Abstract
This study was to investigate the prevalence and death risk of male breast cancer (MBC) patients. The prevalence trend was based on the Surveillance, Epidemiology, and End Results (SEER) database from 1975 to 2017. A competitive risk analysis was performed to analyze the death risk of MBC patients. Hazard ratio (HR) and 95% confidence intervals (CIs) were calculated. The results indicated that the prevalence of MBC after the standardization of the total population increased in 1975–2017 and its annual percentage change (APC) was 0.536% (95% CI = [0.362%, 0.713%]). The prevalence of MBC was rapidly increased in patients aged ≥70 years (APC = 0.780%; 95% CI = [0.491%, 1.076%]) and Grade Ⅱ tumors (APC = 1.462%; 95% CI = [1.260%, 1.686%]). The 1-, 3-, and 5-year cumulative mortality of MBC patients who died of MBC was 2.23% (95% CI = [1.61%, 2.85%]), 7.56% (95% CI = [6.33%, 8.78%]), and 13.10% (95% CI = [11.10%, 11.32%]), respectively. Competitive risk analysis demonstrated that Blacks (HR = 1.76; 95% CI = [1.12, 2.77]), Grade 3 (HR = 2.56; 95% CI = [1.03, 6.35]), AJCC (American Joint Committee on Cancer) Stage Ⅲ (HR = 3.04; 95% CI = [1.76, 5.26]), and AJCC Stage Ⅳ (HR = 7.27; 95% CI = [1.36, 38.83]) were associated with an increased MBC-specific death risk, whereas married status (HR = 0.40; 95% CI = [0.25, 0.64]), surgery (HR = 0.25; 95% CI = [0.12, 0.50]), Luminal A subtype (HR = 0.20; 95% CI = [0.07, 0.53]), and Luminal B subtype (HR = 0.29; 95% CI = [0.10, 0.87]) were related to a reduced MBC-specific death risk. In addition, similar results can be observed in patients with surgery recommended and done (p < .05). This study may provide evidence for the prevalence trend, cumulative mortality, and death risk of MBC patients.
Keywords: male breast cancer, prevalence trend, competitive risk analysis, risk factors of death
Introduction
Male breast cancer (MBC) is a rare and understudied disease, accounting for approximately 1% of all breast cancers (Giordano, 2018). In the United States in 2021, an estimated 2,650 MBC cases will be diagnosed and approximately 530 men are expected to die from this disease (Siegel et al., 2021). Previous studies indicate that the incidence of MBC seems to be increasing (Anderson et al., 2010; Kreiter et al., 2014). Data from the Surveillance, Epidemiology, and End Results (SEER) program indicate that the age-adjusted incidence rate has increased from 0.90 cases per 100,000 men in the general population in 1980 to 1.32 cases per 100,000 in 2017 (SEER Program, 2021).
Factors such as increasing age, BRCA gene mutations, radiation exposure, and elevated estrogen levels may trigger the occurrence of MBC (Giordano, 2018). The incidence risk for MBC increases with age, and compared with women, MBC tends to be diagnosed in the later stages of the disease (Anderson et al., 2010). The lifetime risk of breast cancer for a man is much lower than that for a woman (American Cancer Society, 2018). Due to the lower incidence, clinical studies on MBC always include relatively few cases, which makes a comprehensive analysis of MBC difficult. Research on the epidemiological trends of MBC based on big data samples is needed. In addition, the therapy for MBC is the same as the female breast cancer (Ly et al., 2013), but the prognosis of breast cancer in males was worse than in female patients (Iorfida et al., 2014). Although most studies on the prognosis of MBC have been conducted (Anderson et al., 2010; Wan et al., 2018), many of them ignore the role of other competing death causes in the prognosis of MBC when analyzing the overall survival of MBC.
Herein, we aimed to analyze the epidemiological characteristics of MBC based on the SEER database. Then, we analyzed the death risk of MBC patients and used competitive risk analysis to exclude the effects of other competing death-causing factors.
Method
Study Design and Population
Data of patients with MBC were collected from the SEER database submitted in April 2020. The International Classification of Diseases for Oncology, Third Edition (ICD-O-3) histology codes (8500, 8501, 8502, and 8503, etc.) were used to identify MBC cases. There are three SEER registration systems, including SEER 9, SEER 13, and SEER 18, covering 9.4%, 13.4%, and 27.8% of the entire American population, respectively. To maximize the representativeness of our study, we calculated the incidences of MBC using SEER 9 databases in 1975–1991, SEER 13 in 1992–1999, and SEER 18 in 2000–2017. Except for the incidence analysis (1975-2017), the other analyses were performed based on the SEER 18 databases from 2010-2017 due to the data of the HER2 molecular type were added in the database in 2010. This retrospective study was based on the publicly available de-identified data from the SEER database and did not involve interaction with human subjects or the use of personally identifying information. Therefore, this study did not need to be approved by the Institutional Review Board of the First Affiliated Hospital of Fujian Medical University.
