Meyer et al.,11 Huang et al.48 |
Methyltransferase (writers)
The m6A methyltransferase complex (MTC) is usually composed of various proteins. Among the multisubunit complexes, methyltransferase-like protein 3 (METTL3) is the only core protein with catalytic activity that can transfer methyl groups from S-adenosyl methionine (SAM) to adenine bases of RNA transcripts through the internal SAM-binding domain. Another critical component of m6A MTCs is METTL14, which also contains a SAM motif, but has no catalytic function. It acts as a binding partner of METTL3, maintaining the stability of the METTL3-14 heterodimer and recognizing the m6A-specific sequence (RRACH).20,21 In addition, many cofactors play essential roles in m6A installation, including Wilms tumor 1-associated protein (WTAP), Vir-like m6A methyltransferase-associated (VIRMA), RNA-binding motif protein 15/15B (RBM15/15B), and zinc finger CCCH-type containing 13 (ZC3H13). WTAP has been demonstrated to facilitate m6A modification by guiding METTL3-14 heterodimer localization to nuclear spots.22 VIRMA, also known as KIAA1429, recruits the METTL3/METTL14/WTAP complex to preferentially mediate m6A modification in the 3′ UTR and near the termination codon. In addition, VIRMA can regulate alternative polyadenylation by interacting with cleavage and polyadenylation specificity factor subunit 5 (CPSF5).23 RBM15 and RBM15B are homologous proteins and perform similar functions, which preemptively bind to the U-rich region and guide the METTL3/METTL14/WTAP complex to m6A-specific motifs.24,25 ZC3H13 combines with WTAP to promote MTC deposition in nuclear spots and enhance m6A modification.24 In addition, Cbl proto-oncogene-like 1 (CBLL1, also known as HAKAI) supports efficient methylation through the nuclear location of ZC3H13-WTAP-VIRMA-CBLL1.26 As another zinc finger CCCH-type containing protein, ZCCHC4 has been reported to be a new m6A methyltransferase for human 28S rRNA and involved in translation biology.27 In recent years, the identification of other novel RNA methyltransferases has broadened our horizons. METTL16 is an active m6A methyltransferase that targets U6 small nuclear RNA (snRNA) at position 43, which is important for U6 snRNA to recognize the 5′ splice site during precursor mRNA (pre-mRNA) splicing.28 METTL5 is another newly discovered enzyme that catalyzes m6A installation for 18S rRNA by forming a METTL5-TRMT112 heterodimeric complex to increase metabolic stability, indicating a novel RNA-binding pattern different from the complex of the METTL3-14 heterodimer.29 Even so, there may be more m6A methyltransferases to be explored further.
Demethylases (erasers)
m6A demethylases function as erasers to remove m6A methylation and thus maintain m6A modification as a dynamic and reversible process. To date, only two m6A demethylases, namely, the fat mass and obesity-associated protein (FTO) and α-ketoglutarate-dependent dioxygenase alk B homolog 5 (ALKBH5), have been widely reported. These two proteins belong to the ALKB dioxygenase family and depend on ferrous iron (Fe2+) cofactor and α-ketoglutaric acid to catalyze demethylation. FTO was originally known to be an important obesity-related gene that contributed to increased body mass index and severe obesity in childhood and adults.49,50 The initial link between FTO and m6A was first discovered when it was found that FTO could exhibit efficient oxidative demethylation activity on m6A residues in RNA.30 In acute myeloid leukemia (AML), FTO plays an oncogenic role by regulating the expression of ASB2 and RARA in an m6A-dependent manner.51 However, a recent study demonstrated that in FTO knockouts, m6A peaks were highly enriched in the 5′ UTR, the site of 2′-O-dimethyladenosine (m6Am), indicating that FTO preferentially demethylates m6Am and controls mRNA stability.52 Nevertheless, since the abundance of m6A is much higher than that of m6Am, FTO mainly mediates the demethylation of m6A. In addition, FTO was shown to mediate RNA demethylation of m1A in transfer RNA (tRNA).31 ALKBH5, as an FTO homolog, was the second identified demethylase protein exhibiting m6A demethylase activity.32 Unlike FTO, ALKBH5 can catalyze the removal of m6A methylation without intermediate products and preferentially recognize m6A for demethylation. ALKBH5-mediated m6A demethylation of NANOG mRNA partly induces the breast cancer stem cell phenotype.53 In glioblastoma, ALKBH5 maintains the tumorigenicity of stem-like cells by sustaining FOXM1 expression and the cell proliferation program.54 Recently, ALKBH3 was identified as a novel demethylase protein that preferentially mediates the demethylation of m6A, m1A, and 3-methylcytidine (m3C) on tRNA.33,34
RBPs (readers)
m6A “readers,” a group of RBPs that can recognize m6A modifications, contribute to different biological functions of target RNAs. At present, there are three categories of readers that have been extensively studied, including YT521-B homology (YTH) domain family proteins, heterogeneous nuclear ribonucleoproteins (HNRNP) family and insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs). YTH domain-containing proteins have 5 members: YTH domain containing 1 (YTHDC1), YTH domain containing 2 (YTHDC2), YTH domain family protein 1 (YTHDF1), YTH domain family protein 2 (YTHDF2), and YTH domain family protein 3 (YTHDF3). Of them, YTHDC1 is nuclear enriched and located in nuclear speckles, which can regulate mRNA splicing by recruiting pre-mRNA splicing factors to the binding sites of target RNAs.35 In addition, YTHDC1 mediates the export of m6A-containing mRNA from the nucleus to the cytoplasm by interacting with splicing factors and nuclear export adaptor proteins.36 In the cytoplasm, YTHDC2 recognizes a smaller number of m6A sites, whereas the DF family (YTHDF1–3) can bind all m6A sites in RNA.25 YTHDC2 is essential for the enhancement of translation efficiency37,38 and regulation of mammalian spermatogenesis.39 YTHDF1 was shown to mediate translation promotion via interactions with translation initiation complexes, such as eukaryotic translation initiation factor 3 (eIF3), 4E (eIF4E), 4G (eIF4G), poly (A) binding protein (PABP), and the 40S ribosomal subunit.40 YTHDF2, the most abundant in nearly all types of cells, has been identified to induce instability and accelerate the degradation of m6A-methylated mRNA through interaction with P bodies.41, 42, 43 YTHDF3 facilitates translation and affects the decay of m6A-containing mRNA in synergy with YTHDF1 and YTHDF2.44 Members of the HNRNP family, including HNRNPA2B1, HNRNPC, and HNRNPG, are located in the nucleus. HNRNPA2B1 can bind m6A-bearing RNAs to elicit alternative splicing effects and interact with the miRNA microprocessor complex protein DGCR8 to promote primary miRNA processing.45 Unlike HNRNPA2B1, HNRNPC and HNRNPG cannot directly bind to the sites of m6A. However, due to changes in the local structure of RNA caused by m6A, the binding activities of HNRNPC and HNRNPG are facilitated, thereby affecting the abundance and alternative splicing of target RNAs.46,47 As a distinct family of m6A readers, IGF2BP proteins, including IGF2BP1/2/3, affect gene expression output by promoting mRNA stability and translation in an m6A-dependent manner.48 Moreover, eIF3 has been identified as a novel m6A reader, with the evidence suggesting that it preferentially crosslinks to m6A-bearing mRNA.11 Moreover, eIF3 has been proposed to directly bind to the 5′ UTR of m6A-bearing mRNA and recruit the 43S ribosomal complex to initiate translation in a cap-independent manner.48
circRNA overview
circRNAs are a special kind of RNA, with a covalently closed, single-stranded structure produced from pre-mRNA back-splicing by distinct mechanisms. A number of studies have shown that circRNAs possess many unique characteristics, such as high stability, abundance, species conservation, and tissue- and disease-specific expression patterns, which enable them to perform crucial biological functions.15
The biogenesis and properties of circRNAs
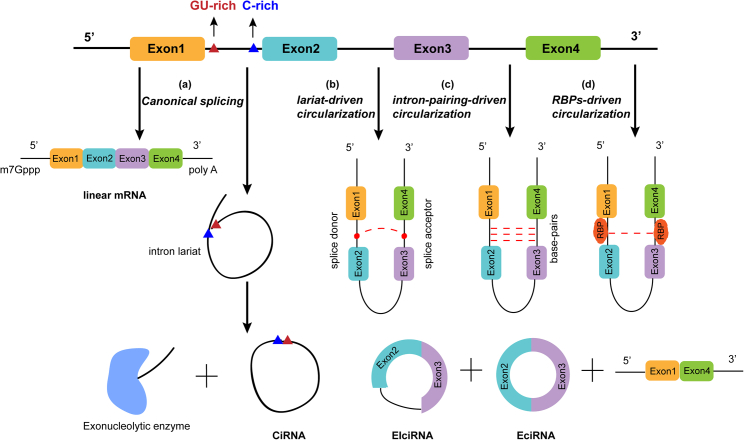
circRNAs were thought to be the result of alternative splicing of pre-mRNA through various mechanisms (Figure 2). Most currently studied circRNAs are generated by back-splicing of exons, in which a downstream 5′ splice donor site is ligated with an upstream 3′ splice acceptor site. According to the different combinations of sequences and domains, circRNAs can be divided into three types: exonic circRNAs (EciRNAs), which originated from exon sequence only;55,56 intronic circRNAs (CiRNAs), which contain intron sequences only;57 and exon-intron circRNAs (EIciRNAs), which consist of exon and intron sequences.58 Two well-known models of EciRNA and EIciRNA biogenesis were proposed by Jeck et al., called lariat-driven circularization and intron-pairing-driven circularization.59 CiRNAs are derived from lariat introns containing a 7-nt GU-rich element near the 5′ splice site and an 11-nt C-rich element at the branchpoint site.57 Recently, some special forms of circRNAs have been identified. Studies have reported that transcribed exons of different genes influenced by translocations could give rise to fusion circRNAs (f-circRNAs), which often function as drivers of tumorigenesis.60, 61, 62 Exon sequencing revealed that readthrough circRNAs (rt-circRNAs) originate from exons in different genes.14,63 In addition, mitochondrial genome-encoded circRNAs (mecciRNAs) play important roles in the mitochondrial entry of proteins.64
Figure 2.
