Abstract
Objectives
The objective of this study was to verify the safety of policosanol supplementation for domestic cats. The effects of raw and encapsulated policosanol were compared with positive (L-carnitine) and negative (no supplementation) controls on outcomes of complete blood count, serum biochemistry, energy expenditure, respiratory quotient and physical activity in healthy young adult cats.
Methods
The study was a replicated 4 × 4 complete Latin square design. Eight cats (four castrated males, four spayed females; mean age 3.0 ± 1.0 years; mean weight 4.36 ± 1.08 kg; mean body condition score 5.4 ± 1.4) were blocked by sex and body weight then randomized to treatment groups: raw policosanol (10 mg/kg body weight), encapsulated policosanol (50 mg/kg body weight), L-carnitine (200 mg/kg body weight) or no supplementation. Treatments were supplemented to a basal diet for 28 days with a 1-week washout between periods. Food was distributed equally between two offerings to ensure complete supplement consumption (first offering) and measure consumption time (second offering). Blood collection (lipid profile, complete blood count, serum biochemistry) and indirect calorimetry (energy expenditure, respiratory quotient) were conducted at days 0, 14 and 28 of each period. Activity monitors were worn 7 days prior to indirect calorimetry and blood collection. Data were analyzed using a repeated measures mixed model (SAS, v.9.4).
Results
Food intake and body weight were similar among treatments. There was no effect of treatment on lipid profile, serum biochemistry, activity, energy expenditure or respiratory quotient (P >0.05); however, time to consume a second meal was greatest in cats fed raw policosanol (P <0.05).
Conclusions and relevance
These data suggest that policosanol is safe for feline consumption. Further studies with cats demonstrating cardiometabolic risk factors are warranted to confirm whether policosanol therapy is an efficacious treatment for hyperlipidemia and obesity.
Keywords: Energy expenditure, hyperlipidemia, obesity, overweight, respiratory quotient
Introduction
In 2017, it was estimated that 60% of cats in the United States were overweight or obese, and the prevalence continues to increase. 1 Being overweight or obese is the most prevalent nutrition-related ailment in domestic cats and is a risk factor for feline type 2 diabetes mellitus and hepatic lipidosis. 2 Overfeeding and inactivity are considered to be the primary contributors to weight gain, and can be accompanied by dyslipidemia, which is characterized in part by elevated triglycerides (TG) and total cholesterol (TC) concentrations. 3
Dietary supplements have been identified as one strategy to induce weight loss in overweight cats. Overweight cats receiving supplemental L-carnitine, an essential cofactor of fatty acid metabolism, had significantly greater energy expenditure (EE) and physical activity as well as a lower respiratory quotient (RQ), indicating greater lipid oxidation.4–6 Furthermore, L-carnitine may lessen weight gain in cats fed diets that exceed their energy requirements. 7 However, given that L-carnitine is responsible for transporting long chain fatty acids into mitochondria for energy metabolism and does not directly affect lipogenesis, 8 its effects on the treatment of feline dyslipidemia have not been investigated.
Policosanol is a dietary supplement composed of higher aliphatic alcohols, predominantly octacosanol, and is commonly extracted from sugar cane or beeswax. Both animal and human models have been used to investigate the effects of octacosanol in treating dyslipidemia via reducing TC and low-density lipoprotein cholesterol (LDL-C), and increasing high-density lipoprotein cholesterol (HDL-C) concentrations.9–13 Policosanol has been found to lower serum cholesterol in pigs, 14 rabbits, 11 rats 15 and humans.16–21 An improvement in lipid profile has been observed in healthy subjects, 16 patients with type II hypercholesterolemia9,11 and dyslipidemia associated with non-insulin dependent (type II) diabetes mellitus. 12 Though the exact mechanism by which policosanol acts is not entirely elucidated, it appears to exert its main effects on the LDL-C fraction, either through the action of inhibiting cholesterol synthesis, enhanced LDL catabolism or both.11,15 In addition, octacosanol has also been shown to increase voluntary physical activity in rats, due to its storage in the muscle for future use. 22 While there is an abundance of research supporting the benefits of policosanol supplementation on dyslipidemia, most of this research was performed by the research group that first developed the supplement. 23 Most external research has been unable to confirm the cholesterol-lowering effects of policosanol in animals24,25 or humans.26,27
To date, no studies have evaluated the safety and efficacy of policosanol supplementation in the domestic cat, whose lipid metabolism differs from herbivores and omnivores, as metabolically, cats tend to utilize more lipids than other mammals, even when fed high-carbohydrate diets. 28 In addition, for cats, lipids released during weight loss are commonly deposited in the liver and may results in hepatic lipidosis. 8 As such, research investigating alternative nutraceuticals to either mitigate weight gain or avoid hepatic lipidosis during weight loss by promoting increased EE and fat oxidation, such as L-carnitine, are needed. The demonstration that subjects have high tolerability and low incidence rate of adverse events with policosanol could make it a promising alternative or adjunctive therapy to L-carnitine. The objective of this study was therefore to investigate the effects of raw and encapsulated policosanol compared with positive (L-carnitine) and negative (no supplementation) controls on complete blood count (CBC), serum biochemistry, EE, RQ and voluntary physical activity in young domestic cats. We hypothesized that raw and encapsulated policosanol would be equivalent in all outcomes to L-carnitine and placebo in adult healthy cats.
