Abstract
Background:
The global prevalence of hypertension in children and adolescents has increased over the past 2 decades and is the strongest predictor of adult hypertension. South Asians have an increased prevalence of metabolic syndrome associated risk factors including abdominal obesity, diabetes, and hypertension. All these factors contribute to their increased cardiovascular disease burden. Accurate and early identification of hypertension in South Asian children is a necessary aspect of cardiovascular disease prevention. Ambulatory blood pressure monitoring (ABPM) is considered the gold-standard for pediatric blood pressure (BP) measurement. However, its utilization is limited due to the lack of validated normative reference data in diverse, multiethnic pediatric populations.
Objective:
The primary objective is to establish normative height-sex and age-sex-specific reference values for 24-h ABPM measurements among South Asian children and adolescents (aged 5-17 years) in Ontario and British Columbia, Canada. Secondary objectives are to evaluate differences in ABPM measurements by body mass index classification, to compare our normative data against pre-existing data from German and Hong Kong cohorts, and to evaluate relationships between habitual movement behaviors, diet quality, and ABPM measurements.
Design:
Cross-sectional study, quasi-representative sample.
Setting:
Participants will be recruited from schools, community centers, and places of worship in Southern Ontario (Greater Toronto and Hamilton area, including the Peel Region) and Greater Vancouver, British Columbia.
Participants:
We aim to recruit 2113 nonoverweight children (aged 5-17 years) for the primary objective. We aim to recruit an additional 633 overweight or obese children to address the secondary objectives.
Measurements:
Ambulatory BP monitoring measurements will be obtained using Spacelabs 90217 ABPM devices, which are validated for pediatric use. The ActiGraph GT3X-BT accelerometer, which has also been validated for pediatric use, will be used to obtain movement behavior data.
Methods:
Following recruitment, eligible children will be fitted with 24-h ABPM and physical activity monitors. Body anthropometrics and questionnaire data regarding medical and family history, medications, diet, physical activity, and substance use will be collected. Ambulatory BP monitoring data will be used to develop height-sex- and age-sex-specific normative reference values for South Asian children. Secondary objectives include evaluating differences in ABPM measures between normal weight, overweight and obese children; and comparing our South Asian ABPM data to existing German and Hong Kong data. We will also use compositional data analysis to evaluate associations between a child’s habitual movement behaviors and ABPM measures.
Limitations:
Bloodwork will not be performed to facilitate recruitment. A non-South Asian comparator cohort will not be included due to feasibility concerns. Using a convenience sampling approach introduces the potential for selection bias.
Conclusions:
Ambulatory BP monitoring is a valuable tool for the identification and follow-up of pediatric hypertension and overcomes many of the limitations of office-based BP measurement. The development of normative ABPM data specific to South Asian children will increase the accuracy of BP measurement and hypertension identification in this at-risk population, providing an additional strategy for primary prevention of cardiovascular disease.
Keywords: hypertension, blood pressure, ambulatory blood pressure monitoring, South Asian, pediatric, children
Abrégé
Contexte:
La prévalence mondiale de l’hypertension chez les enfants et les adolescents a augmenté au cours des deux dernières décennies et constitue le plus important facteur prédictif de l’hypertension chez les adultes. Le syndrome métabolique associé aux facteurs de risque que sont l’obésité abdominale, le diabète et l’hypertension est plus prévalent chez les personnes d’origine sud-asiatique. Tous ces facteurs contribuent à une charge de morbidité cardiovasculaire accrue pour ces personnes. Le dépistage précis et précoce de l’hypertension chez les enfants d’Asie du Sud est un aspect incontournable de la prévention des maladies cardiovasculaires. Le monitoring ambulatoire de la pression artérielle (MAPA) est considéré comme la norme pour la mesure de la pression artérielle chez les enfants. Son utilization est toutefois limitée en raison de l’absence de références normatives validées dans des populations pédiatriques diversifiées et multiethniques.