The models of circRNAs biogenesis
(A) Canonical splicing: The GU-rich element near the 5′ splice site and C-rich element close to the branchpoint site were ligated to form ciRNAs. (B) Lariat-driven circularization: The 5′ splice donor site of exon 4 covalently binds to the 3′ splice acceptor site of exon 1 to form lariat structure and produce EIciRNAs or EciRNAs. (C) Intron-pairing-driven circularization: The complementary base pairs between introns bring splicing sites close to form EIciRNAs or EciRNAs. (D) RBP-driven circularization: RBPs bridge distal splice site to facilitate cyclization.
With the rapid development of next-generation RNA sequencing (RNA-seq) of non-polyadenylated transcriptomes, circRNAs have been extensively investigated and found to abundantly exist in eukaryotic cells.65 In general, EciRNAs contain <5 exons, with the splice lengths ranging from 100 to 4,000 nt.66 Hundreds of human genes give rise to many circRNAs, and the same host gene could produce many different circRNAs due to alternative circularization, which enriches the diversity of circRNAs.55,67 Although the abundance of most circRNAs is less than that of the corresponding linear RNAs, under some circumstances, circRNA expression is dozens of times greater than that of the linear transcripts.59 circRNAs have high stability because of their special covalently closed structure without a polyadenylated tail. According to recent reports, circRNAs can remain resistant to RNA exonucleases and have a longer average half-life than their linear RNAs.68, 69, 70, 71 Although the detailed mechanisms of circRNA degradation have not been elucidated, circRNAs contain miRNA response elements (MREs), which could lead to circRNA degradation by interacting with the AGO protein and forming an RNA-induced silencing complex (RISC).72 Moreover, Park et al. demonstrated that m6A is involved in the degradation process of circRNAs.73 Typically, circRNAs are evolutionarily conserved among different species.74 For instance, 2,121 circRNAs identified in human fibroblasts can be matched to the mouse genome.59 This phenomenon may result from the conservation of splicing regulatory elements and complementary flanking introns such as Alu elements. Furthermore, the expression pattern of circRNAs is cell type specific and occurs in a spatiotemporal manner.75,76
The regulation of circRNA generation depends on several kinds of factors, including spliceosomes, cis-complementary sequences in flanking introns, and special proteins.77 The spliceosome can recognize specific splice sites of the exon flanks, promoting circRNAs biogenesis.78 Back-splicing of circRNA formation is boosted by intronic complementary sequences (ICSs), which are usually mediated by complementary inverted-repeat Alu elements and non-repetitive complementary sequences in human cells.55,59,79 The competition of RNA pairs in the same intron, flanking introns, and different sets of introns significantly influence the efficiency of circRNA production.80, 81, 82 In addition, a number of RBPs regulate the back-splicing of circRNA biogenesis in trans. In one mode, RBPs bridge distal splice sites to facilitate cyclization. The splicing factor muscleblind (Mbl) can modulate circMbl biosynthesis by directly binding to conserved Mbl binding sites in flanking introns.81 Protein quaking (QKI), as an alternative splicing factor, dynamically regulates the production of abundant circRNAs during the epithelial-mesenchymal transition (EMT) process, depending on the QKI recognition motif in introns, which could make splice sites closer together, benefiting the back-splicing of circRNAs.83 Another mode is that RBPs bind to ICSs and stabilize RNA pairs in intron flanks via double-stranded RNA (dsRNA)-binding domains (dsRBDs). For example, the nuclear factor 90 (NF90) and NF110 containing 2 dsRBDs can bind to intronic inverted-repeat Alu elements and promote circRNA formation.84 However, DHX9, a nuclear RNA helicase containing a dsRBD and an RNA helicase domain, can dampen circRNA formation by binding to intronic inverted-repeat Alu elements and disassembling RNA pairs in intron flanks using RNA helicase activity.85 Adenosine deaminase acting on RNA 1 (ADAR1), a kind of RNA-editing enzyme, performs A-to-I editing of inverted-repeat Alu elements, impairing the stability of RNA pairs and negatively regulating circRNA expression.86,87 In recent years, a novel role for m6A in the biogenesis of circRNAs has been uncovered.88
The regulatory mechanisms of circRNAs
Emerging studies have revealed that circRNAs participate in the pathophysiological processes through various regulatory mechanisms at multiple levels. Notably, circRNAs have been reported to serve as miRNA decoys, protein scaffolds, alternative splicing regulators, and translation templates. circRNAs in the cytoplasm may directly bind to miRNAs via complementary miRNA binding sites to regulate downstream target genes. Despite the concerns that most circRNAs possess <10 miRNA binding sites, the role of circRNAs as competing endogenous RNAs (ceRNAs) has still been widely investigated.89,90 Accumulating evidence has demonstrated that miRNA sponging may be the most common mode of action of circRNAs. Apart from interacting with miRNAs, circRNAs can engage with different proteins and function as protein scaffolds. For example, CUT-like homeobox 1 (CUX1)-generated circular RNA circ-CUX1 contributes to aerobic glycolysis and neuroblastoma progression by binding to EWS RBP 1 (EWSR1) to facilitate MYC-associated zinc finger protein (MAZ) transactivation, resulting in transcriptional alteration of CUX1 and other genes associated with tumor progression.91
circRNAs in the nucleus have distinct functions, such as regulation of transcription and alternative splicing. Typically, EIciRNAs can enhance parental gene transcription through interaction with U1 snRNA and RNA polymerase II (RNA Pol II) transcription complex at the promoters of genes.58 The splicing factor Mbl can modulate circMbl biosynthesis by directly binding to conserved Mbl binding sites in flanking introns, which indicates that circRNA production competes with pre-mRNA splicing.81 Recently, research on the function of circRNAs translation has drawn much attention since it was first reported that endogenous circRNAs have the ability to translate proteins or peptides.92 van Heesch et al. have identified hundreds of small peptides translated from lncRNAs and circRNAs by analyzing the translatomes of 80 human hearts and validated the protein products in vivo, supporting the fact that circRNAs can serve as the potential translation templates of proteins.93 To date, the mechanisms of circRNAs translation are internal ribosome entry site (IRES)-driven and m6A-mediated initiation.94
The biological functions of circRNAs
A growing number of circRNAs have been implicated in the initiation and development of various diseases, especially tumors. The functions of circRNAs can be mainly divided into oncogenic and antioncogenic. For example, by adopting ultra-deep RNA-seq and short hairpin RNA (shRNA)-screening libraries, Chen et al. identified a total of 171 circRNAs essential for prostate cancer cell proliferation, whereas their host linear transcripts were not essential.95 This phenomenon reflected that the function of circRNAs may be independent of their parental linear RNAs. However, circYap could enhance apoptosis and restrain the proliferation and metastasis of cancer cells by binding with the translation initiation-associated proteins eIF4G and PABP to antagonize YAP translation.96 In addition, Epstein-Barr virus (EBV)-encoded circLMP2A plays crucial roles in inducing and maintaining stemness phenotypes in EBV-associated gastric cancer. Impaired circLMP2A expression can suppress cell growth and metastasis in vitro and in vivo.97 These studies indicate that different types of circRNAs influence cell transformation properties in various ways.
