Abstract
The prevalence of hepatitis B virus among HIV-seropositive individuals is believed to be high, and yet the disease remains neglected in many areas of the continent. Little is known about occult hepatitis in HIV individuals. This review assessed occult hepatitis B infection and its prevalence in the different regions of the African continent. It also determines its prevalence in the HIV population which is endemic in the region. Studies were searched from the Cochrane, google scholar, PubMed/Medline, and African Journals online. Authors included cross-sectional studies, case controls, and cohorts, from 2010 to January 2021, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Participants, Interventions, Comparisons, Outcomes, and Study design frameworks to develop the search strategy. All studies had participants who were HIV-positive, covering different regions of the continent. Risk ratio was used to measure effect size, and Stata 14 software was used for analysis. Eleven studies met the eligibility criteria, with 2567 participants. Overall prevalence of occult hepatitis B was 11.2%. Regional prevalence was 26.5% for the south, 11% for the north, 9.1% for the east, and 8.5% for the western region. Approximately 10% of HIV-seropositive individuals were co-infected with occult hepatitis B virus. Regionally, the prevalence was highest in the southern region and lowest in the west. The prevalence of occult HBV infection was compared between the southern region and the other regions. It was higher in the south compared to the east (risk ratio = 0.87, 95% confidence interval (0.83–0.91)). It was also higher in the south compared to the north (risk ratio = 0.82, 95% confidence interval (0.79–0.85)), and it was also higher in the south compared to the west (risk ratio = 0.85, 95% confidence interval (0.82–0.87)). Public health measures and interventions are required to raise awareness, increase prevention, and reduce spread of the disease. More evidence-based studies need to be carried out.
Keywords: Hepatitis B, HIV, occult hepatitis B, prevalence
Introduction
Viral hepatitis, ranked the seventh-leading cause of mortality in the world, is also a major cause of morbidity. 1 Hepatitis B virus (HBV) continues to pose a serious threat to public health as it is an endemic in different parts of the world. 2 HBV and HIV frequently co-exist as they share common modes of transmission. 3 At a global level, it is estimated that 3.6% of the world’s population is infected with HBV. 4 In sub-Saharan Africa, an endemic area for HBV, the exposure rate is up to 90%. 5 A carrier of the virus is characterized by chronic hepatitis B surface antigenemia (HBsAg). This is more prevalent in developing nations, such as those in sub-Saharan Africa, Asia, and the Pacific region. 6
The term “occult” hepatitis B infection (OBI) is defined by the presence of HBV DNA in plasma and/or liver tissue of subjects who lack detectable HBsAg. 7 Based on the HBV-specific antibody profiles, OBI may be categorized as seropositive OBI—hepatitis B core antibody (anti-HBc) and/or hepatitis B surface antibody (anti-HBs) positive and seronegative OBI—anti-HBc and anti-HBs negative. 8 Identification of anti-HBc alone can also be a predictive marker of occult HBV infection. 2 OBI was observed in 2% of 400 women who were HIV-seropositive and with antibodies to hepatitis B core (HBc) antigen. 9 Occult HBV has been identified in several cohorts of HIV-infected patients.10,11 Results from the study by Núñez et al. 12 suggested that occult HBV infection is not a frequent phenomenon among both HIV and hepatitis C virus (HCV) co-infected individuals. In a study by de Mendoza et al., 13 it was found that the prevalence of both chronic HBV (8.5%) and OBI (14%) in HIV patients in Ghana was high. A study by Mbangiwa et al. 4 revealed that the prevalence of chronic HBV in pregnant women was 21% and occult HBV was 6.6%. The prevalence rates in the HIV-positive and HIV-negative participants were not different. In Gabon, a study by Bivigou-Mboumba et al. 5 showed OBI (HBV DNA) in one patient irrespective of the HBV serological marker.
Approximately 20% of HIV-seropositive people in sub-Saharan Africa are also infected with HBV. 14 Both diseases are public health threats in the African continent. Clinically, the relevance of occult HBV infection in patients with HIV and/or HCV co-infection is not clear. 15 In this review, our aim was to understand the prevalence of OBI in Africa and also determine regional prevalences. The results from the review will show the burden of the disease in Africa allowing stakeholders to use it as a guide for making informed decisions.
Methodology
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used.16–18 We included studies that reported OBI in HIV participants in Africa. Diagnosis of OBI was laboratory-based in all studies.
