Abstract
Background:
The history of intracranial hemorrhage (ICrH) is considered a contraindication for intravenous thrombolysis (IVT) among patients with acute ischemic stroke (AIS). Objective: This study aimed at comparing the safety of IVT among patients with and without a history of ICrH.
Methods:
We performed a systematic review of the literature. Data regarding all AIS patients with prior ICrH who received IVT were retrieved. Meta-analysis was performed to compare the rate of symptomatic hemorrhagic transformation (sHT), death within 90 days, and favorable and unfavorable 90-day functional outcomes based on modified Rankin Scale (mRS) among stroke patients with and without prior ICrH.
Results:
Out of 13,032 reviewed records, 7 studies were included in the systematic review and meta-analysis. Quantitative synthesis of data regarding the rate of sHT (5068 patients) revealed no significant difference between the two groups [odds ratio, OR: 1.55 (0.77, 3.12); p = 0.22]. However, a significantly higher risk of death within 90 days [OR: 3.91 (2.16, 7.08); p < 0.00001] and a significantly higher 90-day poor functional outcomes (mRS, 4–6) [OR: 1.57 (1.07, 2.30); p = 0.02] were observed among patients with prior ICrH. Likewise, the percentage of 90-day good functional outcomes (mRS, 0–1) was lower in the prior ICrH group [OR: 0.54 (0.35, 0.84); p = 0.06]. Subgroup analyses in patients with a history of ICrH (based on both patients’ medical history and imaging confirmation) revealed no significant between-group differences in rates of sHT. Also, sensitivity analysis consisting of only studies using standard-dose IVT showed no difference in sHT rates and 90-day outcomes between the two groups. There was no evidence of heterogeneity (I2 >50%) among included studies.
Conclusion:
The results of this study indicated that prior history of ICrH does not increase the risk of sHT post-IVT, but it is associated with a higher risk of death and poor functional outcomes in 90 days.
Keywords: intracranial hemorrhages, intravenous thrombolytic therapy, ischemic stroke, mortality, symptomatic hemorrhagic transformation
Introduction
Intravenous thrombolysis (IVT) is one of the most effective therapies for acute ischemic stroke (AIS) 1 and diminishes disability and mortality among stroke patients. 2 However, IVT increases the risk of symptomatic hemorrhagic transformation (sHT) of AIS, which varies based on the patients’ characteristics.3,4 Based on recent surveys in Europe, only a small percentage of patients with AIS (7.3%, on average) receive IVT mainly due to the narrow treatment time window and the long list of contraindications. 5
Several stroke guidelines such as American Heart/Stroke Association (AHA/ASA) categorize a history of intracranial hemorrhage (ICrH) as a contraindication for IVT therapy.6,7 However, the history of ICrH, as a contraindication for IVT, was removed from alteplase prescribing information due to the lack of evidence of harm. 8 In addition, European Stroke Organization (ESO) 2021 guideline makes no particular recommendation on the use of IVT among these patients due to the lack of data. 9
Several studies have investigated outcomes of AIS patients with prior history of ICrH who were either unknowingly or intently treated with IVT.10–13 These studies mostly showed no increment in the rate of adverse outcomes; however, due to the limited number of included patients, there is still no consensus on whether IVT is safe when there is a history of ICrH.
This systematic review and meta-analysis aimed to investigate the safety of IVT among AIS patients with and without a history of ICrH. We further performed a sensitivity analysis of the studies, comparing patients with both history and imaging confirmation of a prior ICrH and the studies which used standard-dose IVT.
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 14
Literature search and selection criteria
To identify the relevant studies, we performed a systematic search in PubMed, Embase, and Cochrane databases in April 2020 with no language or document type restriction. The top search terms included intracranial hemorrhage, subarachnoid hemorrhage, intracerebral hemorrhage, subdural hematoma, epidural hematoma, intravenous, thrombolysis, tissue plasminogen activator, alteplase, ischemic, ischemia, stroke, cerebrovascular accident, CVA, cerebral, infarction, and infarct. We used different Boolean operators and wild cards specific to each database to build the search query. We conducted forward and backward citation tracking and communicated with selected authors to augment the search results.
Outcome measures
The outcome measures were defined as data-driven and as (1) the rate of sHT, (2) 90-day modified Rankin Scale (mRS), and (3) death within 90 days. Prior ICrH includes any history of intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), subdural hematoma (SDH), and epidural hematoma (EDH). ICH was defined either by clinical history, based on imaging, or both. In addition, sHT was defined by an in-hospital clinical deterioration of four points or higher in the National Institutes of Health Stroke Scale (NIHSS) confirmed by imaging modality. There was a moderate variation among studies regarding the ICH and sHT definition. Supplementary Table 1 includes the definitions used in the included studies.
Eligibility criteria
Studies were included if they met the following eligibility criteria: (1) included patients with AIS or imaging findings in favor of prior ICrH, with or without a history of ICrH, prior to the index AIS; (2) included patients who received IVT despite a previous history of ICrH; and (3) provided the outcome measures of our review.
Exclusion criteria consisted of (1) case reports, case series, letters, commentaries, abstract-only reports, review articles, and animal and in vitro studies; (2) original studies that only reported the outcome of patients with microbleeds (i.e. old hemorrhagic lesions containing hemosiderin smaller than 5 mm); 15 and (3) original studies that included patients with other contraindications of thrombolysis therapy.
Study selection was in concordance with PRISMA guidelines. 16 Two authors (M.D. and M.S.) independently assessed the eligibility criteria for the inclusion of studies. In case of any disagreement, the final decision was delegated to a third person (S.S.).
Data extraction
Data extraction was performed based on a predefined protocol by two coauthors (M.D. and E.K.). Disagreements were resolved by a third author (S.S.). The extracted items for the cohort of patients with and without previous ICrH included (1) demographics (i.e. age and sex); (2) stroke-related characteristics, including the NIHSS before receiving IVT, time elapsed from symptom onset to receiving IVT, and type of prior ICrH (i.e. ICH, SAH, SDH, and EDH); (3) presence of comorbidities including diabetes mellitus, hypertension, hyperlipidemia, smoking, coronary artery disease, and atrial fibrillation; (4) related medications such as oral anticoagulants; (5) length of follow-up from receiving IVT to assessing/occurrence of outcomes; and (6) the outcomes including in-hospital sHT, 90-day mRS, and death within 90 days of the index ischemic stroke. Favorable and unfavorable clinical outcomes were defined as 90-day mRS scores 0–1 and mRS scores of 4–6, respectively, based on the data and defined classification in the included studies. The number of patients with or without previous ICrH treated with IVT and the number of patients with favorable and unfavorable outcomes were retrieved from each study.