Objectifs:
L’objectif principal est d’établir des valeurs de référence normatives taille-sexe et âge-sexe pour les mesures de MAPA sur 24 heures chez les enfants et les adolescents d’origine sud-asiatique (âgés de 5-17 ans) de l’Ontario et de Colombie-Britannique (Canada). Les objectifs secondaires sont : 1) d’évaluer les différences dans les mesures de MAPA selon une classification basée sur l’indice de masse corporelle; 2) de comparer nos données normatives aux données préexistantes tirées de cohortes d’Allemagne et de Hong Kong, et 3) d’évaluer les relations entre les comportements actifs habituels, la qualité de l’alimentation et les mesures de MAPA.
Type d’étude:
Étude transversale avec échantillon quasi représentatif.
Cadre:
Les participants seront recrutés dans des écoles, des centers communautaires et des lieux de culte du sud de l’Ontario (région du Grand Toronto et de Hamilton, y compris la région de Peel) et du Grand Vancouver en Colombie-Britannique.
Sujets:
Nous souhaitons recruter 2113 enfants (5 à 17 ans) ne présentant pas de surpoids pour l’objectif principal. Et 633 enfants en surpoids ou obèses pour les objectifs secondaires.
Mesures:
Les mesures de MAPA seront obtenues à l’aide d’appareils Spacelabs 90217 validés pour un usage pédiatrique. L’accéléromètre ActiGraph GT3X-BT, également validé pour un usage pédiatrique, sera utilisé pour colliger des données sur le comportement actif.
Méthodologie:
Après le recrutement, les enfants admissibles seront équipés d’un appareil de MAPA pour 24 heures et de moniteurs d’activité physique. Les caractéristiques anthropométriques et les données d’un questionnaire portant sur les antécédents médicaux et familiaux, la médication, l’alimentation, l’activité physique et la consommation de substances seront recueillies. Les données de MAPA seront utilisées pour établir des valeurs de référence normatives taille-sexe et âge-sexe pour les enfants d’Asie du Sud. Les objectifs secondaires comprennent l’évaluation des différences dans les mesures de MAPA selon que les enfants ont un poids normal, un surpoids ou sont obèses, et la comparaison de nos données de MAPA pour des enfants d’Asie du Sud avec les données existantes en Allemagne et à Hong Kong. Nous procèderons également à l’analyze de composition des données afin d’évaluer les relations entre les comportements actifs habituels de l’enfant et les mesures de MAPA.
Limites:
Pour faciliter le recrutement, les analyses sanguines ne seront pas effectuées. Aucune cohorte de comparaison constituée de sujets non originaires d’Asie du Sud ne sera incluse en raison de problèmes de faisabilité. L’emploi d’une approche d’échantillonnage de commodité introduit un possible biais de sélection.
Conclusion:
Le MAPA est un outil précieux pour le dépistage et le suivi de l’hypertension pédiatrique et elle permet de surmonter plusieurs des limites de la mesure de la PA en cabinet. L’établissement de références normatives de MAPA spécifiques aux enfants d’Asie du Sud permettra d’accroître la précision de la mesure de la PA et le dépistage de l’hypertension dans cette population à risque, fournissant ainsi une stratégie supplémentaire pour la prévention primaire des maladies cardiovasculaires.
Introduction
Hypertension (HTN) is one of the most common causes of preventable death worldwide1,2 and often begins in childhood.3-6 The global prevalence of pediatric HTN has increased over the past 2 decades, from 1.3% (1990-1999) to 6.0% (2010-2014), 7 partly due to the increasing childhood obesity. 8 Childhood blood pressure (BP) remains the strongest predictor of adult HTN and is associated with an increased risk for adult cardiovascular disease (CVD), including ischemic heart disease and stroke.3-6 From a health economics perspective, the costs attributable to HTN in Canada were estimated at $13.9 billion in 2010, rising to $20.5 billion by 2020.9,10
Ambulatory blood pressure monitoring (ABPM) is the reference standard for detecting HTN in children and adults.11-13 It provides a free-living BP assessment over a 24-h period and mitigates many limitations of office-based BP measurement. 14 Despite these advantages, pediatric ABPM interpretation is limited by the continued use of normative data derived over 20 years ago from a homogenous white Caucasian population in Germany. Given that BP is known to vary with ethnicity, 15 this existing normative data may not accurately detect abnormal BP profiles among non-white children. One such population is South Asians (people originating from India, Pakistan, Bangladesh, Nepal and Sri Lanka), which comprise 25% of the world’s population, 16 and are Canada’s fastest-growing non-white ethnic group.
