Abstract
Knee osteoarthritis (KOA) is a debilitating disease characterized by chronic pain, stiffness, and decreased mobility. Intra-articular injectable therapies show good clinical efficacy in improving symptoms; however, these therapies and their comparators (intra-articular saline) have been associated with a large underlying placebo effect. We aimed to describe the existing evidence on the challenges, hypotheses, and potential solutions to mitigate the intra-articular placebo effect in clinical trials in KOA. A targeted literature review was conducted by searching Embase, MEDLINE®, and CENTRAL using predefined study selection criteria. All eligible studies identified were extracted for relevant data, and results were narratively summarized. Forty-three studies were included following screening. Challenges associated with the intra-articular placebo effect included its ability to mask the comparative efficacy of active treatments in trials (n = 7 studies), long-lasting effects (up to 6 months; n = 3), and substantial variation of placebo effect sizes across populations (n = 3). Hypotheses for the mechanism of the placebo effect included aspiration of synovial fluid during administration (n = 6) and dilution of inflammatory mediators (n = 2). Factors affecting the placebo effect size were more invasive routes of administration (e.g., injection versus oral; n = 4) and patient expectations (n = 2). Proposed solutions included the suggestion for readers to weigh the relevance of clinical trial evidence against the presence of large underlying placebo effects (n = 9), discontinuation of intra-articular saline as an appropriate placebo (n = 5), and inclusion of ‘no treatment’ or sham injection as a control (n = 4). The intra-articular placebo effect is a well-documented occurrence in KOA clinical trials, and it is suggested that it be accounted for when designing randomized controlled trials. Awareness and understanding of the intra-articular placebo effect in KOA are required for fair interpretation of clinical trial evidence.
Keywords: knee, osteoarthritis, pain, placebo effect, randomized controlled trial, review
Introduction
Osteoarthritis (OA) was estimated to affect 27 million Americans in 2008. 1 Knees are among the most frequently affected joints, and the global prevalence of knee osteoarthritis (KOA) in adults is estimated to range between 1% and 10%.2,3 The degradation of the joint cartilage and remodeling of the underlying bone lead to symptoms of stiffness, pain, and decreased range of motion. 2 KOA was responsible for over 11 million years lived with disability in 2015 globally 4 and, in combination with hip OA, was ranked as the 11th highest contributor to global disability in 2010. 5 KOA often results in early retirement 6 and is a growing public health issue.
No treatments have been found to slow, prevent, or reverse KOA progression. Total knee replacement is an option for some patients; however, 30- and 90-day mortality rates have recently been estimated globally at 1/500 and 1/250, respectively. 7 Total knee replacement is also associated with life-threatening complications such as venous thromboembolism, bleeding, stroke, and myocardial infarction. 8 Furthermore, lengthy post-operative rehabilitation and lack of suitability for the procedure, among other factors, mitigate against knee arthroplasty in many cases. 9 The mainstay of current treatments for KOA primarily target the symptomatic aspects of the disease. Nonsteroidal anti-inflammatory drugs (NSAIDs), lifestyle adjustments, and intra-articular (IA) injections of corticosteroids have shown efficacy for short term relief of symptoms.6,10 –12 However, for some medications, such as NSAIDs and corticosteroids, the risk of adverse events often outweigh the benefits. 13 There is thus a need for treatment options with favorable risk/benefit profiles, assessed using the gold-standard randomized, placebo-controlled trial. Placebo in clinical trials is necessary to establish a benchmark for the effectiveness of an intervention as well as aid in the experimental blinding of patients and personnel. However, preconceived notions on the effectiveness of the placebo can have detrimental effects on the comparative efficacy of the active intervention.
The placebo effect is a medical phenomenon that describes how one’s positive expectations for a received treatment can cause a clinically significant positive outcome, where these effects are distinct from those caused by discrete therapies. 14 As described by Professor Ted Kaptchuk, who has dedicated his research to this clinical entity, the placebo effect cannot be expected to reduce physical manifestations of the disease such as tumor size in cancer patients or cholesterol levels in those with hyperlipoproteinemia, but rather, placebo acts upon symptoms generated by the brain, such as one’s perception of pain. 15 Studies using functional magnetic resource imaging (MRI) have shown that the placebo response can activate the same areas of the brain involved in pain perception as active treatments. 16 Although a placebo may alleviate symptoms, it will not act as a cure, as the underlying pathology of the ailment remains unchanged.
The placebo effect in KOA was examined in depth through a systematic literature review and meta-analysis, published in 2008, affirming that the measured effect size of placebo was significantly better than no treatment. 17 Reconciling the clinically significant effects of treatments such as IA hyaluronic acid (HA) in routine clinical practice and real-world settings with the measurable effects of placebo on symptom relief in clinical trials requires a nuanced approach to understand better the placebo effect and the factors that influence this effect. For example, as KOA interventions may be administered through different methods (e.g., topical, oral, or IA injection), research has been conducted quantifying the effects of alternative placebo types, which demonstrated that IA placebo provides significantly greater pain relief compared with oral placebo.18,19
Considering these observations, we undertook this review to better understand the existing evidence on the challenges and hypotheses surrounding the IA placebo effect in KOA, along with potential solutions to mitigate the placebo effect in KOA clinical trials.
Methods
A targeted literature review was conducted by an adaptation to the standard methodology for conducting systematic reviews as per guidelines provided by the Cochrane Handbook for Systematic Reviews of Interventions. 20 Study eligibility criteria were defined using a modified PICO (Population, Intervention, Comparator, Outcomes) framework. We searched for publications that included outcomes or discussion points related to the objectives of this review, namely, studies investigating or discussing challenges or hypotheses related to IA injection placebo effects, or proposed solutions to mitigate the IA saline placebo effect in adults with KOA (Table S1 in the Supplementary Materials). Included studies were limited to those published in English.
Searches were designed and executed in MEDLINE®, Embase, and Cochrane Controlled Register of Trials (CENTRAL) for the period covering the database inception to December 17, 2019. Search strategies are provided in Tables S2–S4 of the Supplementary Materials.
After duplicate removal, all publications (titles and abstracts first, followed by full text) were screened for eligibility according to the prespecified eligibility criteria by a senior reviewer. The reviewer extracted all relevant data from the final set of included studies. The following data were extracted where available: (a) study authors, year of publication, country, study setting, blinding, follow-up period, sample size; (b) age, sex, race/ethnicity; and (c) statements on challenges, hypotheses (including mechanisms of action or factors that affect the placebo effect size), or proposed solutions to mitigate the IA placebo effect. Challenges, hypotheses, and solutions from included studies were categorized to facilitate the summary of information from the most frequent categories.