Search methods
Electronic databases such as Cochrane, PubMed/Medline, Google Scholar, and African Journals OnLine (AJOL) were searched so as to identify relevant studies. Occult hepatitis B, hepatitis B, HIV, and Africa were the keywords used. For PubMed, Medical subject’s headings (MeSH) 19 terms were used that were driven from our research question. Boolean operators were also used between the search terms.
Search strategy
The MeSH terms included “HIV” [Text word] OR “AIDS” [MeSH terms] OR “immune-compromised” [MeSH] AND “hepatitis B virus” [Text word] OR Hepatitis [MeSH] OR “Occult hepatitis B” [MeSH terms] OR “occult infection” [MeSH] AND Africa [text word] OR “Sub-Sahara Africa” AND “incidence” [Text word] OR “incidence” [MeSH] OR “epidemiology” [MeSH] OR “epidemiology” [Subheading]
Eligibility criteria
Eligibility was formulated by PICOS (Participants, Interventions, Comparisons, Outcomes, Study design): 20
Population: HIV-positive individuals;
Intervention: testing HBV-DNA in HIV-seropositive participants;
Comparison: the development of OBI or not in participants;
Outcome: positive HBV-DNA test;
Study design: cross-sectional and cohorts from 2005 to 2021.
Study selection
Published articles in English based on the eligibility criteria were selected. The articles were imported from electronic databases into Mendeley reference manager. Screening for titles, abstracts, and full texts was done by two authors independently. Discrepancies in the findings between the authors were resolved by discussion. Quality of studies and risk of bias assessments were done. The Mendeley reference manager was used to remove duplicate studies.
Data extraction
Data extraction was done by all authors. Any disagreements were resolved by discussion among the authors. A form for data extraction was designed in MS Excel that was used to extract data from the studies. Data extracted included author names and the publication year of the article, country, design of the study, total number of HIV participants, and OBI and HIV co-infection.
Quality assessment
Assessment of quality of the studies was done using the Newcastle-Ottawa scale. 21 The scale has the following characteristics: standardized methods of diagnosis confirmation, large enough sample size, multicenter study, appropriate statistical methods that report outcomes, accounting for confounders (demographics, socioeconomic status, previous treatment history), 22 and clear methodology for the selection of participants. Additional sources for retrieving outcomes and participant information, treatment duration, missing data at final analysis, and proof of ethical review of the study were also considered. 22
Statistical analysis
Stata version 14 was used for the meta-analysis. 23 Heterogeneity was calculated using chi-square and I2, with a 95% confidence interval (CI). All of our results were binary outcomes, and the Mantel–Haenszel random-effects method was used in our analysis, with risk ratio (RR) as our effect estimate.
Results
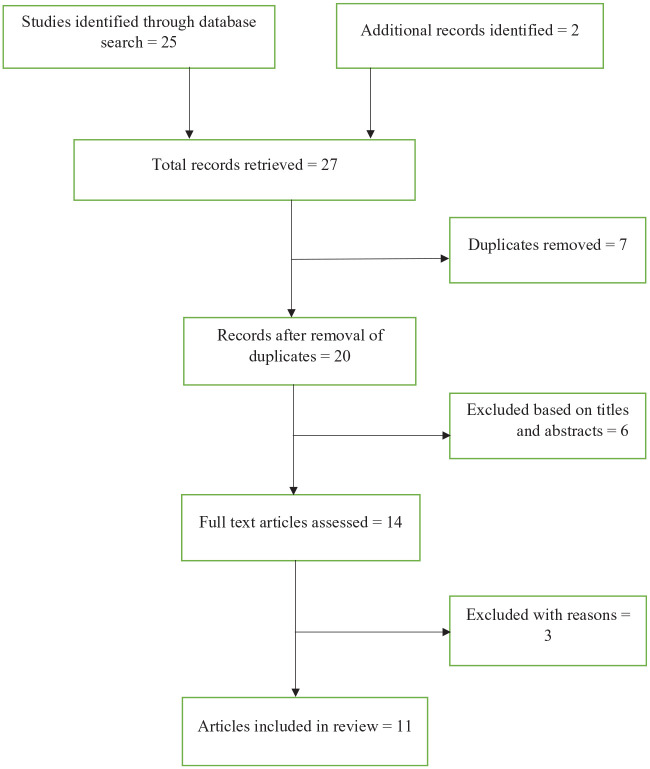
Figure 1 above shows the study search steps highlighting how the studies were retrieved. Total studies identified = 27, and studies included in the final review = 11. All the studies reported OBI in HIV-positive participants. There was a total of 2283 participants in all the studies. Studies were conducted across different regions in Africa. We divided the regions as follows: South (Botswana, South Africa), East (Kenya, Ethiopia), North (Egypt, Sudan), and West (Cameroon, Nigeria, Ivory Coast, Ghana) (Table 1).