Quality assessment, risk of bias, and publication bias assessments
Two authors (M.D. and M.S.) independently assessed the quality of the included studies based on the Newcastle–Ottawa Scale (NOS) for appraisal of case–control and cohort studies in sample selection, comparability of cases and controls, and exposure domains. 17 The total NOS score is 9, where studies with NOS scores higher than 6 are considered at low risk of bias, and those with NOS scores lower than 4 are considered at high risk of bias.
Funnel plots demonstrated if there was a publication bias. A further statistical analysis was not performed due to less than 10 included publications in each analysis. 18 Instead, we used nonstatistical methods, such as searching for unpublished material in clinical trial registries and conference proceedings to prevent publication bias. 19
Data synthesis and meta-analysis
We used Review Manager 5.4.1 for statistical analyses. Odds ratio (OR) was considered the effect size measure for comparing sHT, 90-day mRS scores, and mortality within 90 days of the index ischemic stroke among patients who received IVT between patients with or without previous ICrH. Based on Cochrane guidelines, heterogeneity was assessed by Q statistic tests and the I2 index.20,21 I2 index estimates the percentage of the total variation observed in the studies, attributable to heterogeneity rather than chance. An I2 value of more than 50% is predictive of significant heterogeneity among studies. Forest plots were used for manifesting the heterogeneity among the studies.20,21 In the absence of significant heterogeneity, we used a fixed-effects model for meta-analyses. A p-value of <0.1 for Cochran’s Q test of heterogeneity and a p-value of <0.5 for other analyses were considered statistically significant.
Due to the heterogeneity in the definition of prior ICrH in studies, we further used sensitivity analysis. For this aim, patients with a confirmed prior ICrH based on both medical history and imaging modalities were included in the sensitivity analysis.
Results
Study selection
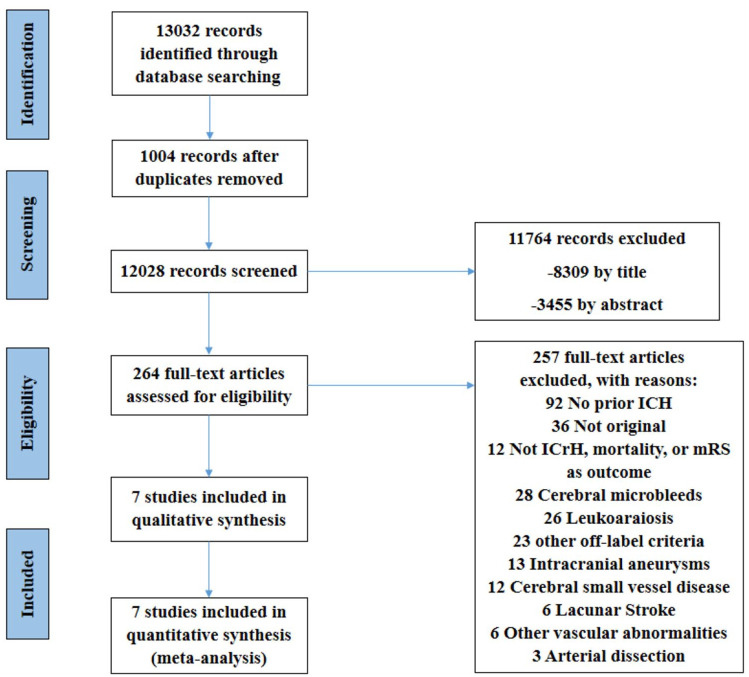
A total of 13,032 reports were identified by systematic search in PubMed, Embase, and Cochrane (Figure 1). After removing duplicates, we screened the title and abstracts of 12,028 reports and excluded 11,764 studies. Of the remaining 264 studies eligible for full-text screening, 7 studies were included in the systematic review. Other studies were excluded due to issues such as different study populations (not including AIS patients with prior ICrH, 92 articles), presence of cerebral microbleeds (CMBs) or leukoaraiosis rather than a history of ICrH (28 and 26 articles, respectively), other off-label criteria (23 articles), different outcome measures (no report of ICrH, mortality, or mRS, 12 articles), and study type other than original (review, letter, etc., 36 articles).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of included studies.
Study characteristics
The details of patients’ characteristics can be found in Tables 1 and 2. A total of seven retrospective observational studies were included in the systematic review. All of the seven included studies reported similar rates of sHT following IVT among patients with and without prior ICrH.10–13,22–24 Four studies reported risk of in-hospital,11,25,26 within 30-day, 22 or within 90-day10,13 death. Zand et al. 11 reported no in-hospital mortalities in the seven patients with prior ICrH. Also, Meretoja et al. 23 reported no death within 30 days in the three patients with prior ICrH. In addition, four studies reported 90-day mRS scores in both groups.10,12,13,23 Among these studies, Lee et al. 10 reported less favorable 90-day functional outcomes in patients with prior ICrH, while others did not show any difference between groups.
Table 1.
Demographic and clinical characteristics of baseline and post-IVT in patients with AIS.