Materials and methods
All procedures were approved by the University of Guelph’s Animal Care Committee (AUP #4015) and were carried out in accordance with the Animals for Research Act and Canadian Council on Animal Care. All cats were deemed healthy upon entering and throughout the study according to standard veterinarian evaluations of health.
Animals and housing
Eight domestic shorthair (n = 6) and longhair (n = 2) cats (four castrated males, four spayed females) with a mean age of 3.0 ± 1.0 years were enrolled in the study. Body condition score (BCS) ranged from 4–8 on a nine-point scale, representing both lean (n = 3) and overweight (n = 5) cats. At baseline (study start), the cats had a mean body weight (BW) of 4.36 ± 1.08 kg, and mean BCS of 5.4 ± 1.4. Measurements of BW as well as visual and physical (feeling of ribcage, spine and abdomen) assessment evaluations of BCS using the nine-point system 29 were performed weekly. For the duration of the study, all cats resided in the Animal Sciences and Nutrition Building at the University of Guelph and were housed in an indoor free-living environment, except for during calorimetry measurements and morning feedings. All cats had daily social interaction with previously familiarized humans for a maximum of 2 h each day that included petting, grooming and playing with restricted access toys. A 12:12 h light cycle that started at 07:00 h was implemented and the room temperature and relative humidity were maintained at 22 ± 1.5°C and 40–70%, respectively. Fresh distilled water was provided daily, and cats had ad libitum access via three large water dishes placed throughout the room.
Diets and treatments
Throughout the entire study period, cats were fed I19 Nutram Ideal Solution Support Skin, Coat and Stomach Cat Food (Elmira; Table 1). Cats were fed ad libitum for 4 weeks prior to the study to promote weight gain, and then for 2 weeks to ensure weight maintenance was achieved. Throughout the 4-week pre-study ad libitum feeding period, the mean BW increased by ~0.25 kg and mean BCS by 0.5. Weight gain was induced during this time to attempt to promote dyslipidemia, as the outcomes of dyslipidemia that are observed in obese humans, such as increases in TC, are similarly observed in obese cats. 3 During the study, cats were fed individually in individual cat condos (91.5 × 60 × 128 cm) once daily at 08:00 h. Following the ad libitum feeding phase, caloric provision was based on historical BW and metabolizable energy (ME) requirements of each individual cat. On average, the ME intake during the ad libitum feeding phase was approximately 15% greater than post-ad libitum.
Table 1.
Nutrient composition and energy density of control diet fed to cats (n = 8) undergoing acclimation
| Nutrient | Guaranteed analysis (as-fed) * |
|---|---|
| Metabolizable energy | 3645 kcal/kg |
| Crude protein | 31.0% |
| Crude fat | 14.0% |
| Crude fiber | 4.0% |
| Moisture | 10.0% |
Guaranteed analysis provided by Nutram (Elmira, ON, Canada); metabolizable energy is calculated; crude protein and crude fat are presented as minimum values; crude fiber and moisture are presented as maximum values
A 4 × 4 replicated complete Latin square design was used in which all cats received all treatments. Each treatment period for each cat consisted of 28 days. A 1-week washout period was implemented between each treatment period wherein all cats were fed the basal diet without supplementation. Treatments and doses were raw policosanol (RP; 10 mg/kg BW), encapsulated policosanol (POLI; 50 mg/kg BW), L-carnitine (carnitine tartrate) (LC; 200 mg/kg BW; positive control) and no supplementation (negative control). The dose of raw policosanol was chosen based on previous studies in healthy and non-healthy human and animal subjects.16,20,21 The policosanol was encapsulated at 20%; therefore, the dose of POLI was increased to 50 mg/kg BW to ensure an isodose relative to RP. The dose of L-carnitine was chosen based on supplemental levels that have been efficacious to promote greater energy expenditure and fat oxidation and reduced adipose accretion.4,5,7 As a note, the diet fed throughout the study period (Table 1) did not contain any L-carnitine supplementation. Cats were initially fed half their daily ration top dressed with each respective treatment and were fed their second half ration once the first half ration was consumed. Intakes were observed and timed to evaluate diet acceptability and indications of satiety. For the first half ration, kibble and treatment were soaked with 10 ml of distilled water to ensure homogeneity and complete consumption of the diet and treatments. The second half ration was only dried kibble. Cats were permitted 1 h to consume the entirety of their daily ration. Orts were then removed, weighed and recorded. Throughout the study period, any health changes or adverse events were recorded.