Inclusion and Exclusion Criteria
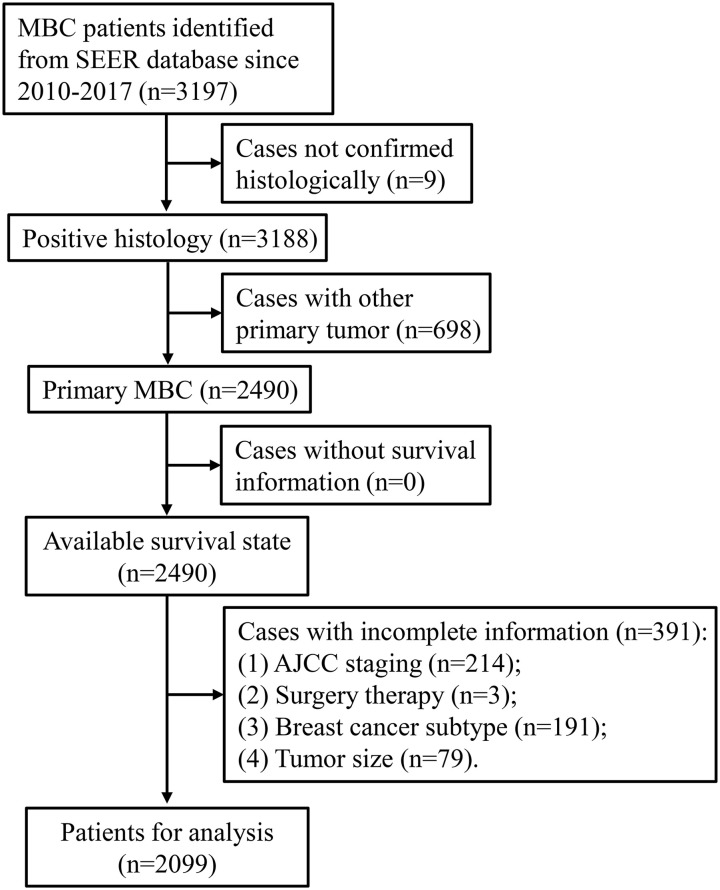
Patients were included when they met the following criteria: (a) MBC patients; (b) confirmed by pathological diagnosis; and (c) primary site tumor. Patients were excluded according to the following criteria: (a) patients without survival information; (b) unknown demographic information, including age and ethnicity; (c) unknown clinical information such as tumor laterality, clinical stage, and grade; and (d) patients with multiple primary tumors. A total of 3,197 MBC patients were selected from the SEER 18 database in 2010–2017 and 2,099 patients were eventually included for other analyses besides the incidence analysis (Figure 1). All included patients were divided into three groups according to the survival information: (a) MBC-specific death, (b) other cause-specific death, and (c) survival.
Figure 1.
The Flowchart of Included Patients.
Note. MBC = male breast cancer; SEER = Surveillance, Epidemiology, and End Results; AJCC = American Joint Committee on Cancer.
Data Collection
Demographic and clinical data of MBC cases were extracted from SEER database. The demographic data of patients include age (≤50, 50–59, 60–69, ≥70 years), marital status (married, unmarried, others), ethnicity (White, Black, Others, unknown), region (metropolis, city, country), and average annual household income (US$). Clinical data include tumor laterality (left, right), clinical grade (1, 2, 3), T stage (0, 1, 2, 3, 4), N stage (0, 1, 2, 3), M stage (0, 1), American Joint Committee on Cancer (AJCC, 7th edition) stage (0, Ⅰ, Ⅱ, Ⅲ, Ⅳ), tumor size (mm), pathological type (invasive ductal carcinoma, intraductal papilloma, adenoma, others), primary site (nipple, central portion, upper-inner quadrant, lower-inner quadrant, upper-outer quadrant, lower-outer quadrant, axillary tail, overlapping lesion, breast-not otherwise specified), surgery (yes, no), surgery recommendations (surgery recommended and done, surgery not recommended, surgery recommended not done), radiation (yes, no, or unknown), molecular subtype (Luminal A, Luminal B, HER2 enriched, Triple negative), and survival months. According to the status of MBC patients at the end of follow-up, all included patients were divided into MBC-specific death group, other cause-specific death group, and survival group.
Statistical Analysis
All statistical analyses were performed using the two-sided test and p < .05 was considered to be statistically significant. SEER * Stat software (Version 8.3.8, Surveillance Research Program, National Cancer Institute) was used to calculate the age-adjusted incidences standardized according to the U.S. standard population in 2000. The variation of MBC prevalence with the Joinpoint Regression Program (Version 4.8, Surveillance Research Program, National Cancer Institute) was assessed by annual percentage changes (APCs) and log-linear models. Continuous variables were described as median and interquartile range and the Kruskal–Wallis test was used for comparison between groups. Categorical variables were described as numbers and percentages, and the chi-square test was used for comparison between groups. The descriptive analysis was performed by SAS software (Version 9.4, SAS Institute, Cary, NC, USA). After descriptive analysis, the Fine-Gray test was used to analyze the differences in the mortality among MBC patients of different ages and the multivariate competitive risk model was utilized to analyze the factors affecting MBC-specific death. In the competitive risk analysis, MBC-specific death and other cause-specific death were two competing endpoint events. The statistical analysis of this part was analyzed by R 4.0.3.
