Abstract
Radiotherapy-related fibrosis remains one of the most challenging treatment related side effects encountered by patients with head and neck cancer. Several established and ongoing novel therapies have been studied with paucity of data in how to best treat these patients. This review aims to provide researchers and health care providers with a comprehensive review on the presentation, etiology, and therapeutic options for this serious condition.
Keywords: Radiotherapy, head and neck radiation, fibrosis, prevention
Introduction
Head and neck cancers are a significant cause of morbidity and mortality worldwide, with more than 650,000 cases and 330,000 deaths per year. 1 Radiation therapy (RT) in the adjuvant or upfront setting, with or without chemotherapy, is a cornerstone of treatment. Nevertheless, RT presents with many acute and long-term complications, several manifesting months to years following treatment. Specifically, fibrosis and scarring of the surrounding skin and musculature is a common adverse effect occurring up to 1 year after completing treatment, and worsening over time. 2 This can be the attributed to a chronic inflammatory process due to repetitive injury induced by RT. The exact mechanisms of fibrosis are not fully clear but may be due to the excess production of fibroblasts and dysregulation of the wound healing processes.3,4 This process can manifest as neck stiffness, trismus, and pain and may be associated with lymphedema often leading to decreased quality of life for long-term cancer survivors of head and neck cancer.5–10
There have been several attempts to decrease the incidence and severity of postradiation fibrosis. These involve improvement in radiation techniques, physical therapy, and topical and systemic treatments.11–21 The aim of this review is to discuss the clinical presentation, underlying pathogenesis and current treatments for patients with postradiation fibrosis in the head and neck area. This review aims to uniquely highlight the up-to-date knowledge on molecular mechanisms and offer clinicians a summary of all historic therapies as well as novel treatment options that have emerged over the past few years.
Methods
A comprehensive literature review was performed using Medline and PubMed search engines using Mesh terms and keywords, such as “radiation therapy,” “head and neck neoplasms,” “adverse events,” “fibrosis,” and other related terms. Additional articles were recommended by colleagues and co-authors of this manuscript. More than 300 articles were retrieved, of which 86 were found relevant and revised for this review. The relevant data was further sorted in the following order (1) signs and symptoms of head and neck fibrosis, (2) the molecular mechanism of radiation-induced fibrosis formation, and (3) suitable treatment strategies
Clinical Presentation
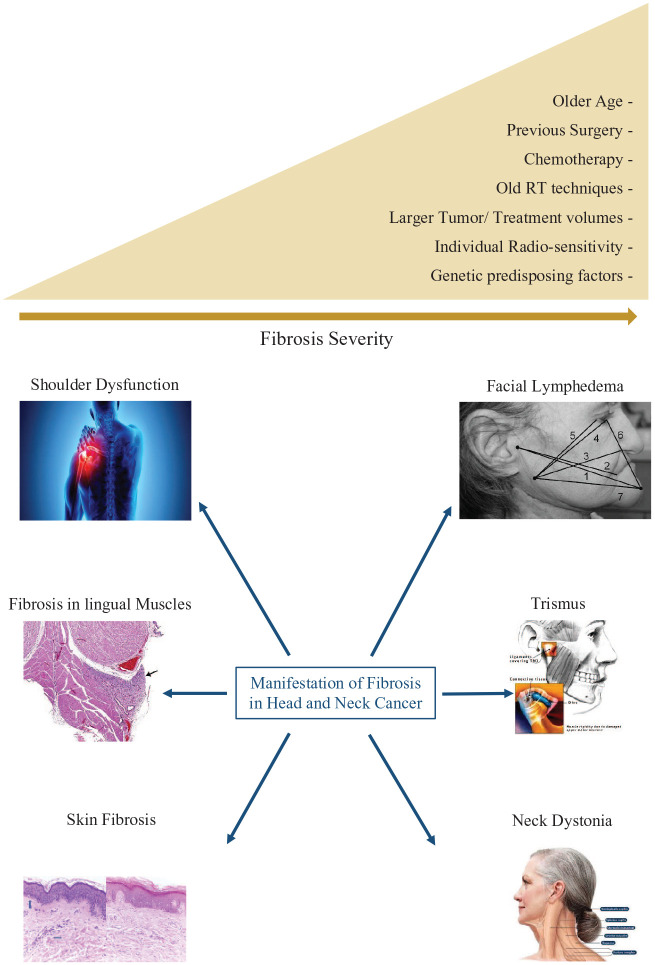
The severity of radiation fibrosis in the head and neck area is affected by several factors and tends to be worse in older patients, larger tumors, higher radiation doses, treatment volume, and in patients who have undergone other treatment modalities such as surgery and chemotherapy.22,23 The incidence of grade-2-or-higher neck fibrosis can sometimes exceed 50% after surgical dissection, and 34% with definitive chemoradiation.16,24 Even in patients treated with modern techniques, such as intensity-modulated radiation therapy (IMRT), the occurrence still remains as high as 30%. 15 A prospective study comparing acute and late side effects of 3D versus IMRT in 60 head and neck cancer patients receiving definitive (chemo)radiation therapy showed a lesser degree of grade-2-or-higher late subcutaneous fibrosis in patients treated with IMRT. 25 The severity of fibrosis in the head and neck area, similar to other disease subsites, is also greatly affected by individual radiosensitivity and the presence of genetic syndromes such as ataxia telangiectasia and others26–29 (Figure 1).
