Abstract
Background:
To evaluate the safety, tolerability, and efficacy of SHR4640, a highly selective urate transporter-1 inhibitor, in combination with febuxostat, in patients with primary hyperuricemia.
Methods:
In this randomized, double-blind, parallel-controlled phase II study, patients whose fasting serum uric acid (sUA) levels were ⩾ 480 μmol/L at screening with gout or sUA levels were ⩾ 420 μmol/L lasting for at least 3 months without gout, either with sUA levels ⩾ 540 μmol/L at screening or sUA levels ⩾ 480 μmol/L with comorbidities at screening, were enrolled. Patients were randomized (1:1:1) to receive SHR4640 10 mg plus febuxostat 80 mg, SHR4640 10 mg plus febuxostat 40 mg, and SHR4640 5 mg plus febuxostat 20 mg orally once daily. The primary end point was the incidence of treatment-emergent adverse events (TEAEs).
Results:
A total of 93 patients were randomized and received treatment. TEAEs occurred in 55.9% of patients. The incidence of TEAEs was comparable among all the groups. Serious TEAEs occurred in one patient (1.1%), with no deaths observed. The proportion of patients who achieved the target sUA levels by week 4 was 79.3%, 96.6%, and 75.0% in the SHR4640 10 mg plus febuxostat 80 mg, SHR4640 10 mg plus febuxostat 40 mg, and SHR4640 5 mg plus febuxostat 20 mg groups, respectively. The mean percent reduction of sUA was 59.7%, 63.7%, and 41.8%, respectively.
Conclusion:
SHR4640 plus febuxostat exhibited a tolerable safety profile and substantial sUA lowering activity in patients with primary hyperuricemia.
Registration:
www.chinadrugtrials.org.cn; CTR 20192429
Keywords: febuxostat, gout, primary hyperuricemia, SHR4640, URAT-1 inhibitor, XOIs
Introduction
Gout is characterized by elevated serum uric acid (sUA) levels and monosodium urate crystal deposition in joint structures and soft tissues, resulting in serious, chronic disabling inflammation. 1 Gout is a prevalent rheumatic disease of adulthood and was reported in 3.9% of adults in the United States and 1.1% in China.2,3 Hyperuricemia, which is clinically defined as a sUA level > 6.8 mg/dL (404 μmol/L), is considered a necessary cause of gout and associated with overproduction of urate or underexcretion of renal urate.4,5
The American College of Rheumatology (ACR) recommends a target sUA level < 6 mg/dL (357 μmol/L) for patients with gout and < 5 mg/dL (297.5 μmol/L) for patients with tophaceous gout. 1 Xanthine oxidase inhibitors (XOIs), including allopurinol and febuxostat, function in uric acid synthesis blocking and are recommended as the first choice for uric acid lowering.1,6 However, 40–70% of patients treated with XOIs cannot reach the target sUA level, and 50–80% of patients failed to maintain the target sUA level.7,8 In addition, patients with allopurinol have a potential risk of developing severe hypersensitivity syndrome, which occurs in 0.7% of patients who receive allopurinol and leads to death in 6% of the hypersensitivity syndrome cases. 9 Notably, Han Chinese and Japanese are high-risk populations for hypersensitivity syndrome due to the common possession of the HLA-B*5801 allele.10,11 Therefore, uricosuric agents that promote uric acid excretion were recommended as alternative drugs for those who failed treatment or were intolerant to XOIs. 1
Approximately 90% of uric acid is reabsorbed by the urate transporter-1 (URAT-1) of the proximal renal tubular cells. Thus, inhibition of the URAT-1 increases uric acid excretion, thereby reducing sUA levels. Several URAT-1 inhibitors, including benzbromarone and probenecid, had been approved. However, benzbromarone was withdrawn from the market in the United States and some European countries due to severe hepatotoxic damage. Probenecid showed ineffective urate lowering activity in patients with renal impairment. 12 The combination of uricosuric agents and XOIs could lower the urate production and increase the urate excretion, representing an attractive therapeutic option for patients who suffer from hyperuricemia and gout. In 2015, lesinurad, a URAT-1 inhibitor, was approved in the United States and the European Union as a combination therapy with an XOI for patients with gout in whom target levels of sUA cannot be reached with a XOI. 13 Effective treatment for patients with hyperuricemia remains in great need.
SHR4640 is a highly selective inhibitor of URAT-1. SHR4640 monotherapy showed a superior sUA lowering effect and a well-tolerated safety profile after a 5-week treatment in patients with hyperuricemia in a previous phase II study. 14 This multicenter, randomized, double-blind, parallel-controlled phase II study was conducted to assess the safety, tolerability, and efficacy of SHR4640 combined with febuxostat in Chinese patients with primary hyperuricemia (with or without gout).