Results
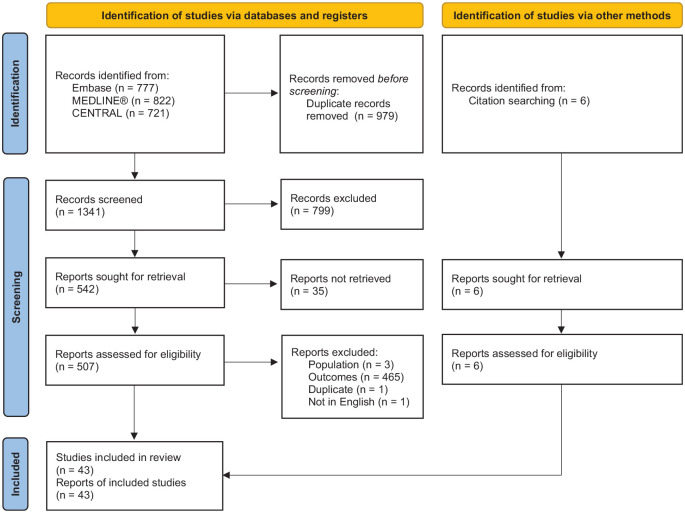
A total of 43 studies were eligible for our qualitative synthesis (Figure 1), consisting of literature reviews and meta-analyses [n = 13 systematic literature reviews18,19,21 –31 (10 of which included a meta-analysis)18,19,21 –23,25,27,28,30,31; n = 8 narrative reviews9,17,32 –37; n = 4 targeted literature reviews38 –41], randomized controlled trials (RCTs; n = 1342–54), and one of each of the following publication types: letter to the editor, 55 consensus statement, 56 protocol for a meta-analysis, 57 pooled analysis of three RCTs, 58 and a single-arm clinical trial (Table S5 in the Supplementary Materials). 59 Most primary studies were single-centered (n = 6)42,48,49,53,54,59 and were conducted in Europe (n = 844,46,48–51,54,59) or North America (n = 643,45,47,52,53,58). Of the clinical trials, 12 utilized a double-blinded study design in which both the participants and investigators were blinded to the study treatment,43 –48,50 –54,58 and one used a single-blind design. 49 Follow-up period of reporting studies ranged from 36 h 54 to 2 years 53 (median: 6 months), and sample size ranged from 36 43 to 588 49 participants (median: n = 210).
Figure 1.
PRISMA flow diagram.
Across primary studies (not including reviews, consensus statements, letters to the editor, or study protocols), mean age ranged from 25.6 49 to 72.1 years, 48 and the proportion of women participants ranged from 39% 44 to 87.0%. 49 Only three trials reported race/ethnicity of participants,45,47,50 in which the proportion of White participants ranged from 81% 47 to 100% 50 across treatment arms.
Five of the included meta-analyses quantified the effect size of the IA placebo effect for pain in KOA.18,19,21,27,31 Two of these studies estimated the change in the IA placebo effect from baseline (i.e., pre- versus post-injection pain scores) as a standardized mean difference (SMD) of 0.68 and 0.61 at ⩽3 months (32 trials, 1705 participants) and 6–12 months (19 trials, 1445 participants), respectively, 21 and a mean difference (visual analogue scale) of 12.10 and 16.62 at 3 months (3 trials, 210 participants) and 6 months (2 trials, 180 participants), respectively. 27 In addition, across two network meta-analyses of RCTs (149 trials, N = 39,814 18 ; 137 trials, N = 33,243 19 ) both by Bannuru et al., authors compared the effect size of IA placebo versus oral placebo, IA HA, and IA corticosteroids at 3 months post-injection, which resulted in significant SMDs of 0.29 (favoring IA placebo),18,19 0.31 (favoring IA HA), 18 and 0.29 (favoring IA corticosteroids), 18 respectively. SMDs between IA placebo and NSAIDs, 18 acetaminophen,18,19 oral plus topical placebo, 18 and topical placebo 18 were not significant. Finally, in a meta-analysis of 215 RCTs (N = 41,392) by Zou et al., 31 authors calculated the effect size ratio between the contextual effect (i.e., effect size of the placebo group) and the overall treatment effect to determine how much of the overall treatment effects could be attributed to placebo effects. Authors found that across all analyzed KOA interventions, an average of 75% of the overall treatment effects for pain experienced could be explained by placebo effects. The proportion of the treatment effect attributable to the placebo effect varied by treatment. Ninety-one percent of the treatment effect of joint lavage was attributable to a placebo effect, while 87%, 85%, and 85% of the treatment effect of paracetamol, topical NSAIDs, and acupuncture were due to placebo effects, respectively.
Challenges
A summary of challenges, hypotheses, and solutions for the placebo effect is provided in Table 1. Seventeen studies described challenges associated with the IA placebo effect in the context of KOA. The most commonly reported challenges were the ability for the placebo effect to diminish or mask the comparative efficacy of active IA therapies (n = 7),18,23,37,44,48,52,57 the long-lasting effects of the placebo (up to 6 months; n = 3),34,41,51 and the tendency for the placebo effect size to vary which is difficult to predict in clinical trials (n = 3).17,38,40
Table 1.
Challenges, hypotheses, and potential solutions to mitigate the intra-articular placebo effect.