Figure 1.
Head and neck radiation-induced fibrosis manifestations and risk factors.
One common side effect of fibrosis in the head and neck area is shoulder dysfunction. This can be attributed to capsulitis as well as tendinitis and hardened tissues limiting the range of motion of the shoulder joint. This is usually managed with physical therapy and steroid injections into the joint to relieve pain and improve range of motion. 30 Radiation may also cause dystonia and spasms of the neck musculature. This can lead to contracture and fixed neck positions, or weakness and difficulty supporting head posture.30–32 Furthermore, fibrosis in lingual muscles and constrictor muscles may limit tongue mobility and swallowing. Trismus, which is a dysfunction in motion of the tempo-mandibular joint (lock jaw) is also a side effect attributed to the assimilation of fibrotic tissue in the joint and surrounding musculature, particularly the lateral pterygoids, often causing poor oral hygiene and nutrition as well as decreased quality of life.33–36 A suggested parameter to define trismus is a mouth opening of less than 35 mm. 37 It is a functional cut-off point for trismus in head and neck cancer patients. Incidence can approach 25% on long-term follow-up and dose to the ipsilateral masseter muscle was noted to be a significant risk factor.14,20,33,38,39 Early physical therapy focusing on range of motion has been shown to decrease the incidence of trismus, and specialized splinting, physical exercises, and botulinum toxins have been used for symptomatic relief.14,20,21,33 Lymphedema is also a common side effect due to radiation fibrosis and may be subdivided into internal and external components. It has been associated with worse quality of life and is more frequent in patients receiving multiple treatment modalities including surgical dissection and re-exploration surgical interventions9,32,40 (Figure 1).
Radiation fibrosis is most evident in the skin tissue and can be a long-term sequelae of radiation dermatitis. Notably, the severity of acute skin reactions at the end of radiation therapy have also been shown to be a prognostic factor for developing radiation fibrosis. 15 Chronic radiation skin fibrosis, in addition to its cosmetic burden, is a predisposing factor for complications due to poor wound healing and compromised vasculature. Fibrosis in the skin can cause xerosis, dyspigmentation, epidermal atrophy, as well as slow healing of painful ulcerations. Also, the skin itself may be a contributing factor to neck stiffness due to contractures and pain.5,27
The RTOG/EORTC late morbidity scoring schema takes into account radiation fibrosis in the grading of subcutaneous tissues but not of the skin. 41 Common terminology criteria for adverse events (CTCAE) do provide a graded clinical scale to measure fibrosis in superficial and deep soft tissues; however, there have been attempts to use more objective image-based severity scales using ultrasound and CT scans11,42 (Figure 1).
Molecular Mechanisms of Radiation Fibrosis
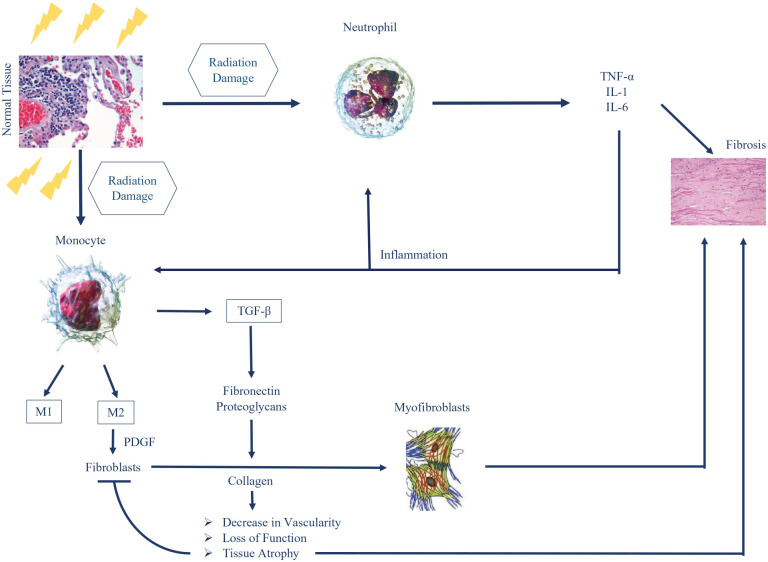
Fibrosis is one of the many RT side effects linked to molecular and cellular events defining radiosensitivity. 43 It is characterized by the induction of DNA damage and tissue inflammation. In fact, irradiation instigates many different types of DNA damage, but studies have shown that DNA double-strand breaks (DSBs) are the most lethal, and most difficult to repair.44–47 DNA DSB can be repaired via different pathways, such as the non-homologous end joining (NHEJ) repair and homologous recombination (HR).46–48 The repair kinetics of DNA DSB is a major factor in determining radiosensitivity, and many studies have highlighted correlation between unrepaired DSB and radio-induced toxicities.43,49–52 In parallel with DNA DSB induction, radiation causes the apparition of reactive oxygen species (ROS) that interacts with water molecules inside the nucleus, leading to the formation of hydroxyl radicals. 53 These radio-induced radicals are responsible for more than 2/3 of DNA DSB in the case of X-rays irradiation but can also damage other cellular components such as proteins, membranes, and RNA 54 (Figure 2).