The roles of circRNAs have been widely investigated in other non-tumor diseases. It has been shown that circRNAs are abundant and conserved in mammalian neurons and synaptogenesis, suggesting that circRNAs play vital roles in regulating neuronal and synaptic functions. Loss of the CDR1as locus in the mouse genome affects synaptic transmission and causes neuropsychiatric disorders.98 In cardiovascular diseases, circFndc3b overexpression in cardiac endothelial cells enhances neovascularization, reduces cardiomyocytes and endothelial cell apoptosis, and improves myocardial functions.99 In adipose biology, Arcinas et al. discovered that circTshz2-1 and circArhgap5-2 serve as indispensable regulators in adipocyte differentiation and metabolism.100
Recently, emerging roles of circRNAs in the immune system have been uncovered. On the one hand, circRNAs have been shown to exhibit cell-type-specific expression in different types of immune cells and induce their differentiation.101,102 On the other hand, circRNAs can regulate the activity of innate immune cells and then facilitate disease development.103 In the absence of viral infection, endogenous circRNAs usually bind to innate immunity proteins and inactivate immunity. Upon infection, these proteins are released to initiate the antiviral immune response by recognizing viral nucleic acids, and the level of endogenous circRNAs globally decreases. As mentioned above, the NF90/NF110 complex can bind to intronic inverted-repeat Alu elements and promote circRNAs formation in the nucleus.84 circRNAs in the cytoplasm can also interact with the NF90/NF110 complex. Once viral infection occurs, nuclear NF90/NF110 rapidly translocates to the cytoplasm to inhibit the translation of viral mRNAs,104 leading to a global reduction in circRNA production. Meanwhile, NF90/NF110 are separated from cytoplasmic circRNAs to interact with viral transcripts and block virus replication.84 In addition, endogenous circRNAs can bind to antiviral protein kinase R (PKR) to prevent innate immune activation. Viral infection triggers the degradation of circRNAs to free PKR and stimulates innate immunity.105 However, foreign circRNAs have opposite effects to endogenous circRNAs. Engineered foreign circRNAs transfected into cells can stimulate innate immunity and inhibit viral infection by activating immune genes such as PKR, melanoma-differentiation-associated gene 5 (MDA5), and retinoic-acid-inducible gene-I (RIG-I).106 Of note, m6A-modified circRNAs also have important roles in innate immunity.
Characteristics of m6A circRNAs
Previous studies have shown that RNase R-resistant (non-linear) RNAs contain a strong m6A signal, suggesting that circRNAs may be modified by m6A.19 METTL3/METTL14 complex is required for the m6A modification of mRNAs, which is also essential for m6A circRNAs. The depletion of METTL3 or METTL14 results in the reduced m6A modification of circRNA-enriched RNA, which is more significant with combined METTL3/METTL14 depletion.19 Similarly, m6A circRNAs are read by the same proteins, such as YTHDF1 and YTHDF2, which interact with mRNAs. YTHDF2 is widely known to regulate the stability of mRNAs by forming a complex with m6A mRNAs.41 However, YTHDF2, which is involved in m6A circRNAs, does not appear to promote the degradation of circRNAs. An interesting phenomenon was observed in which mRNAs methylated on the same exons encoded by the parental genes of m6A circRNAs had the shortest half-lives among all m6A mRNAs, showing that the stability of mRNAs is regulated by YTHDF2 in a process involving the recognition of m6A circRNAs. Furthermore, the m6A circRNA levels are variable across different species and similar to mRNAs in distribution.
Although m6A modification is widespread in circRNAs, unique patterns that are distinct from mRNAs are exhibited. First, m6A circRNAs and m6A peaks in mRNAs across genes have different distributions. m6A sites in mRNAs are most common in the region from the end of the coding sequence to the 3′ end (3′ UTR), but exons encoding m6A circRNAs are often located in the region from the transcription start site (TSS) to the start of the coding sequence (5′ UTR). Thus, the majority of m6A circRNAs are generated from exons without m6A peaks in mRNAs. Second, numerous m6A circRNAs are expressed in a cell-type-specific manner, even though their parental genes or circRNAs are expressed in both cell types, indicating that m6A circRNAs are involved in different biological processes in specific cell types. Third, m6A methylation is more abundant in circRNAs composed of long single exons than multi-exon m6A circRNAs. In addition, transposable elements (TEs) are significantly enriched in the flanking regions of m6A circRNAs, suggesting that the density of TEs flanking circRNAs is associated with m6A modification.
Roles of m6A methylation on circRNAs
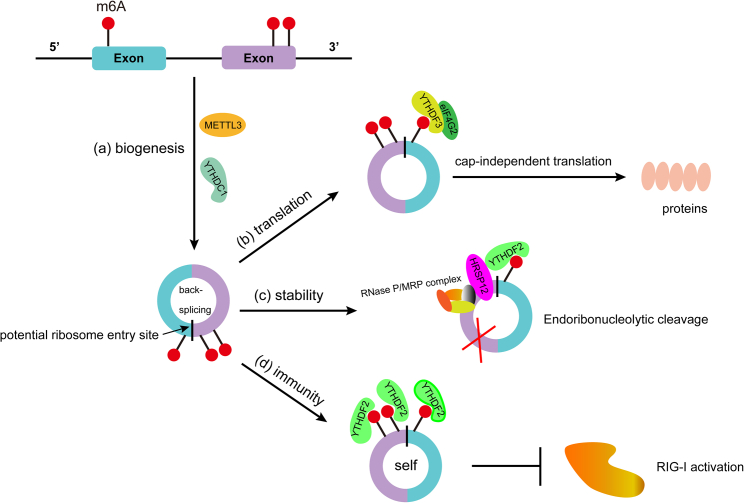
Accumulating studies have identified that m6A modifications have various regulatory effects on circRNAs, including their biogenesis, splicing, localization, transport, and degradation (Figure 3). Here, we describe the roles of m6A modifications in the regulation and function of circRNAs (Table 2).
Figure 3.
Roles of m6A methylation on circRNAs
(A) circRNA production correlates with m6A levels and is modulated by ALKBH5 and METTL3. (B) m6A residues can drive efficient initiation of protein translation from circRNAs in a cap-independent manner. (C) m6A-containing circRNAs are endoribonucleolytically cleaved by YTHDF2-HRSP12-RNase P/MRP complex. (D) m6A suppression of circRNA immunity was mediated by YTHDF2, which can block endogenous circRNAs from activating the RIG-I antiviral pathway.
Table 2.
Roles of m6A methylation on circRNAs
| m6A regulators | circRNA | m6A levels | Effects on circRNAs | Diseases | References |
|---|---|---|---|---|---|
| ALKBH5/METTL3 | a subset of circRNAs | decrease/increase | modulate the biogenesis | spermatogenesis | Tang et al.88 |
| METTL3/YTHDC1 | circ-ZNF609 | increase | modulate the biogenesis | rhabdomyosarcoma tumors | Di Timoteo et al.107 |
| METTL3 | hsa_circ_0029589 | increase | decrease its expression | ACS, AS | Guo et al.108 |
| METTL3/YTHDC1 | circ-ARL3 | increase | promote splicing and biogenesis | HCC | Rao et al.109 |
| METTL3 | circ1662 | increase | induce its expression | CRC | Chen et al.110 |
| METTL3 | circMETTL3 | increase | regulate its expression | breast cancer | Li et al.111 |
| METTL3/YTHDC1 | circHPS5 | increase | promote biogenesis and nucleus-cytoplasm output | HCC | Rong et al.112 |
| KIAA1429 | circDLC1 | increase | regulate its expression | HCC | Liu et al.113 |
| METTL3/FTO/YTHDF1/2 | circRNA-SORE | increase | enhance its stability | HCC | Xu et al.114 |
| METTL3 | circCUX1 | increase | stabilize its expression | hypopharyngeal squamous cell carcinoma | Wu et al.115 |
| METTL3 | circ0000069 | increase | improve its stability | cervical cancer | Chen et al.116 |
| YTHDF3/eIF4G2 | circ-ZNF609 | recognition | increase translation | HeLa cells | Di Timoteo et al.107 |
| METTL3/METTL14 | circE7 | increase | facilitate translation | human papillomavirus | Zhao et al.117 |
| FTO | circRNA ARHGAP35 | decrease | promote translation | HCC | Li et al.118 |
| YTHDF2 | circRNA | recognition | suppress immunity | mammalian cells | Chen et al.119 |
| YTHDC1 | circNSUN2 | recognition | export to cytoplasm from nucleus | CRC | Chen et al.120 |
| METTL14 | circGFRα1 | increase | export to cytoplasm from nucleus | FGSCs | Li et al.121 |
| METTL3/METTL14 | circNDUFB2 | increase | impair interaction with proteins | NSCLC | Li et al.122 |
m6A methylation modulates the biogenesis of circRNAs
Recently, the role of m6A in the biogenesis of circRNAs has been investigated. Studies have reported that circRNAs are abundantly synthesized in male germ cells during spermatogenesis.88 Junction points of these circRNAs appear to occur in m6A-enriched sites, which are usually located around the start and stop codons of linear mRNAs.123 This evidence implies that circRNA production correlates with m6A levels, which are modulated by ALKBH5 and METTL3.88 In addition, half of these circRNAs contain large open reading frames (ORFs) with m6A-modified start codons and have coding potential, revealing a novel mechanism by which circRNAs can maintain the continuous and stable production of proteins in the absence of linear transcripts.88 Another study discovered that the biogenesis of circ-ZNF609 was modulated by METTL3 and YTHDC1, which displayed specific m6A signatures that controlled its accumulation.107 This feature is shared with other circRNAs because of a significant direct correlation with METTL3 requirement, YTHDC1 binding, and the ability of m6A exons to undergo circularization.