Alarmingly, emerging data suggests an impending epidemic of HTN among South Asian children. In a recent comparison of office-based BP from seven countries, South Asian children had the highest and second-highest median diastolic and systolic BP measurements, respectively.17-19 Our previous work has identified that South Asian adults living in Canada have a higher prevalence of HTN; twice the prevalence of type 2 diabetes; higher body fat; and lower levels of physical activity compared to white Caucasians, all of which contribute to their higher CVD burden.20-24 Many of these cardiometabolic risk factors are also present in South Asian children, who are more likely to have excess adiposity compared to white Caucasian children.25-27
As the South Asian population grows in Canada, efforts to identify and mitigate CVD risk factors at an early stage are of critical importance. Thus, accurate identification of HTN by ABPM among South Asian children should be a priority. However, before ABPM can be applied in routine pediatric practice, it first needs to be validated with normative data representative of multi-ethnic populations. As an initial step to address this critical knowledge gap in research and clinical care, ASHA (Deriving Normative 24-hour Ambulatory Blood Pressure Monitoring Data Among SoutH Asian Children) study has been designed to establish normative ABPM data among a large cohort of South Asian children living in 2 of Canada’s most populated provinces, Ontario, and British Columbia (BC).
Study Objectives
Primary Objective
Establish normative height-sex and age-sex-specific reference values for 24-h ABPM measurements among non-overweight South Asian children and adolescents (aged 5-17 years) living in Ontario and BC.
Secondary Objectives
Determine the extent to which various ABPM parameters differ across body mass index (BMI) classifications (normal vs. overweight vs. obese)
Determine the extent to which South Asian normative ABPM data differ from existing white Caucasian (German) and East Asian (Hong Kong) normative data
Evaluate the association between children’s habitual movement behaviors (physical activity, sedentary time, and sleep) and ABPM parameters
Evaluate the association between diet quality and ABPM parameters, using a simple diet score accounting for major dietary contributors to pediatric BP
Hypothesis
The overarching goal is to improve cardiovascular health of South Asian children in North America and HTN is an immediately targetable risk factor. For this reason, accurate measurement of BP by ABPM among South Asians is critical. Hence, the primary objective is not directly hypothesis-driven but aimed at generating the required 24-h ABPM normative data. The secondary objectives are hypothesis-driven and aimed at internally validating this ABPM normative data.
The working hypothesis is that overweight or obese South Asian children will have higher ABPM measures than normal weight individuals; South Asian children will have higher BP compared to white Caucasians and East Asians; children with optimal movement behaviors (ie, high physical activity, low sedentary time, adequate sleep) and those with an optimal diet (ie, high intake of fruits and vegetables, dairy, and whole grains and low intake of sugary drinks, processed salty foods, soft drinks, and sweets) will demonstrate healthier ABPM profiles.
Methods
Study Design
This is a cross-sectional study with a quasi-representative sample of South Asian children living in Southern Ontario (Greater Toronto and Hamilton area, including the Peel Region) and Greater Vancouver, British Columbia (including Surrey). Hamilton Integrated Research Ethics Board approval for this study has been obtained.
Eligibility Criteria
Children (aged 5-17 years) will be included if they have at least three South Asian grandparents.28,29 This age cut off was chosen because ABPM is recommended for children ≥5 years old. Children with pre-existing medical conditions (Table 1) or using specific medications known to affect BP will be excluded.
Table 1.
Inclusion and Exclusion Criteria for the ASHA Study.