Energy expenditure
Indirect calorimetry was performed on days 0 (baseline), 4 and 28 of each study period). Cats were considered successfully acclimated to the indirect calorimetry chambers according to the 11-week acclimation procedure. 30 Cats were placed in the chambers at approximately 06:00 h and fed at 08:00 h. Cats remained in the chamber for approximately 23 h. Calorimetry chambers had an open-circuit, flow-through design. The rate of air flow into the chambers ranged from 5–8 l/min and was adjusted for the individual cat. Chamber air was dried using Drierite (Xenia), and dried again with a smaller Drierite prior to use of gas analyzers. Calorimetry data were collected using Qubit calorimetry software (Qubit C950-MCGES; Qubit Systems). Data collection occurred in 5-min intervals, every 25 mins. For each interval, the last 3 mins of data was averaged. For fasting measures, three intervals were completed before feeding, cats were fed, and gas was sampled for 10–12 more intervals. Carbon dioxide (CO2) and oxygen (O2) sensors were recalibrated before data collection, every 8 h, and when reference gas and atmospheric pressure values varied by >5%. EE was calculated using the abbreviated Weir equation 31 and RQ was calculated using the ratio of CO2 production to O2 consumption.
Blood collection
Fasted (22 h postprandial) blood samples (5 ml/cat) were collected in each study period on days 0, 14 and 28. Blood samples were taken prior to the morning feeding (07:00 h) by jugular venipuncture into vacutainers (Fisher Scientific). Samples were immediately submitted to the University of Guelph’s Animal Health Laboratory for analysis. Whole blood samples analyzed for hematological indices (CBC) using a Siemens hematology analyzer (Siemens Healthcare). Serum was isolated by centrifugation (2000 g for 10 mins) and used to measure serum biochemical components using a Cobas biochemistry analyzer (Roche Diagnostics). LDL-C was quantified as the difference between TC and HDL-C concentrations. Fatty acid profiles were measured by gas chromatography and mass spectrometry at Lipid Analytical Laboratories.
Activity measurement
Voluntary physical activity of the cats was measured using previously validated 32 ActiCal activity monitors that use an omni-directional accelerometer. The monitors were attached to harnesses placed on the cats parallel to the ribs. Activity levels were measured for 7 consecutive days for three intervals during each period: days −7 to 0, 7–14 and 21–28. Activity monitors were removed prior to indirect calorimetry and blood collection.
Statistics
With regard to sample size, previous studies include 8–10 cats per treatment and resulted in adequate statistical power.3-5,7 Based on these reports, the sample size of eight was determined to be adequate to detect differences with 80% power and 95% confidence. In addition, a crossover design was used to ensure less variability within and between cats, and so that each cat would serve as its own control, therefore reducing the number of cats needed for this study.
All data were analyzed using a repeated measures mixed model (SAS v.9.4; SAS Institute). Cat was considered a random effect, and treatment, period, time and treatment × time interaction were considered fixed effects. If a fixed effect was not significant, it was removed from the model. Day was treated as a repeated measure. When treatment or treatment × time interaction was significant, means were separated using pdiff. Results were considered statistically significant at P <0.05.
Results
Body weight and food intake
All cats remained healthy throughout the study. There were no changes in BW or BCS from baseline to the study end, and no effect of day or period. Treatments had no effect on BCS or food intake (P >0.10, data not shown). The time to consume the second food allotment was not affected by day or period (P >0.10); however, cats consuming the RP diet took significantly more time than for CON, LC or POLI diets (P <0.05; Figure 1).
Figure 1.
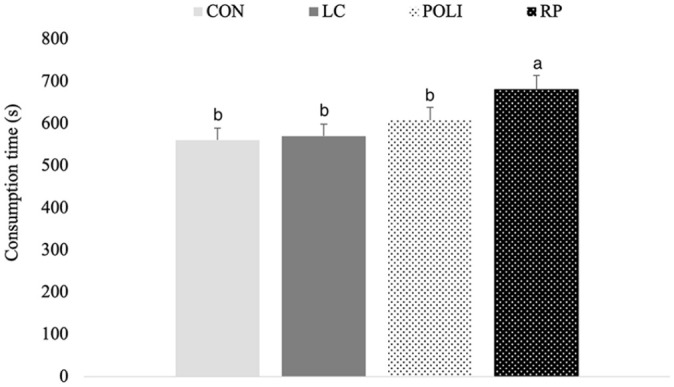
Consumption time of second ration (mean ± SEM) in cats (n = 8) fed CON, +LC, +POLI or +RP diets. CON = control diet with no supplementation; +LC = control diet supplemented with L-carnitine; +POLI = control diet supplemented with encapsulated policosanol; +RP = control diet supplemented with raw policosanol. a–bMean values with different superscripts are significantly different (P <0.05)
Lipid profile and blood parameters
Treatment, day nor period affected endpoint lipid concentrations, or percentage change from baseline (P >0.10, Table 2). Treatment had no effect on hematological indices or serum biochemistry values (P >0.10; data not shown) and all values fell within standard reference intervals as determined by the Animal Health Laboratory. Period affected mean cell volume, mean cell hemoglobin, red cell distribution width, calcium, phosphorus, potassium and urea (P <0.05; Table 3); however, all were within normal reference intervals.
Table 2.