| Evidence type | Outcome category | Outcome subcategory | Studies |
|---|---|---|---|
| Challenges | Impact on clinical trials (n = 10) |
Long-lasting | 334,41,51 |
| Varying placebo effect | 317,38,40 | ||
| Blinding may be unsuccessful | 1 33 | ||
| Difficult to estimate | 1 27 | ||
| Difficult to predict outcomes | 1 36 | ||
| Heterogeneity in placebo type | 1 57 | ||
| Publication bias; smaller placebo effect more likely to be published | 1 17 | ||
| Impact on active treatments (n = 8) |
Diminish/mask comparative efficacy of intra-articular therapies | 718,23,37,44,48,52,57 | |
| Difficult to market treatment options | 1 57 | ||
| Inflates strength of treatment arm | 1 29 | ||
| Other (n = 1) | Mechanism unknown | 1 27 | |
| Hypotheses | Biological (n = 8) |
Aspiration of synovial fluid | 622,39,40,46,50,56 |
| Dilution of inflammatory mediators | 218,27 | ||
| Psychological effect | 239,50 | ||
| Patient-related factors (n = 9) |
Patient education | 325,39,50 | |
| Patient expectation/beliefs a | 318,19,31 | ||
| Baseline pain a | 318,30,31 | ||
| Disease severity (high placebo effect in low Kellgren–Lawrence grade) | 1 45 | ||
| Patient knowledge of high-tech equipment | 1 9 | ||
| Patient perception of therapy as innovative | 1 9 | ||
| Treatment-related factors (n = 5) |
Route of administration a | 518,19,30,31,57 | |
| Frequency of treatment administration | 1 57 | ||
| Duration of treatment | 1 31 | ||
| Strength of active treatment | 1 30 | ||
| Confounders and trial design factors (n = 9) |
Comedications | 418,24,51,56 | |
| Clinical study setting and provider type | 418,31,47,51 | ||
| Large sample size a | 318,30,31 | ||
| Appropriate footwear | 1 25 | ||
| Assistive devices for ambulation | 1 25 | ||
| Lack of washout period | 1 53 | ||
| Occupational therapy or physiotherapy | 1 25 | ||
| Publicly funded trial | 1 31 | ||
| Rescue medication | 1 24 | ||
| Statistical methods cannot detect differences | 1 51 | ||
| Weight loss | 1 25 | ||
| Patient–physician interaction (n = 3) |
Physician confidence | 29,17 | |
| Culture | 1 17 | ||
| Personal attention factor | 1 48 | ||
| Physician optimism of treatment | 1 17 | ||
| Religion | 1 17 | ||
| Solutions | Clinical trial design and interpretation of results (n = 18) |
Awareness/weighing relevance of trials | 99,18,19,25,32,34,35,42,54 |
| Intra-articular saline not considered a placebo | 521,25,27,55,58 | ||
| Inclusion of a ‘no treatment’ arm or sham injection as control | 427,32,49,57 | ||
| Active treatment instead of placebo for control | 1 26 | ||
| Blinding needed | 1 28 | ||
| Inclusion of joint aspiration in placebo arm | 1 59 | ||
| Standardization across trials | 1 57 | ||
| Further research (n = 4) |
Use of individual patient data to provide insight into different predictors of placebo effect | 231,57 | |
| Research of mechanism of action | 1 25 | ||
| Research to identify key contextual elements that can be delivered by physicians to enhance treatment effects | 1 17 | ||
| Harnessing the placebo effect (n = 2) |
Make use of beneficial effects of placebo effect in chronic distressing conditions | 1 32 | |
| Placebo as an active comparator | 1 43 |
At least one of the cited studies reported the relationship between the placebo effect and the factor that was investigated to affect the placebo effect as nonsignificant.
Hypotheses
Twenty-one studies investigated or described hypotheses for the IA placebo effect (Table 1). Hypotheses could be broken down into several broad categories, including patient-related factors (n = 9),9,18,19,25,30,31,39,45,50 confounders and factors related to trial design (n = 9),18,24,25,30,31,47,51,53,56 factors related to the KOA treatment (n = 5),18,19,30,31,57 biological mechanisms of the IA placebo effect (n = 8),18,22,27,39,40,46,50,56 and factors related to patient–physician interactions (n = 3).9,17,48
Patient-related factors that impacted the placebo effect included baseline pain (n = 2), where increases in the placebo effect size were associated with higher baseline pain scores (i.e., a regression to the mean effect).18,30 One study found that baseline pain was not a significant determinant of the placebo effect (p = 0.150). 31 However, the latter study included patients with several types of OA, including hip, spine, foot, temporomandibular joint, and mixed joints (although over 70% of the data was from KOA patients, and thus was considered relevant for this review). It is unknown how this may have affected the results of their meta-regression. Patient education on managing their KOA symptoms (n = 3) was also cited as a potential nonpharmacological factor that may explain the high placebo effect seen across studies.25,39,50 Patient expectations or beliefs (n = 2)19,31 as well as patient knowledge of high-tech equipment and their perception of therapy as innovative 9 were also reported. For example, in the study by Altman et al., 21 different placebo effect sizes were seen in subgroup analyses of the specific IA therapy investigated (i.e., HA, corticosteroid, and platelet-rich plasma), with the highest placebo effect demonstrated in trials on HA, which may have been considered more innovative. Notably, one study refuted this hypothesis by reporting that the effect of placebo was not influenced by the patient’s expectation of being randomly assigned to an active treatment. 18 Authors provided no further explanation for this insignificant result.
For hypotheses related to confounding factors or trial-related factors that impacted the placebo effect, potential confounders included use of comedications (n = 4)18,24,51,56 or rescue medications during the trial, 24 use of appropriate footwear during the trial, 25 use of assistive walking devices, 25 concurrent occupational therapy or physiotherapy, 25 or weight loss during follow-up. 25 Although not directly related to the placebo itself, these factors were stated as potential explanations for why we may see that patients in the placebo arm performed better than expected compared to the treatment arm. This was hypothesized to be a result of imbalances in the treatment arms that may confound the effect of the treatment.
Trial-related factors described by authors included a lack of a washout period of NSAIDs and other analgesics prior to the initiation of the trial. 18 Authors stated that it was possible that their patient sample had better than expected pain control and function at baseline due to prior medications, which may have resulted in an underestimation of the effect of the study treatments. Clinical study setting (e.g., community-based outpatient setting versus hospital-based outpatient care and recruitment), study site, and provider type (e.g., primary care practitioners, rheumatologists, orthopedic surgeons) were also described as significant factors affecting the placebo effect (n = 317,46,50; one study reported this factor as nonsignificant 31 ).