Methods
Participants
Patients [aged 18–65 years, 18 kg/m2 ⩽ body mass index (BMI) ⩽ 32 kg/m2] with hyperuricemia requiring a long-term urate-lowering therapy were enrolled. Eligible patients also need to meet any of the following criteria: fasting sUA levels ⩾ 480 μmol/L at screening with a diagnosis of gout according to the criteria of ACR15,16; fasting sUA levels ⩾ 420 μmol/L for at least 3 months with a diagnosis of hyperuricemia, either had fasting sUA levels ⩾ 540 μmol/L at screening or had fasting sUA levels ⩾ 480 μmol/L with comorbidities (hypertension, hyperlipidemia, or type 2 diabetes mellitus under stable medication doses for at least 3 months). Exclusion criteria included the following: estimated glomerular filtration rate (eGFR) < 60 mL/min [calculated by the Modification of Diet in Renal Disease (MDRD) formula]; gout attacks within 2 weeks before randomization; evidence or suspicion of kidney stones within 3 weeks of randomization. Complete exclusion criteria are presented in the Supplementary Materials.
Study design and treatment
This multicenter, randomized, double-blind, parallel-controlled phase II trial consisted of a 3-week screening period, a 4-week double-blind treatment period, and a 2-week safety follow-up period (www.chinadrugtrials.org.cn, clinical registration no. CTR20192429).
To reduce gout flares, eligible patients were given colchicine (0.5 mg once daily) at least 2 weeks before randomization until the end of the treatment period. Eligible patients were randomly assigned in a 1:1:1 ratio to receive SHR4640 10 mg plus febuxostat 80 mg, SHR4640 10 mg plus febuxostat 40 mg, or SHR4640 5 mg plus febuxostat 20 mg orally once daily (Supplementary Figure S1). Patients were encouraged to drink plenty of water. Randomization was stratified according to renal function (60 mL/min ⩽ eGFR < 90 mL/min and eGFR ⩾ 90 mL/min). Allocation at all the study sites was done via an interactive web-response system, and all the investigators, patients, and the sponsor of the study were masked to the treatment group. Medication adherence was assessed on the basis of returned medication counts, medication dispensing records, and inquiry into use and loss of medication of each patient.
The study protocol was reviewed and approved by the independent ethics committee at each site (Ethics Committee of West China Hospital, Sichuan University; Ethics Committee of Jilin Province People’s Hospital; Ethics Committee of The First Affiliated Hospital of Chongqing Medical University; Ethics Committee of Shengjing Hospital of China Medical University; Ethics Committee of Shanxi Provincial People’s Hospital; Ethics Committee of The First Affiliated Hospital of Clinical Medicine of Guangdong Pharmaceutical University; Ethics Committee of Maanshan People’s Hospital; Ethics Committee of Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School; Ethics Committee of The First Affiliated Hospital of Chengdu Medical College). The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization/Good Clinical Practice, local laws, and regulatory requirements. Written informed consent was obtained from each patient before participation. The study was conducted from 19 December 2019 to 17 July 2020.
Evaluation
The primary end point was the incidence of treatment-emergent adverse events (TEAEs). The secondary end points included the proportion of patients who achieved a sUA level ⩽ 360 μmol/L at weeks 2 and 4; the absolute and relative change in sUA levels from baseline at weeks 2 and 4; and safety assessments (including laboratory investigations, vital signs, and 12-lead electrocardiograms).
Statistical analyses
Sample size calculation was based on safety and efficacy. Assuming a 1:1:1 randomization among the treatment groups, a sample size of 25 patients per group provided a 64% probability to detect at least one adverse event with an underlying occurrence rate of ⩾ 4%. Combining three groups, a sample size of 75 patients provided a 95% probability to observe at least one adverse event with an underlying occurrence rate of ⩾ 4%. Assuming that the proportion of patients with a sUA level ⩽ 360 μmol/L at week 4 was 80% in all the groups, a sample size of 25 patients per group provided a 90% power to declare that the lower bound of the 95% confidence interval (CI) of the response rate exceeded 50%. Considering a dropout rate of 10%, 28 patients per group were required (84 patients in total).
Safety was assessed based on the safety analysis set, including all the patients who were randomized, received at least one dose of study medication, and had completed at least one post-treatment safety assessment. TEAEs were classified as related, possibly related, unlikely related, not assessable, and not related to study drugs by the investigators at each site. Treatment-related TEAEs included those judged as related, possibly related, and not assessable. Efficacy was analyzed based on the full analysis set, including all the randomized patients who received at least one dose of study medication and had completed the baseline sUA assessment and at least one post-randomization sUA evaluation.