Figure 2.
Molecular pathogenisis of radiation induced fibrosis.
Once damaged, cells release different types of molecules that stimulate the migration of inflammatory cells, such as neutrophils, monocytes, and lymphocytes. Neutrophils are known to be the first inflammatory cells recruited to the site of injured cells. 55 Their presence will lead to the release of several types of cytokines: tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), and 6 (IL-6). These cytokines have the capacity to exaggerate inflammation and are considered to be a direct cause of the onset of fibrosis. On the other hand, monocytes, once at the damaged site, will be differentiated into M1 and M2 macrophages. Platelet-derived growth factors (PDGFs) are then released by the M2 macrophages and will stimulate the migration of fibroblasts to the damaged region.56,57 More importantly, M2 will also secrete the transforming growth factor beta (TGF-β), a major player in radiation fibrosis. 27 There are 3 different isoforms of TGF-β, called TGF-β1 to β3, with TGF-β1being the most implicated in fibroproliferative diseases. 58
In fact, TGF-β is a protein that can regulate fibrogenesis by inducing the proliferation of postmitotic fibrocytes from progenitor fibroblasts. These fibrocytes have the capacity to produce collagen. TGF-β can also induce a more excessive extracellular matrix deposition by (1) upregulating tissue inhibitors of metalloproteinases (TIMPs) and (2) dysregulating matrix metalloproteinase (MMP) activity (MMP-2 and MMP-9). Finally, TGF-β induces the secretion of more collagen, fibronectin, and proteoglycans by leading to the differentiation of fibroblasts into myofibroblasts. These secretions are known to cause the increased tissue rigidity and thickness.59,60 Myofibroblasts are the major actor in fibrogenesis and are directly associated with the repair of tissues and fibrosis. 61 Moreover, excess collagen leads to a reduced vascularity over time. 62 This change in vascularity makes fibrotic regions more susceptible to loss of function, necrosis, tissue atrophy, and decrease in the number of fibroblasts. 3
Many studies have shown that the levels of TGF-β proteins, mainly TGF-β1, increases after irradiation in human and animal models, while they remain unchanged in unirradiated tissues. 63 TGF-β levels also increase with the additional doses of RT. 64 Moreover, patients showing a higher TGF-β1 plasma level are more at risk of developing radiation-induced fibrosis. The high levels of TGF-β might even remain detectable for months and years after the end of the treatment. 58 Interestingly, when TGF-β was administered to nonirradiated healthy animals, tissue fibrosis was observed. On the other hand, when patients were treated with liposomal Cu/Zn superoxide dismutase, an agent that downregulates TGF-β, the radiation-induced fibrosis was reversed. 65
Current Treatment Strategies
There is a paucity of data in the treatment of radiation fibrosis in the head and neck area with most clinicians extrapolating treatment from treatments applied to other subsites such as breast. The treatment approach consists of either systemic treatments, topical treatments, or mechanical treatments in addition to palliative measures. Palliative treatments can include botulinum toxin to relieve spasms as well as corticosteroids and nonsteroidal inflammatory drugs as well as other analgesics.12,21 Mechanical maneuvers are often used preventatively; however, studies on their efficacy as treatment of trismus are not consistent.14,19,20,66–69
Pentoxifylline, a xanthine derivative, has been used off-label in combination with Vitamin E (tocopherol), a free radical scavenger, to treat of radiation-induced fibrosis. Pentoxifylline has favorable vascular properties, such as improving microvasculature, increasing oxygen release to tissues, and having multiple immunomodulatory features that drive anti-fibrotic properties. Pentoxifylline is involved in downregulating protein kinases and other inflammatory cytokines. It also inhibits intracellular signaling to TFG-β, a pathway significantly involved in radiation fibrosis.70–72 Pentoxifylline, with and without tocopherol, displayed promising results in the treatment of fibrosis, osteonecrosis, and radiculopathy of different affected sites.12,17,73–77 A randomized study in breast cancer patients showed superiority of combination treatment of pentoxifylline with tocopherol over monotherapy. 75 In the head and neck region, its use was focused on treating trismus in nasopharyngeal cancer patients, with modest results of 4 mm mean gap improvement in a pilot study (n = 20). 17 In a randomized trial recruiting head and neck (n = 7) and other tumors (n = 36), pentoxifylline (800 mg), and Vit E (1000 mg) were recorded to significantly reduce fibrosis severity in all treated patients. Notably, treatment effect was realized in the first 6 months without any additional benefit at 12 months. 73 Another small randomized trial of 78 patients used pentoxifylline as monotherapy prophylaxis and showed lower incidence of late fibrosis as compared with the control arm. 78