Serum lipid profile (mean ± SEM, or ratios) in cats (n = 8) fed CON, +LC, +POLI or +RP diets* at days 0, 14 and 28
| CON | +LC | +POLI | +RP | SEM | P value | ||
|---|---|---|---|---|---|---|---|
| TC (mmol/l) | Day 0 | 3.20 | 3.36 | 3.00 | 3.12 | 0.33 | 0.909 |
| Day 14 | 3.10 | 2.84 | 2.97 | 3.07 | 0.36 | 0.926 | |
| Day 28 | 2.96 | 3.18 | 3.19 | 3.04 | 0.33 | 0.936 | |
| P value | 0.824 | 0.607 | 0.861 | 0.981 | |||
| LDL-C (mmol/l) | Day 0 | 0.46 | 0.43 | 0.41 | 0.39 | 0.07 | 0.876 |
| Day 14 | 0.42 | 0.35 | 0.35 | 0.37 | 0.08 | 0.899 | |
| Day 28 | 0.37 | 0.35 | 0.39 | 0.35 | 0.07 | 0.945 | |
| P value | 0.645 | 0.614 | 0.861 | 0.889 | |||
| HDL-C (mmol/l) | Day 0 | 2.74 | 2.93 | 2.59 | 2.73 | 0.28 | 0.892 |
| Day 14 | 2.68 | 2.49 | 2.62 | 2.70 | 0.31 | 0.937 | |
| Day 28 | 2.59 | 2.83 | 2.80 | 2.70 | 0.28 | 0.916 | |
| P value | 0.895 | 0.632 | 0.832 | 0.994 | |||
| TG (mmol/l) | Day 0 | 0.40 | 0.47 | 0.47 | 0.46 | 0.31 | 0.939 |
| Day 14 | 0.46 | 0.40 | 0.41 | 0.44 | 0.21 | 0.888 | |
| Day 28 | 0.43 | 0.42 | 0.43 | 0.38 | 0.18 | 0.859 | |
| P value | 0.793 | 0.847 | 0.869 | 0.658 | |||
| TC:HDL-C | Day 0 | 3.1:2.7 | 3.4:2.9 | 1.5:1.3 | 3.0:2.7 | 0.614 | |
| Day 14 | 3.0:2.7 | 1.5:1.2 | 1.5:1.3 | 1.5:1.3 | 0.543 | ||
| Day 28 | 1.5:1.3 | 3.2:2.8 | 3.2:2.8 | 1.5:1.3 | 0.885 | ||
| P value | 0.473 | 0.696 | 0.353 | 0.782 | |||
| LDL-C:HDL-C | Day 0 | 0.23:1.4 | 0.22:1.5 | 0.20:1.3 | 0.20:1.4 | 0.614 | |
| Day 14 | 0.21:1.3 | 0.18:1.2 | 0.17:1.3 | 0.19:1.3 | 0.542 | ||
| Day 28 | 0.18:1.3 | 0.17:1.4 | 0.19:1.4 | 0.17:1.3 | 0.885 | ||
| P value | 0.473 | 0.696 | 0.353 | 0.775 |
CON = control diet with no supplementation; +LC = control diet supplemented with L-carnitine; +POLI = control diet supplemented with encapsulated policosanol; +RP = control diet supplemented with raw policosanol; TC = total cholesterol (reference interval [RI] 1.00–4.40 mmol/l; LDL-C = low-density lipoprotein cholesterol, reference interval of 0.00–1.40 mmol/l); HDL-C = high-density lipoprotein cholesterol (RI 2.00–3.30 mmol/l); TG = triglycerides (RI of 0.20–1.10 mmol/l); TC:HDL-C = ratio of triglycerides to high-density lipoprotein cholesterol; LDL-C:HDL-C = ratio of low-density lipoprotein cholesterol to high-density lipoprotein cholesterol. RIs determined by the Lipid Analytical Laboratories
Table 3.
Blood parameters (mean ± SEM) in cats (n = 8) fed CON, +LC, +POLI or +RP diets* that significantly differed among 28-day treatment periods
| Parameter | Reference interval * | Period 1 | Period 2 | Period 3 | Period 4 | SEM | P value |
|---|---|---|---|---|---|---|---|
| MCV (fl) | 39.0–52.0 | 48 a | 45 b | 44 b | 44 b | 1.0 | <0.01 |
| MCH (pg) | 13.0–17.0 | 16 a | 15 a b | 15 b | 14 b | 0.35 | <0.01 |
| MCHC (g/l) | 317.0–350.0 | 329 b | 336 b | 328 b | 310 a | 2.35 | <0.01 |
| RDW (%) | 13.0–17.0 | 14 b | 14 b | 15 a | 15 a | 0.16 | <0.01 |
| Ca (mmol/l) | 2.22–2.78 | 2.40 c | 2.43 b c | 2.42 b c | 2.48 b | 0.018 | 0.03 |
| P (mmol/l) | 0.80–2.29 | 1.65 a | 1.53 b c | 1.58 c | 1.53 b c | 0.031 | 0.04 |
| Mg (mmol/l) | 0.80–1.10 | 0.96 b | 0.91 b | 0.91 b | 0.87 a | 0.016 | <0.01 |
| K (mmol/l) | 3.6–5.2 | 4.7 a | 4.4 b | 4.7 a | 4.5 b | 0.081 | 0.04 |
| Urea (mmol/l) | 6.0–12.0 | 7.9 a | 7.7 b | 7.3 b c | 7.2 b c | 0.15 | <0.01 |
CON = control diet with no supplementation; +LC = control diet supplemented with L-carnitine; +POLI = control diet supplemented with encapsulated policosanol; +RP = control diet supplemented with raw policosanol; MCV = mean cell volume; MCH = mean cell hemoglobin; MCHC = mean cell hemoglobin concentration; RDW = red cell distribution width; Ca = calcium; P = phosphorus; Mg = magnesium; K = potassium
Reference interval determined by the Animal Health Laboratory (Guelph, Ontario, Canada)
Values in a row with different superscript letters are significantly different (P <0.05)
Voluntary physical activity
There was no effect of treatment, day or period on total activity, mean activity in the light, or mean activity in the dark (P >0.10; Table 4).