The transcriptome-wide map of m6A circRNAs in hypoxia-mediated pulmonary hypertension (HPH) showed that m6A circRNAs were derived mainly from genes spanning single exons in the control and HPH groups. The expression of m6A-circRNAs tended to be reduced in comparison with that of non-m6A circRNAs in HPH, suggesting that m6A methylation could influence the expression of circRNAs.124 This characteristic was also observed in lens epithelium cells from age-related cataracts.125 In addition, Guo et al. found that the decreased expression of hsa_circ_0029589 through elevated m6A levels promoted by METTL3 may facilitate macrophage pyroptosis in acute coronary syndrome (ACS) and atherosclerosis (AS).108 The expression of METTL3 upregulated by HBx proteins in hepatitis B virus-associated hepatocellular carcinoma increased the level of m6A circ-ARL3, which YTHDC1 bound to promote its reverse splicing and biogenesis.109 Also, METTL3-induced circ1662 expression by binding its flanking sequences and installing m6A modifications could promote colorectal carcinoma (CRC) metastasis by accelerating YAP1 nuclear transport.110 As the host gene of circMETTL3, METTL3 may regulate its expression in an m6A-dependent manner to facilitate breast cancer progression.111 In addition, Rong et al. illustrated that METTL3 facilitated the m6A modification process and controlled the accumulation of circHPS5.112 The nucleus-cytoplasm output of circHPS5 could be mediated by recruiting YTHDC1, which promoted the EMT and cancer stem-like cell (CSC) phenotypes in hepatocellular carcinoma (HCC).112 Furthermore, as a key component of the m6A methyltransferase complex, KIAA1429 could regulate the expression of circDLC1, contributing to the inhibition of HCC progression.113 These results suggest that m6A methylation affects the biogenesis and expression of circRNAs in different ways.
m6A methylation regulates the stability of circRNAs
circRNAs remain resistant to RNA exonuclease and have a longer average half-life than linear RNAs due to their special covalently closed structure without a polyadenylated tail.68, 69, 70, 71 However, the detailed mechanisms of circRNAs degradation have not been elucidated. A previous study reported that circRNAs with miRNA response elements (MREs) could lead to their degradation by interacting with the AGO protein and forming RISC.72 Recently, a new mechanism was uncovered in which m6A-containing linear RNAs and circRNAs are endoribonucleolytically cleaved by the YTHDF2-HRSP12-RNase P/MRP (mitochondrial RNA processing) complex.73 HRSP12 acts as a bridge to link YTHDF2 and RNase P/MRP. HRSP12 and RNase P/MRP can destabilize YTHDF2-bound transcripts. circRNAs associated with YTHDF2 in a HRSP12-dependent manner are preferentially reduced by RNase P/MRP. Thus, these findings demonstrate that m6A is involved in the degradation process of circRNAs.
m6A modification has also been considered to elevate the expression of circRNAs, resulting partially from increased RNA stability. For example, m6A methylation enhanced the stability of circRNA-SORE, which was upregulated in sorafenib-resistant HCC cells and induced sorafenib resistance.114 METTL3-mediated m6A modification stabilized the expression of circCUX1, which conferred radioresistance in hypopharyngeal squamous cell carcinoma.115 m6A modification improved circ0000069 transcript stability and promoted cervical cancer cell proliferation and migration.116 These studies hint that m6A methylation could regulate the stability of circRNAs to affect their biological functions.
m6A methylation facilitates the translation of circRNAs
Due to the lack of a 5′ 7-methylguanosine cap structure and poly(A) tail, two factors that are required for mRNAs translation, circRNAs have been classified as ncRNAs without the ability to encode proteins. However, accumulated evidence suggests that circRNAs may be used as templates for protein synthesis. Artificial circRNAs can be translated in a cap-independent manner through IRES elements directly binding initiation factors or the ribosome.126,127 Subsequently, Yang et al. discovered that m6A residues are abundant in circRNAs and can drive efficient initiation of protein translation from circRNAs.18 The m6A-induced translation of circRNAs could be increased by various cellular stresses, such as heat shock or upregulation of the methyltransferase METTL3/14, but could be decreased by the demethylase FTO.18 In addition, the eukaryotic translation initiation factor eIF4G2 and the m6A reader YTHDF3 are essential for circRNA translation driven by m6A, with a possible role of YTHDF3 in recruiting eIF4G2 to the m6A-containing RNA.18 Bozzoni et al. identified that circ-ZNF609 translation is modulated through recognition by YTHDF3 and eIF4G2.107 Overexpression of either of these two factors resulted in the increased translation of circ-ZNF609. YTHDF3 and eIF4G2 were physically associated with endogenous circ-ZNF609, indicating the relevance of both of these factors in the translational control of circ-ZNF609.107
circE7, derived from human papillomavirus 16 (HPV16), is associated with polysomes and translated to produce the E7 oncoprotein. Zhao et al. found that circE7 possessed multiple potential m6A sites and was confirmed to be methylated.117 Knockdown of METTL3/14 resulted in a decrease in circE7 expression after m6A immunoprecipitation. Mutation of the m6A motifs also significantly decreased the abundance of circE7 and inhibited E7 oncoprotein expression.117 These results indicated that m6A modification facilitates the cap-independent translation of circE7. Similarly, a novel circRNA ARHGAP35 promotes cancer cell progression through translation into an oncogenic protein driven by m6A modification. Overexpression of the m6A demethylase FTO significantly reduced the abundance of m6A and translation of the oncogenic protein, confirming that m6A modification is essential for the efficient protein translation from circARHGAP35.118
m6A modification controls circRNA immunity
circRNAs have important biological roles in immune cells and immune response.128 A recent study revealed that in the absence of viral infection, endogenous circRNAs possessing 16- to 26-bp imperfect RNA duplexes could interact with PKR monomers to suppress their activity. Upon viral infection, endonuclease RNase L degrades endogenous circRNAs to release PKR to initiate the innate immune response.105 These findings show that endogenous circRNAs function as suppressors of immune stimulation. In contrast, exogenous circRNAs potently induce immune signaling by interacting with RIG-I in the presence of K63-polyubiquitin to cause mitochondrial antiviral signaling (MAVS) filamentation, IRF3 dimerization, and interferon production.106,119 In addition, exogenous circRNAs could induce antigen-specific T and B cell activation, antibody production, and antitumor immunity in vivo.119
The different roles of endogenous and exogenous circRNAs in immune signaling are partly dependent on m6A modification.119 Yan et al. identified that endogenous circRNAs marked by m6A modification could escape immunological surveillance and avoid eliciting an immune response.128 They also demonstrated that m6A suppression of circRNA immunity was mediated by the RBP YTHDF2, which could recruit m6A-modified RNAs into phase-separated condensates via their N-terminal disordered domains and block endogenous circRNAs from activating the RIG-I antiviral pathway.128 Collectively, this strong evidence suggests that m6A modification is a key regulator of circRNAs immunity.
m6A methylation affects the biological functions of circRNAs
The effects of m6A modification on the biological functions of circRNAs are also reflected in other aspects. Chen et al. identified that an m6A-modified circNSUN2 was elevated in CRC patients with liver metastasis and predicted poorer survival.120 The nucleus export to the cytoplasm of circNSUN2 was mediated by YTHDC1 in an m6A-dependent manner. Increased cytoplasmic circNSUN2 stabilized high-mobility group AT-hook (HMGA2) mRNA and interacted with IGF2BP2 to promote colorectal liver metastasis.120 In the same way, m6A-modified circGFRα1 (glial cell line-derived neurotrophic factor [GDNF] receptor α1) was highly abundant in female germline stem cells (FGSCs). The m6A writer METTL14 facilitated circGFRα1 export to the cytoplasm from the nucleus in an m6A-dependent manner. Cytoplasmic circGFRα1 sponges miR-449 to enhance GFRα1 expression and activate the GDNF signaling pathway, ultimately promoting FGSC self-renewal.121 In addition, Li et al. revealed that circNDUFB2 (NADH:ubiquinone oxidoreductase subunit B2) inhibits the growth and metastasis of non-small cell lung carcinoma (NSCLC) cells via a mechanism in which the TRIM25/circNDUFB2/IGF2BPs ternary complex facilitates the ubiquitination and degradation of IGF2BPs.122 In this study, m6A modification was abundant in circNDUFB2, and the interactions between circNDUFB2 and IGF2BPs were significantly impaired, with a reduction in m6A levels caused by METTL3/14 knockdown.122 Clearly, these investigations demonstrate that m6A modification plays a key role in the biological functions of circRNAs.