Superoxide dismutase is a metalloenzyme found in many tissues that plays a role in the conversion of toxic superoxide radicals. 79 Topical use had shown promising results in patients with radiation fibrosis in the breast. 80 However, a recent randomized clinical trial studying 68 head and neck patients with radiation fibrosis treated with twice daily application of superoxide dismutase cream did not show any significant benefit as compared with the group receiving placebo. Of note, both the treatment and placebo arms had around 40% benefit at the end of the 3-month period, which may have been attributable to the massage/physical therapy that all patients received. 13
Statins have been shown in in-vitro and vivo studies to downregulate the fibrotic cascade. 81 The PRAVACUR trial was a prospective phase II trial that included 60 head and neck squamous cell carcinoma patients with cutaneous and subcutaneous fibrosis. Patients received pravastatin 40 mg daily for 1 year and were assessed based on skin thickness changes measured using high-frequency ultrasonography. The intervention was well tolerated and uncommon toxicities included myalgias and arthralgias. Results were promising with one-third of the patients having more than 30% reduction in skin thickness, and overall 50% of patients had clinical decrease in severity of fibrosis. 16 These studies have been summarized in the table form (Table 1).
Table 1.
Select studies in the treatment of radiation fibrosis in the head and neck.
| Study | Intervention | Study design and population | Results |
|---|---|---|---|
| Delanian et al. 73 | A combination of PTX (800 mg/d) and Vit E (1000 IU/d) was administered orally for at least 6 months | Phase 2 trial | 53% mean regression of fibrosis surface areas |
| Forty-three patients presenting with 50 distinct zones of chronic radiotherapy damage | Mean linear dimensions diminished from 6.5–4.5 cm | ||
| Mean subjective objective medical management and analytic (SOMA) scores improved significantly, from 13.2 to 6.9 | |||
| Chua et al. 17 | 8-week course of pentoxifylline at a dose of 400 mg two to three times daily | Pilot study | The mean dental gap before treatment was 12.5 mm compared with 16.5 mm at the end of treatment (P = .023) |
| Sixteen patients diagnosed with severe trismus (dental gap ⩽25 mm) after finishing radiotherapy for nasopharyngeal carcinoma | |||
| Hartl et al. 21 | Botox (Allergan) or 250 units of Dysport (Ipsen) * injected transcutaneously into the masseters | Prospective, nonrandomized | No improvement in trismus at 1 month |
| Nineteen patients in complete remission with radiation-induced pain and trismus with or without masticator spasms | Improvement in the function (P = .004), pain (P = .002), and cramps (P = .004) | ||
| No side effects occurred. | |||
| Landeen et al. 13 | Sodermix cream (280 IU/g superoxide dismutase) once daily on fibrotic area or placebo | Prospective, blinded study d | 46.4% Improvement in study arm vs 43.3% in placebo (Not statistically significant) |
| Randomized, prospective, blinded. | |||
| ⩾18 years, H&N radiation therapy-induced fibrosis | |||
| Zatarain et al. 67 | Stretching exercises with or without Dynasplint device during treatment and 3 months post-treatment | Phase 3, randomized 40 patients undergoing radiotherapy treatment for head and neck cancer | No benefit from using Dynasplint device regarding jaw opening (only 25% compliance in intervention arm) |
| Bourgier et al. 16 | Pravastatin 40 mg/d for 12 months | Phase 2 trial | 35.7% had ⩾30% decrease in thickness |
| Sixty NSCC cases in remission | 50% had decrease in severity | ||
| Grade ⩾2 cutaneous and subcutaneous neck radiation-induced fibrosis |
Dysport ipsen: abobotulinumtoxinA injection Dynasplint® Dynasplint systems company, Maryland, USA.
Future Directions
At the time of writing this review, a search on clinicaltrials.gov was conducted for “radiation toxicity.” Of the 234 trials shown, most trials listed were studying interventions, such as radio protectors, to decrease acute side effects. One active trial studying the effect of hyperbaric oxygen in the treatment of chronic radiation effects in breast (NCT04193722) was found. Notably hyperbaric oxygen has been studied for the treatment of other head and neck RT complications, such as osteonecrosis and xerostomia, with promising results despite its high costs and low availability.82–84 In our search, there were no identified trials for interventions in the treatment radiation fibrosis in head and neck patients.