Table 4.
Voluntary physical activity count (mean ± SEM) in cats (n = 8) fed CON, +LC, +POLI or +RP diets at days 0, 14 and 28
| CON | +LC | +POLI | +RP | SEM | P value | ||
|---|---|---|---|---|---|---|---|
| Total activity | Day 0 | 10.73 | 9.49 | 11.39 | 11.20 | 2.51 | 0.679 |
| Day 14 | 12.21 | 10.36 | 16.31 | 14.06 | 2.51 | 0.469 | |
| Day 28 | 12.74 | 13.65 | 13.35 | 10.30 | 2.51 | 0.723 | |
| P value | 0.472 | 0.246 | 0.663 | 0.252 | |||
| Total activity during dark hours | Day 0 | 13.05 | 12.24 | 14.53 | 14.73 | 2.89 | 0.830 |
| Day 14 | 14.27 | 13.26 | 15.92 | 18.58 | 2.89 | 0.542 | |
| Day 28 | 16.37 | 15.75 | 16.68 | 12.98 | 2.89 | 0.851 | |
| P value | 0.554 | 0.507 | 0.913 | 0.385 | |||
| Total activity during light hours | Day 0 | 8.50 | 6.20 | 8.33 | 7.82 | 2.60 | 0.710 |
| Day 14 | 8.98 | 7.36 | 15.24 | 10.87 | 2.60 | 0.341 | |
| Day 28 | 9.08 | 11.52 | 9.86 | 8.46 | 2.60 | 0.815 | |
| P value | 0.971 | 0.166 | 0.431 | 0.289 |
Energy expenditure
There was no effect of treatment, day or period on EE (kcal/kg/day) or RQ in the fasted or postprandial state (P >0.10, Table 5). Food intake on calorimetry days was similar among treatments, days and periods (P >0.10; data not shown).
Table 5.
Fasted and postprandial energy expenditure and respiratory quotient (mean ± SEM) in cats (n = 8) fed CON, +LC, +POLI or +RP diets at days 0, 14 and 28
| CON | +LC | +POLI | +RP | SEM | P value | ||
|---|---|---|---|---|---|---|---|
| Fasted EE (kcal/kg/day) | Day 0 | 29.62 | 30.87 | 29.52 | 29.25 | 3.54 | 0.989 |
| Day 14 | 31.25 | 29.00 | 30.58 | 30.89 | 3.54 | 0.975 | |
| Day 28 | 30.90 | 28.04 | 28.85 | 28.57 | 3.54 | 0.934 | |
| P value | 0.919 | 0.868 | 0.957 | 0.860 | |||
| Postprandial EE (kcal/kg/day) | Day 0 | 28.00 | 27.5 | 28.59 | 27.72 | 3.42 | 0.996 |
| Day 14 | 30.74 | 29.41 | 30.85 | 30.32 | 3.42 | 0.992 | |
| Day 28 | 30.73 | 28.38 | 27.64 | 28.15 | 3.42 | 0.911 | |
| P value | 0.796 | 0.936 | 0.839 | 0.763 | |||
| Fasted RQ | Day 0 | 0.8225 | 0.8158 | 0.8186 | 0.8222 | 0.0092 | 0.906 |
| Day 14 | 0.7980 | 0.7933 | 0.7920 | 0.7960 | 0.0092 | 0.967 | |
| Day 28 | 0.8268 | 0.8112 | 0.8042 | 0.8133 | 0.0092 | 0.504 | |
| P value | 0.153 | 0.218 | 0.259 | 0.109 | |||
| Postprandial RQ | Day 0 | 0.8033 | 0.8033 | 0.8002 | 0.8018 | 0.0089 | 0.991 |
| Day 14 | 0.7886 | 0.7937 | 0.7850 | 0.7898 | 0.0089 | 0.895 | |
| Day 28 | 0.7877 | 0.7871 | 0.7805 | 0.7825 | 0.0089 | 0.954 | |
| P value | 0.329 | 0.465 | 0.244 | 0.409 |
CON = control diet with no supplementation; +LC = control diet supplemented with L-carnitine; +POLI = control diet supplemented with encapsulated policosanol; +RP = control diet supplemented with raw policosanol; EE = energy expenditure; RQ = respiratory quotient
Discussion
Results from this study indicate that doses of raw and encapsulated policosanol that correspond to therapeutic concentrations in other animal models are well tolerated and can be safely consumed by domestic cats. Supplementation of POLI and RP had no effect on CBC and serum biochemistry, suggesting both are safe for use with domestic cats. Results presented herein mirror safety assessments in rats, rabbits, dogs and monkeys that have reported no adverse metabolic effects with acute and chronic supplementation of policosanol at concentrations as high as 5000 mg/kg BW/day.11,33–35
While policosanol supplementation did not affect diet intake when compared with other treatments, the time to consume the second offering of food was greatest in RP when compared with all other treatments. However, given that there was no change in orts with RP (data not shown), this was likely not a result of satiation. As an aliphatic hydrocarbon, policosanol is highly hydrophobic and has a tacky consistency; thus, the RP treatments may have changed the hedonic or physiochemical characteristic of the food, which may have affected rate of consumption. To the authors’ knowledge, this is the first time an RP has been fed to domestic cats and research on feeding behaviour and gastrointestinal hormonal response may be of interest.