| Inclusion criteria: |
| 1. Aged 5-17 years at the time of the study enrollment 2. South Asian descent (defined as having at least 3 of 4 grandparents who had ancestral origins in India, Pakistan, Bangladesh, Sri Lanka, or Nepal). |
| Exclusion criteria: |
| 1. Inability or unwillingness to provide informed or surrogate consent 2. History of previously diagnosed hypertension from a healthcare provider or previous treatment with lifestyle measures or antihypertensives (we will not exclude children with previously elevated BP readings that have not been diagnosed with hypertension and/or have not had any recommendations for lifestyle of medication management) 3. History of cardiovascular, renal, or endocrine disorders - Cardiovascular: aortic coarctation, any congenital heart disease, and cardiomyopathy - Renal: renal artery stenosis/abnormalities, renal vein thrombosis, acute glomerulonephritis, nephrotic syndrome, long-term kidney disease, end-stage renal disease (dialysis or transplantation), polycystic kidney disease, congenital anomalies of the kidneys and urinary tract (CAKUT) - Endocrine: thyroid disorders, congenital adrenal hyperplasia, Cushing syndrome, hyperaldosteronism, hyperparathyroidism, hypercalcemia, diabetes mellitus (type 1 or 2), diabetes insipidus, and pheochromocytoma - Other: malignancy, systemic lupus erythematosus, systemic juvenile idiopathic arthritis, Kawasaki disease, neurofibromatosis, tuberous sclerosis, and fibromuscular dysplasia 4. History of clinically relevant inborn errors of metabolism or pathological genetic disorders 5. Current use of medications known to affect BP, regardless of dose/duration or indication for use (defined as any ACE inhibitor, angiotensin-II receptor blocker, calcium channel blocker, thiazide-like diuretic, potassium-sparing diuretic, alpha-blocker, or beta-blocker) 6. Current use of oral/intravenous steroids and/or ≥1 month of cumulative oral/intravenous steroid use in the past 12 months regardless of dose (includes prednisone, prednisolone, dexamethasone, and hydrocortisone). This does not include inhaled, intranasal, ophthalmic, or topical corticosteroids. 7. Current use of any stimulant medication (includes amphetamine/dextroamphetamine, dextroamphetamine) 8. Actively pregnant or breastfeeding |
Note. ASHA = South Asian cHildren in Canada; BP = blood pressure; ACE = Angiotensin-converting enzyme.
For our primary objective, only nonoverweight children will be included, consistent with 2017 American Academy of Pediatrics (AAP) HTN guidelines. 30 For the secondary objectives, additional overweight/obese children will be enrolled for comparison with nonoverweight children.
Participant Recruitment
A comprehensive list of local schools, places of worship (ie, temples, mosques, and churches), and community centers within the Greater Toronto and Hamilton area and Greater Vancouver will be compiled. All institutions will be invited to participate. After approval from school boards, invitation packages will be distributed (either in person or electronically) to the parents/guardians of all students enrolled at participating schools. These packages will include an invitation letter, study description, and required consent forms. Study information will also be disseminated in the newsletters of participating schools, and information stands will be set up at school events to advertise the study to parents/guardians.
After obtaining consent, study information leaflets that are accessible in multiple languages (English, Hindi, Punjabi, Gujrati, and Urdu) will be distributed. In addition, with the required permission, research staff will be made available at booths during health fairs and public events, distributing study packages with the pertinent contact information. Individuals that are interested in participating will be able to communicate with a research coordinator (via phone or email) to discuss the study and arrange an appointment to be fitted for the ABPM and accelerometer devices. Participant health card numbers will be recorded for future linkage with Ontario administrative databases housed at ICES.
Questionnaire and data management
Parents/guardians (for children <12 years) and adolescents (≥12 years) will be asked to complete questionnaires on covariates (including age/sex), physical activity, sedentary and sleep behaviors. Children ≥12 years will be asked confidentially about smoking, vaping, and alcohol use. 31 Children’s diet quality (reflecting intake of fruits and vegetables, dairy, whole grains; and processed foods, salty snacks, sugary drinks, and sweets) will also be assessed, adapting a diet quality score that has previously been published by the research team. 32 Briefly, diet quality will be scored as follows: 1 point is given for consuming more than the study population median of green vegetables, fruits, whole grains, and so on.; or less than the study population median of sugary snacks, sweets, and so on. The final score ranges from 0 to 6; diet quality is classified as low (0 or 1), intermediate (2 or 3), or high.4-6 Information on annual household income, parenteral employment, and marital status will also be collected to derive a validated social disadvantage index; which combines social and economic factors into a single measure and has previously been published by the team. 33 The social disadvantage score ranges from 0 to 5, with 5 representing the greatest social disadvantage. Among children ≥12 years, we will also use a validated perceived stress scale (PSS-10) 34 to measure perception of psychological stress, which can also affect BP. 35 Score ranges from 0 to 40, and higher scores correspond to a higher level of perceived stress.