For factors related to the treatments administered during the trial, five studies reported that the invasiveness of the treatment corresponded to the placebo effect size.18,19,30,31,57 More invasive treatments such as surgery, injection, or acupuncture resulted in a stronger placebo effect compared with less invasive treatments such as topical ointments or oral pills. However, one of these studies reported that route of administration (IA injection versus oral) only significantly affected the placebo effect size for pain-related outcomes, but not outcomes for stiffness or function. 19 In-line with that hypothesis, higher frequency of administration as well as longer duration of treatment were also described to increase the placebo effect.31,57 Strength of active treatment was described as a significant determinant of the placebo effect as well, where perceived or anticipated stronger active treatments were associated with stronger placebo effects. 30 The placebo effect for pain was thus larger for drugs compared with nondrugs (e.g., herbals, vitamins, fish oil) and even greater for drugs given by injection (e.g., IA HA). 30
The most commonly mentioned plausible biological mechanism of the IA placebo effect was aspiration of synovial fluid prior to the injection of the IA treatment (n = 6).22,39,40,46,50,56 Although not described in further detail by the authors themselves, aspiration of synovial fluid may remove some of the pro-inflammatory cytokines in the joint, leading to an enhanced IA placebo effect. All injectable IA treatments require removal of fluid from the joint (i.e., aspiration or arthrocentesis) prior to administration of the injectable medication (or saline placebo in the case of RCTs). Despite a scarcity of data on the impact of this procedure alone for improving pain and functional outcomes in KOA, there is some evidence suggesting that arthrocentesis on its own may be considered a beneficial treatment. Aspiration of the temporomandibular joint has been shown to be associated with reduced levels of inflammatory cytokines 2 weeks post-procedure. 60 Along these same lines, dilution of inflammatory markers by IA saline in KOA was another biological hypothesis, which was reported in two studies.18,27 Dilution of inflammatory mediators (e.g., cytokines) within the knee was hypothesized to provide relief of patient pain and stiffness of the joint.
Physician-related factors were less commonly described in the context of KOA, but these could generally be attributable to the physician’s personability. The patient’s perception of their physician’s confidence,9,17 optimism for the outcome of the treatment, 17 and ‘personal attention factor’ 48 were considered as perhaps influencing the placebo effect. The latter of these is a term commonly used in the customer service industry, describing how a service employee’s manner of relating to the customer (or patient) on a human level while exhibiting warmth, politeness, courtesy, and friendliness can increase customer satisfaction. 61 This positive interaction between the patient and physician is expected to increase the patient’s expectations for the treatment, thereby increasing the placebo effect. Culture and religion of the patient were also described as factors that may impact the placebo effect. 17 Authors stated that in clinical practice, placebo (or ‘nocebo’) effects can depend on interactions between a patient and their physician. For example, a ‘doctor’s touch’ may be beneficial to some in expressing warmth, optimism, or caring but may be perceived negatively by others who may not like to be touched. 17
Proposed solutions
A total of 21 studies described potential solutions to mitigate the IA placebo effect in KOA (Table 1). Among these studies, the most commonly reported solution (n = 9) involved raising awareness among researchers, physicians, or other readers to exercise appropriate caution when interpreting the results of KOA trials investigating injectable IA treatments.9,18,19,25,32,34,35,42,54 In this regard, the trial results should be weighed appropriately in light of the known large placebo effect when determining the efficacy of the active treatment and whether it is suitable for use in treating patients. It has been pointed out that there is a large discrepancy between the small additional benefits of IA treatments compared with IA placebo observed in the clinical trial setting and the substantial effectiveness of these treatments in real-world practice.9,17
Five studies concluded that IA saline should not be considered as a viable placebo in KOA clinical trials at all.21,25,27,55,58 In one systematic literature review and network meta-analysis of determinants of the placebo effect in KOA, 30 the authors opted to use the term ‘saline vehicle control’ instead of ‘placebo control’. This change in terminology was adopted to make a clear distinction between IA saline, which has been speculated to potentially produce therapeutic effects at the biomechanical level, and the traditional ‘sugar pill’, which is not expected to produce therapeutic effects biomechanically. 55 In line with these possible solutions, several other studies proposed that the IA saline treatment as a placebo be replaced with an active treatment comparator 26 or supplemented with an additional sham injection arm (i.e., no treatment at all).27,32,49,57 By including a sham injection control arm where no fluid is injected into the joint, in addition to a separate IA saline control arm, potential active effects of the saline such as joint lubrication and dilution of inflammatory mediators may become distinguishable from the ‘true’ placebo effects (due to patient expectations/beliefs) as seen in the sham injection arm.
Two studies proposed solutions that pertained to harnessing the power of the placebo effect.32,43 In the first study, authors described that placebo and contextual effects (e.g., patient’s perception that the practitioner is experienced, competent, optimistic, and eager to monitor progress) should be acknowledged and optimized in clinical practice, particularly for the treatment of chronic conditions for which available treatments options are poor. 32 The second went beyond the context of clinical practice to state that IA saline may even be more appropriately considered as an active comparator instead of a placebo. 43
Finally, further research into the mechanism underlying the placebo effect was highlighted in four studies.17,25,31,57 One study underscored the importance of elucidating the mechanism of action of IA saline injections, as this would allow for a better understanding of how to treat KOA more efficiently. 25 One review highlighted the need for research to identify key contextual elements (e.g., clinician communication style, framing treatment outcome expectations positively) that can be utilized by physicians to potentially increase the treatment effect (i.e., increase the placebo effect to benefit the patient). 17 In the third and fourth publications, the authors suggested that in lieu of aggregate-level data, analysis of individual patient data may help to provide further insight into the various predictors of the placebo effect.31,57
Discussion
This review describes the existing evidence on the challenges, hypotheses, and potential solutions concerning the IA placebo effect in KOA clinical trials. We identified several issues related to the IA placebo effect that bear consideration by trial investigators, clinicians, and policy makers when interpreting RCT results in KOA.
Key challenges associated with the IA placebo effect were the long-lasting effects of placebo (can last up to 6 months or more) and that it varies substantially across clinical trials. This is particularly problematic for KOA trial investigators, as it makes it difficult to evaluate the true clinical effects of active IA treatments. These identified challenges may be present in many KOA clinical trials. Take, for example, two recent randomized, multicenter, quadruple-blinded, 26-week, placebo-controlled trials investigating the efficacy and safety of the same IA HA in adults with KOA, with nearly identical study designs apart from geographic location.46,62 In the first trial, conducted in Europe, the primary efficacy endpoint of change in the Western Ontario and McMaster Univiersities Osteoarthritis Index (WOMAC) A from baseline to 26 weeks was significantly better for the active treatment compared with placebo (IA HA −0.84 versus IA saline −0.69; p = 0.047). 11 However, in the trial conducted in China, the primary efficacy endpoint of change in WOMAC A1 from baseline to 26 weeks between the IA HA and the placebo did not reach statistical significance (IA HA −2.146 versus IA saline −2.271; p = 0.36). 12 Authors of the Chinese study attributed this finding to an inflated placebo response that led to nonstatistically significant difference between IA HA and IA saline. In other words, a large underlying placebo effect may have caused a diminishing treatment effect size relative to the placebo effect. In line with this, one of the most commonly reported challenges associated with the IA placebo effect in the present review was the ability for IA placebo to diminish or mask the comparative efficacy of IA therapies. At least four other studies experienced this same outcome with other IA HA treatments and attributed their findings to a similarly large and unexpected placebo effect.23,44,48,52 This highlights a substantial issue that many clinical trial investigators are facing when determining the efficacy of their active therapy.