| Author | Condition | Sample size | Age median (IQR) or mean (SD) | Gender (%male) | NIHSS median (IQR) or mean (SD) | Length of follow-up | Adverse outcome |
Difference between group | ||
|---|---|---|---|---|---|---|---|---|---|---|
| mRS (n, %) | ICrH (n, %) | Death (n, %) | ||||||||
| AbdelRazek et al. 24 | w/ pv ICH (IPH) | 11 | 75 (12.4) | 70.4 | – | 90 days | – | sICH: 1, 9.9%, aICH: 2,19.8% | – | Similar sICH (OR, 1.00; 95% CI, 0.06–17.5, p = 0.69) |
| w/o pv ICH (IPH) | 613 | – | – | – | sICH: 25, 4.03% | – | ||||
| Aoki et al. 12 | w/ aICH on T2 | 25 | 77 (70–83) | 48 | 12 (6–18) | 24 h + 90 days | 0–1 (6, 24%) 4–6 (15, 59%) |
sICH: 1, 4% aICH: 6, 24% |
– | Similar aICH (OR, 1.19; 95% CI, 0.40–3.54, p = 0.753), sICH (OR, 0.95; 95% CI, 0.08–11.90, p = 0.970), similar mRS 0–1 (p = 0.300), and mRS 4–6 (p = 0.202) |
| w/o aICH on T2 | 275 | 76 (68–83) | 59 | 0–1 (105, 38%), 4–6 (113, 41%) | sICH: 6, 2.18% aICH: 92, 33.5% |
– | ||||
| Kvistad et al. 22 | w/ pv ICH | 3 | – | – | – | 30 days | – | sICH: 0 | – | Similar sICH (OR, 3.26; 95% CI, 0.15–71.19) |
| w/o pv ICH | 130 | 63.7 (12.2) | 29.2 | 7 (3–13) | 0–1 (56, 43.4%) | sICH: 5, 3.9% | At day 7: 3 (2.3%) at day 30: 6 (4.6%) | |||
| Lee et al. 10 | w/ pv ICH | 73 | 72 (9.3) | 65.8 | 11 (6–16) | 24 h + 90 days | 0–1 (14, 19.4%), 4–6 (34, 46.8%) | sICH: 5, 6.8% | 14 (19.4%) | Similar sICH or sHT (p = 0.39) (OR, 1.08, 95% CI: 0.39–2.96), at discharge (p values > 0.4), less favorable outcomes at 3 months (mRS 0–1 (p = 0.01), mRS 0–2 (p = 0.07), and mortality (p = 0.07)) |
| w/o pv ICH | 1422 | 67.9 (12.7) | 58.5 | 10 (6–16) | 0–1 (501, 35.2%), 4–6 (516, 6.3% | sICH: 66, 4.6% | 170 (11.9%) | |||
| Meretoja et al. 23 | w/ pv ICrH (ICH or SAH) | 3 a | 80.0 (4.6) | – | 9 (5.5) | 90 days | 0–1 (177, 37.6%), 4–6 (118, 25.1 %) | sICH b : 0 | 0 (0%) | Similar sICH (OR, 1.23; 95% CI, 0.06–24.25), and mortality (OR, 1.48; 95% CI, 0.08–29.12) |
| w/o pv ICrH | 486 | 64.5 (11.2) | – | 12 (5.1) | 0–1 (2, 66.6%), 4–6 (0, 0%) | sICH b : 50, 10.3% | 41 (8.7%) | |||
| Zand et al. 11 | All patients | 1212 | 62 (14) | 52 | Median: 7 | Hospital stay | – | sICH: 44, 3.6% | 73 (6.02%) | Similar sICH (0% versus 3.6%, p = 0.61) (OR, 1.75; 95% CI, 0.10–31.13) and in-hospital mortality (0% versus 6.0%, p = 0.50) (OR, 1.03; 95% CI, 0.06–18.27) |
| w/ pv ICH | 7 | 72 (11) | 57 | Median: 5 | – | sICH: 0 aICH: 1, CMB: 1, multi-CMB: 2 | 0 (0%) | |||
| Zhao et al. 13 | w/ pv ICH | 12 | 66 (60–73) | 58.3 | 13 (10–16) | 90 days | 0–1 (5, 41.7%), 4–6 (3, 25%) | sICH: 1, 8.3% | 1 (8.3%) | Higher sICH (8.3% versus 4.3%, p = 0.039 OR, 2.03; 95% CI, 0.25–16.18), 90-d mRS scores of 0–1 (41.7% versus 43.6%, p = 0.530), and similar 90-day mortality rate (8.3% versus 6.5%, p = 0.946, OR, 1.30; 95% CI, 0.16–10.23) |
| w/o pv ICH | 793 | 66 (60–72) | 61.8 | 12 (10–15) | 0–1 (346, 43.6%), 4–6 (174, 21.9%) | sICH: 34, 4.3% | 52 (6.5%) | |||
a, asymptomatic; AIS, acute ischemic stroke; CI, confidence interval; CMB, cerebral microbleed; HT, hemorrhagic transformation; ICH, intracerebral hemorrhage; ICrH, intracranial hemorrhage; IPH, intraparenchymal hemorrhage; IQR, interquartile range; IVT, intravenous thrombolysis; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; NINDS, Neurological Disorders and Stroke; OR, odds ratio; pv, previous; s, symptomatic; SAH, subarachnoid hemorrhage; SD, standard deviation; w/, with; w/o, without.
One case of ICH and two cases of SAH.
Based on NINDS definition (not European Cooperative Acute Stroke Study (ECASS) or Safe Implementation of Treatments in Stroke (SITS) criteria).
Table 2.
Vascular risk factors in patients with and without prior intracranial hemorrhage.