The 95% CIs for the proportion of patients with a sUA level ⩽ 360 μmol/L were calculated using the Clopper–Pearson method. The differences in the response rates among the treatment groups were estimated, with the corresponding 95% CIs calculated using the normal approximation method. The absolute and relative changes in sUA levels from baseline were summarized descriptively. The last observation carried forward (LOCF) method was used to impute missing sUA values. Sensitivity analyses were performed based on the observed cases (OCs) and non-responder imputation (NRI) method (for binary variables only). All statistical analyses were performed using SAS version 9.4.
Results
Distribution of study participants
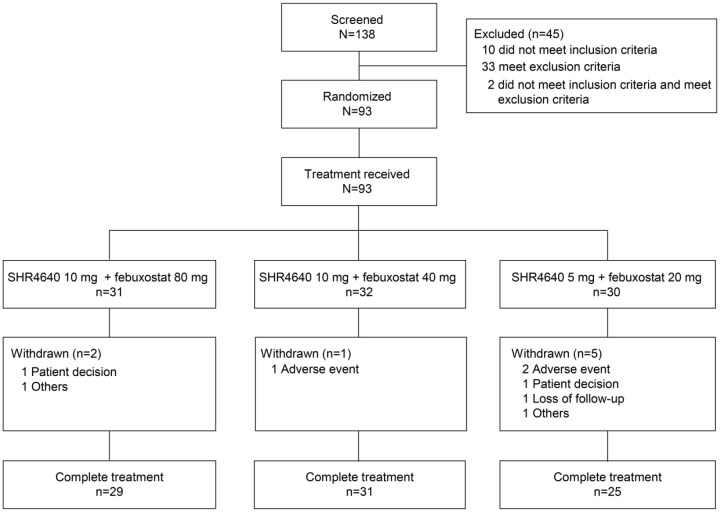
A total of 138 patients were screened, and 93 patients were randomized and received study treatment at 10 sites in China (31 patients to the SHR4640 10 mg plus febuxostat 80 mg group, 32 patients to the SHR4640 10 mg plus febuxostat 40 mg group, and 30 patients to the SHR4640 5 mg plus febuxostat 20 mg group). Of the 93 patients, 8 did not complete the study treatment (Figure 1). All 93 patients were included in the safety analysis set. Seven patients did not complete at least one post-randomization sUA assessments (two with SHR4640 10 mg plus febuxostat 80 mg, three with SHR4640 10 mg plus febuxostat 40 mg, and two with SHR4640 5 mg plus febuxostat 20 mg), therefore, 86 patients were included in the full analysis set.
Figure 1.
Distribution of study participants.
Demographic and clinical characteristics
The demographic and baseline characteristics of the patients were generally balanced among all the treatment groups (Table 1). Of the 86 patients, 85 (98.8%) were male, with a mean (SD) age of 36.8 (10.0) years and a mean (SD) BMI of 26.2 (2.8) kg/m2. All the patients enrolled had gout history.
Table 1.
Demographic and baseline disease characteristics (full analysis set).
| Characteristics | SHR4640 10 mg + febuxostat 80 mg (n = 29) |
SHR4640 10 mg + febuxostat 40 mg (n = 29) |
SHR4640 5 mg + febuxostat 20 mg (n = 28) |
Total (n = 86) |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 35.3 (9.6) | 39.3 (11.1) | 35.7 (9.1) | 36.8 (10.0) |
| Median | 34.0 | 37.0 | 37.0 | 36.5 |
| Range | 21–61 | 21–64 | 23–56 | 21–64 |
| Sex, n (%) | ||||
| Male | 28 (96.6) | 29 (100.0) | 28 (100.0) | 85 (98.8) |
| Female | 1 (3.4) | 0 | 0 | 1 (1.2) |
| Ethnicity, n (%) | ||||
| Han Chinese | 26 (89.7) | 25 (86.2) | 26 (92.9) | 77 (89.5) |
| Others | 3 (10.3) | 4 (13.8) | 2 (7.1) | 9 (10.5) |
| Body mass index, kg/m2 | ||||
| Mean (SD) | 25.7 (2.9) | 26.5 (2.9) | 26.3 (2.4) | 26.2 (2.8) |
| Median | 25.2 | 26.1 | 26.5 | 26.2 |
| Range | 20–31 | 21–32 | 21–31 | 20–32 |
| Body weight, kg | ||||
| Mean (SD) | 77.1 (13.4) | 78.9 (10.7) | 77.5 (9.6) | 77.8 (11.3) |
| Median | 76.0 | 78.0 | 77.5 | 77.0 |
| Range | 48–106 | 60–99 | 61–104 | 48–106 |
| Drinking habits, n (%) | ||||
| Never | 8 (27.6) | 6 (20.7) | 13 (46.4) | 27 (31.4) |
| Stopped drinking | 11 (37.9) | 11 (37.9) | 7 (25.0) | 29 (33.7) |
| Drinking | 10 (34.5) | 12 (41.1) | 8 (28.6) | 30 (34.9) |
| sUA, μmol/L | ||||
| Mean (SD) | 617.3 (97.6) | 589.8 (89.0) | 601.0 (133.5) | 602.7 (107.4) |
| Median | 599.0 | 574.0 | 597.5 | 587.0 |
| Range | 374–880 | 394–800 | 147–817 | 147–880 |
| Renal function, n (%) | ||||
| 60 ⩽ eGFR < 90 mL/min | 16 (55.2) | 15 (51.7) | 16 (57.1) | 47 (54.7) |
| eGFR ⩾ 90 mL/min | 13 (44.8) | 14 (48.3) | 12 (42.9) | 39 (45.3) |
| Gout history, n (%) | 29 (100) | 29 (100) | 28 (100) | 86 (100) |
| Cardiovascular comorbidity or disease, n (%) | ||||
| Hypertension | 3 (10.3) | 4 (13.8) | 2 (7.1) | 9 (10.5) |
| Hypercholesterolemia | 8 (27.6) | 3 (10.3) | 10 (35.7) | 21 (24.4) |
| Hypertriglyceridemia | 1 (3.5) | 0 | 2 (7.1) | 3 (3.5) |
| Type 2 diabetes mellitus | 2 (6.9) | 0 | 0 | 2 (2.3) |
| Renal disease, n (%) | 4 (13.8) | 1 (3.5) | 0 | 5 (5.8) |
eGFR, estimated glomerular filtration rate; SD, standard deviation; sUA, serum uric acid.