Roles of circRNAs in the regulation of m6A modification
Studies have shown that circRNAs can affect m6A modification by regulating m6A regulators (Table 3). However, circRNAs competitively bind to m6A regulators to affect m6A levels. For example, circSTAG1 was significantly downregulated in the peripheral blood of patients with major depressive disorder. Overexpressed circSTAG1 captured the demethylase ALKBH5, which increased m6A levels of fatty acid amide hydrolase (FAAH) mRNA by reducing the translocation of ALKBH5 into the nucleus and promoted the degradation of FAAH in astrocytes with subsequent attenuation of depressive-like behaviors.129 Liu et al. reported that circZbtb20 was highly expressed in group 3 innate lymphoid cells (ILC3s) and was required for their function.130 circZbtb20 enhances the interaction of ALKBH5 with Nr4a1 mRNA, leading to a reduction in the m6A modification of Nr4a1 mRNA to improve its stability. Subsequently, Nr4a1 initiates Notch2 signaling activation to maintain ILC3 homeostasis.130 In NSCLC, circNOTCH1 was upregulated and competed with NOTCH1 mRNA for METTL14 binding, causing decreased m6A methylation on NOTCH1 mRNA and elevating its expression by maintaining stability.131 circPTPRA is an important tumor suppressor in bladder cancer that abolishes the promotion of cell proliferation and metastasis induced by IGF2BP1. Ectopic expression of circPTPRA could downregulate the stability of FSCN1 and MYC mRNA through interaction with KH domains of IGF2BP1 and blocking its recognition of downstream m6A-modified mRNAs.132 In bladder cancer, circ0008399 bound with WTAP to promote the formation of the WTAP/METTL3/METTL14 m6A methyltransferase complex, which upregulated the expression of tumor necrosis factor-α (TNF-α)-induced protein 3 (TNFAIP3) by increasing its mRNA stability in an m6A-dependent manner.133 However, circRNAs directly regulate the expression of m6A regulators to modulate m6A levels. circRAB11FIP1, overexpressed in ovarian cancer, directly bound to FTO mRNA and promoted its expression, which decreased the m6A levels of ATG5 and ATG7 mRNA and altered their expression.134 In addition, circRNA could act as an miRNAs sponge to indirectly modulate m6A regulators and methylation. For instance, circ_0072083 interacts with miR-1252-5p to upregulate demethylase ALKBH5 expression, which increases NANOG mRNA stability by reducing m6A levels of NANOG mRNA, leading to enhanced temozolomide resistance in glioma.135 Chi et al. confirmed that circMAP2K4 could act as miR-139-5p sponge to upregulate the expression and activity of YTHDF1 and promote HCC proliferation.136 These findings enrich our understanding of the roles of RNA epigenetic regulation patterns.
Table 3.
Roles of circRNAs in the regulation of m6A modification
| Role mode | circRNA | m6A regulators | m6A levels | Function | Diseases | References |
|---|---|---|---|---|---|---|
| Combined with m6A regulators | circSTAG1 | ALKBH5 | increase | promote the degradation of FAAH | depressive disorder | Huang et al.129 |
| circZbtb20 | ALKBH5 | decrease | improve Nr4a1 stability | ILC3 homeostasis | Liu et al.130 | |
| circNOTCH1 | METTL14 | decrease | maintain NOTCH1 stability | NSCLC | Shen et al.131 | |
| circPTPRA | IGF2BP1 | block recognition of m6A | downregulate the stability of FSCN1 and MYC | bladder cancer | Xie et al.132 | |
| circ0008399 | WTAP | increase | upregulate TNFAIP3 expression | bladder cancer | Wei et al.133 | |
| Directly regulate m6A regulators | circRAB11FIP1 | FTO | decrease | alter ATG5 and ATG7 expression | ovarian cancer | Zhang et al.134 |
| Indirectly regulate m6A regulators | circ_0072083 | ALKBH5 | decrease | increase NANOG stability | glioma | Ding et al.135 |
| circMAP2K4 | YTHDF1 | affect recognition of m6A | – | HCC | Chi et al.136 |
Limitations of m6A-modified circRNAs
Although current research on m6A-modified RNAs is in full swing, our understanding is only beginning, especially regarding m6A-modified circRNAs. There are many technical obstacles and challenges in studying circRNA-specific m6A modifications. First, it is difficult and complicated to accurately detect the m6A levels of specific circRNAs in disease-specific tissue samples and body fluids due to their low abundance, which hinders their clinical application. Second, the detection methods must be improved to precisely identify the specific sites of m6A-modified circRNAs, which is beneficial in exploring their regulatory mechanisms and biological functions in diseases. Third, m6A modification mediates the different roles of endogenous and exogenous circRNAs in immune signaling. More advanced technologies need to be developed to apply m6A-modified circRNAs to the immunotherapy of diseases.
Conclusions and prospects
In this review, we comprehensively summarized the characteristics and biological functions of m6A and circRNAs and focused on recent advances in the interactive effects of m6A modification and circRNAs, which provides a new perspective for understanding the complex regulatory network between RNA transcriptomics and epigenetic modifications.
Following the rapid development of biological methods and informatics technologies, numerous circRNAs have been identified to stably exist in body fluids, such as serum, plasma, and urine.137 Due to their high stability and conservation, circRNAs are ideal candidates for liquid biopsy biomarkers in many human diseases, such as cancers, autoimmune diseases, and cardiovascular diseases.138 The specific circRNAs activated by m6A present differential expression in special tissues and developmental stages. It is possible that more m6A-regulated circRNAs will be developed as biomarkers in body fluids. However, the technical problem of detecting m6A levels of specific circRNAs or measuring the content of circRNAs with low abundance remains to be solved.
In addition, given that circRNAs are usually expressed in a tissue- or cell-type-specific manner, they are considered potentially effective therapeutic targets. Recently, several tools that can effectively target circRNAs have been developed. Synthetic circRNAs that enhance circRNA expression in vitro could be delivered to target cells. However, these foreign circRNAs can also induce immune system activation in vivo.106 Strategies that introduce chemical modifications such as m6A modification have been explored to reduce synthetic circRNA immunogenicity.139 Therefore, m6A-modified circRNAs further broaden their clinical applications.
Accumulating studies have reported that m6A and its regulators serve as new biomarkers for diagnosis and prognosis in various diseases. These core members of m6A methylation usually exert important functions in many biological processes and represent effective therapeutic targets. Recently, m6A inhibitors have been developed and widely applied in clinical treatment, including the FTO inhibitors MO-I-500, R-2-hydroxyglutarate (R-2HG), FB23-2, FTO-04, meclofenamic acid (MA), and the ALKBH5 inhibitor MV1035. However, few inhibitors targeting m6A writers and readers have been reported to date, which may be a research direction for future studies. In addition, circRNAs could affect m6A levels by competitively binding to or directly targeting m6A regulatory proteins. The interactions between m6A and circRNAs provide novel insights into the mechanisms controlling physiological and pathological processes and promote the identification of critical targets for the diagnosis and treatment of diseases.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (grant nos. 81972806, 82073288, and 81802093), the Nanjing Medical and Health Scientific Research Project (grant no. YKK21137), the Jiangsu Provincial Key Research and Development Plan (grant no. BE2019614), the Elderly Health Research Project of Jiangsu Province (grant no. LR2021017), and the Specialized Cohort Research Project of Nanjing Medical University (NMUC2020035).
Author contributions
T.X., B.H., and H.S. conceived the study, drafted the manuscript, and drew the diagrams. M.X. and J.N. screened the literature and created the tables. S.W. and Y.P. performed the quality assessment, supervised the study, and revised the manuscript. All of the authors approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Shukui Wang, Email: sk_wang@njmu.edu.cn.
Yuqin Pan, Email: panyuqin01@163.com.