Also noteworthy in these two example RCTs, the outcome of failing to reach their superiority endpoint in the Chinese trial may have been mitigated had there been knowledge that some regions or cultures may experience a substantially larger placebo effect than others. Variation of placebo effects across study sites and culture were only briefly described as potential factors that could impact the placebo effect in our review.17,47 However, geographic and cultural disparities have also been described to impact the placebo effect outside of the context of KOA.63 –65 Participants’ expectations or beliefs in treatments can vary across cultures, and it is important for clinical trial investigators to be aware of potential biases that may arise when including participants who have strong preconceived notions toward the effectiveness of one intervention over another. 63 Further research into cultural and regional variation of the placebo effect in KOA trials is warranted to elucidate their effects in KOA.
In terms of biological hypotheses for the IA placebo effect in KOA, aspiration of synovial fluid was among the top commonly reported reasons for why some patients may experience real physical benefit from placebo. Unlike a traditional placebo which is anticipated to induce no physiological changes upon administration, the IA saline placebo disrupts the joint space in a way that may be clinically beneficial to the patient. By removing some of the fluid in the joint space, the patient may benefit from reduced levels of inflammatory cytokines. 60 Another characteristic of IA saline is that it requires the injection of a fluid into the joint space. The injection of saline solution may dilute inflammatory mediators, resulting in pain relief. These underlying biological mechanisms may be some of the key reasons why some authors do not consider IA saline to be a suitable placebo at all;21,25,55,58 as unlike inert placebo treatments, there appears to be active treatment effects at work. However, although these hypothetical biological effects of IA saline suggest a solid mechanism of action, it is important to note that there are fundamental differences between the mechanism of action of IA saline and that of pharmacological KOA treatments such as IA HA. While IA saline may temporarily dilute inflammatory markers in the joint and provide lubrication, benefits from IA HA are believed to be based on multiple well-studied mechanisms. IA HA has been shown to decrease chondrocyte apoptosis and increase chondrocyte proliferation66,67 as well as increase synthesis of stimulated proteoglycan, delaying the progression of OA.68,69 Mechanisms based on anti-inflammatory properties, mechanical benefits (e.g., joint lubrication), effects on the subchondral bone, and analgesic effects (reduction of joint nociceptors) have also been described in OA. 70 Further research needs to be conducted on the full mechanism of action of IA saline in KOA to better understand the key differences between these injectables.
Perhaps one of the most important take-aways from our review were the proposed solutions to mitigate the IA placebo effect in knee OA trials. A number of authors described that it is imperative to weigh the relevance of clinical trial evidence when making decisions about a treatment’s efficacy or whether it may be suitable for clinical practice.9,18,19,25,32,34,35,42,54 This may be directed at clinical practice guideline groups, policy makers, or practicing clinicians and refers to the need for readers of clinical trial evidence to take into account the underlying placebo effect before making a judgment on the active treatment’s relative clinical effectiveness. In this way, available therapies that generally have promising safety and efficacy profiles but also have some evidence that shows no superiority over placebo may still be considered as beneficial to these patients, who otherwise have few effective treatment options.
Another interesting solution was that the beneficial clinical effects of IA saline should be realized and put to good use. It was suggested that IA saline, which has been demonstrated time and time again to show good efficacy in reducing symptoms of pain in patients with knee OA in RCTs, could potentially be harnessed as an active treatment itself 43 and could be made useful in patients who suffer from chronic distressing conditions for which there is no cure. 32 Afterall, the large placebo effect that may be considered a burden to clinical trial researchers may conversely work in the favor of a treating physician who simply wants to provide their patient with pain relief and improved quality of life. However, it should be noted that the notion of considering IA saline as an active comparator may yield some ethical concerns. The biological mechanism of action for IA saline is not currently well established. Marketing of a ‘placebo’ that has no biological mechanism of action aside from that based on patient expectations would not be appropriate. Further confirmation of the underlying biological mechanisms (e.g., aspiration of synovial fluid or dilution of inflammatory markers) of IA saline, if any, is needed before it may be considered as a viable treatment. It is also worth noting that IA saline may not be as effective in the real world outside the context of clinical trials. Participation into an RCT alone has been shown to heighten the effect of placebo, 71 which is due to patient expectations that the treatment they receive may be an active treatment, and therefore may benefit them. This scenario is in contrast to a real-world setting, where if a patient were to receive IA saline presented as mere ‘placebo’, the effects of the IA saline would depend mainly on any underlying biological mechanisms, while the additive ‘true’ placebo effect would be diminished due to lowered patient expectations. Hence, we cannot expect the effectiveness of IA saline to be the same in a clinical trial versus a real-world setting. Further research into the mechanisms of injectable IA saline may elucidate any active treatment effects that set this intervention apart from a ‘true’ placebo. In particular, research investigating the changes in synovial fluid and serum biomarkers following IA saline will provide crucial information to better understand how it provides symptomatic relief in patients with KOA.72 –74 Whether or not the IA saline placebo should be removed completely from the KOA research landscape or harnessed for its potential therapeutic benefits is still up for debate.