Study compliance
Participants in this study exhibited good compliance to study medication, with a mean compliance of 98.7% in the SHR4640 10 mg plus febuxostat 80 mg group, 98.9% in the SHR4640 10 mg plus febuxostat 40 mg group, and 99.0% in the SHR4640 5 mg plus febuxostat 20 mg group (Supplementary Table S1). The concomitant medication use known to affect sUA levels is listed in Supplementary Table S2.
Safety evaluation
The duration of medication exposure was comparable among the three treatment groups (Supplementary Table S1). TEAEs were observed in 51.6% of patients in the SHR4640 10 mg plus febuxostat 80 mg group, 56.3% of patients in the SHR4640 10 mg plus febuxostat 40 mg group, and 60.0% of patients in the SHR4640 5 mg plus febuxostat 20 mg group (Table 2). Most of the TEAEs were mild or moderate. No deaths were observed. TEAEs observed in 29.0%, 31.3%, and 30.0% of patients were considered to be related to study medication. Serious TEAEs occurred in one patient (3.1%) in the SHR4640 10 mg plus febuxostat 40 mg group (acute kidney injury), which were considered to be related to study treatment. Study treatment was discontinued due to the occurrence of TEAEs in one patient (3.1%) in the SHR4640 10 mg plus febuxostat 40 mg group and two patients (6.7%) in the SHR4640 5 mg plus febuxostat 20 mg group. The most commonly reported TEAEs were gout flares (22.6%) and increased alanine aminotransferase (15.1%) in all patients (Table 3).
Table 2.
Summary of TEAEs (safety analysis set).
| Adverse events | SHR4640 10 mg + febuxostat 80 mg (n = 31) |
SHR4640 10 mg + febuxostat 40 mg (n = 32) |
SHR4640 5 mg + febuxostat 20 mg (n = 30) |
Total (n = 93) |
|---|---|---|---|---|
| Any TEAE | 16 (51.6) | 18 (56.3) | 18 (60.0) | 52 (55.9) |
| Treatment-related TEAEs | 9 (29.0) | 10 (31.3) | 9 (30.0) | 28 (30.1) |
| Any serious TEAEs | 0 | 1 (3.1) | 0 | 1 (1.1) |
| Any treatment-related serious TEAEs | 0 | 1 (3.1) | 0 | 1 (1.1) |
| TEAEs leading to study discontinuation | 0 | 1 (3.1) | 2 (6.7) | 3 (3.2) |
| Treatment-related TEAEs leading to study discontinuation | 0 | 1 (3.1) | 2 (6.7) | 3 (3.2) |
TEAEs, treatment-emergent adverse events.
Data are shown as n (%).
Table 3.
TEAEs occurring in at least 5% of patients in any treatment group (safety analysis set).