| Author | Condition | HTN | DM | Stroke | AF | HLP | Coronary heart diseases | Smoking | SBP (mmHg) | DBP (mmHg) | Statin use | Antiplatelet use | Anticoagulants use | Pre-stroke mRS | Stroke etiology | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Small vessel disease | Atherosclerosis | Cardioembolism | |||||||||||||||
| AbdelRazek et al. 24 | w/ prior ICrH | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Aoki et al. 12 | w/ prior ICrH | 18/25 | 7/25 | 10/25 | 16/25 | 4/25 | 1/25 | – | 160 | 86 | – | 9/25 | 3/25 | – | 0/25 | 2/25 | 16/25 |
| w/o prior ICrH | 189/275 | 65/275 | 24/275 | 125/275 | 75/275 | 19/275 | – | 153 | 82 | – | 59/275 | 24/275 | – | 15/275 | 30/275 | 136/275 | |
| Difference | p = 0.824 | p = 0.628 | p < 0.001* | p = 0.094 | p = 0.342 | p = 1.000 | – | p = 0.35 | p = 0.83 | – | p = 0.131 | p = 0.481 | – | p = 0.623 | p = 1.000 | p = 0.211 | |
| Kvistad et al. 22 | w/o prior ICrH | 58/130 | 15/130 | 13/130 | 22/130 | – | – | – | – | – | – | – | – | – | 6/130 | 19/130 | 37/130 |
| Lee et al. 10 | w/ prior ICrH | 59/73 | 24/73 | 42/73 | 33/73 | 21/73 | – | 9/73 | 150.5 (29.7) | 85.2 (17.3) | 19/73 | – | 34/73 | ⩾1: 23/73 | 23/73 | 17/73 | 33/73 |
| w/o prior ICrH | 826/1422 | 311/1422 | 204/1422 | 552/1422 | 214/1422 | – | 345/1422 | 146.4 (27.1) | 85.7 (16.5) | 199/1422 | – | 451/1422 | ⩾1: 226/1422 | 477/1422 | 405/1422 | 570/1422 | |
| Difference | p < 0.001* | p = 0.03* | p < 0.001* | p = 0.28 | p = 0.002* | – | p = 0.02* | p = 0.2 | p = 0.8 | p = 0.004* | – | p = 0.01* | p = 0.001* | ToAST classification (p = 0.57) | |||
| Meretoja et al. 23 | With prior ICrH | 3/3 | 0/3 | 0/3 | 1/3 | – | – | – | 169 (16.9) | – | 2/3 | 3/3 | – | 2–5: 0/3 | – | – | – |
| W/o prior ICrH | 261/486 | 63/486 | 44/486 | 124/486 | – | – | – | 151 (19.9) | – | 117/486 | 184/486 | – | 2–5: 31/486 | – | – | – | |
| Zand et al. 11 | With prior ICrH | 100% | 28% | 43% | – | – | – | – | – | – | – | – | – | – | – | – | – |
| All patients | 77% | 34% | 24% | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Difference | p < 0.01* | p > 0.05 | p < 0.05* | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Zhao et al. 13 | With prior ICrH | 10/12 | 2/12 | – | 3/12 | 4/12 | 5/12 | 5/12 | – | – | – | – | – | 0–1: 11/12 | 4/12 | 5/12 | 2/12 |
| All patients | 492/793 | 153/793 | – | 191/793 | 301/793 | 260/793 | 350/793 | – | – | – | – | – | 0–1: 737/793 | 270/793 | 305/793 | 176/793 | |
| Difference | p = 0.028* | p = 0.561 | – | p = 0.486 | p = 0.837 | p = 0.157 | p = 0.302 | – | – | – | – | – | p = 0.611 | p = 0.761 | p = 0.809 | p = 0.297 | |
a, asymptomatic; AF, atrial fibrillation; DBP, diastolic blood pressure; DM, diabetes mellitus; HLP, hyperlipidemia; HTN, hypertension; ICrH, intracranial hemorrhage; mRS, modified Rankin scale; Pv, previous; s, symptomatic; SBP, systolic blood pressure; TOAST, Trial of Org 10172 in Acute Stroke Treatment; w/, with; w/o, without.
Significant between-group difference.
Also, two studies compared the outcomes of patients with prior ICrH who were treated with IVT to those who had not received IVT.10,13 Zhao et al. 13 showed similar rates of sHT, death within 90 days, and significantly better functional outcomes in patients who received IVT than those who did not. Lee et al., 10 however, observed no significant difference between these groups.
Seven articles10–13,22–24 were eligible for meta-analysis. All of the included studies in the meta-analyses were observational. Four of the included studies in the meta-analysis required findings in favor of previous ICrH according to magnetic resonance imaging (MRI) images acquired at their initial admission to the hospital, or previous head computed tomography (CT) scans associated with prior history of ICrH11,13 or not.12,24 Two studies, however, only relied on the history of ICrH, as claimed by their families.22,23 In the study by Lee et al., 10 a group of patients had both history and imaging confirmation, while other patients had either a history or imaging finding indicating prior ICrH.
Rate of sHT following IVT in patients with and without a history of ICrH
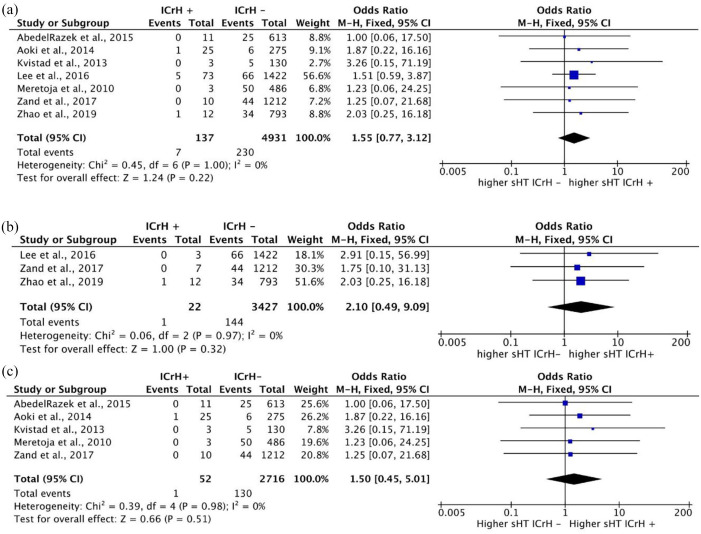
None of the seven included studies showed a significant difference between prior ICrH and no prior ICrH groups regarding the rate of sHT. The crude percentages of sHT were 5.1% (7 of 137 cases) among patients with ICrH and 4.6% (230 of 4931 cases) among those without prior ICrH. Quantitative synthesis using a fixed-effects model and in the absence of heterogeneity showed no significant difference in the rate of sHT between patients with and without a history of ICrH [OR: 1.55 (0.77, 3.12); p = 0.22] (Figure 2(a)).
Figure 2.
Forest plots for the meta-analysis comparing the rate of sHT. (a) Rate of sHT in patients with prior ICrH versus patients without prior ICrH. (b) Rate of sHT in patients with prior ICrH versus patients without prior ICrH (based on patients’ medical history AND imaging confirmation). (c) Rate of sHT in patients with prior ICrH versus patients without prior ICrH in studies using standard-dose IVT.
For sensitivity analysis, we performed a subgroup analysis of the three studies, which compared the rate of sHT in a subgroup of patients with a positive medical history in addition to imaging confirmation of ICrH. Quantitative synthesis consisting of these studies in the absence of heterogeneity revealed no significant between-group difference in the rates of sHT [OR: 2.10 (0.49, 9.09); p = 0.32] (Figure 2(b)). In addition, a subgroup analysis of studies using standard-dose IVT showed no significant difference in the rates of sHT [OR: 1.50 (0.45, 5.01); p = 0.51] (Figure 2(c)).