Conclusion
Radiation fibrosis is a common adverse event in patients treated with radiation therapy for head and neck malignancies. The pathogenesis is complex and involves dysregulation of the wound healing processes and upregulation of TGF-β and inflammatory cytokines. Clinical presentation is variable but can include trismus, neck stiffness, and fibrosis of cutaneous and subcutaneous tissues. There is a paucity of data for treating this condition; however, promising results have been displayed in small studies using pentoxifylline and Vitamin E as well as pravastatin. This condition continues to be understudied and trials based on molecular understanding should be conducted to better improve patients’ therapeutic options.
Acknowledgments
Authors would like to acknowledge and thank Ms. Faryal Iqbal for helping with the figures.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: All the authors contirbuted equally in writing, reviewing and analyzing the manuscript.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. O’Sullivan B, Levin W. Late radiation-related fibrosis: pathogenesis, manifestations, and current management. Semin Radiat Oncol. 2003;13:274-289. [DOI] [PubMed] [Google Scholar]
- 3. Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141:1985-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Citrin DE, Prasanna PGS, Walker AJ, et al. Radiation-induced fibrosis: mechanisms and opportunities to mitigate. Report of an NCI workshop, September 19, 2016. Radiat Res. 2017;188:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baudelet M, Van den Steen L, Tomassen P, et al. Very late xerostomia, dysphagia, and neck fibrosis after head and neck radiotherapy. Head Neck. 2019;41:3594-3603. [DOI] [PubMed] [Google Scholar]
- 6. Shah S, Har-El G, Rosenfeld RM. Short-term and long-term quality of life after neck dissection. Head Neck. 2001;23:954-961. [DOI] [PubMed] [Google Scholar]
- 7. Sciubba JJ, Goldenberg D. Oral complications of radiotherapy. Lancet Oncol. 2006;7:175-183. [DOI] [PubMed] [Google Scholar]
- 8. Huang TL, Chien CY, Tsai WL, et al. Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head Neck. 2016;38 Suppl 1:E1026-E1032. [DOI] [PubMed] [Google Scholar]
- 9. Tyker A, Franco J, Massa ST, Desai SC, Walen SG. Treatment for lymphedema following head and neck cancer therapy: a systematic review. Am J Otolaryngol. 2019;40:761-769. [DOI] [PubMed] [Google Scholar]
- 10. Daugaard R, Kjaer T, Johansen C, et al. Association between late effects assessed by physicians and quality of life reported by head-and-neck cancer survivors. Acta Oncol. 2017;56:342-347. [DOI] [PubMed] [Google Scholar]
- 11. Shaw SM, Skoretz SA, O’Sullivan B, Hope A, Liu LW, Martino R. Valid and reliable techniques for measuring fibrosis in patients with head and neck cancer postradiotherapy: a systematic review. Head Neck. 2016;38 Suppl 1:E2322-E2334. [DOI] [PubMed] [Google Scholar]
- 12. Patel V, McGurk M. Use of pentoxifylline and tocopherol in radiation-induced fibrosis and fibroatrophy. Br J Oral Maxillofac Surg. 2017;55:235-241. [DOI] [PubMed] [Google Scholar]
- 13. Landeen KC, Spanos WC, Gromer L. Topical superoxide dismutase in posttreatment fibrosis in patients with head and neck cancer. Head Neck. 2018;40:1400-1405. [DOI] [PubMed] [Google Scholar]
- 14. Kamstra JI, Roodenburg JL, Beurskens CH, Reintsema H, Dijkstra PU. TheraBite exercises to treat trismus secondary to head and neck cancer. Support Care Cancer 2013;21:951-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nevens D, Duprez F, Daisne JF, Laenen A, De Neve W, Nuyts S. Radiotherapy induced dermatitis is a strong predictor for late fibrosis in head and neck cancer. The development of a predictive model for late fibrosis. Radiother Oncol. 2017;122:212-216. [DOI] [PubMed] [Google Scholar]
- 16. Bourgier C, Auperin A, Rivera S, et al. Pravastatin reverses established radiation-induced cutaneous and subcutaneous fibrosis in patients with head and neck cancer: results of the biology-driven phase 2 clinical trial pravacur. Int J Radiat Oncol Biol Phys. 2019;104:365-373. [DOI] [PubMed] [Google Scholar]
- 17. Chua DT, Lo C, Yuen J, Foo YC. A pilot study of pentoxifylline in the treatment of radiation-induced trismus. Am J Clin Oncol. 2001;24:366-369. [DOI] [PubMed] [Google Scholar]