| Adverse events | SHR4640 10 mg + febuxostat 80 mg (n = 31) |
SHR4640 10 mg + febuxostat 40 mg (n = 32) |
SHR4640 5 mg + febuxostat 20 mg (n = 30) |
Total (n = 93) |
|---|---|---|---|---|
| Gout flares | 8 (25.8) | 8 (25.0) | 5 (16.7) | 21 (22.6) |
| Increased alanine aminotransferase | 3 (9.7) | 5 (15.6) | 6 (20.0) | 14 (15.1) |
| Diarrhea | 2 (6.5) | 1 (3.1) | 1 (3.3) | 4 (4.3) |
| Upper respiratory tract infection | 0 | 3 (9.4) | 1 (3.3) | 4 (4.3) |
| Increased γ-glutamyltransferase | 0 | 1 (3.1) | 3 (10.0) | 4 (4.3) |
| Hematuria | 3 (9.7) | 0 | 0 | 3 (3.2) |
| Asthenia | 2 (6.5) | 1 (3.1) | 0 | 3 (3.2) |
| Increased blood creatinine | 0 | 1 (3.1) | 2 (6.7) | 3 (3.2) |
| Noninfective gingivitis | 0 | 2 (6.3) | 0 | 2 (2.2) |
| Back pain | 0 | 0 | 2 (6.7) | 2 (2.2) |
| Chest pain | 0 | 0 | 2 (6.7) | 2 (2.2) |
TEAEs, treatment-emergent adverse events.
Data are shown as n (%).
Efficacy evaluation
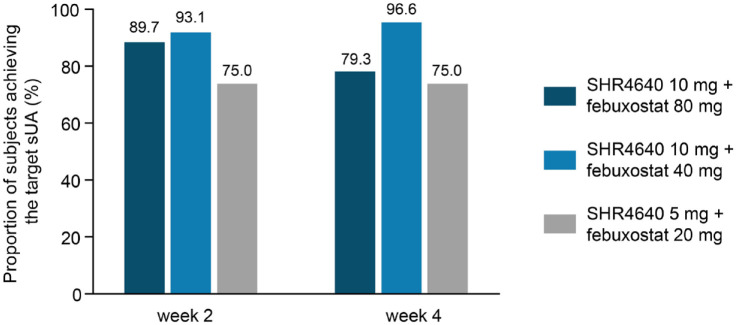
Based on the LOCF analysis, the proportion of patients who achieved sUA ⩽ 360 μmol/L by week 2 was 89.7% (95% CI: 72.6–97.8) in the SHR4640 10 mg plus febuxostat 80 mg group, 93.1% (95% CI: 77.2–99.2) in the SHR4640 10 mg plus febuxostat 40 mg group, and 75.0% (95% CI: 55.1–89.3) in the SHR4640 5 mg plus febuxostat 20 mg group. The proportion of patients who achieved the target sUA level by week 4 was 79.3% (95% CI: 60.3–92.0), 96.6% (95% CI: 82.2–99.9), and 75.0% (95% CI: 55.1–89.3), respectively (Figure 2). The lower bounds of 95% CIs for response rates among all the treatment groups were higher than 50%, demonstrating the effectiveness of SHR4640 plus febuxostat in patients with primary hyperuricemia with or without gout. In addition, prespecified sensitivity analyses using the OC method and NRI analysis showed similar results (Supplementary Tables S3 and S4).
Figure 2.
Proportion of patients who achieved a sUA target level ⩽ 360 μmol/L by visit in the full analysis set (LOCF imputation).
sUA, serum uric acid.
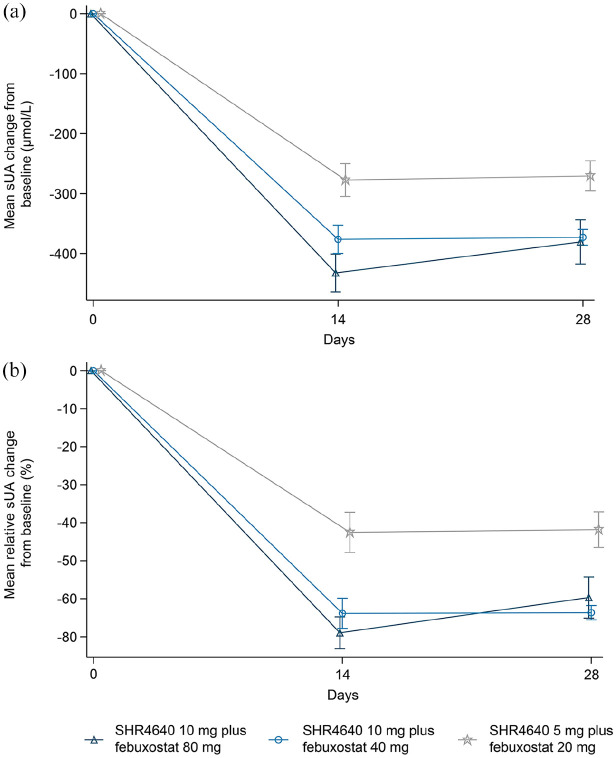
The mean absolute and relative changes in sUA levels are presented in Figure 3. The mean absolute reduction (SD) in sUA levels from baseline at week 2 was greater in the SHR4640 10 mg plus febuxostat 80 mg group [432.3 (169.7) μmol/L] and SHR4640 10 mg plus febuxostat 40 mg group [376.3 (127.1) μmol/L] than that in the SHR4640 5 mg plus febuxostat 20 mg group [277.3 (144.5) μmol/L] using the LOCF analysis. The mean absolute reduction (SD) in sUA levels from baseline at week 4 showed consistent results, with 380.7 (198.3) μmol/L, 373.0 (70.6) μmol/L, and 270.5 (132.9) μmol/L reductions, respectively (Supplementary Table S5). Based on the LOCF analysis, the mean percent (SD) change in sUA levels from baseline at week 2 was −69.0% (22.6%) in the SHR4640 10 mg plus febuxostat 80 mg group, −63.8% (21.4%) in the SHR4640 10 mg plus febuxostat 40 mg group, and −42.5% (28.0%) in the SHR4640 5 mg plus febuxostat 20 mg group. In the week 4 assessment, the mean percent (SD) change was −59.7% (29.1%), −63.7% (10.2%), and −41.8% (24.8%) among all three treatment groups, respectively (Supplementary Table S5). Results evaluated using the OC method were similar to the LOCF results (Supplementary Table S6).