Results
Prevalence Trend of MBC
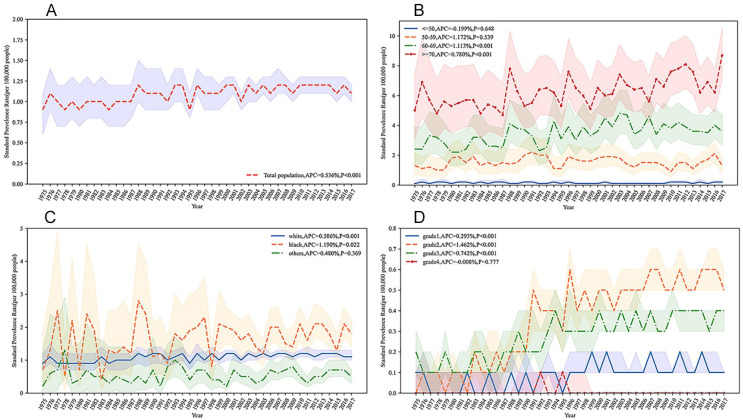
The prevalence of MBC after the standardization of the total population increased in 1975–2017, and its APC was 0.536% (95% confidence interval [CI] = [0.362%, 0.713%]; Figure 2A). Based on age group, the prevalence of MBC patients aged < 50 years and 50 to 59 years remained stable and their APCs were −0.199% (95% CI = [−1.04%, 0.65%]) and 1.172% (95% CI = [−0.371%, 0.719%]), respectively. The prevalence of MBC was increased in patients aged 60 to 69 years and ≥70 years and their APCs were 1.113% (95% CI = [0.731%, 1.55%]) and 0.780% (95% CI = [0.491%, 1.076%]), respectively (Figure 2B). In the terms of race, the prevalence of MBC for Whites and Blacks was also increased, with APC of 0.586% (95% CI = [0.378%, 0.796%]) and 1.190% (95% CI = [0.207%, 2.197%]), respectively, whereas the prevalence of other races remained stable (APC = 0.480%; 95% CI = [−0.555%, 1.529%]; Figure 2C). For clinical grade, except for Grade Ⅳ (APC = −0.008%; 95% CI = [−0.060%, 0.044%]), the prevalence of other grades was all increased. Among which, the prevalence was from high to low for Grade Ⅱ (APC = 1.462%; 95% CI = [1.260%, 1.686%]), Grade Ⅲ (APC = 0.742%; 95% CI = [0.609%, 0.879%]), and Grade Ⅰ (APC = 0.293%; 95% CI = [0.173%, 0.414%]; Figure 2D).
Figure 2.
Standardized Annual Incidence of Male Breast Cancer From 1975 to 2017. (A) Total Standardized Annual Incidence of Male Breast Cancer; (B) Standardized Annual Incidence of Male Breast Cancer by Age; (C) Standardized Annual Incidence of Male Breast Cancer by Race; (D) Standardized Annual Incidence of Male Breast Cancer by Grade.
Note. APC = annual percentage change.
Comparison of the Characteristics Between MBC-Specific Death, Other Cause-Specific Death, and Survival Groups
The results of the analysis indicated that there was statistical significance in patient’s age (χ2 = 83.387, p < .001), marital status (χ2 = 32.607, p < .001), ethnicity (χ2 = 24.645, p < .001), average annual household income (H = 17.573, p < .001), clinical grade (χ2 = 19.886, p < .001), T stage (χ2 = 33.261, p < .001), N stage (χ2 = 26.205, p < .001), AJCC stage (χ2 = 53.505, p < .001), tumor size (H = 32.941, p < .001), surgery (χ2 = 28.712, p < .001), surgery recommendations (χ2 = 28.951, p < .001), molecular subtypes (χ2 = 18.751, p < .001), and survival months (H = 6.382, p < .041) in the three groups (Table 1).
Table 1.
Comparison the Baseline Characteristics of Patients in the MBC-Specific Death Group, Other Cause-Specific Death Group, and Alive Group.