- 18. McGarvey AC, Chiarelli PE, Osmotherly PG, Hoffman GR. Physiotherapy for accessory nerve shoulder dysfunction following neck dissection surgery: a literature review. Head Neck. 2011;33:274-280. [DOI] [PubMed] [Google Scholar]
- 19. Grandi G, Silva ML, Streit C, Wagner JC. A mobilization regimen to prevent mandibular hypomobility in irradiated patients: an analysis and comparison of two techniques. Med Oral Patol Oral Cir Bucal. 2007;12:E105-E109. [PubMed] [Google Scholar]
- 20. Barañano CF, Rosenthal EL, Morgan BA, McColloch NL, Magnuson JS. Dynasplint for the management of trismus after treatment of upper aerodigestive tract cancer: a retrospective study. Ear Nose Throat J. 2011;90:584-590. [DOI] [PubMed] [Google Scholar]
- 21. Hartl DM, Cohen M, Juliéron M, Marandas P, Janot F, Bourhis J. Botulinum toxin for radiation-induced facial pain and trismus. Otolaryngol Head Neck Surg. 2008;138:459-463. [DOI] [PubMed] [Google Scholar]
- 22. Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys. 2007;68:750-757. [DOI] [PubMed] [Google Scholar]
- 24. Hirota S, Tsujino K, Oshitani T, et al. Subcutaneous fibrosis after whole neck irradiation. Int J Radiat Oncol Biol Phys. 2002;52:937-943. [DOI] [PubMed] [Google Scholar]
- 25. Gupta T, Agarwal J, Jain S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol. 2012;104:343-348. [DOI] [PubMed] [Google Scholar]
- 26. Suarez EM, Knackstedt RJ, Jenrette JM. Significant fibrosis after radiation therapy in a patient with Marfan syndrome. Radiat Oncol J. 2014;32:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bray FN, Simmons BJ, Wolfson AH, Nouri K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther. 2016;6:185-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheuk IW, Yip SP, Kwong DL, Wu VW. Association of XRCC1 and XRCC3 gene haplotypes with the development of radiation-induced fibrosis in patients with nasopharyngeal carcinoma. Mol Clin Oncol. 2014;2:553-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiong H, Liao Z, Liu Z, et al. ATM polymorphisms predict severe radiation pneumonitis in patients with non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:1066-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DiFrancesco T, Khanna A, Stubblefield MD. Clinical evaluation and management of cancer survivors with radiation fibrosis syndrome. Semin Oncol Nurs. 2020;36:150982. [DOI] [PubMed] [Google Scholar]
- 31. Appels C, Goekoop R. Dropped-head syndrome due to high-dose irradiation. J Rheumatol. 2009;36:2316. [DOI] [PubMed] [Google Scholar]
- 32. Seidel C, Kuhnt T, Kortmann RD, Hering K. Radiation-induced camptocormia and dropped head syndrome: review and case report of radiation-induced movement disorders. Strahlenther Onkol. 2015;191:765-770. [DOI] [PubMed] [Google Scholar]
- 33. Nagaraja S, Kadam SA, Selvaraj K, Ahmed I, Javarappa R. Trismus in head and neck cancer patients treated by telecobalt and effect of early rehabilitation measures. J Cancer Res Ther. 2016;12:685-688. [DOI] [PubMed] [Google Scholar]
- 34. Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, Brennan MT. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer. 2010;18:1033-1038. [DOI] [PubMed] [Google Scholar]
- 35. Lee LY, Chen SC, Chen WC, Huang BS, Lin CY. Postradiation trismus and its impact on quality of life in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:187-195. [DOI] [PubMed] [Google Scholar]
- 36. Sroussi HY, Epstein JB, Bensadoun RJ, et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6:2918-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35:337-342. [DOI] [PubMed] [Google Scholar]
- 38. Gebre-Medhin M, Haghanegi M, Robért L, Kjellén E, Nilsson P. Dose-volume analysis of radiation-induced trismus in head and neck cancer patients. Acta Oncol. 2016;55:1313-1317. [DOI] [PubMed] [Google Scholar]
- 39. Lindblom U, Gärskog O, Kjellén E, et al. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta Oncol. 2014;53:620-627. [DOI] [PubMed] [Google Scholar]
- 40. Deng J, Ridner SH, Dietrich MS, et al. Factors associated with external and internal lymphedema in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;84:e319-e328. [DOI] [PubMed] [Google Scholar]
- 41. Hoeller U, Tribius S, Kuhlmey A, Grader K, Fehlauer F, Alberti W. Increasing the rate of late toxicity by changing the score? A comparison of RTOG/EORTC and LENT/SOMA scores. Int J Radiat Oncol Biol Phys. 2003;55(4):1013-1018. doi:10.1016/s0360-3016(02)04202-5 [DOI] [PubMed] [Google Scholar]