Figure 3.
Absolute and relative changes from baseline in sUA levels at each visit in the full analysis set (LOCF imputation). (a) Mean (SE) absolute sUA change from baseline. (b) Mean (SE) relative sUA change from baseline.
sUA, serum uric acid.
Discussion
In this multicenter, randomized, double-blind, parallel-controlled trial, we investigated the safety, tolerability, and efficacy of once daily SHR4640 plus febuxostat in patients with primary hyperuricemia with or without gout. This study defined sUA levels ⩽ 360 μmol/L as the target on the basis of the recommendation of ACR guideline. 1 SHR4640 10 mg plus febuxostat (80 mg or 40 mg) and SHR4640 5 mg plus febuxostat 20 mg showed a manageable safety profile and substantial sUA lowering effect in patients with gout or asymptomatic hyperuricemia, with 79.3%, 96.6%, and 75.0% of patients reaching the target sUA level after 4 weeks of treatment, respectively.
The combination of SHR4640 and febuxostat was well tolerated. The majority of TEAEs were mild or moderate, and all the TEAEs were recovered or clinically stable with or without intervention. The proportion of patients experiencing TEAEs were generally comparable among the treatment groups. Three patients (3.2%) discontinued study treatment due to TEAEs: one was recovering and the other two had relived at the end of the study. It seemed that dose increase was not accompanied by elevated TEAEs in this study. The most commonly reported TEAEs were gout flares (22.6%) and increased alanine aminotransferase (15.1%). Concerns have been raised regarding the high incidence of increased alanine aminotransferase. In our trial, all the increased alanine aminotransferase was mild in severity, and no patients discontinued study treatment or had dose reduction because of this specific adverse event. In the FOCUS study, the incidence of increased alanine aminotransferase was much lower (5.1%) with SHR4640 monotherapy than that reported in this trial. 14 In addition, the most common adverse events reported with febuxostat include elevated liver function tests. 17 Therefore, we assumed that the high incidence of increased alanine aminotransferase might mainly be attributed to febuxostat. The individual liver function tests in this study are listed in Supplementary Table S7. The safety profile was similar to that in the FOCUS study in which patients were treated with SHR4640 monotherapy, and was also comparable with the previous reports with the combination of lesinurad and febuxostat.14,18
Uricosuric agents increase the excretion of uric acid, which may promote the formation of microcrystallization of uric acid in the renal tubules, leading to injury in the epithelium of renal cells. 18 Therefore, renal-related TEAEs should be closely monitored during study treatment. In this study, one patient (1.1%) in the SHR4640 10 mg plus febuxostat 40 mg group experienced serious TEAEs (acute kidney injury), with an elevation in serum creatinine ⩾ 3 times of the baseline level. However, the elevation of serum creatinine resolved after adequate hydration and alkalization of urine. Elevations in other patients were mild (NCI-CTCAE grade 1), and most of them had grade 1 creatinine elevation at baseline.
SHR4640 (10 mg) plus febuxostat (40 mg or 80 mg) and SHR4640 (5 mg) plus febuxostat (20 mg) substantially reduced the sUA levels. The proportion of patients who achieved the target sUA level ⩽ 360 μmol/L by week 4 was 79.3%, 96.6%, and 75.0% in the SHR4640 10 mg plus febuxostat 80 mg, SHR4640 10 mg plus febuxostat 40 mg, and SHR4640 5 mg plus febuxostat 20 mg groups, respectively. In the FOCUS study, 72.5% of patients in the SHR4640 10 mg group achieved the target sUA level at week 5, 14 while in this study, addition of febuxostat to SHR4640 increased the proportion of patients who achieved the target sUA level of 96.6% in the SHR4640 10 mg plus febuxostat 40 mg group. Although the between-study comparison should be interpreted cautiously, our results further confirmed the superior sUA lowering effect of the combination of XOIs and the URAT-1 inhibitor compared with the URAT-1 inhibitor alone. 19 It seemed that SHR4640 10 mg plus febuxostat (40 mg or 80 mg) had a better sUA lowering effect than SHR4640 5 mg plus febuxostat 20 mg. As SHR4640 10 mg plus febuxostat 80 mg showed a comparable sUA lowering effect compared with SHR4640 10 mg plus febuxostat 40 mg, the latter dosage might represent a potential regimen in treating patients with primary hyperuricemia.