Study questionnaires will be completed by parents/guardians electronically using REDCap Surveys. Participants who are unable to complete the electronic survey will complete paper questionnaires, that will be entered by research coordinators into a secure REDCap database. Ambulatory BP monitoring software outputs and movement behavior data for each participant will also be entered into REDCap and will be linked using unique identifiers to these questionnaires and forms. REDCap databases will be housed on a secure centralized server at McMaster University.
Anthropometric Measurements
A team of trained research staff will collect anthropometric data at the ABPM fitting visit. Standing height and body weight will be measured. There is no consensus on which anthropometric method is best to define overweight/obesity among children. Each of the commonly used indicators of childhood obesity (ie, body mass index [BMI], waist and hip circumference, waist-to-height ratio, and body fat percentage) are associated with cardiometabolic risk.36,37 Moreover, this research group has previously shown that all of these adiposity indicators are strongly associated with HTN among South Asian children. 22 Hence, overweight or obesity in the study population will be defined if any of the following criteria are met: BMI: Overweight if BMI >85th percentile, obesity as BMI >97th percentile, using World Health Organization (WHO) reference data. 38 Waist circumference: Obesity will be classified as sex-specific waist circumference >90th percentile. Waist-to-height ratio (WHtR): calculated as (waist circumference [cm]/height [cm]) and transformed into WHtR z-score; obesity defined as a ratio of >0.5. Body fat percentage: estimated by bioelectrical impedance analysis (BIA); Overweight if body fat >85th percentile, obesity if >95th percentile based on existing normative data. 39 Casual BP will be measured from right arm by auscultatory method as per AAP 2017 guidelines. 30 Three readings, at least 3 minutes apart, will be taken and averaged. Participants will be asked to perform the hand grip strength test using Takei Handgrip Dynamometer. Urine samples will be collected from participants for storage and future analysis for creatinine, sodium, and potassium.
Ambulatory BP monitoring
All ABPM measurements will be taken from the nondominant arm. All measurements will be performed according to 2014 American Heart Association ABPM guidelines. 40 The Spacelabs 90217 ABPM device has been validated for use in children and can measure heart rate, systolic and diastolic BP by oscillometric method. 41 The validation study enrolled 112 American children aged 6 to 17 from diverse ethnic backgrounds, finding that the Spacelab 90217 ABPM device accurately measured BP, compared against a British Hypertension Society protocol. Ambulatory BP monitoring readings will be obtained every 30 min during the day and 30 min during the night for a 24-h period. All participants will be instructed to maintain their usual activity level but will be requested to avoid swimming or bathing for the 24-h period. Furthermore, participants and parents/guardians will be instructed to record bedtime and wake time in a diary, which will be used to categorize daytime and nighttime readings.
Ambulatory BP monitoring profiles with less than 50% of the proposed daily or nighttime readings will be excluded. Profiles where there was device disconnection for ≥1.5 consecutive hours will also be excluded. In these instances, with consent from parents/guardians, an ABPM device will be re-fitted, and data will be re-collected for 24-h. The initial recording will be excluded from the study. If, however, during the second data collection process, an adequate profile is not obtained, the subject will be excluded from the study. In instances where the individual is unable to tolerate the device resulting in early removal, the participant will not undergo data re-collection. In the event of device loss or damage, the participant will also not undergo data re-collection. If there is a significant discrepancy (≥3 h physical activity time) between reported physical activity (by questionnaire) and physical activity monitor measurements, the family will be contacted for clarification. If it is determined that the child’s physical activity during the measurement period was significantly different from normal, data re-collection will be offered, and the initial recording will be excluded from the study.
ABPM Parameters
The following ABPM parameters will be measured: mean systolic BP (mmHg), mean diastolic BP (mmHg), mean arterial pressure (mmHg), systolic and diastolic BP load (percentage of abnormal readings; >95th percentile), and nocturnal dipping (percentage of BP reduction while sleeping compared to daytime; normal >10%). Except for nocturnal dipping, these outcomes will be quantified for 3 time periods: overall 24-h, daytime, and night-time periods.