| Characteristics | Group | Statistics | p | ||
|---|---|---|---|---|---|
| MBC-specific death (n = 109) |
Other cause-specific death (n = 206) |
Survival (n = 1,784) |
|||
| Age (years), n (%) | χ2 = 83.387 | <.001 | |||
| ≤50 | 12 (11.01) | 2 (0.97) | 185 (10.37) | ||
| 50–59 | 17 (15.06) | 20 (9.71) | 388 (21.75) | ||
| 60–69 | 42 (38.53) | 51 (24.76) | 601 (33.69) | ||
| ≥70 | 38 (34.86) | 133 (64.56) | 610 (34.19) | ||
| Marital status, n (%) | χ2 = 32.607 | <.001 | |||
| Married | 47 (43.12) | 131 (63.59) | 1,193 (66.87) | ||
| Unmarried | 34 (31.19) | 28 (13.59) | 253 (14.18) | ||
| Others | 28 (25.69) | 47 (22.82) | 338 (18.95) | ||
| Ethnicity, n (%) | χ2 = 24.645 | <.001 | |||
| White | 77 (70.64) | 176 (85.44) | 1,413 (79.20) | ||
| Black | 30 (27.52) | 24 (11.65) | 250 (14.01) | ||
| Others | 2 (1.83) | 6 (2.91) | 108 (6.05) | ||
| Unknown | 0 (0.00) | 0 (0.00) | 13 (0.73) | ||
| Region, n (%) | χ2 = 4.885 | .299 | |||
| Metropolis | 67 (61.47) | 126 (61.17) | 1,166 (65.36) | ||
| City | 30 (27.52) | 66 (32.04) | 462 (25.90) | ||
| Country | 12 (11.01) | 14 (6.80) | 156 (8.74) | ||
| Average annual household income ($), M (Q1, Q3) | 6,827 (5,612, 7,795) | 7,170 (6,169, 8,657) | 7,443 (6,317, 9,136) | H = 17.573 | <.001 |
| Laterality, n (%) | χ2 = 2.145 | .342 | |||
| Left | 66 (60.55) | 110 (53.40) | 952 (53.36) | ||
| Right | 43 (39.45) | 96 (46.60) | 832 (46.64) | ||
| Grade, n (%) | χ2 = 19.886 | <.001 | |||
| 1 | 6 (5.50) | 25 (12.14) | 224 (12.56) | ||
| 2 | 45 (41.28) | 102 (49.51) | 962 (53.92) | ||
| 3 | 58 (53.21) | 79 (38.35) | 598 (33.52) | ||
| T stage, n (%) | χ2 = 33.261 | <.001 | |||
| 0 | 0 (0.00) | 0 (0.00) | 1 (0.06) | ||
| 1 | 30 (27.52) | 80 (38.83) | 837 (46.92) | ||
| 2 | 60 (55.05) | 92 (44.66) | 785 (44.00) | ||
| 3 | 4 (3.67) | 8 (3.88) | 55 (3.08) | ||
| 4 | 15 (13.76) | 26 (12.62) | 106 (5.94) | ||
| N stage, n (%) | χ2 = 26.205 | <.001 | |||
| 0 | 42 (38.53) | 117 (56.80) | 1,015 (56.89) | ||
| 1 | 40 (36.70) | 59 (28.64) | 540 (30.27) | ||
| 2 | 14 (12.84) | 24 (11.65) | 153 (8.58) | ||
| 3 | 13 (11.93) | 6 (2.91) | 76 (4.62) | ||
| M stage, n (%) | χ2 = 2.941 | .230 | |||
| 0 | 107 (98.17) | 206 (100.00) | 1,763 (98.82) | ||
| 1 | 2 (1.83) | 0 (0.00) | 21 (1.18) | ||
| AJCC stage, n (%) | χ2 = 53.505 | <.001 | |||
| 0 | 0 (0.00) | 0 (0.00) | 1 (0.06) | ||
| I | 21 (19.27) | 62 (30.10) | 618 (34.64) | ||
| II | 47 (43.12) | 98 (47.57) | 888 (49.78) | ||
| III | 39 (35.78) | 46 (22.33) | 240 (13.45) | ||
| IV | 2 (1.83) | 0 (0.00) | 37 (2.07) | ||
| Tumor size (mm), M (Q1, Q3) | 25.00 (20.00, 35.00) | 24.50 (18.00, 34.00) | 21.00 (15.00, 29.00) | H = 32.941 | <.001 |
| Pathological type, n (%) | χ2 = 6.522 | .367 | |||
| Invasive ductal carcinoma | 1 (0.49) | 1 (0.92) | 26 (1.46) | ||
| Intraductal papilloma | 185 (89.81) | 102 (93.58) | 1,610 (90.25) | ||
| Adenoma | 2 (0.97) | 0 (0.00) | 4 (0.22) | ||
| Others | 18 (8.74) | 6 (5.50) | 144 (8.07) | ||
| Primary site, n (%) | χ2 = 20.781 | .187 | |||
| Nipple | 4 (3.67) | 14 (6.80) | 90 (5.04) | ||
| Central portion | 52 (47.71) | 89 (43.20) | 764 (42.83) | ||
| Upper-inner quadrant | 5 (4.59) | 5 (2.43) | 79 (4.43) | ||
| Lower-inner quadrant | 1 (0.92) | 4 (1.94) | 28 (1.57) | ||
| Upper-outer quadrant | 8 (7.34) | 26 (12.62) | 222 (12.44) | ||
| Lower-outer quadrant | 4 (3.67) | 7 (3.40) | 74 (4.15) | ||
| Axillary tail | 0 (0.00) | 1 (0.49) | 2 (0.11) | ||
| Overlapping lesion, n (%) | 15 (16.59) | 21 (10.19) | 296 (16.59) | ||
| Breast, NOS | 20 (12.84) | 39 (18.93) | 229 (12.84) | ||
| Surgery, n (%) | χ2 = 28.712 | <.001 | |||
| Yes | 97 (88.99) | 188 (91.26) | 1,728 (96.86) | ||
| No | 12 (11.01) | 18 (8.74) | 56 (3.14) | ||
| Surgery recommendations, n (%) | χ2 = 28.951 | <.001 | |||
| Surgery recommended and done | 97 (88.99) | 188 (91.26) | 1,728 (96.86) | ||
| Surgery not recommended | 8 (7.34) | 11 (5.24) | 36 (2.02) | ||
| Surgery recommended not done | 4 (3.67) | 7 (3.40) | 20 (1.12) | ||
| Radiation, n (%) | χ2 = 4.507 | .105 | |||
| Yes | 81 (74.31) | 159 (77.18) | 526 (29.48) | ||
| No or unknown | 28 (25.69) | 47 (22.82) | 1,258 (70.52) | ||
| Subtype, n (%) | χ2 = 18.751 | <.001 | |||
| Luminal A | 83 (85.44) | 176 (85.44) | 1,541 (86.38) | ||
| Luminal B | 20 (18.35) | 30 (14.56) | 206 (11.55) | ||
| HER2 enriched | 1 (0.92) | 0 (0.00) | 13 (0.73) | ||
| Triple negative | 5 (4.59) | 0 (0.00) | 24 (1.35) | ||
| Survival months, M (Q1, Q3) | 31.00 (17.00, 44.00) | 26.50 (12.00, 47.00) | 32.00 (13.00, 56.00) | H = 6.382 | <.041 |
Note. Metropolis, population over 1 million. MBC = male breast cancer; AJCC = American Joint Committee on Cancer; NOS = not otherwise specified.