Figure 1.
A PRISMA flow diagram of the retrieval process for the searched studies.
Table 1.
Characteristics of the studies that were included.
| Study author and publication year | Study country | Study design | HIV-positive individuals in the study | OBI and HIV co-infection |
|---|---|---|---|---|
| Mudawi et al. (2014) 24 | Sudan | Cross-sectional | 358 | 54 |
| Abdelaziz et al. (2019) 25 | Egypt | Cross-sectional | 197 | 7 |
| Attiku et al. (2021) 26 | Ghana | Longitudinal purposive | 113 | 4 |
| N’Dri-Yoman et al. (2010) 27 | Ivory Coast | Cross-sectional | 495 | 51 |
| Opaleye et al. (2014) 28 | Nigeria | Retrospective analysis | 188 | 21 |
| Patel et al. (2020) 29 | Ethiopia | Cross-sectional | 115 | 22 |
| Salyani et al. 2021 30 | Kenya | Cross-sectional | 208 | 11 |
| Gachara et al. (2017) 31 | Cameroon | Cross-sectional retrospective | 337 | 20 |
| Ryan et al. (2021) 32 | Botswana | Retrospective analysis | 272 | 72 |
| Ayana et al. (2020) 33 | Ethiopia | Cohort | 117 | 7 |
| Mphahlele et al. (2006) 34 | South Africa | Case control | 167 | 50 |
OBI: occult hepatitis B infection.
Table 2.
Summary of risk of bias in each study using the Newcastle-Ottawa quality assessment scale.
| Standardized methods confirming OBI | Large enough sample size | Multicenter study | Appropriate statistical methods that report outcomes | Accounting for confounders | Clear methodology for the selection of participants | Representativeness of infected population | |
|---|---|---|---|---|---|---|---|
| Mudawi et al. (2014) 24 |
|
||||||
| Abdelaziz et al. (2019) 25 | |||||||
| Attiku et al. 2021 26 | |||||||
| N’Dri-Yoman et al. (2010) 27 | |||||||
| Opaleye et al. (2014) 28 | |||||||
| Patel et al. (2020) 29 | |||||||
| Salyani et al. 2021 30 | |||||||
| Gachara et al. (2017) 31 | |||||||
| Ryan et al. (2021) 32 | |||||||
| Ayana et al. (2020) 33 | |||||||
| Mphahlele et al. (2006) 34 | |||||||
OBI: occult hepatitis B infection.
Green cells indicate low risk of bias. Red cells indicate high risk of bias. Blank cells indicate unknown risk.
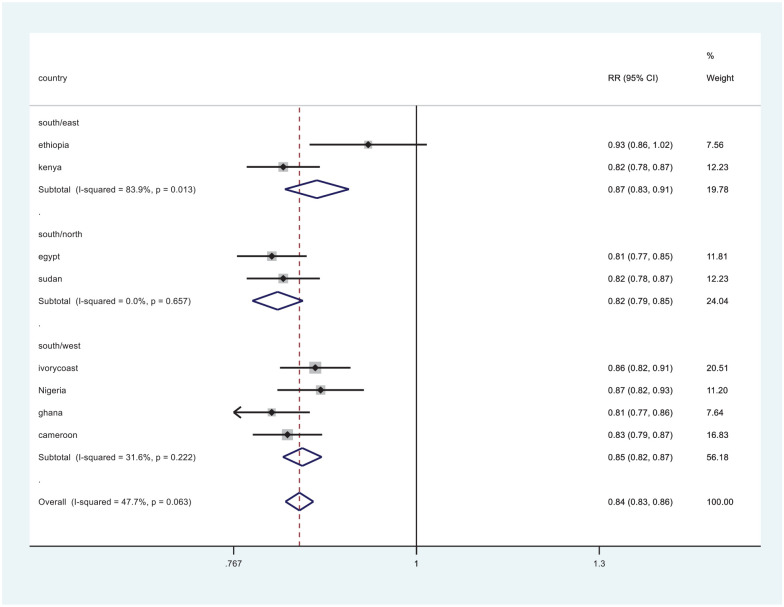
Table 1 characteristically summarizes the reviewed studies. Participants aged 18 years and above were included. Overall, the prevalence of occult hepatitis B was 12.4% (319 of 2567 total participants). Regionally, the prevalence in the South was 27.7%, East 9.1%, North 11%, and West 8.5% (Figure 2).