Movement Behaviour Assessment
During the 24-h ABPM period, participant activity levels will simultaneously be tracked using a wearable physical activity monitoring device. Accelerometry is an objective method of assessing free-living activity and has become the method of choice for the measurement of physical activity, sedentary, and sleep time in children. 42 We will use the ActiGraph GT3X-BT accelerometer, which is a small, unobtrusive device validated for pediatric use.43-46 The ActiGraph and ABPM devices will be time-synced and worn over the same 24-h period. All participants will be instructed to maintain their usual activity levels during this 24-h recording period. Physical activity data will be retrieved from the devices and stored together with ABPM data. If the activity monitor recording is inadequate (due to device disconnection, battery failure, etc.), the recording will be excluded but the participants ABPM data will still be included in the study.
To accurately estimate habitual movement behaviors, participants will then continue wearing the ActiGraph device 24-h per day (with the exception of water activities) for a total of 7 consecutive days, as recommended by Trost et al. 47 Participants will be asked to record any device removals, wake and sleep times. Accelerometers will record accelerations at a sampling frequency of 30 Hz, which will then be filtered and converted using a proprietary algorithm into activity counts in 3-s epochs (sampling intervals) to capture the typical “stop-and-go” activity patterns observed in children. 48 Only participants with ≥4 days with ≥10 h of average wear time per day will be included, which has been shown to provide a highly reliable (~92%) estimate of movement behaviors. 49 Using established Evenson cut-points, 43 the daily minutes of sedentary time (defined as time spent with activity counts ≤25), all physical activity (time spent with activity counts >25), light-intensity activity (time spent with activity counts between 26-573), and moderate-to-vigorous physical activity (time spent with activity counts ≥574) will be calculated, which is the focus of public health recommendations. 50
Total sleep time will be quantified using the refined Tudor-Locke algorithm, which has been validated for use in children. 51 This 2-step process first uses the Sadeh sleep-wake algorithm to establish the probability of sleep, where probability of sleep for a given epoch = 7.601 − (0.065 × mean activity counts for 5 min before the epoch) − ln(1.08) − (0.056 × standard deviation activity counts for 6 minutes after the epoch) − 0.703 × ln (activity count of the epoch). If the probability of sleep is ≥0, then the given epoch is scored as sleep; if the probability of sleep is <0, the epoch is scored as awake. 52 This algorithm has been validated using polysomnography data. Next, all epochs scored as sleep are verified against accelerometer-based measures of relative position. When probability of sleep and relative position both indicate sleep, the epoch will be assigned a final label of sleep. 53 Sleep epochs for each day of accelerometer wear will be summed, and reported as average time spent sleeping per day, in minutes.
Safety Monitoring
Ambulatory BP monitoring is a relatively noninvasive tool; however, in the event, a participant experiences intolerance to the device (eg, due to discomfort), parents will be advised to remove the BP cuff for a period of up to 1 h. If they are unable to re-fit the cuff after this time and/or the participant remains intolerant to the device, it will be recommended that they disconnect and return the device.
All participants/families will be notified of the BP classification of their clinic BP readings (as per AAP guidelines) during their final study visit (24-h after the initial readings). They will be provided a handout documenting their AAP BP classification and recommended timing of BP re-measurement. They will be asked to contact their primary-care physician for follow-up of abnormal readings, if necessary. If the participant has evidence of hypertensive urgency or emergency (ie, symptomatic HTN, BP >30mmHg above the 95th percentile, or >150/90mmHg in an adolescent) during their “office-based” readings, they will not proceed with ABPM monitoring, and it will recommend that they seek immediate care in an emergency department setting. If the ABPM reading results in a more severe BP classification than previously recorded during “office-based” readings, the family will be contacted to notify them of this difference and to update the recommendations made regarding BP and medical follow-up.