Analysis on the Cumulative Mortality of MBC Patients
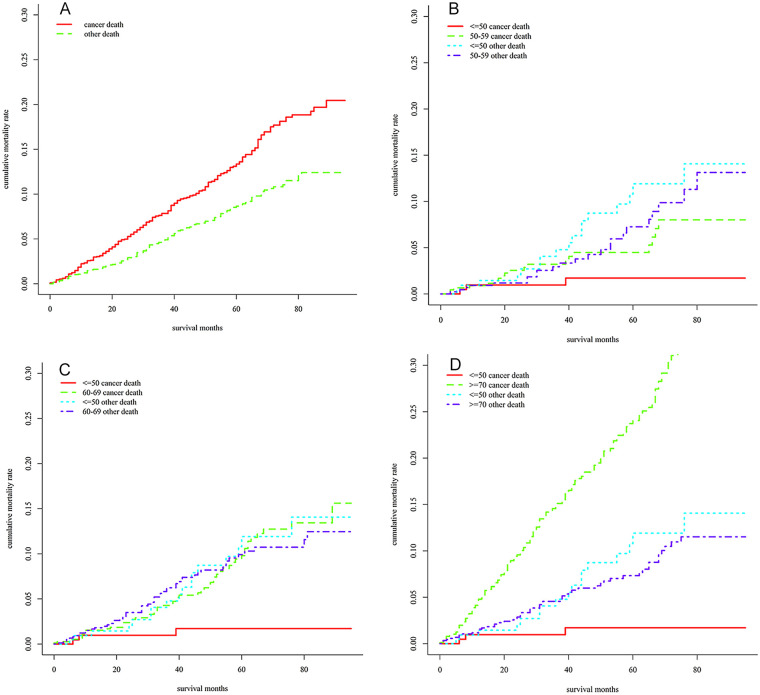
The 1-, 3-, and 5-year cumulative mortality of patients who died of MBC was 2.23% (95% CI = [1.61%, 2.85%]), 7.56% (95% CI = [6.33%, 8.78%]), and 13.10% (95% CI = [11.10%, 11.32%]), respectively, whereas the 1-, 3-, and 5-year cumulative mortality of MBC patients died of other diseases was 1.46% (95% CI = [0.96%, 1.96%]), 5.59% (95% CI = [4.49%, 6.70%]), and 9.79% (95% CI = [8.10%, 11.47%]), respectively (Figure 3A).
Figure 3.
Cumulative Mortality of Male Breast Cancer Patients. (A) Total Cumulative Mortality; (B) Comparison of Cumulative Mortality Between ≤ 50 Years Group and 50 to 59 Years Group; (C) 60 to 69 Years Group; (D) ≥ 70 Years Group.
Further analysis about the cumulative mortality of MBC patients at different ages was performed. Compared with patients ≤50 years old, patients at the age of 50 to 59 (χ2 = 4.294, p = .038; Figure 3B), 60 to 69 (χ2 = 11.302, p < .001; Figure 3C), and ≥70 (χ2 = 40.221, p < .001; Figure 3D) had a higher MBC-specific cumulative mortality, after adjusting for death from other diseases.
Competitive Death Risk Analysis of MBC Patients
A competitive risk model was constructed to analyze the factors affecting the MBC-specific death of MBC patients. The results demonstrated that Blacks (hazard ratio [HR] = 1.76; 95% CI = [1.12, 2.77]), Grade 3 (HR = 2.56; 95% CI = [1.03, 6.35]), AJCC Stage Ⅲ (HR =3.04; 95% CI = [1.76, 5.26]), and AJCC Stage Ⅳ (HR = 7.27; 95% CI = [1.36, 38.83]) were associated with an increased MBC-specific death risk in overall MBC patients, whereas married status (HR = 0.40; 95% CI = [0.25, 0.64]), surgery (HR = 0.25; 95% CI = [0.12, 0.50]), Luminal A subtype (HR = 0.20; 95% CI = [0.07, 0.53]), and Luminal B subtype (HR = 0.29; 95% CI = [0.10, 0.87]) were related to decreased MBC-specific death risk. Among patients with surgery recommended and done, similar results can be observed (p < .05), and radiation (HR = 0.61; 95% CI = [0.37, 0.99]) was also associated with a reduced MBC-specific death risk (Table 2).
Table 2.