In humans, policosanol is a traditional dietary supplement for the treatment of dyslipidemia. This study reported no effect of policosanol (raw or encapsulated) on lipid profile in either lean (n = 3; BCS ⩽5) or overweight (n = 5; BCS >5), healthy domestic cats. Various mechanisms of action have been proposed for policosanol’s cholesterol-lowering efficacy, including decreasing beta-hydroxy-beta-methylglutaryl coenzyme A reductase activity 36 and increasing LDL-C receptor activity. 11 Studies in animal models including rats, rabbits, dogs and monkeys all conclude that policosanol significantly reduces concentrations of TC (12–23%) and LDL-C (11–30%), with dosages ranging from 5 to 500 mg/kg BW/day.10,15,35,37 It is worthwhile noting that studies reporting the cholesterol-lowering efficacy of policosanol in humans and various animal models have been primarily conducted in a single laboratory, while studies outside of that group in Cuba have failed to show effects of the same magnitude. 23 The original policosanol research group has suggested that the positive results are due to a different mixture or purity of their specific policosanol. 38 However, a study by Kassis et al 25 found no differences between the original Cuban policosanol (Dalmer) and the alternative policosanol (Degussa Bioactives), as neither elicited a positive change in lipid profile in hamsters. Similarly, other studies conducted outside of Cuba in rabbits and hamsters found no change in lipid profiles with policosanol supplementation.24,39 However, high-cholesterol diets in both studies may have confounded these results.
Additionally, it should be noted that the cats in this study did not present with a dyslipidemia upon enrollment into the study, which may have affected the ability for policosanol to lower LDL-C and TG levels. For future studies, body fat stores should also be considered along with BW and BCS, as these cats only had a mean BCS of 5.4 at baseline and largely gained weight over a short period of time during the 4-week ad libitum feeding prior to baseline. It may take longer for cats to display characteristically dyslipidemic blood, as they have increased fat utilization as obligate carnivores. Genetics may also play a part in the relationship between lipid metabolism and weight gain, as there were some cats that had an overweight BCS but did not have characteristically dyslipidemic blood and experienced little to no changes with any dietary treatments, or vice versa. Thus, pre-screening of cats based on dyslipidemia and level of obesity would be useful in studies moving forward, as these factors likely impact the level of effectiveness of supplementation.
Similar to dyslipidemia, there was no effect of treatment on voluntary physical activity, EE and RQ. The lack of change in voluntary physical activity in LC was interesting, as previous research has reported an increase in play motivation and a decrease in RQ in overweight cats, but not lean ones, supplemented with L-carnitine. 5 However, increased play motivation may not coincide with voluntary physical activity, and the cats’ perceived motivation to play during socialization was not measured in the current study. Furthermore, while most cats were classified as overweight (BCS of 6), their weight gain occurred in a relatively short period of time prior to the study and thus their activity patterns may not have been impacted by LC.
Previous research has demonstrated that L-carnitine increases the RQ in overweight and obese cats;4,5 however, EE and RQ were not affected by LC in the present study. However, postprandial RQ decreased similarly across all treatments, potentially indicating some longer-term effects of supplementation. These observations warrant further research into policosanol supplementation with an alternative experimental design, such as a 2 × 2 Latin square to investigate the direct effects of encapsulated policosanol against a control to investigate the potential effects of supplementation in domestic cats. Finally, it was not surprising that there was no treatment difference in EE, as resting EE is largely controlled by LBM 40 and so is unlikely to change unless body composition was altered. Given that cats in the present study were fed to maintain BW, LBM would likely not change and thus would not affect resting EE.
Conclusions
This is the first study to demonstrate that supplemental policosanol can be safely administered to domestic cats. No disturbances in biochemical or hematological parameters indicate a similar safety margin to what has been demonstrated in previous animal studies, which is essential for moving forward with clinical studies. However, as cats naturally have a positive lipid profile with a higher proportion of HDL-C, supplementing policosanol is likely only of significant benefit in overweight, dyslipidemic cats, and those with diabetes mellitus or hepatic lipidosis. Future research should therefore focus on cats with these characteristics. In addition, future research could consider utilizing a parallel design rather than crossover to more practically investigate the longer-term effects of supplementation; however, this may require a larger sample size of subjects.