Study Procedure and Timelines
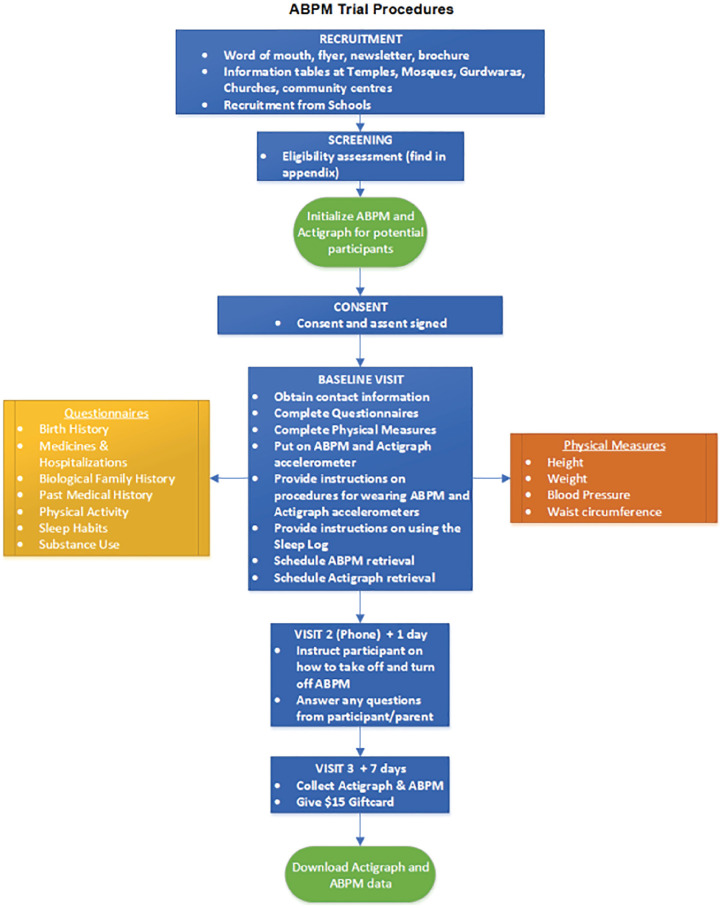
All participants will have a baseline visit (visit 1) which will include completing questionnaires (electronically or paper), collecting anthropometric data, providing instructions on ABPM and ActiGraph, and attachment of both devices. The research coordinator will call the family 24-h later (visit 2) to instruct them to take-off the ABPM device and to answer any questions they may have. The final visit (visit 3) will be in-person on day 8, where ABPM and ActiGraph devices will be returned (Figure 1). The aim is to complete recruitment within 4 years, followed by knowledge translation activities. Based on our assessment of the eligible population size, established support from local recruitment sites (eg, school boards, places of worship, and community centers), our study team’s past experience recruiting within this population, and the resources available for recruitment, we believe that target recruitment is feasible within this timeframe.
Figure 1.
Participant flow diagram.
Note. ABPM = ambulatory blood pressure monitoring.
Sample Size Calculation
Height-sex specific ABPM curves ranging from 120 to 185 cm for boys and to 175 cm for girls, at intervals of 5 cm will be created. Assuming a standard deviation (SD) in mean systolic BP of 8 mmHg by ABPM 54 and a desired precision of measurement of ±2.5 mmHg (95% CI), 70 nonoverweight participants within each height and sex group (a total of 980 boys between 120-185 cm and 840 girls between 120-175 cm) will be required for acceptable precision (SAS software, version 9.4; SAS Institute; Cary, NC). Oversampling will be required to account for 15% loss of participants due to inadequate ABPM recordings (machine malfunction, accidental cuff removal, etc.). This estimate is based on reported frequencies of inadequate pediatric ABPM readings: 8% of children (German study), 11% (Hong Kong study), and 13% to 17% (other pediatric cohorts).55-59 Therefore, a total of 2113 nonoverweight participants are required for the primary objective.
This study is also adequately powered for age-sex-specific ABPM curves. Keeping the same assumptions as above, a sample size of 1932 children will be required, which includes 70 normal weight participants within each age (5-17 years) and sex group and 15% data loss. This sample size is also consistent with recommendations to include at least 50 to 100 individuals in each LMS analysis group, and with previous German (1141 participants) and Hong Kong (1445 participants) studies.
According to Public Health Agency of Canada, around 30% Canadian children are overweight or have obesity. 60 Hence, we will enroll 633 additional children to have adequate power for the secondary objective. Therefore, the total sample size for the study is 2746 (2113 + 633) children.