Rate of mortality following IVT in patients with and without a history of ICrH
Two studies reported death within 90 days as an outcome. The crude percentages of death within 90 days were 17.6% (15 of 85 cases) among patients with a history of ICrH and 5.5% (122 of 2215 cases) in patients without prior ICrH. Meta-analysis revealed a significant difference in frequency of death between these two groups [OR: 3.91 (2.16, 7.08); p < 0.00001] (Figure 3).
Figure 3.
Forest plots for the meta-analysis comparing overall mortality rate within 90 days in patients with prior ICrH versus patients without prior ICrH.
90-day mRS following IVT in patients with and without a history of ICrH
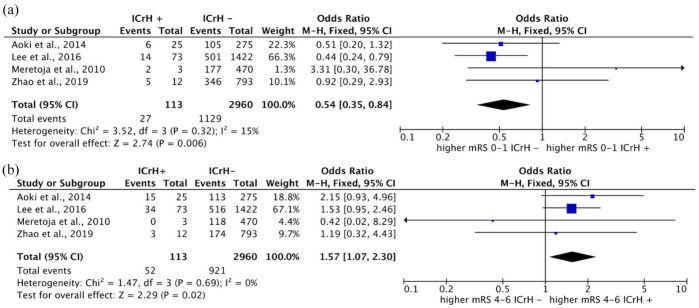
Four studies included data on 90-day mRS scores following IVT in patients with ICrH history. Overall, 23.8% (27 of 113) and 46.0% (52 of 113) of patients with prior ICrH and 38.1% (1129 of 2960) and 31.1% (921 of 2960) of the patients without previous history of ICrH showed 90-day mRS scores of 0–1 and 4–6, respectively. Meta-analysis of the four studies with available data regarding 90-day mRS scores, consisting of 113 and 2960 patients with and without prior ICrH, revealed significantly lower rates of favorable outcomes (mRS, 0–1) in patients with prior ICrH compared with those without a history of ICrH (OR: 0.54; 95% CI, 0.35–0.84; p = 0.006) (Figure 4(a)). Similarly, we observed higher rates of unfavorable outcomes (mRS, 4–6) in patients with prior ICrH compared with those without prior ICrH [OR: 1.57 (1.07, 2.30); p = 0.02] (Figure 4(b)).
Figure 4.
Forest plots for the meta-analysis based on 90-day mRS scores in patients with prior ICrH versus patients without prior ICrH: (a) favorable outcomes (mRS, 0–1) and (b) unfavorable outcomes (mRS, 4–6).
Furthermore, sensitivity analyses including two studies using standard-does IVT showed no significant difference between groups in rates of favorable [OR: 0.93 (0.17, 5.09); p = 0.93] (Figure 5(a)) and unfavorable outcomes [OR: 1.83 (0.84, 3.96); p = 0.13] (Figure 5(b)).
Figure 5.
Forest plots for the meta-analysis based on 90-day mRS scores in patients with prior ICrH versus patients without prior ICrH in studies using standard-dose IVT: (a) favorable outcomes (mRS, 0–1) and (b) unfavorable outcomes (mRS, 4–6).
Quality assessment and risk of bias
A summary of quality assessment based on NOS is provided (Supplementary Table 2). Overall, three of the included studies (42.7%) in the meta-analyses were subject to selection bias in terms of representativeness of the cohorts.11,22,23 Still, all of the studies met the quality assessment criteria based on exposure and outcome presence to select the cases and controls. Also, in all studies, the cases were not comparable to controls (at least in one domain) except for Aoki et al. 12 and Zhao et al. 13
A summary of the risk of bias assessment is provided (Supplementary Table 3). All of the included studies used nonrandom selection and allocation and thus were subject to a high risk of selection bias. The latter was especially the case for the study by Kvistad et al. and Merejota et al., which included a low number of patients with prior ICrH. For instance, the allocation of the patients with prior ICrH into intervention and nonintervention groups was not random in some studies, for example, based on their families’ wishes or clinical judgment. For instance, in the study by Lee et al., the patients deprived of IVT tended to have lower NIHSS scores and higher pre-stroke disabilities. 10 Only the study by Aoki et al. reported blinding of the outcome assessment. In some studies,10,13,22 the prior ICrH and no prior ICrH groups were significantly different in clinical characteristics other than the history of ICrH, such as age, prior history of stroke, and NIHSS scores.
In the studies by Zhao et al. and Lee et al., a low-dose tissue plasminogen activator (tPA) was used to treat all or some of the patients with prior ICrH for safety reasons, while other studies used a standard dose. All of the included studies, however, were of low risk of attrition bias or reporting bias.
Funnel plots on rates of sHT, mortality, and mRS scores did not present publication bias (Supplementary Figures 1–5).
Discussion
Our results suggest that IVT among AIS patients with prior history of ICrH is not associated with higher rates of sHT, but is associated with a higher risk of death within 90 days and a higher risk of 90-day unfavorable functional outcomes compared with those without a history of ICrH.
According to this meta-analysis, sHT rate among patients with no prior ICrH was 4.6% (230 of 4931 cases). An analysis of the third International Stroke Trial (IST-3) demonstrated that 6.8% (104 of 1515) of patients treated with IVT developed a sHT. 27 Overall, the rate of sHT in clinical trials is estimated to be 7.4%. In contrast, it is estimated to be 3.5% in registries, which signifies the variability caused by case definition in determining the rate of sHT. 3 In addition, this meta-analysis showed the risk of death within 90 days among patients without a history of ICrH to be 5.5% (122 of 2215 cases).
Similarly, the study by Liao et al. 28 showed that the mortality rate in patients who received a standard dose of alteplase was 7.36%. In another study, the mortality rate was estimated to be 7.7% in the patients receiving alteplase within 3–4.5 h of symptoms onset. 29 These reports on mortality rates are comparable to the results of our meta-analysis.