Finally, one noteworthy mention within a study on hip OA by Saltzman et al. 27 was the observation that the placebo effect is present for continuous subjective measures of disease activity (e.g., stiffness, self-reported function, and physician’s global assessment) but not for objective outcomes such as quadriceps strength, knee swelling, range of motion, and radiographic joint space narrowing, as seen in a meta-analysis by Bannuru et al. 18 The use of objective physical examination or laboratory measures (such as 6-min walk test, knee swelling, and range of motion and radiographic joint space narrowing) in KOA RCTs may be another avenue to circumvent the substantial placebo effect and tease apart the active treatment effects. 75
Our review has several strengths. To our knowledge, this is the first literature review to summarize the existing evidence and expert opinions on challenges, hypotheses, and potential solutions to mitigate the IA placebo effect in KOA trials. This is expected to shed light on the unique challenges that researchers experience in this research space, and the summary of proposed solutions may help to improve the interpretation of clinical trial evidence. Second, we searched three major electronic databases to capture our studies, resulting in an evidence base that is comprehensive and representative of the existing evidence on this topic. Third, an in-depth hand-searching of citations cited by relevant reviews was carried out to ensure that studies that may have been missed in the searches were captured. Limitations of our review included the nonsystematic methodological approach to conducting this review and therefore the inability to completely rule out of the possibility that some relevant studies were missed. In addition, although some studies described additional mechanisms of action of the placebo effect, evidence was only available in animal studies and was not summarized here. 76 It is worth noting that there are a number of biological hypotheses for the placebo effect that have been investigated outside of the context of KOA and therefore beyond the scope of this review. Although these hypotheses have been primarily investigated in healthy participants, they may warrant consideration as potential hypotheses for the IA placebo effect in KOA. Examples of such hypotheses include placebo-treated participants experiencing a release of dopamine77 –80 or endogenous opioids77 –82 that may contribute to pain relief, involvement of subcortical pain transmission centers in the placebo effect,77,79,83 genetics as an explanation for placebo effect variability, 84 placebo’s impact on the neurologic pain signature, and a multivariate brain pattern tracking nociceptive pain seen on functional neuroimaging. 85 Further research in the context of KOA specifically is needed to confirm the applicability of these hypotheses to this disease area. In addition, the nature of the present review was primarily qualitative, as well as the number of included clinical trials was small. Although we did not restrict our inclusion criteria to any particular study design, the fact that we did not encounter many clinical trials reporting challenges, hypotheses, or potential solutions to mitigate the IA placebo effect may reflect a paucity of high-quality published literature on this topic.
Based on the available evidence, one can conclude that the IA placebo effect is real and omnipresent in IA saline-controlled RCTs in KOA and should be adequately accounted for in clinical trial research. There is a need for further research into the mechanism of action of IA saline in KOA as well as other patient-, trial-, and treatment-related factors that may impact the placebo effect size, particularly those related to culture and geographic region. However, increased awareness and understanding of the placebo effect in KOA is important for fair interpretation of clinical trial evidence in this space.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X211066689 for Intra-articular placebo effect in the treatment of knee osteoarthritis: a survey of the current clinical evidence by Mir Sohail Fazeli, Louis McIntyre, Yili Huang and Xavier Chevalier in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors would like to thank Wilson Wai Ngai of Sanofi (Bridgewater, NJ, USA) for coordinating the development of this manuscript and facilitating author discussions, Kimberly Hofer and Christopher Vannabouathong of Evidinno Outcomes Research Inc. (Vancouver, BC, Canada) for medical writing support funded by Sanofi, and Christopher Crotty of Evidinno Outcomes Research Inc. (Vancouver, BC, Canada) for providing review and copy-editing funded by Sanofi.
Footnotes
Author contributions: Mir Sohail Fazeli: Conceptualization; Data curation; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Louis McIntyre: Methodology; Supervision; Writing – review & editing.
Yili Huang: Methodology; Supervision; Writing – review & editing.
Xavier Chevalier: Methodology; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.S.F. reports employment with Evidinno Outcomes Research Inc., which was contracted by Sanofi to conduct this study. X.C. reports nonfinancial support from Novartis, and personal fees from IBSA, Sanofi, Flexion Therapeutics, and Macopharma outside the submitted work. Y.H. and L.M. report personal fees from Sanofi.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Sanofi.
ORCID iD: Mir Sohail Fazeli  https://orcid.org/0000-0002-0369-3848
https://orcid.org/0000-0002-0369-3848
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mir Sohail Fazeli, Evidinno Outcomes Research Inc., 1750 Davie Street, Suites 601 & 602, Vancouver, BC V6G 1W3, Canada.
Louis McIntyre, U.S. Orthopaedic Partners, Jackson, MS, USA.
Yili Huang, Northwell Health, New Hyde Park, NY, USA.
Xavier Chevalier, Hôpital Henri Mondor, Université Paris XII, UPEC, Créteil, France.
References
- 1. Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol 2012; 8: 77–89. [DOI] [PubMed] [Google Scholar]
- 2. Michael JW-P, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int 2010; 107: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum 2008; 58: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 6. Bannuru RR, Natov NS, Obadan IE, et al. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum 2009; 61: 1704–1711. [DOI] [PubMed] [Google Scholar]
- 7. Berstock JR, Beswick AD, López-López JA, et al. Mortality after total knee arthroplasty: a systematic review of incidence, temporal trends, and risk factors. J Bone Joint Surg Am 2018; 100: 1064–1070. [DOI] [PubMed] [Google Scholar]
- 8. Pedersen AB, Mehnert F, Sorensen HT, et al. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15-year retrospective cohort study of routine clinical practice. Bone Joint J 2014; 96-B: 479–485. [DOI] [PubMed] [Google Scholar]
- 9. Richardson C, Plaas A, Block JA. Intra-articular hyaluronan therapy for symptomatic knee osteoarthritis. Rheum Dis Clin North Am 2019; 45: 439–451. [DOI] [PubMed] [Google Scholar]
- 10. Persson MSM, Sarmanova A, Doherty M, et al. Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology 2018; 57: 1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin KW. Treatment of knee osteoarthritis. Am Fam Physician 2018; 98: 603–606. [PubMed] [Google Scholar]
- 12. Braithwaite GJC, Daley MJ, Toledo-Velasquez D. Rheological and molecular weight comparisons of approved hyaluronic acid products – preliminary standards for establishing class III medical device equivalence. J Biomater Sci Polym Ed 2016; 27: 235–246. [DOI] [PubMed] [Google Scholar]
- 13. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 2007; 15: 981–1000. [DOI] [PubMed] [Google Scholar]
- 14. Kaptchuk TJ, Miller FG. Placebo effects in medicine. N Engl J Med 2015; 373: 8–9. [DOI] [PubMed] [Google Scholar]
- 15. The power of the placebo effect: treating yourself with your mind is possible, but there is more to the placebo effect than positive thinking, https://www.health.harvard.edu/mental-health/the-power-of-the-placebo-effect (2019, accessed 10 October 2019).