Our study has some limitations. The first limitation is the short duration of study treatment. Second, a small number of female patients did not provide any firm conclusion in this patient population. The third limitation is the lack of a control or placebo group, which limits the direct comparison analysis. Future long-term, controlled studies are warranted to ascertain the benefits of SHR4640 combined with febuxostat.
The results of this study showed that SHR4640 combined with febuxostat was well tolerated, with substantial urate lowering activity in patients with primary hyperuricemia with or without gout. Future studies with a large sample size are warranted to investigate the long-term efficacy of this combination and determine the most effective dosage.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X211067304 for Safety and efficacy of SHR4640 combined with febuxostat for primary hyperuricemia: a multicenter, randomized, double-blind, phase II study by Honghu Tang, Beibei Cui, Yiyu Chen, Lin Chen, Zhihong Wang, Ning Zhang, Yanlan Yang, Xiaodong Wang, Xiangliang Xie, Lingyun Sun, Wantai Dang, Xianyang Wang, Runzi Li, Jianjun Zou, Yi Zhao and Yi Liu in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X211067304 for Safety and efficacy of SHR4640 combined with febuxostat for primary hyperuricemia: a multicenter, randomized, double-blind, phase II study by Honghu Tang, Beibei Cui, Yiyu Chen, Lin Chen, Zhihong Wang, Ning Zhang, Yanlan Yang, Xiaodong Wang, Xiangliang Xie, Lingyun Sun, Wantai Dang, Xianyang Wang, Runzi Li, Jianjun Zou, Yi Zhao and Yi Liu in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors thank the patients and their families for their participation in this study, as well as the study teams at each study site. They would also like to acknowledge Yanwen Wang (PhD, a medical writer at Jiangsu Hengrui Pharmaceuticals) for medical writing support according to Good Publication Practice Guidelines.
Footnotes
Author contributions: Honghu Tang: Data curation; Investigation; Writing – original draft; Writing – review & editing.
Beibei Cui: Data curation; Investigation; Writing – review & editing.
Yiyu Chen: Data curation; Investigation; Writing – review & editing.
Lin Chen: Data curation; Investigation; Writing – review & editing.
Zhihong Wang: Data curation; Investigation; Writing – review & editing.
Ning Zhang: Data curation; Investigation; Writing – review & editing.
Yanlan Yang: Data curation; Investigation; Writing – review & editing.
Xiaodong Wang: Data curation; Investigation; Writing – review & editing.
Xiangliang Xie: Data curation; Investigation; Writing – review & editing.
Lingyun Sun: Data curation; Investigation; Writing – review & editing.
Wantai Dang: Data curation; Investigation; Writing – review & editing.
Xianyang Wang: Data curation; Methodology; Project administration; Writing – original draft; Writing – review & editing
Runzi Li: Formal analysis; Writing – original draft; Writing – review & editing.
Jianjun Zou: Conceptualization; Methodology; Writing – review & editing.
Yi Zhao: Conceptualization; Data curation; Investigation; Methodology; Writing – review & editing.
Yi Liu: Conceptualization; Data curation; Investigation; Methodology; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: XYW, RL, and JZ are employees of Jiangsu Hengrui Pharmaceuticals. The other authors declared no conflict of interests.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Jiangsu Hengrui Pharmaceuticals Co., Ltd.
ORCID iD: Yi Liu  https://orcid.org/0000-0002-9234-5457
https://orcid.org/0000-0002-9234-5457
Data availability: The data of this article can be shared on reasonable request to the corresponding author.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Honghu Tang, Department of Rheumatology and Immunology, West China Hospital, Sichuan University, Chengdu, China.
Beibei Cui, Department of Rheumatology and Immunology, West China Hospital, Sichuan University, Chengdu, China.
Yiyu Chen, Department of Rheumatology and Immunology, West China Hospital, Sichuan University, Chengdu, China.
Lin Chen, Department of Rheumatology and Immunology, Jilin Province People’s Hospital, Changchun, China.
Zhihong Wang, Department of Endocrinology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Ning Zhang, Department of Rheumatology and Immunology, Shengjing Hospital of China Medical University, Shenyang, China.
Yanlan Yang, Department of Endocrinology, Shanxi Provincial People’s Hospital, Taiyuan, China.