The Results of Competitive Death Risk Analysis in MBC Patients.
| Factors | All MBC patients | MBC patients with surgery recommended and done | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Marital status | ||||
| Unmarried | Reference | Reference | ||
| Married | 0.40 [0.25, 0.64] | <.001 | 0.43 [0.27, 0.70] | <.001 |
| Others | 0.78 [0.46, 1.32] | .348 | 0.78 [0.45, 1.35] | .376 |
| Race | ||||
| Whites | Reference | Reference | ||
| Blacks | 1.76 [1.12, 2.77] | .0147 | 1.91 [1.18, 3.09] | .009 |
| Others | 0.48 [0.11, 2.00] | .3120 | 0.24 [0.03, 1.82] | .168 |
| Grade | ||||
| 1 | Reference | Reference | ||
| 2 | 1.49 [0.61, 3.63] | .377 | 1.53 [0.56, 4.16] | .408 |
| 3 | 2.56 [1.03, 6.35] | .043 | 2.89 [1.04, 8.05] | .043 |
| AJCC stage | ||||
| Ⅰ | Reference | Reference | ||
| Ⅱ | 1.45 [0.84, 2.51] | .185 | 1.80 [0.97, 3.36] | .064 |
| Ⅲ | 3.04 [1.76, 5.26] | <.001 | 4.71 [2.48, 8.95] | <.001 |
| Ⅳ | 7.27 [1.36, 38.83] | .020 | 28.43 [3.26, 248.18] | .003 |
| Surgery | ||||
| No | Reference | |||
| Yes | 0.25 [0.12, 0.50] | <.001 | — | — |
| Radiation | ||||
| No | — | — | Reference | |
| Yes | — | — | 0.61 [0.37, 0.99] | .043 |
| Subtype | ||||
| Triple negative | Reference | |||
| Luminal A | 0.20 [0.07, 0.53] | .001 | 0.18 [0.06, 0.55] | .016 |
| Luminal B | 0.29 [0.10, 0.87] | .027 | 0.23 [0.07, 0.76] | .003 |
| HER2 enriched | 0.32 [0.03, 3.34] | .342 | 0.31 [0.03, 3.66] | .350 |
Note. MBC = male breast cancer; HR = hazard ratio; CI = confidence interval; AJCC = American Joint Committee on Cancer.
Discussion
In this study, we analyzed the prevalence trend of MBC from 1975 to 2017 based on SEER databases. The prevalence of MBC has increased gradually with APC of 0.536%, and it was rapidly increased in patients aged ≥ 70 years or at Grade Ⅱ. The 1-, 3-, and 5-year MBC-specific cumulative mortality of patients was 2.23%, 7.56%, and 13.10%, respectively. The MBC-specific cumulative mortality of patients in the 50 to 59 years group, 60 to 69 years group, and ≥70 years group was higher than those in the ≤50 years group. In addition, we conducted a competitive risk analysis on the factors affecting the MBC-specific death and found that Blacks, Grade 3, AJCC Stage Ⅲ, AJCC stage Ⅳ, married status, surgery, and Luminal A and B subtype subtypes possibly affected the MBC-specific death of patients.
The occurrence of MBC may be related to many factors, including demographic factors (age, race, etc.), genetic factors (BRCA1 and 2, etc.), environmental factors (radiation exposure), and hormonal factors (increased serum estradiol, liver disease, obesity, etc.; Giordano, 2018). MBC is an age-related disease, the incidence rates of which raise steadily with age (American Cancer Society, 2018). The BRCA mutation is one of the clearest risk factors for MBC (Antoniou et al., 2003). Previous studies have shown that some of MBC patients (0%–4%) have BRCA1 mutations and some (4%–16%) have BRCA2 mutations (Basham et al., 2002; Ding et al., 2011; Ottini et al., 2003). In addition, radiation exposure has been reported as a risk factor for breast in men (Little & McElvenny, 2017). Importantly, MBC is highly sensitive to hormonal changes. The occurrence risk of MBC may be increased by some diseases related to abnormal estrogen exposure or imbalance of estrogen/androgen ratio, such as Klinefelter syndrome, obesity, and liver disease (Brinton et al., 2014; Ottini, 2014). This study analyzed the prevalence trend of MBC from 1975 to 2017 based on SEER databases. The results showed that the prevalence of MBC has increased gradually with APC of 0.536%. In particular, it was rapidly increased in patients aged ≥ 70 years or at Grade Ⅱ. The prevalence of patients with Grade Ⅱ and Ⅲ has increased significantly, especially for patients with Grade Ⅱ, whereas the prevalence of patients with Grade Ⅳ and Ⅰ increased slowly. The possible reason for the higher detection rate of early-stage tumors may be related to the development of detection technology (Gao et al., 2018).
We used the competitive risk analysis to explore the factors that affected the MBC-specific death of MBC patients. Our results suggested that Blacks, Grade 3, AJCC Stage Ⅲ, and AJCC Stage Ⅳ were related to an increased MBC-specific risk, whereas married status, surgery, and Luminal A and B subtypes were associated with reduced MBC-specific risk. Taylor et al. reported that the relative survival rate of Blacks with MBC was lower than that of Whites and Hispanics (Ellington et al., 2020). In terms of marital status, Adekolujo et al. found that married men with Stage Ⅱ, Ⅲ, and Ⅳ MBC had significantly better 5-year cancer-specific survival rates compared with unmarried men (Adekolujo et al., 2017). The main reason seems to be that married people can get more psychological support (Baine et al., 2011). Sarmiento et al. (2020) revealed that surgery can significantly improve survival in MBC patients. Furthermore, the association between shorter surgery time and improved survival is well recognized (Bleicher et al., 2016; Mansfield et al., 2017; Polverini et al., 2016). Among different molecular types of MBC, patients with Luminal A type exhibited a lower death risk than those with other types. Another study also supported that Luminal A type was associated with better overall survival (Sánchez-Muñoz et al., 2018; Schildhaus et al., 2013; Yu et al., 2013).