Recently, the myriad of contraindications for IVT in AIS, which limit its application, has been questioned. Recent large-scale studies and meta-analyses have reached some results in favor of using off-label IVT. 30 For instance, a meta-analysis on six studies comprising nearly 900 patients with a history of prior stroke within 3 months of index stroke who were treated with IVT showed similar rates of sHT and death and similar 3-month neurological improvement compared with the patients without such history. 31 However, the data regarding the severity of the stroke and the time between prior and index stroke were not determined. Furthermore, a meta-analysis comparing IVT outcomes in AIS patients who were pretreated with oral anticoagulants to those not receiving oral anticoagulation showed no significant difference in sHT, early mortality, and functional outcomes. 32 In this meta-analysis, we observed that IVT in AIS patients with prior history of ICrH does not increase the risk of sHT. At the same time, it is associated with higher mortality rates within 90 days and significantly higher rates of unfavorable 90-day functional outcomes. Similarly, a recent individual patient data meta-analysis by Charidimou et al. 33 has shown that the presence of incidental CMBs on imaging is not associated with increased risk of sHT but is associated with higher unfavorable outcomes in 3–6 months. However, this study showed that the risk of sHT increases when the CMB burden is high. 33 Other meta-analyses on CMBs have shown a significantly higher risk of sHT and poor functional outcomes associated with CMBs, especially when the CMB burden is high.34–36 Multiple hemorrhages/microbleeds, similar to prior history of ICrH, might signify an underlying vascular pathology, which increases the risk for severe stroke, poor functional outcomes, and the susceptibility to developing sHT. 37 It is noteworthy that small vessel diseases, including CMBs, white matter hyperintensities, lacunae, and cerebral amyloid angiopathy, are recognized to contribute to the occurrence of spontaneous ICH. 38 Patients with small vessel diseases are at risk for increased fragility of blood vessels, which along with mechanisms such as matrix metalloproteinase upregulation and hypertension, contribute to the increased risk for sHT. 39 Nevertheless, it has been shown that patients with underlying small vessel disease might benefit from IVT, and this should not be considered an exclusion criterion. 40 In this regard, taking into account the overall vascular pathologies on admission MRI and the prior history of ICrH, hypertension or stroke might be beneficial in individual decision-making.
Taking the importance of vascular risk factors into account, the versatile premorbid status of patients and the underlying causes of prior ICrH or stroke could have affected our results. Only one study in this meta-analysis provided data on underlying mechanism of prior ICrH. More data were presented on the underlying vascular pathologies for the index stroke, showing similar distribution of stroke etiologies among patients with and without ICrH history. However, most of the included studies reported significantly higher history of stroke or hypertension in patients with an ICrH history (see Table 2), a fact that might have confounded the results of this meta-analysis.
In this study, we performed a sensitivity analysis based on the definition of prior ICrH. Patients with a confirmed history of ICrH by both imaging and medical history were analyzed, and no difference was observed regarding the rates of sHT between groups. This may signify that imaging findings in favor of prior ICrH, even asymptomatic ICrH detected by admission MRI, pose no lower risk for mortality within 90 days of IVT in patients with AIS than a true history of ICrH. However, the limited number of studies/patients in these subgroup analyses may not provide the necessary power to detect the between-group differences.
Importantly, all patients with prior ICrH in the study by Zhao et al. and some patients with prior ICrH in the study by Lee et al. received low-dose IV r-tPA (0.6 mg/kg). Some studies have recommended low-dose IVT in AIS, as it might be accompanied by noninferior efficacy in reducing mRS scores, lower or similar ICH rates, lower mortality rates,41,42 and similar overall outcomes, which is especially the case in Asian population or patients taking antiplatelets.43–45 However, several studies have shown less favorable/inferior functional outcomes despite similar or lower sHT and mortality rates associated with low-dose IVT.45–47 We performed a subgroup analysis on studies with standard-dose IVT to check for sensitivity of the meta-analysis to IVT dosage. Contrary to the primary results, which showed higher rates of unfavorable outcomes in patients with ICrH history, sensitivity analysis showed similar 90-day mRS scores in patients with and without prior ICrH. Although these studies do not suffice for a conclusion, it can be inferred that low-dose IVT is associated with no lower unfavorable outcomes rates than standard-dose IVT in patients with ICrH history.
Furthermore, to determine whether IVT should be considered an appropriate measure in the treatment of AIS patients with prior ICrH, comparing the outcomes of patients with prior ICrH who have received IVT to similar patients who have been deprived of this treatment should be studied. For instance, old age (above 80 years) was once believed to be a contraindication for receiving IVT. Older patients had less favorable outcomes than younger patients after receiving IVT. Later, it was observed that the proportion of favorable outcomes in AIS patients above 80 years who had received IVT was higher than those who did not receive IVT, and the mortality rates were similar. Therefore, old age is no longer considered a contraindication for IVT. 48 Due to the limited number of studies and insufficient data, we could not perform a quantitative synthesis to compare the outcomes of receiving IVT or deprivation from IVT in patients with a prior history of ICrH. Zhao et al. showed that the prior ICrH group who underwent low-dose IVT had similar rates of sHT and 90-day mortality rates compared with the group receiving conventional therapy. However, the group treated with IVT had lower 90-day mRS scores and better functional outcomes. 13 Lee et al. 10 also showed similar clinical outcomes in patients with prior ICrH treated with IVT or not.
Although most of the contraindications according to AHA/ASA guidelines are experimented within large, standard trials and meta-analyses, some contraindications for receiving IVT, including prior history of ICrH, are not tested rigorously. In this study, we systematically reviewed the evidence in favor of IVT in patients with a previous history of ICrH or against it, from multiple aspects, including cerebral hemorrhage, functional outcome, and mortality, for the first time. A myriad of factors, however, might have compromised the results of this meta-analysis. First, two abstracts (with no full-texts) from the same research group were excluded from this meta-analysis because they had not undergone peer review.25,26 In addition, these two studies may have used similar cohorts. These two abstracts reported significantly higher mortality rates in patients with prior history of ICrH, unlike the studies included in this meta-analysis. Second, mortality analysis in this study comprised only two studies by Lee et al. and Zhao et al., on Asian population that applied both standard-dose and low-dose IVT, causing a potential risk of bias. Third, in the study by Zhao et al., some of the patients with ICrH other than ICH (like SDH, SAH) were excluded, 13 while some of the studies included in this meta-analysis included all types of ICrH. 23 Fourth, only some included studies reported the location, number, and size of the incident ICrH after thrombolysis and whether it had occurred in the previous site or not. Nevertheless, the study by Lee et al. 10 did reveal that the rate of sHT in patients with multiple hemorrhagic lesions is not significantly higher than those with single lesions. Fifth, other potential sources of heterogeneity could be the etiology of prior ICrH, and the time interval between the prior hemorrhage and the index stroke. However, only one study provided the relevant data. Sixth, in some studies, the two comparison groups were significantly different in some factors such as history of stroke or hypertension, or allocation of patients into treatment groups was nonrandom. Finally, whether the previous hemorrhage was intra-parenchymal or not was a source of heterogeneity. In addition, asymptomatic hemorrhage was reported as an outcome in some studies, while in some studies, it was not. Some studies reported asymptomatic hemorrhagic transformation (aHT) in both prior ICrH and no prior ICrH groups, while some only reported aHT in the group with prior ICrH. Thus, we could not consider aHT as an outcome as selective reporting of outcomes in these groups was probable. Therefore, we could not determine whether or not there is any significant difference in the rate of aHT or overall HT between patients with and without a history of ICrH.