Xiaodong Wang, Department of Rheumatology and Osteology, The First Affiliated Hospital of Clinical Medicine of Guangdong Pharmaceutical University, Guangzhou, China.
Xiangliang Xie, Department of Rheumatology and Immunology, Maanshan People’s Hospital, Maanshan, China.
Lingyun Sun, Department of Rheumatology and Immunology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China.
Wantai Dang, Department of Rheumatology and Immunology, The First Affiliated Hospital of Chengdu Medical College, Chengdu, China.
Xianyang Wang, Clinical Research and Development, Jiangsu Hengrui Pharmaceuticals Co., Ltd. (formerly Jiangsu Hengrui Medicine Co., Ltd.), Shanghai, China.
Runzi Li, Clinical Research and Development, Jiangsu Hengrui Pharmaceuticals Co., Ltd. (formerly Jiangsu Hengrui Medicine Co., Ltd.), Shanghai, China.
Jianjun Zou, Clinical Research and Development, Jiangsu Hengrui Pharmaceuticals Co., Ltd. (formerly Jiangsu Hengrui Medicine Co., Ltd.), Shanghai, China.
Yi Zhao, Department of Rheumatology and Immunology, West China Hospital, Sichuan University, No. 37 Guoxue Lane, Wuhou District, Chengdu 610041, China.
Yi Liu, Department of Rheumatology and Immunology, West China Hospital, Sichuan University, No. 37 Guoxue Lane, Wuhou District, Chengdu 610041, China.
References
- 1. Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res 2012; 64: 1431–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum 2011; 63: 3136–3141. [DOI] [PubMed] [Google Scholar]
- 3. Liu R, Han C, Wu D, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int 2015; 2015: 762820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol 2010; 6: 30–38. [DOI] [PubMed] [Google Scholar]
- 5. Neogi T. Gout. N Engl J Med 2011; 364: 443–452. [DOI] [PubMed] [Google Scholar]
- 6. Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 2006; 58: 87–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010; 12: R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schumacher HR, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 2008; 59: 1540–1548. [DOI] [PubMed] [Google Scholar]
- 9. Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum 2012; 64: 2529–2536. [DOI] [PubMed] [Google Scholar]
- 10. Hung S, Chung W, Liou L, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA 2005; 102: 4134–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jung J, Song W, Kim Y, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant 2011; 26: 3567–3572. [DOI] [PubMed] [Google Scholar]
- 12. Reinders M, van Roon EN, Jansen TL, et al. Efficacy and tolerability of urate-lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Ann Rheum Dis 2009; 68: 51–56. [DOI] [PubMed] [Google Scholar]
- 13.Zurampic (lesinurad) prescribing information. Wilmington, DE: AstraZeneca, 2015. [Google Scholar]
- 14. Lin Y, Chen X, Ding H, et al. Efficacy and safety of a selective URAT1 inhibitor SHR4640 in Chinese subjects with hyperuricemia: a randomized controlled phase II study. Rheumatology 2021; 60: 5089–5097, https://pubmed.ncbi.nlm.nih.gov/33693494 [DOI] [PubMed] [Google Scholar]
- 15. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977; 20: 895–900. [DOI] [PubMed] [Google Scholar]
- 16. Neogi T, Jansen TLTA, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol 2015; 67: 2557–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grewal HK, Martinez JR, Espinoza LR. Febuxostat: drug review and update. Expert Opin Drug Metab Toxicol 2014; 10: 747–758. [DOI] [PubMed] [Google Scholar]
- 18. Dalbeth N, Jones G, Terkeltaub R, et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with febuxostat in patients with tophaceous gout: findings of a phase III clinical trial. Arthritis Rheumatol 2017; 69: 1903–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bardin T, Keenan RT, Khanna PP, et al. Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study). Ann Rheum Dis 2017; 76: 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X211067304 for Safety and efficacy of SHR4640 combined with febuxostat for primary hyperuricemia: a multicenter, randomized, double-blind, phase II study by Honghu Tang, Beibei Cui, Yiyu Chen, Lin Chen, Zhihong Wang, Ning Zhang, Yanlan Yang, Xiaodong Wang, Xiangliang Xie, Lingyun Sun, Wantai Dang, Xianyang Wang, Runzi Li, Jianjun Zou, Yi Zhao and Yi Liu in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X211067304 for Safety and efficacy of SHR4640 combined with febuxostat for primary hyperuricemia: a multicenter, randomized, double-blind, phase II study by Honghu Tang, Beibei Cui, Yiyu Chen, Lin Chen, Zhihong Wang, Ning Zhang, Yanlan Yang, Xiaodong Wang, Xiangliang Xie, Lingyun Sun, Wantai Dang, Xianyang Wang, Runzi Li, Jianjun Zou, Yi Zhao and Yi Liu in Therapeutic Advances in Musculoskeletal Disease