Footnotes
Accepted: 7 April 2021
Author note: Data associated with this report are archived in the University of Guelph Agri-environmental Research Data Repository and are currently accessible via a private URL: https://dataverse.scholarsportal.info/privateurl.xhtml?token=461e4fca-8e2a-4b47-a7ed-d52f34c1ae2f. Also available at: DOI: 10.5683/SP2/FGS3VO.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AB is an employee of the Pancosma SA; and CPFM is an employee of Pulse Canada. JRT, KH, AC, AV and AKS declare no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Pancosma SA (Rolle, Switzerland) [grant number 053634].
Ethical approval: This work involved the use of experimental animals and the study therefore had ethical approval from an established committee as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: James R Templeman  https://orcid.org/0000-0003-2048-6127
https://orcid.org/0000-0003-2048-6127
References
- 1. Ward E. 2017 pet obesity survey results. Association for pet obesity prevention. https://petobesityprevention.org/2017/ (2018, accessed May 12, 2020).
- 2. Kopelman PG. Obesity as a medical problem. Nature 2000; 404: 635–643. [DOI] [PubMed] [Google Scholar]
- 3. Blanchard G, Paragon BM, Milliat F, et al. Dietary L-carnitine supplementation in obese cats alters carnitine metabolism and decreases ketosis during fasting and induced hepatic lipidosis. J Nutr 2002; 132: 204–210. [DOI] [PubMed] [Google Scholar]
- 4. Center SA, Warner KL, Randolph JF, et al. Influence of dietary supplementation with L-carnitine on metabolic rate, fatty acid oxidation, body condition, and weight loss in overweight cats. Am J Vet Res 2012; 73: 1002–1015. [DOI] [PubMed] [Google Scholar]
- 5. Shoveller AK, Minikhiem DL, Carnagey K, et al. Low level of supplemental dietary L-carnitine increases energy expenditure in overweight, but not lean, cats fed a moderate energy density diet to maintain body weight. Intern J Appl Res Vet Med 2014; 12: 33–43. [Google Scholar]
- 6. de Godoy MR, Shoveller AK. Overweight adult cats have significantly lower voluntary physical activity than adult lean cats. J Feline Med Surg 2017; 19: 1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gooding MA, Minikhiem DL, Shoveller AK. Cats in positive energy balance have lower rates of adipose gain when fed diets containing 188 versus 121 ppm L-carnitine. Sci World J 2016; 2016: 2649093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Center SA, Harte J, Watrous D, et al. The clinical and metabolic effects of rapid weight loss in obese pet cats and the influence of supplemental oral L-carnitine. J Vet Intern Med 2000; 14: 598–608. [DOI] [PubMed] [Google Scholar]
- 9. Pons P, Rodríguez M, Más R, et al. One-year efficacy and safety of policosanol in patients with type II hypercholesterolemia. Curr Ther Res 1994; 55: 1084–1092. [Google Scholar]
- 10. Rodriguez-Echenique C, Mesa R, Mas R, et al. Effects of policosanol chronically administered in male monkeys (Macaca arctoides). Food Chem Toxicol 1994; 32: 565–575. [DOI] [PubMed] [Google Scholar]
- 11. Menéndez R, Arruzazabala L, Más R, et al. Cholesterol-lowering effect of policosanol on rabbits with hypercholesterolaemia induced by a wheat starch-casein diet. Br J Nutr 1997; 77: 923–932. [DOI] [PubMed] [Google Scholar]
- 12. Castano G, Mas R, Fernandez L, et al. Effects of policosanol 20 versus 40 mg/day in the treatment of patients with type II hypercholesterolemia: a 6-month double-blind study. Int J Clin Pharmocol Res 2001; 21: 43–57. [PubMed] [Google Scholar]
- 13. Gouni-Berthold I, Berthold HK. Policosanol: clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am Heart J 2002; 143: 356–365. [DOI] [PubMed] [Google Scholar]
- 14. Cruz-Bustillo D, Mederos D, Más R, et al. Cholesterol-lowering effect of Ateromixol (PPG) on fattening hogs. Rev CENIC Cienc Biol 1991; 22: 62–63. [Google Scholar]
- 15. Menéndez R, Amor AM, Gonzalez RM, et al. Effect of policosanol on the hepatic cholesterol biosynthesis of normocholesterolemic rats. Biol Res 1996; 29: 253–258. [PubMed] [Google Scholar]
- 16. Hernandez F, Illnait J, Mas R, et al. Effect of policosanol on serum lipids and lipoproteins in healthy volunteers. Curr Ther Res 1992; 51: 568–575. [Google Scholar]
- 17. Pons P, Mas R, Illnait J, et al. Efficacy and safety of policosanol in patients with primary hypercholesterolemia. Curr Ther Res 1992; 52: 507–513. [Google Scholar]