Figure 2.
Comparison of OBI in the southern and other regions (eastern, northern, and western regions).
Discussion
The main focus of this review was to determine the prevalence of OBI in HIV-positive individuals. To the awareness of the authors, this is the first meta-analysis on OBI and HIV co-infection in the African setting. There was a generally high prevalence of OBI and HIV co-infection of 12%. Notably, there was a higher prevalence in the southern region. Heterogeneity was substantial in the studies. This could be attributed to the different study settings and the anti-retroviral therapy (ART) status of the individuals. Two studies, one by Mudawi et al. 24 and the other by N’Dri-Yoman et al., 27 had HIV-positive treatment-naïve participants.
A cohort study from Italy by Tramuto et al. 7 investigated how impactful occult HBV infection can be in HIV-infected individuals in Sicily and found the overall prevalence of 5.9% (95% CI: 3.8%–8.7%). Another cohort by Filippini et al. 35 revealed a 20% prevalence of OBI in HIV-positive and HBsAg-negative patients, who were not on antiretroviral therapy. Hepatic flare was more frequently seen in patients with occult HBV infection than in those without. 35 A retrospective analysis was performed on laboratory data in India and indicated that HIV-infected patients are at a higher risk of HBV co-infection in their set-up, as illustrated by the high prevalence of HBsAg (7.28%) in HIV-positive patients in comparison with HIV-negative patients (1.4%). 3 The study also showed a very high persistence of HBV genome (overall 37.5%) even in those patients who are HBsAg-negative. 3 In a study by Azadmanesh et al., 36 the HBV-DNA was found in 13.6% of Iranian HIV-positive patients with isolated anti-HBc. There are several explanations for the persistence of HBV-DNA with the absence of HBsAg. 36 These reasons could be presence of HBV-DNA at a low copy number, genetic variations in the S gene and the presence of immune complexes in which HBsAg may be hidden. 36 A study by de Mendoza et al. 13 revealed a 14.2% prevalence of OBI in the general population in Ghana. Soriano et al. 37 found that OBI was negligible in the HIV population.
There is lack of a clear description of the clinical impact of occult HBV in HIV-seropositive patients in Africa. 38 There was an increase in liver complications measured by elevated liver transaminases in HIV-seropositive patients who were co-infected with occult HBV in Italy. 38 Another study found the cause of hepatocellular carcinoma to be linked to OBI. 15 Tsui et al. revealed that OBI was more likely to reduce the CD4 cell count to <200 cells/mm3, without elevated aminotransferase levels being associated with detectable HBV-DNA. 9 OBI has also had public health implications, especially in terms of blood donations and transfusions. A study in Spain in seronegative participants deduced that 0.003% of blood donors were found with OBI, the majority being immigrants. 39
In most African settings and HIV clinics, testing for HBV or HCV is not done. It is also not part of the recommendations of most country guidelines. So, our only hope is that people are initiated on ARTs early enough, to benefit from the early antiretroviral (ARV) treatments. With these results, recommendations on screening and testing for HBV and HBV-DNA is necessary. In countries like the United States and Canada, it is part of the comprehensive care in HIV clinics to screen for OBI, and their co-infection rates are low. 40 Recommendations on immunization of HIV and hepatitis-negative persons, as a priority group. 40 Vaccination and molecular OBI diagnostics may not be feasible due to financial reasons and other logistical reasons. So, we strongly recommend screening of the HIV patients.
Some study limitations were the fact that we failed to find enough studies to represent some of the regions. The southern, eastern, and northern regions were represented by two studies. Regardless, the available studies are well sourced and discussed.
Conclusion
Approximately 12% of HIV-seropositive patients have OBI. Screening for OBI is necessary as the review reports high rates of disease in Africa. Without screening, OBI may cause transmission of HBV as many settings test for HBsAg only. Clinical significance and implications of OBI need to be further examined, as it is a potential threat.