Conclusion
Prior ICrH has been considered a contraindication for IVT in AIS. Based on the current systematic review and meta-analysis, the rate of sHT in patients with prior ICrH is no more than those without prior ICrH. However, the rate of mortality within 90 days and unfavorable functional outcomes are higher in patients with prior ICrH. The current relevant studies are mostly nonrandomized and suffer from bias and limitations in their design. To reach a more robust consensus on whether these groups of patients might benefit from IVT, it is pivotal to perform large-scale, randomized controlled studies, especially comparing the outcomes of IVT and conventional treatment in patients with prior history of ICrH. Furthermore, comparing the outcomes of low-dose and standard-dose IVT in patients undergoing off-label thrombolysis would be helpful. Multiple factors, including other comorbidities, age, time between prior ICrH and incident stroke, and NIHSS, should be considered to make individualized decisions.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864221074144 for Intravenous thrombolysis in ischemic stroke patients with a prior intracranial hemorrhage: a meta-analysis by Mahsa Dolatshahi, Mohammadmahdi Sabahi, Shima Shahjouei, Eric Koza, Vida Abedi and Ramin Zand in Therapeutic Advances in Neurological Disorders
Footnotes
Author contributions: Mahsa Dolatshahi: Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing – original draft; Writing – review & editing.
Mohammadmahdi Sabahi: Data curation; Formal analysis; Visualization; Writing – original draft.
Shima Shahjouei: Conceptualization; Methodology; Project administration; Supervision; Writing – review & editing.
Eric Koza: Data curation; Writing – review & editing.
Vida Abedi: Conceptualization; Project administration; Writing – review & editing.
Ramin Zand: Conceptualization; Investigation; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ramin Zand  https://orcid.org/0000-0002-9477-0094
https://orcid.org/0000-0002-9477-0094
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mahsa Dolatshahi, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran Neurology Department, Neuroscience Institute, Geisinger Health System, Danville, PA, USA.
Mohammadmahdi Sabahi, Neurology Department, Neuroscience Institute, Geisinger Health System, Danville, PA, USANeurosurgery Research Group (NRG), Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran.
Shima Shahjouei, Neurology Department, Neuroscience Institute, Geisinger Health System, Danville, PA, USA.
Eric Koza, Geisinger Commonwealth School of Medicine, Scranton, PA, USA.
Vida Abedi, Neuroscience Institute, College of Medicine, The Pennsylvania State University, Hershey, PA, USA.
Ramin Zand, Neurology Department, Neuroscience Institute, Geisinger Health System, 100 North Academy Avenue, Danville, PA 17822, USA. Neuroscience Institute, College of Medicine, The Pennsylvania State University, Hershey, PA, USA.
References
- 1. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 2. Wardlaw JM, Murray V, Berge E, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2014; 2014: CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Modrego PJ. The risk of symptomatic intracranial hemorrhage after thrombolysis for acute stroke: current concepts and perspectives. Ann Indian Acad Neurol 2019; 22: 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seet RCS, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: a critical review of case definitions. Cerebrovasc Dis 2012; 34: 106–114. [DOI] [PubMed] [Google Scholar]
- 5. Aguiar de Sousa D, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J 2019; 4: 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 7. Fugate JE, Rabinstein AA. Absolute and relative contraindications to IV rt-PA for acute ischemic stroke. Neurohospitalist 2015; 5: 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: 581–641. [DOI] [PubMed] [Google Scholar]
- 9. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021; 6: I–LXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee S-H, Kim BJ, Han M-K, et al. Should we exclude acute stroke patients with previous intracerebral hemorrhage from receiving intravenous thrombolysis? Int J Stroke 2016; 11: 783–790. [DOI] [PubMed] [Google Scholar]
- 11. Zand R, Tsivgoulis G, Sadighi A, et al. Safety of intravenous thrombolysis in chronic intracranial hemorrhage: a five-year multicenter study. J Stroke Cerebrovasc Dis 2018; 27: 620–624. [DOI] [PubMed] [Google Scholar]
- 12. Aoki J, Shibazaki K, Saji N, et al. Risk of intracerebral hemorrhage after thrombolysis in patients with asymptomatic hemorrhage on T2*. Cerebrovasc Dis 2014; 38: 107–116. [DOI] [PubMed] [Google Scholar]
- 13. Zhao G-J, Wang Z-R, Lin F-Z, et al. The safety and efficacy of tPA intravenous thrombolysis for treating acute ischemic stroke patients with a history of cerebral hemorrhage. Braz J Med Biol Res 2019; 52: e7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenberg SM, Nandigam RN, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke 2009; 40: 2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 18. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000; 53: 1119–1129. [DOI] [PubMed] [Google Scholar]
- 19. Lin L, Chu H, Murad MH, et al. Empirical comparison of publication bias tests in meta-analysis. J Gen Intern Med 2018; 33: 1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP. Cochrane handbook for systematic reviews of interventions version 5.0.1. The Cochrane Collaboration, 2008, http://www.cochrane-handbook.org
- 21. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kvistad CE, Logallo N, Thomassen L, et al. Safety of off-label stroke treatment with tissue plasminogen activator. Acta Neurol Scand 2013; 128: 48–53. [DOI] [PubMed] [Google Scholar]