- 42. Deng J, Dietrich MS, Ridner SH, Fleischer AC, Wells N, Murphy BA. Preliminary evaluation of reliability and validity of head and neck external lymphedema and fibrosis assessment criteria. Eur J Oncol Nurs. 2016;22:63-70. [DOI] [PubMed] [Google Scholar]
- 43. Granzotto A, Benadjaoud MA, Vogin G, et al. Influence of nucleoshuttling of the ATM protein in the healthy tissues response to radiation therapy: toward a molecular classification of human radiosensitivity. Int J Radiat Oncol Biol Phys. 2016;94:450-460. [DOI] [PubMed] [Google Scholar]
- 44. Foray N, Arlett CF, Malaise EP. Radiation-induced DNA double-strand breaks and the radiosensitivity of human cells: a closer look. Biochimie. 1997;79:567-575. [DOI] [PubMed] [Google Scholar]
- 45. Foray N, Monroco C, Marples B, et al. Repair of radiation-induced DNA double-strand breaks in human fibroblasts is consistent with a continuous spectrum of repair probability. Int J Radiat Biol. 1998;74:551-560. [DOI] [PubMed] [Google Scholar]
- 46. Bodgi L, Canet A, Pujo-Menjouet L, Lesne A, Victor JM, Foray N. Mathematical models of radiation action on living cells: from the target theory to the modern approaches. A historical and critical review. J Theor Biol. 2016;394:93-101. [DOI] [PubMed] [Google Scholar]
- 47. Bodgi L, Granzotto A, Devic C, et al. A single formula to describe radiation-induced protein relocalization: towards a mathematical definition of individual radiosensitivity. J Theor Biol. 2013;333:135-145. [DOI] [PubMed] [Google Scholar]
- 48. Bodgi L, Foray N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: resolution of the linear-quadratic model. Int J Radiat Biol. 2016;92:117-131. [DOI] [PubMed] [Google Scholar]
- 49. Ferlazzo ML, Bourguignon M, Foray N. Functional assays for individual radiosensitivity: a critical review. Semin Radiat Oncol. 2017;27:310-315. [DOI] [PubMed] [Google Scholar]
- 50. Gatti RA. The inherited basis of human radiosensitivity. Acta Oncol. 2001;40:702-711. [DOI] [PubMed] [Google Scholar]
- 51. Massart C, Joubert A, Granzotto A, et al. Prediction of the human radiosensitivity: what is the most relevant endpoint? Gene expressions, mutations or functions? In: Kocsis A, Molna H, eds. Genotoxicity: Evaluation, Testing and Prediction. New York, NY: Nova Science Publishers, Inc., 2009. [Google Scholar]
- 52. Vogin G, Bastogne T, Bodgi L, et al. The phosphorylated ATM immunofluorescence assay: a high-performance radiosensitivity assay to predict postradiation therapy overreactions. Int J Radiat Oncol Biol Phys. 2018;101:690-693. [DOI] [PubMed] [Google Scholar]
- 53. Tak JK, Park J-W. The use of ebselen for radioprotection in cultured cells and mice. Free Radic Biol Med. 2009;46:1177-1185. [DOI] [PubMed] [Google Scholar]
- 54. Terasaki Y, Ohsawa I, Terasaki M, et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2011;301:L415-L426. [DOI] [PubMed] [Google Scholar]
- 55. Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453-4460. [DOI] [PubMed] [Google Scholar]
- 56. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604. [DOI] [PubMed] [Google Scholar]
- 57. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549-555. [DOI] [PubMed] [Google Scholar]
- 58. Martin M, Lefaix J-L, Delanian S. TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277-290. [DOI] [PubMed] [Google Scholar]
- 59. Shaw SM, Skoretz SA, O’Sullivan B, Hope A, Liu LWC, Martino R. Valid and reliable techniques for measuring fibrosis in patients with head and neck cancer postradiotherapy: a systematic review. Head Neck. 2016;38:E2322-E2334. [DOI] [PubMed] [Google Scholar]
- 60. Deng J, Wulff-Burchfield EM, Murphy BA. Late soft tissue complications of head and neck cancer therapy: lymphedema and fibrosis. JNCI Monogr. 2019. [DOI] [PubMed] [Google Scholar]
- 61. Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136-143. [DOI] [PubMed] [Google Scholar]
- 62. Lefaix JL, Daburon F. Diagnosis of acute localized irradiation lesions: review of the French experimental experience. Health Phys. 1998;75:375-384. [DOI] [PubMed] [Google Scholar]
- 63. Pohlers D, Brenmoehl J, Löffler I, et al. TGF-β and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746-756. [DOI] [PubMed] [Google Scholar]
- 64. de Andrade CBV, Ramos IPR, de Moraes ACN, et al. Radiotherapy-induced skin reactions induce fibrosis mediated by TGF-β1 cytokine. Dose Response. 2017;15:1559325817705019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lewanski CR, Gullick WJ. Radiotherapy and cellular signalling. Lancet Oncol. 2001;2:366-370. [DOI] [PubMed] [Google Scholar]