Acknowledgments
Thanks to Mafia District hospital for the support during the process of this work.
Footnotes
Author’s contributions: V.D.K. and S.S.S. were responsible for study design. V.D.K., S.S.S., and S.G. were responsible for literature search. V.D.K. and S.G. were responsible for methodology, data analysis, and synthesis. V.D.K., S.S.S., and S.G. prepared the article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Violet Dismas Kajogoo  https://orcid.org/0000-0002-6122-3828
https://orcid.org/0000-0002-6122-3828
References
- 1. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2013; 388: 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shire NJ, Rouster SD, Rajicic N, et al. Occult hepatitis B in HIV-infected patients. J Acquir Immune Defic Syndr 2004; 36: 869–875. [DOI] [PubMed] [Google Scholar]
- 3. Gupta S, Singh S. Occult hepatitis B virus infection in ART-Naive HIV-infected patients seen at a tertiary care centre in North India. BMC Infect Dis 2010; 10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mbangiwa T, Id IK, Anderson M, et al. Chronic and occult hepatitis B virus infection in pregnant women in Botswana. Genes 2018; 9: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bivigou-Mboumba B, Amougou-Atsama M, Zoa-Assoumou S, et al. Hepatitis B infection among HIV infected individuals in Gabon: occult hepatitis B enhances HBV DNA prevalence. PLoS ONE 2018; 13(1): e0190592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- 7. Tramuto F, Maida CM, Colomba GME, et al. Prevalence of occult hepatitis B virus infection in a cohort of HIV-positive patients resident in Sicily, Italy. Biomed Res Int 2013; 2013: 859583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raimondo G, Locarnini S, Pollicino T, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol 2019; 71(2): 397–408. [DOI] [PubMed] [Google Scholar]
- 9. Tsui JI, French AL, Seaberg EC, et al. Prevalence and long-term effects of occult hepatitis B virus infection in HIV-infected women. Clin Infect Dis 2007; 45: 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hofer M, Joller-Jemelka HI, Grob PJ, et al. Frequent chronic hepatitis B virus infection in HIV-infected patients positive for antibody to hepatitis B core antigen only. Eur J Clin Microbiol Infect Dis 1998; 17(1): 6–13. [DOI] [PubMed] [Google Scholar]
- 11. Ismail H, Soliman M, Nahed Ismail N. Occult hepatitis B virus infection in Egyptian hemodialysis patients with or without hepatitis C virus infection. Pathol Lab Med Int 2010; 2: 113–120. [Google Scholar]
- 12. Núñez M, Ríos P, Pérez-Olmeda M, et al. Lack of “occult” hepatitis B virus infection in HIV-infected patients. AIDS 2002; 16: 2099–2101. [DOI] [PubMed] [Google Scholar]
- 13. de Mendoza C, Bautista JM, Perez-Benavente S, et al. Screening for retroviruses and hepatitis viruses using dried blood spots reveals a high prevalence of occult hepatitis B in Ghana. Ther Adv Infect Dis 2019; 6: 2049936119851464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chadwicka D, Stanleya A, Sarfo S, et al. Response to antiretroviral therapy in occult hepatitis B and HIV co-infection in West Africa. AIDS 2013; 27: 139–144. [DOI] [PubMed] [Google Scholar]
- 15. Moreno S, Rockstroh J, Bra N. Occult hepatitis B virus infection in the setting of hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infection: clinically relevant or a diagnostic problem? J Med Virol 2007; 700: 694–700. [DOI] [PubMed] [Google Scholar]
- 16. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: elaboration and explanation. BMJ 2015; 349: g7647. [DOI] [PubMed] [Google Scholar]
- 17. Zinicola R, Nascimbeni R, Cirocchi R, et al. The impact of transanal local excision of early rectal cancer on completion rectal resection without neoadjuvant chemoradiotherapy: a systematic review. Tech Coloproctol 2021; 25(9): 997–1010. [DOI] [PubMed] [Google Scholar]
- 18. Kajogoo VD, Gorret Atim M, Amare D, et al. HIV protease inhibitors and insulin sensitivity: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol 2021; 12: 635089–635089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baumann N. How to use the medical subject headings (MeSH). Int J Clin Pract 2016; 70: 171–174. [DOI] [PubMed] [Google Scholar]
- 20. Gebrie D, Getnet D, Manyazewal T. Efficacy of remdesivir in patients with COVID-19: a protocol for systematic review and meta-analysis of randomised controlled trials. BMJ Open 2020; 10: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stubbs B. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal 2017; 5: 80–84. [Google Scholar]