- 23. Meretoja A, Putaala J, Tatlisumak T, et al. Off-label thrombolysis is not associated with poor outcome in patients with stroke. Stroke 2010; 41: 1450–1458. [DOI] [PubMed] [Google Scholar]
- 24. AbdelRazek MA, Mowla A, Hojnacki D, et al. Prior asymptomatic parenchymal hemorrhage does not increase the risk for intracranial hemorrhage after intravenous thrombolysis. Cerebrovasc Dis 2015; 40: 201–204. [DOI] [PubMed] [Google Scholar]
- 25. Chu SY, Sommaruga S, Hwang D, et al. Abstract TMP14: thrombolysis in ischemic stroke patients with prior history of intracranial hemorrhage. Stroke 2018; 49: ATMP14. [Google Scholar]
- 26. Sommaruga S, Chu S, Hwang D, et al. History of intracranial hemorrhage is associated with in-hospital mortality in ischemic stroke patients treated with intravenous thrombolytics. J Neurol Surg A Cent Eur Neurosurg 2018; 79: P30. [Google Scholar]
- 27. Whiteley WN, Thompson D, Murray G, et al. Targeting recombinant tissue-type plasminogen activator in acute ischemic stroke based on risk of intracranial hemorrhage or poor functional outcome: an analysis of the third international stroke trial. Stroke 2014; 45: 1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liao X, Wang Y, Pan Y, et al. Standard-dose intravenous tissue-type plasminogen activator for stroke is better than low doses. Stroke 2014; 45: 2354–2358. [DOI] [PubMed] [Google Scholar]
- 29. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 30. Tsivgoulis G, Kargiotis O, De Marchis G, et al. Off-label use of intravenous thrombolysis for acute ischemic stroke: a critical appraisal of randomized and real-world evidence. Ther Adv Neurol Disord 2021; 14: 1756286421997368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsivgoulis G, Katsanos AH, Schellinger PD, et al. Intravenous thrombolysis in patients with acute ischaemic stroke with history of prior ischaemic stroke within 3 months. J Neurol Neurosurg Psychiatry 2019; 90: 1383–1385. [DOI] [PubMed] [Google Scholar]
- 32. Shahjouei S, Tsivgoulis G, Goyal N, et al. Safety of intravenous thrombolysis among patients taking direct oral anticoagulants: a systematic review and meta-analysis. Stroke 2020; 51: 533–541. [DOI] [PubMed] [Google Scholar]
- 33. Charidimou A, Turc G, Oppenheim C, et al. Microbleeds, cerebral hemorrhage, and functional outcome after stroke thrombolysis. Stroke 2017; 48: 2084–2090. [DOI] [PubMed] [Google Scholar]
- 34. Tsivgoulis G, Zand R, Katsanos AH, et al. Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: a meta-analysis. JAMA Neurol 2016; 73: 675–683. [DOI] [PubMed] [Google Scholar]
- 35. Yan J, Qiu J, Wu X, et al. Pretreatment cerebral microbleeds and symptomatic intracerebral hemorrhage post-thrombolysis: a systematic review and meta-analysis. J Neurol 2020; 267: 301–307. [DOI] [PubMed] [Google Scholar]
- 36. Wang S, Lv Y, Zheng X, et al. The impact of cerebral microbleeds on intracerebral hemorrhage and poor functional outcome of acute ischemic stroke patients treated with intravenous thrombolysis: a systematic review and meta-analysis. J Neurol 2017; 264: 1309–1319. [DOI] [PubMed] [Google Scholar]
- 37. Liu X, Li T, Diao S, et al. The global burden of cerebral small vessel disease related to neurological deficit severity and clinical outcomes of acute ischemic stroke after IV rt-PA treatment. Neurol Sci 2019; 40: 1157–1166. [DOI] [PubMed] [Google Scholar]
- 38. Park YS, Chung MS, Choi BS. MRI assessment of cerebral small vessel disease in patients with spontaneous intracerebral hemorrhage. Yonsei Med J 2019; 60: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Derex L, Nighoghossian N. Intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: an update. J Neurol Neurosurg Psychiatry 2008; 79: 1093–1099. [DOI] [PubMed] [Google Scholar]
- 40. Pantoni L, Fierini F, Poggesi A. Thrombolysis in acute stroke patients with cerebral small vessel disease. Cerebrovasc Dis 2014; 37: 5–13. [DOI] [PubMed] [Google Scholar]
- 41. Chao AC, Han K, Lin SF, et al. Low-dose versus standard-dose intravenous alteplase for octogenerian acute ischemic stroke patients: a multicenter prospective cohort study. J Neurol Sci 2019; 399: 76–81. [DOI] [PubMed] [Google Scholar]
- 42. Nguyen TH, Truong ALT, Ngo MB, et al. Patients with thrombolysed stroke in Vietnam have an excellent outcome: results from the Vietnam Thrombolysis Registry. Eur J Neurol 2010; 17: 1188–1192. [DOI] [PubMed] [Google Scholar]
- 43. Wang X, Robinson TG, Lee T-H, et al. Low-dose vs standard-dose alteplase for patients with acute ischemic stroke: secondary analysis of the ENCHANTED randomized clinical trial. JAMA Neurol 2017; 74: 1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen CH, Hsieh CY, Lai TB, et al. Optimal dose for stroke thrombolysis in Asians: low dose may have similar safety and efficacy as standard dose. J Thromb Haemost 2012; 10: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 45. Schellinger PD, Tsivgoulis G. Another enchantment from ENCHANTED (Enhanced Control of Hypertension and Thrombolysis Stroke Study). Stroke 2017; 48: 1720–1722. [DOI] [PubMed] [Google Scholar]
- 46. Zhou XY, Wang SS, Collins ML, et al. Efficacy and safety of different doses of intravenous tissue plasminogen activator in Chinese patients with ischemic stroke. J Clin Neurosci 2010; 17: 988–992. [DOI] [PubMed] [Google Scholar]
- 47. Anderson CS, Robinson T, Lindley RI, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med 2016; 374: 2313–2323. [DOI] [PubMed] [Google Scholar]
- 48. Bluhmki E, Danays T, Biegert G, et al. Alteplase for acute ischemic stroke in patients aged > 80 years. Stroke 2020; 51: 2322–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864221074144 for Intravenous thrombolysis in ischemic stroke patients with a prior intracranial hemorrhage: a meta-analysis by Mahsa Dolatshahi, Mohammadmahdi Sabahi, Shima Shahjouei, Eric Koza, Vida Abedi and Ramin Zand in Therapeutic Advances in Neurological Disorders