Age played an important role in the occurrence and prognosis of MBC. It was reported that the average age of man diagnosed with breast cancer is approximately 5 years older than that of women (67 vs. 62 years; Giordano et al., 2004). Our results displayed that the MBC-specific cumulative mortality of MBC patients in the 50 to 59 years group, 60 to 69 years group, and ≥70 years group was higher than those in the ≤50 years group. According to reports, approximately one third of MBC patients were aged <60, one third aged 60 to 69, and one third aged ≥70 (Ellington et al., 2020). Our results also demonstrated that age may not be related to the MBC-specific death risk. Previous studies also supported our results that age was not associated with the MBC-specific death but with all-cause death in MBC patients (Cardoso et al., 2018; Cronin et al., 2018).
Limitations and Strengths
We analyzed the prevalence trend of MBC from 1975 to 2017 by using SEER database from and explored the factors affecting the death of MBC patients through competitive risk analysis. The cumulative mortality of MBC at 1, 3, and 5 years was calculated and further analyzed based on age. However, this study has some limitations. First, the biochemical indicators that might cause the death of MBC patients were not explored because of their deficiency in the SEER database. Second, except the analysis of prevalence trends, other analyses were based on the data from 2010 to 2017 because the data of HER2 molecular type in the SEER database did not exist until 2010. Third, the database lacked other detailed treatment information, such as information on the use of drugs during chemotherapy.
Conclusion
The prevalence of MBC has increased gradually and it was rapidly increased in patients aged ≥ 70 or at Grade Ⅱ. The MBC-specific cumulative mortality of MBC patients in the 50 to 59 years group, 60 to 69 years group, and ≥70 years group were higher than those in the ≤ 50 years group. The competitive risk analysis showed that Blacks, Grade 3, AJCC Stage Ⅲ, AJCC Stage Ⅳ, married status, surgery, and Luminal A and B subtypes were possible factors affecting the death of MBC patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Xiaofei Cui  https://orcid.org/0000-0002-3726-0370
https://orcid.org/0000-0002-3726-0370
References
- Adekolujo O. S., Tadisina S., Koduru U., Gernand J., Smith S. J., Kakarala R. R. (2017). Impact of marital status on tumor stage at diagnosis and on survival in male breast cancer. American Journal of Men’s Health, 11(4), 1190–1199. 10.1177/1557988316669044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. (2018). Key statistics for breast cancer in men: Cancer facts & figures 2018. https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
- Anderson W. F., Jatoi I., Tse J., Rosenberg P. S. (2010). Male breast cancer: A population-based comparison with female breast cancer. Journal of Clinical Oncology, 28(2), 232–239. 10.1200/jco.2009.23.8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou A., Pharoah P. D., Narod S., Risch H. A., Eyfjord J. E., Hopper J. L., . . . Easton D. F. (2003). Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. American Journal of Human Genetics, 72(5), 1117–1130. 10.1086/375033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baine M., Sahak F., Lin C., Chakraborty S., Lyden E., Batra S. K. (2011). Marital status and survival in pancreatic cancer patients: A SEER based analysis. PLOS ONE, 6(6), Article e21052. 10.1371/journal.pone.0021052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham V. M., Lipscombe J. M., Ward J. M., Gayther S. A., Ponder B. A., Easton D. F., Pharoah P. D. (2002). BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast Cancer Research, 4(1), R2. 10.1186/bcr419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicher R. J., Ruth K., Sigurdson E. R., Beck J. R., Ross E., Wong Y. N., . . . Egleston B. L. (2016). Time to surgery and breast cancer survival in the United States. JAMA Oncology, 2(3), 330–339. 10.1001/jamaoncol.2015.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton L. A., Cook M. B., McCormack V., Johnson K. C., Olsson H., Casagrande J. T., . . . Thomas D. B. (2014). Anthropometric and hormonal risk factors for male breast cancer: Male breast cancer pooling project results. Journal of the National Cancer Institute, 106(3), djt465. 10.1093/jnci/djt465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F., Bartlett J. M. S., Slaets L., van Deurzen C. H. M., van Leeuwen-Stok E., Porter P., . . . Giordano S. H. (2018). Characterization of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Annals of Oncology, 29(2), 405–417. 10.1093/annonc/mdx651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin P. A., Romanoff A., Zabor E. C., Stempel M., Eaton A., Smyth L. M., . . . Gemignani M. L. (2018). Influence of age on the clinical outcome of breast cancer for men and the development of second primary cancers. Annals of Surgical Oncology, 25(13), 3858–3866. 