- 16. Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci 2015; 16: 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang W. The powerful placebo effect in osteoarthritis. Clin Exp Rheumatol 2019; 37(Suppl. 120): 118–123. [PubMed] [Google Scholar]
- 18. Bannuru RR, McAlindon TE, Sullivan MC, et al. Effectiveness and implications of alternative placebo treatments: a systematic review and network meta-analysis of osteoarthritis trials. Ann Intern Med 2015; 163: 365–372. [DOI] [PubMed] [Google Scholar]
- 19. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015; 162: 46–54. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions, version 6.1, 2020, www.training.cochrane.org/handbook
- 21. Altman RD, Devji T, Bhandari M, et al. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum 2016; 46: 151–159. [DOI] [PubMed] [Google Scholar]
- 22. Bannuru RR, Natov NS, Dasi UR, et al. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis-meta-analysis. Osteoarthritis Cartilage 2011; 19: 611–619. [DOI] [PubMed] [Google Scholar]
- 23. Bannuru RR, Vaysbrot EE, Sullivan MC, et al. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2014; 43: 593–599. [DOI] [PubMed] [Google Scholar]
- 24. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2005; 2: CD005321. [DOI] [PubMed] [Google Scholar]
- 25. Colen S, van den Bekerom MP, Mulier M, et al. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012; 26: 257–268. [DOI] [PubMed] [Google Scholar]
- 26. Jevsevar D, Donnelly P, Brown GA, et al. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am 2015; 97: 2047–2060. [DOI] [PubMed] [Google Scholar]
- 27. Saltzman BM, Leroux T, Meyer MA, et al. The therapeutic effect of intra-articular normal saline injections for knee osteoarthritis: a meta-analysis of evidence level 1 studies. Am J Sports Med 2017; 45: 2647–2653. [DOI] [PubMed] [Google Scholar]
- 28. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg 2017; 12: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vannabouathong C, Bhandari M, Bedi A, et al. Nonoperative treatments for knee osteoarthritis: an evaluation of treatment characteristics and the intra-articular placebo effect: a systematic review. JBJS Rev 2018; 6: e5. [DOI] [PubMed] [Google Scholar]
- 30. Zhang W, Robertson J, Jones AC, et al. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis 2008; 67: 1716–1723. [DOI] [PubMed] [Google Scholar]
- 31. Zou K, Wong J, Abdullah N, et al. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis 2016; 75: 1964–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abhishek A, Doherty M. Mechanisms of the placebo response in pain in osteoarthritis. Osteoarthritis Cartilage 2013; 21: 1229–1235. [DOI] [PubMed] [Google Scholar]
- 33. Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep 2017; 19: 24. [DOI] [PubMed] [Google Scholar]
- 34. Brandt KD, Smith GN, Jr, Simon LS. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum 2000; 43: 1192–1203. [DOI] [PubMed] [Google Scholar]
- 35. Hameed F, Ihm J. Injectable medications for osteoarthritis. PM R 2012; 4(Suppl. 5): S75–S81. [DOI] [PubMed] [Google Scholar]
- 36. Hunter JA, Blyth TH. A risk-benefit assessment of intra-articular corticosteroids in rheumatic disorders. Drug Saf 1999; 21: 353–365. [DOI] [PubMed] [Google Scholar]
- 37. Jones IA, Togashi R, Wilson ML, et al. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol 2019; 15: 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boutron I, Tubach F, Giraudeau B, et al. Methodological differences in clinical trials evaluating nonpharmacological and pharmacological treatments of hip and knee osteoarthritis. JAMA 2003; 290: 1062–1070. [DOI] [PubMed] [Google Scholar]
- 39. Curran MP. Hyaluronic acid (Supartz): a review of its use in osteoarthritis of the knee. Drugs Aging 2010; 27: 925–941. [DOI] [PubMed] [Google Scholar]
- 40. Kirwan JR, Rankin E. Intra-articular therapy in osteoarthritis. Baillieres Clin Rheumatol 1997; 11: 769–794. [DOI] [PubMed] [Google Scholar]
- 41. Kirwan J. Is there a place for intra-articular hyaluronate in osteoarthritis of the knee? Knee 2001; 8: 93–101. [DOI] [PubMed] [Google Scholar]
- 42. Abate M, Schiavone C, Di Gregorio P, et al. Comparison between hyaluronic acid and platelet rich plasma in the treatment of hip and knee osteoarthritis: preliminary results. J Orthop Traumatol 2013; 14: S17. [DOI] [PubMed] [Google Scholar]
- 43. Altman RD, Rosen JE, Bloch DA, et al. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin Arthritis Rheum 2009; 39: 1–9. [DOI] [PubMed] [Google Scholar]
- 44. Auw Yang KG, Raijmakers NJ, van Arkel ER, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis Cartilage 2008; 16: 498–505. [DOI] [PubMed] [Google Scholar]
- 45. Bar-Or D, Salottolo KM, Loose H, et al. A randomized clinical trial to evaluate two doses of an intra-articular injection of LMWF-5A in adults with pain due to osteoarthritis of the knee. PLoS ONE 2014; 9: e87910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis 2010; 69: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Conaghan PG, Cohen SB, Berenbaum F, et al. A phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol 2018; 70: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henderson EB, Smith EC, Pegley F, et al. Intra-articular injections of 750 kD hyaluronan in the treatment of osteoarthritis: a randomised single centre double-blind placebo-controlled trial of 91 patients demonstrating lack of efficacy. Ann Rheum Dis 1994; 53: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Henriksen M, Graven-Nielsen T, Aaboe J, et al. Gait changes in patients with knee osteoarthritis are replicated by experimental knee pain. Arthritis Care Res 2010; 62: 501–509. [DOI] [PubMed] [Google Scholar]
- 50. Jorgensen A, Stengaard-Pedersen K, Simonsen O, et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: a multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann Rheum Dis 2010; 69: 1097–1102. [DOI] [PubMed] [Google Scholar]
- 51. Karlsson J, Sjogren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology 2002; 41: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 52. Neustadt D, Caldwell J, Bell M, et al. Clinical effects of intraarticular injection of high molecular weight hyaluronan (Orthovisc) in osteoarthritis of the knee: a randomized, controlled, multicenter trial. J Rheumatol 2005; 32: 1928–1936. [PubMed] [Google Scholar]
- 53. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2003; 48: 370–377. [DOI] [PubMed] [Google Scholar]