- 22. Chem ED, Hout MC, Van Hope V. Treatment outcomes and antiretroviral uptake in multidrug-resistant tuberculosis and HIV co-infected patients in sub Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis 2019; 19: 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costa SM, Martins CC, Pinto MQC, et al. Socioeconomic factors and caries in people between 19 and 60 years of age: an update of a systematic review and meta-analysis of observational studies. Int J Environ Res Public Health 2018; 15: 1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mudawi H, Hussein W, Mukhtar M, et al. Overt and occult hepatitis B virus infection in adult Sudanese HIV patients. Int J Infect Dis 2014; 29: 65–70. [DOI] [PubMed] [Google Scholar]
- 25. Abdelaziz AF, Fekry MM, Hashish MH. Occult hepatitis B virus infection in Egyptian HIV- infected patients with isolated Anti-HBc. J High Inst Public Health 2019; 49: 162–167. [Google Scholar]
- 26. Attiku K, Bonney J, Agbosu E, et al. Circulation of hepatitis delta virus and occult hepatitis B virus infection amongst HIV/HBV co-infected patients in Korle-Bu, Ghana. PLoS ONE 2021; 16(1): e0244507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. N’Dri-Yoman T, Anglaret X, Attia A, et al. Short communication: occult HBV infection in untreated HIV-infected adults in Côte d’Ivoire. Antivir Ther 2010; 1034: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 28. Opaleye OO, Oluremi AS, Atiba AB, et al. Occult hepatitis B virus infection among HIV positive patients in Nigeria. J Trop Med 2014; 2014: 796121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel NH, Meier-Stephenson V, Genetu M, et al. Prevalence and genetic variability of occult hepatitis B virus in a human immunodeficiency virus positive patient cohort in Gondar, Ethiopia. PLoS ONE 2020; 15(11): e0242577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salyani A, Shah J, Adam R, et al. Occult hepatitis B virus infection in a Kenyan cohort of HIV infected anti-retroviral therapy naïve adults. PLoS ONE 2021; 16: e0244947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gachara G, Magoro T, Mavhandu L, et al. Characterization of occult hepatitis B virus infection among HIV positive patients in Cameroon. AIDS Res Ther 2017; 14: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryan K, Anderson M, Gyurova I, et al. High rates of occult Hepatitis B virus infection in HIV-positive individuals initiating antiretroviral therapy in Botswana. Open Forum Infect Dis 2017; 4(4): ofx195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ayana DA, Mulu A, Mihret A, et al. Occult hepatitis B virus infection among HIV negative and positive isolated anti-HBc individuals in eastern Ethiopia. Sci Rep 2020; 10: 22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mphahlele MJ, Lukhwareni A, Burnett RJ, et al. High risk of occult hepatitis B virus infection in HIV-positive patients from South Africa. J Clin Virol 2006; 35(1): 14–20. [DOI] [PubMed] [Google Scholar]
- 35. Filippini P, Coppola N, Pisapia R, et al. Impact of occult hepatitis B virus infection in HIV patients naive for antiretroviral therapy. AIDS 2006; 20: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 36. Azadmanesh K, Mohraz M, Aghakhani A, et al. Occult hepatitis B virus infection in HIV-infected patients with isolated Hepatitis B core antibody. Intervirology 2008; 51(4): 270–274. [DOI] [PubMed] [Google Scholar]
- 37. Soriano V, Aguilera A, Gonzalez R, et al. Occult hepatitis B and HIV infection. Europ J Gastroenterol Hepatol 2019; 31: 1403–1407. [DOI] [PubMed] [Google Scholar]
- 38. Firnhaber C, Viana R, Reyneke A, et al. Occult hepatitis B virus infection in patients with isolated core antibody and HIV co-infection in an urban clinic in Johannesburg, South Africa. Int J Infect Dis 2009; 13(4): 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. González R, Barea L, Arruga A, et al. Overt and occult hepatitis B among immigrants and native blood donors in Madrid, Spain. Ther Adv Infect Dis 2020; 7: 2049936120982122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agyeman AA, Asenso RO. Prevalence of HIV and hepatitis B coinfection in Ghana: a systematic review and meta‑analysis. AIDS Res Ther 2016; 13: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]