- 18. Pons P, Rodriguez M, Mas R, et al. One year efficacy and safety of policosanol in patients with type II hypercholesterolemia. Curr Ther Res 1994; 55: 1084–1092. [Google Scholar]
- 19. Aneiros E, Mas R, Calderon B, et al. Effect of policosanol in lowering cholesterol levels in patients with type II hypercholesterolemia. Curr Ther Res 1995; 56: 176–182. [Google Scholar]
- 20. Canetti M, Moreira M, Illnait J, et al. One-year study of the effect of policosanol on lipid profile in patients with type II hypercholesterolemia. Adv Ther 1995; 12: 245–254. [Google Scholar]
- 21. Canetti M, Moreira M, Mas R, et al. A two-year study on the efficacy and tolerability of policosanol in patients with type II hyperlipoproteinaemia. Int J Clin Pharm Res 1995; 15: 159–165. [PubMed] [Google Scholar]
- 22. Kabir Y, Kimura S. Biodistribution and metabolism of orally administered octacosanol in rats. Ann Nutr Metab 1993; 37: 33–38. [DOI] [PubMed] [Google Scholar]
- 23. Marinangeli CPF, Jones PJ, Kassis AN, et al. Policosanols as nutraceuticals: Fact or fiction. Crit Rev Food Sci Nutr 2010; 50: 259–267. [DOI] [PubMed] [Google Scholar]
- 24. Murphy KJ, Saint DA, Howe PR. Lack of effect of sugarcane and sunflower seed policosanols on plasma cholesterol in rabbits. J Am Coll Nutr 2008; 27: 476–484. [DOI] [PubMed] [Google Scholar]
- 25. Kassis AN, Marinangeli CP, Jain D, et al. Lack of effect of sugarcane policosanol on plasma cholesterol in Golden Syrian hamsters. Atherosclerosis 2007; 194: 153–158. [DOI] [PubMed] [Google Scholar]
- 26. Berthold HK, Unverdorben S, Degenhardt R, et al. Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: a randomized controlled trial. JAMA 2006; 295: 2262–2269. [DOI] [PubMed] [Google Scholar]
- 27. Francini-Pesenti F, Beltramolli D, Dall’Acqua S, et al. Effect of sugar cane policosanol on lipid profile in primary hypercholesterolemia. Phytother Res 2008; 22: 318–322. [DOI] [PubMed] [Google Scholar]
- 28. Montague MJ, Li G, Gandolfi B, et al. Comparative analysis of the domestic cat genome reveals genetic signatures underlying feline biology and domestication. Proc Nat Acad Sci 2014; 111: 17230–17235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laflamme DP. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 1997; 25: 13–18. [Google Scholar]
- 30. Gooding MA, Atkinson JL, Duncan IJ, et al. Dietary fat and carbohydrate have different effects on body weight, energy expenditure, glucose homeostasis and behaviour in adult cats fed to energy requirement. J Nutr Sci 2015; 4: E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weir J. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol-London 1949; 109: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andrews CJ, Potter MA, Thomas DG. Quantification of activity in domestic cats (Felis catus) by accelerometry. Appl Anim Behav Sci 2015; 173: 17–21. [Google Scholar]
- 33. Aleman CL, Más R, Hernández C, et al. A 12-month study of policosanol oral toxicity in Sprague Dawley rats. Toxicol Lett 1994; 70: 77–87. [DOI] [PubMed] [Google Scholar]
- 34. Gamez R, Alemán CL, Más R, et al. A 6-month study on the toxicity of high doses of policosanol orally administered to Sprague-Dawley rats. J Med Food 2010; 4: 57–65. [DOI] [PubMed] [Google Scholar]
- 35. Mesa ADR, Mas R, Noa M, et al. Toxicity of policosanol in Beagle dogs: one-year study. Toxicol Lett 1994; 73: 81–90. [DOI] [PubMed] [Google Scholar]
- 36. Singh DK, Li L, Porter TD. Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J Pharmacol Exp Ther 2006; 318: 1020–1026. [DOI] [PubMed] [Google Scholar]
- 37. Arruzazabala MDL, Carbajal D, Mas R, et al. Cholesterol-lowering effects of policosanol in rabbits. Biol Res 1994; 27: 205–208. [PubMed] [Google Scholar]
- 38. Castano G, Fernandez L, Mas R, et al. Comparison of the efficacy, safety and tolerability of original policosanol versus other mixtures of higher aliphatic primary alcohols in patients with type II hypercholesterolemia. Int J Clin Pharmacol Res 2002; 22: 55–66. [PubMed] [Google Scholar]
- 39. Wang YW, Jones PJH, Pischel I, et al. Effects of policosanols and phytosterols on lipid levels and cholesterol biosynthesis in hamsters. Lipids 2003; 38: 165–170. [DOI] [PubMed] [Google Scholar]
- 40. Center SA, Warner KL, Randolph JF, et al. Resting energy expenditure per lean body mass determined by indirect calorimetry and bioelectrical impedance analysis in cats. J Vet Intern Med 2011; 25: 1341–1350. [DOI] [PubMed] [Google Scholar]