- 54. Rosseland LA, Helgesen KG, Breivik H, et al. Moderate-to-severe pain after knee arthroscopy is relieved by intraarticular saline: a randomized controlled trial. Anesth Analg 2004; 98: 1546–1551. [DOI] [PubMed] [Google Scholar]
- 55. Bar-Or D, Salottolo K. Comment on ‘clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials’. Semin Arthritis Rheum 2017; 46: e20. [DOI] [PubMed] [Google Scholar]
- 56. Dougados M, Leclaire P, van der Heijde D, et al. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage 2000; 8: 395–403. [DOI] [PubMed] [Google Scholar]
- 57. Yu SP, Ferreira ML, van Middelkoop M, et al. Predictors of placebo response to local (intra-articular) therapy in osteoarthritis: an individual patient data meta-analysis protocol. BMJ Open 2019; 9: e027372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cole B, McGrath B, Salottolo K, et al. LMWF-5A for the treatment of severe osteoarthritis of the knee: integrated analysis of safety and efficacy. Orthopedics 2018; 41: e77–e83. [DOI] [PubMed] [Google Scholar]
- 59. Clarke S, Lock V, Duddy J, et al. Intra-articular hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK). Knee 2005; 12: 57–62. [DOI] [PubMed] [Google Scholar]
- 60. Gulen H, Ataoglu H, Haliloglu S, et al. Proinflammatory cytokines in temporomandibular joint synovial fluid before and after arthrocentesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: e1–e4. [DOI] [PubMed] [Google Scholar]
- 61. Medrano N, Olarte-Pascual C, Pelegrín-Borondo J, et al. Consumer behavior in shopping streets: the importance of the salesperson’s professional personal attention. Front Psychol 2016; 7: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ke Y, Jiang W, Xu Y, et al. Efficacy and safety of a single intra-articular injection of 6 ml Hylan G-F 20 compared to placebo in Chinese patients with symptomatic knee osteoarthritis: C-SOUND study, a 26-week multicenter double-blind randomized placebo-controlled trial in China. BMC Musculoskelet Disord 2021; 22: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fregni F, Imamura M, Chien HF, et al. Challenges and recommendations for placebo controls in randomized trials in physical and rehabilitation medicine: a report of the international placebo symposium working group. Am J Phys Med Rehabil 2010; 89: 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moerman DE. Cultural variations in the placebo effect: ulcers, anxiety, and blood pressure. Med Anthropol Q 2000; 14: 51–72. [DOI] [PubMed] [Google Scholar]
- 65. Vieta E, Pappadopulos E, Mandel FS, et al. Impact of geographical and cultural factors on clinical trials in acute mania: lessons from a ziprasidone and haloperidol placebo-controlled study. Int J Neuropsychopharmacol 2011; 14: 1017–1027. [DOI] [PubMed] [Google Scholar]
- 66. Brun P, Zavan B, Vindigni V, et al. In vitro response of osteoarthritic chondrocytes and fibroblast-like synoviocytes to a 500-730 kDa hyaluronan amide derivative. J Biomed Mater Res B Appl Biomater 2012; 100: 2073–2081. [DOI] [PubMed] [Google Scholar]
- 67. Brun P, Panfilo S, Daga Gordini D, et al. The effect of hyaluronan on CD44-mediated survival of normal and hydroxyl radical-damaged chondrocytes. Osteoarthritis Cartilage 2003; 11: 208–216. [DOI] [PubMed] [Google Scholar]
- 68. Williams JM, Zhang J, Kang H, et al. The effects of hyaluronic acid on fibronectin fragment mediated cartilage chondrolysis in skeletally mature rabbits. Osteoarthritis Cartilage 2003; 11: 44–49. [DOI] [PubMed] [Google Scholar]
- 69. Han F, Ishiguro N, Ito T, et al. Effects of sodium hyaluronate on experimental osteoarthritis in rabbit knee joints. Nagoya J Med Sci 1999; 62: 115–126. [PubMed] [Google Scholar]
- 70. Altman RD, Manjoo A, Fierlinger A, et al. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord 2015; 16: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008; 336: 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Catterall JB, Stabler TV, Flannery CR, et al. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res Ther 2010; 12: R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kokebie R, Aggarwal R, Lidder S, et al. The role of synovial fluid markers of catabolism and anabolism in osteoarthritis, rheumatoid arthritis and asymptomatic organ donors. Arthritis Res Ther 2011; 13: R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vollert J, Cook NR, Kaptchuk TJ, et al. Assessment of placebo response in objective and subjective outcome measures in rheumatoid arthritis clinical trials. JAMA Netw Open 2020; 3: e2013196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brander V, Skrepnik N, Petrella RJ, et al. Evaluating the use of intra-articular injections as a treatment for painful hip osteoarthritis: a randomized, double-blind, multicenter, parallel-group study comparing a single 6-mL injection of hylan G-F 20 with saline. Osteoarthritis Cartilage 2019; 27: 59–70. [DOI] [PubMed] [Google Scholar]
- 76. Hunziker EB, Kapfinger E. Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells. J Bone Joint Surg Br 1998; 80: 144–150. [DOI] [PubMed] [Google Scholar]
- 77. Eippert F, Bingel U, Schoell ED, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009; 63: 533–543. [DOI] [PubMed] [Google Scholar]
- 78. Sauro MD, Greenberg RP. Endogenous opiates and the placebo effect: a meta-analytic review. J Psychosom Res 2005; 58: 115–120. [DOI] [PubMed] [Google Scholar]
- 79. Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA 2007; 104: 11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zubieta JK, Bueller JA, Jackson LR, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 2005; 25: 7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999; 19: 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Peck C, Coleman G. Implications of placebo theory for clinical research and practice in pain management. Theor Med 1991; 12: 247–270. [DOI] [PubMed] [Google Scholar]
- 83. Bingel U, Lorenz J, Schoell E, et al. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 2006; 120: 8–15. [DOI] [PubMed] [Google Scholar]
- 84. Hall KT, Loscalzo J, Kaptchuk TJ. Genetics and the placebo effect: the placebome. Trends Mol Med 2015; 21: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zunhammer M, Bingel U, Wager TD. Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol 2018; 75: 1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X211066689 for Intra-articular placebo effect in the treatment of knee osteoarthritis: a survey of the current clinical evidence by Mir Sohail Fazeli, Louis McIntyre, Yili Huang and Xavier Chevalier in Therapeutic Advances in Musculoskeletal Disease