10.1245/s10434-018-6767-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y. C., Steele L., Kuan C. J., Greilac S., Neuhausen S. L. (2011). Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Research and Treatment, 126(3), 771–778. 10.1007/s10549-010-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington T. D., Henley S. J., Wilson R. J., Miller J. W. (2020). Breast cancer survival among males by race, ethnicity, age, geographic region, and stage: United States, 2007-2016. Morbidity and Mortality Weekly Report, 69(41), 1481–1484. 10.15585/mmwr.mm6941a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Heller S. L., Moy L. (2018). Male breast cancer in the age of genetic testing: An opportunity for early detection, tailored therapy, and surveillance. Radiographics, 38(5), 1289–1311. 10.1148/rg.2018180013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano S. H. (2018). Breast cancer in men. New England Journal of Medicine, 378(24), 2311–2320. 10.1056/NEJMra1707939 [DOI] [PubMed] [Google Scholar]
- Giordano S. H., Cohen D. S., Buzdar A. U., Perkins G., Hortobagyi G. N. (2004). Breast carcinoma in men: A population-based study. Cancer, 101(1), 51–57. 10.1002/cncr.20312 [DOI] [PubMed] [Google Scholar]
- Iorfida M., Bagnardi V., Rotmensz N., Munzone E., Bonanni B., Viale G., . . . Colleoni M. (2014). Outcome of male breast cancer: A matched single-institution series. Clinical Breast Cancer, 14(5), 371–377. 10.1016/j.clbc.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Kreiter E., Richardson A., Potter J., Yasui Y. (2014). Breast cancer: Trends in international incidence in men and women. British Journal of Cancer, 110(7), 1891–1897. 10.1038/bjc.2014.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M. P., McElvenny D. M. (2017). Male breast cancer incidence and mortality risk in the Japanese atomic bomb survivors: Differences in excess relative and absolute risk from female breast cancer. Environmental Health Perspectives, 125(2), 223–229. 10.1289/ehp151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly D., Forman D., Ferlay J., Brinton L. A., Cook M. B. (2013). An international comparison of male and female breast cancer incidence rates. International Journal of Cancer, 132(8), 1918–1926. 10.1002/ijc.27841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield S. A., Abdel-Rasoul M., Terando A. M., Agnese D. M. (2017). Timing of breast cancer surgery: How much does it matter? Breast Journal, 23(4), 444–451. 10.1111/tbj.12758 [DOI] [PubMed] [Google Scholar]
- Ottini L. (2014). Male breast cancer: A rare disease that might uncover underlying pathways of breast cancer. Nature Reviews Cancer, 14(10), Article 643. 10.1038/nrc3806 [DOI] [PubMed] [Google Scholar]
- Ottini L., Masala G., D’Amico C., Mancini B., Saieva C., Aceto G., . . . Palli D. (2003). BRCA1 and BRCA2 mutation status and tumor characteristics in male breast cancer: A population-based study in Italy. Cancer Research, 63(2), 342–347. [PubMed] [Google Scholar]
- Polverini A. C., Nelson R. A., Marcinkowski E., Jones V. C., Lai L., Mortimer J. E., . . . Kruper L. (2016). Time to treatment: Measuring quality breast cancer care. Annals of Surgical Oncology, 23(10), 3392–3402. 10.1245/s10434-016-5486-7 [DOI] [PubMed] [Google Scholar]
- Sánchez-Muñoz A., Vicioso L., Santonja A., Álvarez M., Plata-Fernández Y., Miramón J., . . . Alba E. (2018). Male breast cancer: Correlation between immunohistochemical subtyping and PAM50 intrinsic subtypes, and the subsequent clinical outcomes. Modern Pathology, 31(2), 299–306. 10.1038/modpathol.2017.129 [DOI] [PubMed] [Google Scholar]
- Sarmiento S., McColl M., Musavi L., Gani F., Canner J. K., Jacobs L., . . . Habibi M. (2020). Male breast cancer: A closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Research and Treatment, 180(2), 471–479. 10.1007/s10549-020-05556-y [DOI] [PubMed] [Google Scholar]
- Schildhaus H. U., Schroeder L., Merkelbach-Bruse S., Binot E., Büttner R., Kuhn W., Rudlowski C. (2013). Therapeutic strategies in male breast cancer: Clinical implications of chromosome 17 gene alterations and molecular subtypes. Breast, 22(6), 1066–1071. 10.1016/j.breast.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Fuchs H. E., Jemal A. (2021). Cancer statistics, 2021. CA: A Cancer Journal for Clinicians, 71(1), 7–33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- Surveillance, Epidemiology, and End Results Program. (2021). SEER cancer statistics review (CSR) 1975-2017. https://seer.cancer.gov/csr/1975_2017/
- Wan B. A., Ganesh V., Zhang L., Sousa P., Drost L., Lorentz J., . . . Chow E. (2018). Treatment outcomes in male breast cancer: A retrospective analysis of 161 patients. Clinical Oncology, 30(6), 354–365. 10.1016/j.clon.2018.02.026 [DOI] [PubMed] [Google Scholar]
- Yu X. F., Feng W. L., Miao L. L., Chen B., Yang H. J. (2013). The prognostic significance of molecular subtype for male breast cancer: A 10-year retrospective study. Breast, 22(5), 824–827. 10.1016/j.breast.2013.02.005 [DOI] [PubMed] [Google Scholar]