References
- 1.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao B.S., Roundtree I.A., He C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helm M., Motorin Y. Detecting RNA modifications in the epitranscriptome: predict and validate. Nat. Rev. Genet. 2017;18:275–291. doi: 10.1038/nrg.2016.169. [DOI] [PubMed] [Google Scholar]
- 4.Song P., Tayier S., Cai Z., Jia G. RNA methylation in mammalian development and cancer. Cell Biol. Toxicol. 2021;37:811–831. doi: 10.1007/s10565-021-09627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Y., Dominissini D., Rechavi G., He C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat. Rev. Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y., Hsu P.J., Chen Y.S., Yang Y.G. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–624. doi: 10.1038/s41422-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X., Wu R., Liu Y., Zhao Y., Bi Z., Yao Y., Liu Q., Shi H., Wang F., Wang Y. m(6)A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy. 2020;16:1221–1235. doi: 10.1080/15548627.2019.1659617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge L., Zhang N., Chen Z., Song J., Wu Y., Li Z., Chen F., Wu J., Li D., Li J., et al. Level of N6-methyladenosine in peripheral blood RNA: a novel predictive biomarker for gastric cancer. Clin. Chem. 2020;66:342–351. doi: 10.1093/clinchem/hvz004. [DOI] [PubMed] [Google Scholar]
- 9.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., Cesarkas K., Jacob-Hirsch J., Amariglio N., Kupiec M., et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 10.Deng X., Su R., Weng H., Huang H., Li Z., Chen J. RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507–517. doi: 10.1038/s41422-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer K.D., Patil D.P., Zhou J., Zinoviev A., Skabkin M.A., Elemento O., Pestova T.V., Qian S.B., Jaffrey S.R. 5' UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X., Xu Y., Qian Z., Zheng W., Wu Q., Chen Y., Zhu G., Liu Y., Bian Z., Xu W., et al. circRNA_104075 stimulates YAP-dependent tumorigenesis through the regulation of HNF4a and may serve as a diagnostic marker in hepatocellular carcinoma. Cell Death Dis. 2018;9:1091. doi: 10.1038/s41419-018-1132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng J., Xu L., Deng L., Xue L., Meng Q., Wei F., Wang J. RNA N(6)-methyladenosine modification is required for miR-98/MYCN axis-mediated inhibition of neuroblastoma progression. Sci. Rep. 2020;10:13624. doi: 10.1038/s41598-020-64682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vo J.N., Cieslik M., Zhang Y., Shukla S., Xiao L., Zhang Y., Wu Y.M., Dhanasekaran S.M., Engelke C.G., Cao X., et al. The landscape of circular RNA in cancer. Cell. 2019;176:869–881.e13. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Yang L., Chen L.L. The biogenesis, functions, and challenges of circular RNAs. Mol. Cell. 2018;71:428–442. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Patop I.L., Wust S., Kadener S. Past, present, and future of circRNAs. EMBO J. 2019;38:e100836. doi: 10.15252/embj.2018100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baassiri A., Nassar F., Mukherji D., Shamseddine A., Nasr R., Temraz S. Exosomal non coding RNA in LIQUID biopsies as a promising biomarker for colorectal cancer. Int. J. Mol. Sci. 2020;21:1398. doi: 10.3390/ijms21041398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y., Fan X., Mao M., Song X., Wu P., Zhang Y., Jin Y., Yang Y., Chen L.L., Wang Y., et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27:626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou C., Molinie B., Daneshvar K., Pondick J.V., Wang J., Van Wittenberghe N., Xing Y., Giallourakis C.C., Mullen A.C. Genome-wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20:2262–2276. doi: 10.1016/j.celrep.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Feng J., Xue Y., Guan Z., Zhang D., Liu Z., Gong Z., Wang Q., Huang J., Tang C., et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 21.Wang P., Doxtader K.A., Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol. Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ping X.L., Sun B.F., Wang L., Xiao W., Yang X., Wang W.J., Adhikari S., Shi Y., Lv Y., Chen Y.S., et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue Y., Liu J., Cui X., Cao J., Luo G., Zhang Z., Cheng T., Gao M., Shu X., Ma H., et al. VIRMA mediates preferential m(6)A mRNA methylation in 3'UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10. doi: 10.1038/s41421-018-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knuckles P., Lence T., Haussmann I.U., Jacob D., Kreim N., Carl S.H., Masiello I., Hares T., Villasenor R., Hess D., et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–429. doi: 10.1101/gad.309146.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patil D.P., Chen C.K., Pickering B.F., Chow A., Jackson C., Guttman M., Jaffrey S.R. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–373. doi: 10.1038/nature19342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen J., Lv R., Ma H., Shen H., He C., Wang J., Jiao F., Liu H., Yang P., Tan L., et al. Zc3h13 regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol. Cell. 2018;69:1028–1038.e6. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma H., Wang X., Cai J., Dai Q., Natchiar S.K., Lv R., Chen K., Lu Z., Chen H., Shi Y.G., et al. N(6-)Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat. Chem. Biol. 2019;15:88–94. doi: 10.1038/s41589-018-0184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warda A.S., Kretschmer J., Hackert P., Lenz C., Urlaub H., Hobartner C., Sloan K.E., Bohnsack M.T. Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18:2004–2014. doi: 10.15252/embr.201744940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Tran N., Ernst F.G.M., Hawley B.R., Zorbas C., Ulryck N., Hackert P., Bohnsack K.E., Bohnsack M.T., Jaffrey S.R., Graille M., et al. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 2019;47:7719–7733. doi: 10.1093/nar/gkz619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., Yi C., Lindahl T., Pan T., Yang Y.G., et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J., Liu F., Lu Z., Fei Q., Ai Y., He P.C., Shi H., Cui X., Su R., Klungland A., et al. Differential m(6)A, m(6)Am, and m(1)A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol. Cell. 2018;71:973–985.e5. doi: 10.1016/j.molcel.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.M., Li C.J., Vagbo C.B., Shi Y., Wang W.L., Song S.H., et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda Y., Ooshio I., Fusamae Y., Kitae K., Kawaguchi M., Jingushi K., Hase H., Harada K., Hirata K., Tsujikawa K. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci. Rep. 2017;7:42271. doi: 10.1038/srep42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z., Qi M., Shen B., Luo G., Wu Y., Li J., Lu Z., Zheng Z., Dai Q., Wang H. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–2545. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao W., Adhikari S., Dahal U., Chen Y.S., Hao Y.J., Sun B.F., Sun H.Y., Li A., Ping X.L., Lai W.Y., et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol. Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Roundtree I.A., Luo G.Z., Zhang Z., Wang X., Zhou T., Cui Y., Sha J., Huang X., Guerrero L., Xie P., et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 2017;6:e31311. doi: 10.7554/eLife.31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanabe A., Tanikawa K., Tsunetomi M., Takai K., Ikeda H., Konno J., Torigoe T., Maeda H., Kutomi G., Okita K., et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1alpha mRNA is translated. Cancer Lett. 2016;376:34–42. doi: 10.1016/j.canlet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Mao Y., Dong L., Liu X.M., Guo J., Ma H., Shen B., Qian S.B. m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat. Commun. 2019;10:5332. doi: 10.1038/s41467-019-13317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu P.J., Zhu Y., Ma H., Guo Y., Shi X., Liu Y., Qi M., Lu Z., Shi H., Wang J., et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–1127. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X., Zhao B.S., Roundtree I.A., Lu Z., Han D., Ma H., Weng X., Chen K., Shi H., He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X., Lu Z., Gomez A., Hon G.C., Yue Y., Han D., Fu Y., Parisien M., Dai Q., Jia G., et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batista P.J., Molinie B., Wang J., Qu K., Zhang J., Li L., Bouley D.M., Lujan E., Haddad B., Daneshvar K., et al. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geula S., Moshitch-Moshkovitz S., Dominissini D., Mansour A.A., Kol N., Salmon-Divon M., Hershkovitz V., Peer E., Mor N., Manor Y.S., et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 44.Shi H., Wang X., Lu Z., Zhao B.S., Ma H., Hsu P.J., Liu C., He C. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alarcon C.R., Goodarzi H., Lee H., Liu X., Tavazoie S., Tavazoie S.F. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu N., Dai Q., Zheng G., He C., Parisien M., Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu N., Zhou K.I., Parisien M., Dai Q., Diatchenko L., Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–6063. doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H., Weng H., Sun W., Qin X., Shi H., Wu H., Zhao B.S., Mesquita A., Liu C., Yuan C.L., et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dina C., Meyre D., Gallina S., Durand E., Korner A., Jacobson P., Carlsson L.M., Kiess W., Vatin V., Lecoeur C., et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 50.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., Perry J.R., Elliott K.S., Lango H., Rayner N.W., et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z., Weng H., Su R., Weng X., Zuo Z., Li C., Huang H., Nachtergaele S., Dong L., Hu C., et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mauer J., Luo X., Blanjoie A., Jiao X., Grozhik A.V., Patil D.P., Linder B., Pickering B.F., Vasseur J.J., Chen Q., et al. Reversible methylation of m(6)Am in the 5' cap controls mRNA stability. Nature. 2017;541:371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C., Samanta D., Lu H., Bullen J.W., Zhang H., Chen I., He X., Semenza G.L. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc. Natl. Acad. Sci. U S A. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S., Zhao B.S., Zhou A., Lin K., Zheng S., Lu Z., Chen Y., Sulman E.P., Xie K., Bogler O., et al. m(6)A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606.e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X.O., Wang H.B., Zhang Y., Lu X., Chen L.L., Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Chen I., Chen C.Y., Chuang T.J. Biogenesis, identification, and function of exonic circular RNAs. Wiley Interdiscip. Rev. RNA. 2015;6:563–579. doi: 10.1002/wrna.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y., Zhang X.O., Chen T., Xiang J.F., Yin Q.F., Xing Y.H., Zhu S., Yang L., Chen L.L. Circular intronic long noncoding RNAs. Mol. Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 58.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 59.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guarnerio J., Bezzi M., Jeong J.C., Paffenholz S.V., Berry K., Naldini M.M., Lo-Coco F., Tay Y., Beck A.H., Pandolfi P.P. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 61.Tan S., Sun D., Pu W., Gou Q., Guo C., Gong Y., Li J., Wei Y.Q., Liu L., Zhao Y., et al. Circular RNA F-circEA-2a derived from EML4-ALK fusion gene promotes cell migration and invasion in non-small cell lung cancer. Mol. Cancer. 2018;17:138. doi: 10.1186/s12943-018-0887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu K., Liao X., Gong Y., He J., Zhou J.K., Tan S., Pu W., Huang C., Wei Y.Q., Peng Y. Circular RNA F-circSR derived from SLC34A2-ROS1 fusion gene promotes cell migration in non-small cell lung cancer. Mol. Cancer. 2019;18:98. doi: 10.1186/s12943-019-1028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang D., Tatomer D.C., Luo Z., Wu H., Yang L., Chen L.L., Cherry S., Wilusz J.E. The output of protein-coding genes shifts to circular RNAs when the pre-mRNA processing machinery is limiting. Mol. Cell. 2017;68:940–954.e3. doi: 10.1016/j.molcel.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X., Wang X., Li J., Hu S., Deng Y., Yin H., Bao X., Zhang Q.C., Wang G., Wang B., et al. Identification of mecciRNAs and their roles in the mitochondrial entry of proteins. Sci. China Life Sci. 2020;63:1429–1449. doi: 10.1007/s11427-020-1631-9. [DOI] [PubMed] [Google Scholar]
- 65.Glazar P., Papavasileiou P., Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20:1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lasda E., Parker R. Circular RNAs: diversity of form and function. RNA. 2014;20:1829–1842. doi: 10.1261/rna.047126.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salzman J., Gawad C., Wang P.L., Lacayo N., Brown P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki H., Zuo Y., Wang J., Zhang M.Q., Malhotra A., Mayeda A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006;34:e63. doi: 10.1093/nar/gkl151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzuki H., Tsukahara T. A view of pre-mRNA splicing from RNase R resistant RNAs. Int. J. Mol. Sci. 2014;15:9331–9342. doi: 10.3390/ijms15069331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Enuka Y., Lauriola M., Feldman M.E., Sas-Chen A., Ulitsky I., Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44:1370–1383. doi: 10.1093/nar/gkv1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansen T.B., Wiklund E.D., Bramsen J.B., Villadsen S.B., Statham A.L., Clark S.J., Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park O.H., Ha H., Lee Y., Boo S.H., Kwon D.H., Song H.K., Kim Y.K. Endoribonucleolytic cleavage of m(6)A-containing RNAs by RNase P/MRP complex. Mol. Cell. 2019;74:494–507.e8. doi: 10.1016/j.molcel.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 74.Wang P.L., Bao Y., Yee M.C., Barrett S.P., Hogan G.J., Olsen M.N., Dinneny J.R., Brown P.O., Salzman J. Circular RNA is expressed across the eukaryotic tree of life. PLoS One. 2014;9:e90859. doi: 10.1371/journal.pone.0090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salzman J., Chen R.E., Olsen M.N., Wang P.L., Brown P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veno M.T., Hansen T.B., Veno S.T., Clausen B.H., Grebing M., Finsen B., Holm I.E., Kjems J. Spatio-temporal regulation of circular RNA expression during porcine embryonic brain development. Genome Biol. 2015;16:245. doi: 10.1186/s13059-015-0801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen L.L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 2016;17:205–211. doi: 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 78.Starke S., Jost I., Rossbach O., Schneider T., Schreiner S., Hung L.H., Bindereif A. Exon circularization requires canonical splice signals. Cell Rep. 2015;10:103–111. doi: 10.1016/j.celrep.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Liang D., Wilusz J.E. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014;28:2233–2247. doi: 10.1101/gad.251926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X.O., Dong R., Zhang Y., Zhang J.L., Luo Z., Zhang J., Chen L.L., Yang L. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016;26:1277–1287. doi: 10.1101/gr.202895.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ashwal-Fluss R., Meyer M., Pamudurti N.R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 82.Kelly S., Greenman C., Cook P.R., Papantonis A. Exon skipping is correlated with exon circularization. J. Mol. Biol. 2015;427:2414–2417. doi: 10.1016/j.jmb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 83.Conn S.J., Pillman K.A., Toubia J., Conn V.M., Salmanidis M., Phillips C.A., Roslan S., Schreiber A.W., Gregory P.A., Goodall G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 84.Li X., Liu C.X., Xue W., Zhang Y., Jiang S., Yin Q.F., Wei J., Yao R.W., Yang L., Chen L.L. Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol. Cell. 2017;67:214–227.e7. doi: 10.1016/j.molcel.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 85.Aktas T., Avsar Ilik I., Maticzka D., Bhardwaj V., Pessoa Rodrigues C., Mittler G., Manke T., Backofen R., Akhtar A. DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome. Nature. 2017;544:115–119. doi: 10.1038/nature21715. [DOI] [PubMed] [Google Scholar]
- 86.Ivanov A., Memczak S., Wyler E., Torti F., Porath H.T., Orejuela M.R., Piechotta M., Levanon E.Y., Landthaler M., Dieterich C., et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 87.Rybak-Wolf A., Stottmeister C., Glazar P., Jens M., Pino N., Giusti S., Hanan M., Behm M., Bartok O., Ashwal-Fluss R., et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 88.Tang C., Xie Y., Yu T., Liu N., Wang Z., Woolsey R.J., Tang Y., Zhang X., Qin W., Zhang Y., et al. m(6)A-dependent biogenesis of circular RNAs in male germ cells. Cell Res. 2020;30:211–228. doi: 10.1038/s41422-020-0279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bosson A.D., Zamudio J.R., Sharp P.A. Endogenous miRNA and target concentrations determine susceptibility to potential ceRNA competition. Mol. Cell. 2014;56:347–359. doi: 10.1016/j.molcel.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Denzler R., Agarwal V., Stefano J., Bartel D.P., Stoffel M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell. 2014;54:766–776. doi: 10.1016/j.molcel.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H., Yang F., Hu A., Wang X., Fang E., Chen Y., Li D., Song H., Wang J., Guo Y., et al. Therapeutic targeting of circ-CUX1/EWSR1/MAZ axis inhibits glycolysis and neuroblastoma progression. EMBO Mol. Med. 2019;11:e10835. doi: 10.15252/emmm.201910835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abe N., Matsumoto K., Nishihara M., Nakano Y., Shibata A., Maruyama H., Shuto S., Matsuda A., Yoshida M., Ito Y., et al. Rolling circle translation of circular RNA in living human cells. Sci. Rep. 2015;5:16435. doi: 10.1038/srep16435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Heesch S., Witte F., Schneider-Lunitz V., Schulz J.F., Adami E., Faber A.B., Kirchner M., Maatz H., Blachut S., Sandmann C.L., et al. The translational landscape of the human heart. Cell. 2019;178:242–260.e29. doi: 10.1016/j.cell.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 94.Kong S., Tao M., Shen X., Ju S. Translatable circRNAs and lncRNAs: driving mechanisms and functions of their translation products. Cancer Lett. 2020;483:59–65. doi: 10.1016/j.canlet.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 95.Chen S., Huang V., Xu X., Livingstone J., Soares F., Jeon J., Zeng Y., Hua J.T., Petricca J., Guo H., et al. Widespread and functional RNA circularization in localized prostate cancer. Cell. 2019;176:831–843.e22. doi: 10.1016/j.cell.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 96.Wu N., Yuan Z., Du K.Y., Fang L., Lyu J., Zhang C., He A., Eshaghi E., Zeng K., Ma J., et al. Translation of yes-associated protein (YAP) was antagonized by its circular RNA via suppressing the assembly of the translation initiation machinery. Cell Death Differ. 2019;26:2758–2773. doi: 10.1038/s41418-019-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gong L.P., Chen J.N., Dong M., Xiao Z.D., Feng Z.Y., Pan Y.H., Zhang Y., Du Y., Zhang J.Y., Bi Y.H., et al. Epstein-Barr virus-derived circular RNA LMP2A induces stemness in EBV-associated gastric cancer. EMBO Rep. 2020;21:e49689. doi: 10.15252/embr.201949689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piwecka M., Glazar P., Hernandez-Miranda L.R., Memczak S., Wolf S.A., Rybak-Wolf A., Filipchyk A., Klironomos F., Cerda Jara C.A., Fenske P., et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science. 2017;357:eaam8526. doi: 10.1126/science.aam8526. [DOI] [PubMed] [Google Scholar]
- 99.Garikipati V.N.S., Verma S.K., Cheng Z., Liang D., Truongcao M.M., Cimini M., Yue Y., Huang G., Wang C., Benedict C., et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat. Commun. 2019;10:4317. doi: 10.1038/s41467-019-11777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arcinas C., Tan W., Fang W., Desai T.P., Teh D.C.S., Degirmenci U., Xu D., Foo R., Sun L. Adipose circular RNAs exhibit dynamic regulation in obesity and functional role in adipogenesis. Nat. Metab. 2019;1:688–703. doi: 10.1038/s42255-019-0078-z. [DOI] [PubMed] [Google Scholar]