- 66. Marrafon CS, Matos LL, Simões-Zenari M, Cernea CR, Nemr K. Speech-language therapy program for mouth opening in patients with oral and oropharyngeal cancer undergoing adjuvant radiotherapy: a pilot study. Codas. 2018;30:e20160221. [DOI] [PubMed] [Google Scholar]
- 67. Zatarain LA, Smith DK, Deng J, et al. A randomized feasibility trial to evaluate use of the jaw dynasplint to prevent trismus in patients with head and neck cancer receiving primary or adjuvant radiation-based therapy. Integr Cancer Ther. 2018;17:960-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee R, Yeo ST, Rogers SN, et al. Randomised feasibility study to compare the use of Therabite® with wooden spatulas to relieve and prevent trismus in patients with cancer of the head and neck. Br J Oral Maxillofac Surg. 2018;56:283-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cohen EG, Deschler DG, Walsh K, Hayden RE. Early use of a mechanical stretching device to improve mandibular mobility after composite resection: a pilot study. Arch Phys Med Rehabil. 2005;86:1416-1419. [DOI] [PubMed] [Google Scholar]
- 70. Lee JG, Shim S, Kim MJ, et al. Pentoxifylline regulates plasminogen activator inhibitor-1 expression and protein kinase A phosphorylation in radiation-induced lung fibrosis. BioMed Res Int. 2017;2017:1279280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hamama S, Gilbert-Sirieix M, Vozenin MC, Delanian S. Radiation-induced enteropathy: molecular basis of pentoxifylline-vitamin E anti-fibrotic effect involved TGF-β1 cascade inhibition. Radiother Oncol. 2012;105:305-312. [DOI] [PubMed] [Google Scholar]
- 72. Packer L, Weber SU, Rimbach G. Molecular aspects of α-tocotrienol antioxidant action and cell signalling. J Nutr. 2001;131:369S–373S. [DOI] [PubMed] [Google Scholar]
- 73. Delanian S, Balla-Mekias S, Lefaix J-L. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol. 1999;17:3283-3290. [DOI] [PubMed] [Google Scholar]
- 74. Delanian S, Lefaix J-L, Maisonobe T, Salachas F, Pradat P-F. Significant clinical improvement in radiation-induced lumbosacral polyradiculopathy by a treatment combining pentoxifylline, tocopherol, and clodronate (Pentoclo). J Neurol Sci. 2008;275:164-166. [DOI] [PubMed] [Google Scholar]
- 75. Delanian S, Porcher R, Balla-Mekias S, Lefaix J-L. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21:2545-2550. [DOI] [PubMed] [Google Scholar]
- 76. Dion MW, Hussey DH, Doornbos JF, Vigliotti AP, Wen B-C, Anderson B. Preliminary results of a pilot study of pentoxifylline in the treatment of late radiation soft tissue necrosis. Int J Radiat Oncol Biol Phys. 1990;19:401-407. [DOI] [PubMed] [Google Scholar]
- 77. Delanian S, Chatel C, Porcher R, Depondt J, Lefaix J-L. Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherol-clodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80:832-839. [DOI] [PubMed] [Google Scholar]
- 78. Aygenc E, Celikkanat S, Kaymakci M, Aksaray F, Ozdem C. Prophylactic effect of pentoxifylline on radiotherapy complications: a clinical study. Otolaryngol Head Neck Surg. 2004;130:351-356. [DOI] [PubMed] [Google Scholar]
- 79. Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59-62. [DOI] [PubMed] [Google Scholar]
- 80. Campana F, Zervoudis S, Perdereau B, et al. Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis. J Cell Mol Med. 2004;8:109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Haydont V, Bourgier C, Pocard M, et al. Pravastatin inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res. 2007;13:5331-5340. [DOI] [PubMed] [Google Scholar]
- 82. Ravi P, Vaishnavi D, Gnanam A, Krishnakumar Raja VB. The role of hyperbaric oxygen therapy in the prevention and management of radiation-induced complications of the head and neck – a systematic review of literature. J Stomatol Oral Maxillofac Surg. 2017;118:359-362. [DOI] [PubMed] [Google Scholar]
- 83. Teguh DN, Bol Raap R, Struikmans H, et al. Hyperbaric oxygen therapy for late radiation-induced tissue toxicity: prospectively patient-reported outcome measures in breast cancer patients. Radiat Oncol. 2016;11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cankar K, Finderle Z, Jan J. The effect of hyperbaric oxygenation on postradiation xerostomia and saliva in patients with head and neck tumours. Caries Res. 2011;45:136-141. [DOI] [PubMed] [Google Scholar]