- 101.Nicolet B.P., Engels S., Aglialoro F., van den Akker E., von Lindern M., Wolkers M.C. Circular RNA expression in human hematopoietic cells is widespread and cell-type specific. Nucleic Acids Res. 2018;46:8168–8180. doi: 10.1093/nar/gky721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen X., Ouyang Z., Shen Y., Liu B., Zhang Q., Wan L., Yin Z., Zhu W., Li S., Peng D. CircRNA_28313/miR-195a/CSF1 axis modulates osteoclast differentiation to affect OVX-induced bone absorption in mice. RNA Biol. 2019;16:1249–1262. doi: 10.1080/15476286.2019.1624470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paramasivam A., Vijayashree Priyadharsini J. Novel insights into m6A modification in circular RNA and implications for immunity. Cell Mol. Immunol. 2020;17:668–669. doi: 10.1038/s41423-020-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harashima A., Guettouche T., Barber G.N. Phosphorylation of the NFAR proteins by the dsRNA-dependent protein kinase PKR constitutes a novel mechanism of translational regulation and cellular defense. Genes Dev. 2010;24:2640–2653. doi: 10.1101/gad.1965010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu C.X., Li X., Nan F., Jiang S., Gao X., Guo S.K., Xue W., Cui Y., Dong K., Ding H., et al. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell. 2019;177:865–880.e21. doi: 10.1016/j.cell.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 106.Chen Y.G., Kim M.V., Chen X., Batista P.J., Aoyama S., Wilusz J.E., Iwasaki A., Chang H.Y. Sensing self and foreign circular RNAs by intron identity. Mol. Cell. 2017;67:228–238.e5. doi: 10.1016/j.molcel.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Di Timoteo G., Dattilo D., Centron-Broco A., Colantoni A., Guarnacci M., Rossi F., Incarnato D., Oliviero S., Fatica A., Morlando M., et al. Modulation of circRNA metabolism by m(6)A modification. Cell Rep. 2020;31:107641. doi: 10.1016/j.celrep.2020.107641. [DOI] [PubMed] [Google Scholar]
- 108.Guo M., Yan R., Ji Q., Yao H., Sun M., Duan L., Xue Z., Jia Y. IFN regulatory Factor-1 induced macrophage pyroptosis by modulating m6A modification of circ_0029589 in patients with acute coronary syndrome. Int. Immunopharmacol. 2020;86:106800. doi: 10.1016/j.intimp.2020.106800. [DOI] [PubMed] [Google Scholar]
- 109.Rao X., Lai L., Li X., Wang L., Li A., Yang Q. N(6) -methyladenosine modification of circular RNA circ-ARL3 facilitates Hepatitis B virus-associated hepatocellular carcinoma via sponging miR-1305. IUBMB Life. 2021;73:408–417. doi: 10.1002/iub.2438. [DOI] [PubMed] [Google Scholar]
- 110.Chen C., Yuan W., Zhou Q., Shao B., Guo Y., Wang W., Yang S., Guo Y., Zhao L., Dang Q., et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11:4298–4315. doi: 10.7150/thno.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Z., Yang H.Y., Dai X.Y., Zhang X., Huang Y.Z., Shi L., Wei J.F., Ding Q. CircMETTL3, upregulated in a m6A-dependent manner, promotes breast cancer progression. Int. J. Biol. Sci. 2021;17:1178–1190. doi: 10.7150/ijbs.57783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rong D., Wu F., Lu C., Sun G., Shi X., Chen X., Dai Y., Zhong W., Hao X., Zhou J., et al. m6A modification of circHPS5 and hepatocellular carcinoma progression through HMGA2 expression. Mol. Ther. Nucleic Acids. 2021;26:637–648. doi: 10.1016/j.omtn.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu H., Lan T., Li H., Xu L., Chen X., Liao H., Chen X., Du J., Cai Y., Wang J., et al. Circular RNA circDLC1 inhibits MMP1-mediated liver cancer progression via interaction with HuR. Theranostics. 2021;11:1396–1411. doi: 10.7150/thno.53227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu J., Wan Z., Tang M., Lin Z., Jiang S., Ji L., Gorshkov K., Mao Q., Xia S., Cen D., et al. N(6)-methyladenosine-modified CircRNA-SORE sustains sorafenib resistance in hepatocellular carcinoma by regulating beta-catenin signaling. Mol. Cancer. 2020;19:163. doi: 10.1186/s12943-020-01281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu P., Fang X., Liu Y., Tang Y., Wang W., Li X., Fan Y. N6-methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis. 2021;12:298. doi: 10.1038/s41419-021-03558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Z., Ling K., Zhu Y., Deng L., Li Y., Liang Z. circ0000069 promotes cervical cancer cell proliferation and migration by inhibiting miR-4426. Biochem. Biophys. Res. Commun. 2021;551:114–120. doi: 10.1016/j.bbrc.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 117.Zhao J., Lee E.E., Kim J., Yang R., Chamseddin B., Ni C., Gusho E., Xie Y., Chiang C.M., Buszczak M., et al. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat. Commun. 2019;10:2300. doi: 10.1038/s41467-019-10246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y., Chen B., Zhao J., Li Q., Chen S., Guo T., Li Y., Lai H., Chen Z., Meng Z., et al. HNRNPL circularizes ARHGAP35 to produce an oncogenic protein. Adv. Sci. (Weinh). 2021;8:2001701. doi: 10.1002/advs.202001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Y.G., Chen R., Ahmad S., Verma R., Kasturi S.P., Amaya L., Broughton J.P., Kim J., Cadena C., Pulendran B., et al. N6-Methyladenosine modification controls circular RNA immunity. Mol. Cell. 2019;76:96–109.e9. doi: 10.1016/j.molcel.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen R.X., Chen X., Xia L.P., Zhang J.X., Pan Z.Z., Ma X.D., Han K., Chen J.W., Judde J.G., Deas O., et al. N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat. Commun. 2019;10:4695. doi: 10.1038/s41467-019-12651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X., Tian G., Wu J. Novel circGFRalpha1 promotes self-renewal of female germline stem cells mediated by m(6)A writer METTL14. Front. Cell Dev. Biol. 2021;9:640402. doi: 10.3389/fcell.2021.640402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li B., Zhu L., Lu C., Wang C., Wang H., Jin H., Ma X., Cheng Z., Yu C., Wang S., et al. circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat. Commun. 2021;12:295. doi: 10.1038/s41467-020-20527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tang C., Klukovich R., Peng H., Wang Z., Yu T., Zhang Y., Zheng H., Klungland A., Yan W. ALKBH5-dependent m6A demethylation controls splicing and stability of long 3'-UTR mRNAs in male germ cells. Proc. Natl. Acad. Sci. U S A. 2018;115:E325–E333. doi: 10.1073/pnas.1717794115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Su H., Wang G., Wu L., Ma X., Ying K., Zhang R. Transcriptome-wide map of m(6)A circRNAs identified in a rat model of hypoxia mediated pulmonary hypertension. BMC Genomics. 2020;21:39. doi: 10.1186/s12864-020-6462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li P., Yu H., Zhang G., Kang L., Qin B., Cao Y., Luo J., Chen X., Wang Y., Qin M., et al. Identification and characterization of N6-methyladenosine CircRNAs and methyltransferases in the lens epithelium cells from age-related cataract. Invest. Ophthalmol. Vis. Sci. 2020;61:13. doi: 10.1167/iovs.61.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen C.Y., Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- 127.Wang Y., Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21:172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan L., Chen Y.G. Circular RNAs in immune response and viral infection. Trends Biochem. Sci. 2020;45:1022–1034. doi: 10.1016/j.tibs.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang R., Zhang Y., Bai Y., Han B., Ju M., Chen B., Yang L., Wang Y., Zhang H., Zhang H., et al. N(6)-Methyladenosine modification of fatty acid amide hydrolase messenger RNA in circular RNA STAG1-regulated astrocyte dysfunction and depressive-like behaviors. Biol. Psychiatry. 2020;88:392–404. doi: 10.1016/j.biopsych.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 130.Liu B., Liu N., Zhu X., Yang L., Ye B., Li H., Zhu P., Lu T., Tian Y., Fan Z. Circular RNA circZbtb20 maintains ILC3 homeostasis and function via Alkbh5-dependent m(6)A demethylation of Nr4a1 mRNA. Cell Mol. Immunol. 2021;18:1412–1424. doi: 10.1038/s41423-021-00680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shen Y., Li C., Zhou L., Huang J.A. G protein-coupled oestrogen receptor promotes cell growth of non-small cell lung cancer cells via YAP1/QKI/circNOTCH1/m6A methylated NOTCH1 signalling. J. Cell Mol. Med. 2021;25:284–296. doi: 10.1111/jcmm.15997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xie F., Huang C., Liu F., Zhang H., Xiao X., Sun J., Zhang X., Jiang G. CircPTPRA blocks the recognition of RNA N(6)-methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression. Mol. Cancer. 2021;20:68. doi: 10.1186/s12943-021-01359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei W., Sun J., Zhang H., Xiao X., Huang C., Wang L., Zhong H., Jiang Y., Zhang X., Jiang G. Circ0008399 interaction with WTAP promotes assembly and activity of the m6A methyltransferase complex and promotes cisplatin resistance in bladder cancer. Cancer Res. 2021;81:6142–6156. doi: 10.1158/0008-5472.CAN-21-1518. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Z., Zhu H., Hu J. CircRAB11FIP1 promoted autophagy flux of ovarian cancer through DSC1 and miR-129. Cell Death Dis. 2021;12:219. doi: 10.1038/s41419-021-03486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ding C., Yi X., Chen X., Wu Z., You H., Chen X., Zhang G., Sun Y., Bu X., Wu X., et al. Warburg effect-promoted exosomal circ_0072083 releasing up-regulates NANGO expression through multiple pathways and enhances temozolomide resistance in glioma. J. Exp. Clin. Cancer Res. 2021;40:164. doi: 10.1186/s13046-021-01942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chi F., Cao Y., Chen Y. Analysis and validation of circRNA-miRNA network in regulating m(6)A RNA methylation modulators reveals CircMAP2K4/miR-139-5p/YTHDF1 axis involving the proliferation of hepatocellular carcinoma. Front. Oncol. 2021;11:560506. doi: 10.3389/fonc.2021.560506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang S., Zhang K., Tan S., Xin J., Yuan Q., Xu H., Xu X., Liang Q., Christiani D.C., Wang M., et al. Circular RNAs in body fluids as cancer biomarkers: the new frontier of liquid biopsies. Mol. Cancer. 2021;20:13. doi: 10.1186/s12943-020-01298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wen G., Zhou T., Gu W. The potential of using blood circular RNA as liquid biopsy biomarker for human diseases. Protein Cell. 2020;12:911–946. doi: 10.1007/s13238-020-00799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holdt L.M., Kohlmaier A., Teupser D. Circular RNAs as therapeutic agents and targets. Front. Physiol. 2018;9:1262. doi: 10.3389/fphys.2018.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]