Statistical Analysis for the Primary Objective
Among nonoverweight children, height-sex, and age-sex-specific centile curves (5th, 50th, 75th, 90th and 95th) for mean systolic BP, mean diastolic BP, and mean arterial pressure will be created. Based on the findings from the German and Hong Kong studies, it is expected that the distributions will be skewed. For this reason, the LMS method will be applied. 61 This approach allows the incorporation of any degree and direction of skewness in the distributions. First, to normalize the skewed distribution data, the Box-Cox power transformation will be applied for each height-sex-specific level. By incorporating this Box-Cox power curve (L), a smoothed median curve (M), and a smoothed coefficient of variation curve (S) of the distribution, height-sex-specific percentile curves will be created. This analysis will be performed using LMS Program (version 12.43; Institute of Child Health, London, UK). The LMS method was also used in previous German and Hong Kong studies, which will allow for easier comparisons between cohorts.54,56 This method will be repeated to determine age-sex-specific centiles. These analyses will be performed for all three time periods (overall 24-h, daytime, and nighttime periods). All statistical tests will be 2-sided, with a P value < .05 considered to be statistically significant.
Statistical Analysis for Secondary Objectives
All secondary objective analyses will be performed for the three time periods: overall 24-h, daytime, and nighttime periods.
To determine mean differences in ABPM measures across BMI classification
Multivariable regression analysis adjusting for age and other covariates (diet quality score, social disadvantage index, perceived stress scores) will be performed. To investigate potential interactions between obesity and sex, sex will individually be added into the model alongside an interaction term. The interaction P value will determine if there is a significant difference in ABPM measures among overweight/obese children and sex.
To descriptively compare our normative data against the German and Hong Kong cohorts
We will plot the height-sex and age-sex-specific 5th, 50th, and 95th centile curves of each cohort alongside each other for three ABPM parameters: mean systolic BP, mean diastolic BP, and mean arterial pressure. Mann Whitney U-test will be used to determine differences between our data with each of the other 2 cohorts (Germany and Hong Kong).
To evaluate the association between child’s habitual movement behaviors and ABPM measures
Compositional data analysis will be used, as outlined by Chastin et al. 62 The advantage of this approach is that it accounts for the fact that physical activity, sedentary time, and sleep time are interconnected behaviors that make up a whole day (ie, sedentary time + physical activity + sleep = 24-h day). The specific habitual movement behaviors of interest include average daily sedentary time (minutes per day), time spent in light-intensity physical activity, moderate-to-vigorous intensity physical activity and sleep, all measured by accelerometry. Briefly, the geometric mean of movement behaviors will first be calculated, which will provide an estimate of the proportion of time spent in each movement behavior per day—the key exposure variables (Table 2). Next, the dispersion of the compositions will be estimated using a variation matrix. 62 The composition, which is summarized by the geometric mean and its variation matrix, will be transformed using the isometric log-ratio transformation and incorporated into regression analyses.
Table 2.
Key Variables on Which Data Will Be Collected Through Validated Questionaries.
| Variable | Parent | Child |
|---|---|---|
| Ethnicity | X | |
| Country of Origin (India, Sri Lanka, Pakistan, Nepal, and Bangladesh) | X | |
| Education | X | |
| Employment | X | |
| Income | X | |
| Family history of CVD, diabetes, HTN | X | |
| Past medical history | x | |
| Sleep time a | x | |
| Physical activity a | x | |
| Office BP (auscultatory method) | x | |
| Diet quality score (consumption of fruits and vegetables, processed foods/salty snacks, low-fat dairy, whole grains, sugary drinks, and sweets) | X | x |
| For children 13 years and older: smoking, vaping, alcohol For children 12 years and older: perceived stress score |
x | |
| Height, weight, waist and hip circumference, body fat, hand grip strength, urine sample | x | |
| Age and sex | x |
Note. CVD = cardiovascular disease; HTN = hypertension; BP = blood pressure.
The questionnaire data for these variables will be used for quality control purposes.
Regression models will be used to estimate:
The independent contribution of each movement behavior to ABPM outcomes (eg, what is the relationship between sedentary time and ABPM parameters?)
The combined effect of the composition of movement behaviors on ABPM outcomes, adjusted for age and BMI (eg, how do the collective movement behaviors predict ABPM parameters?)
The effect of displacing time from one behavior into another on ABPM outcomes, adjusted for age BMI (eg, what is the effect of replacing 30 min of sedentary time with sleep on ABPM parameters?)
To evaluate the association between child’s diet quality and ABPM measures
Univariate and multivariable regression analyses will be used to determine the association of the diet quality score (categorized as low, intermediate, and high) with the ABPM parameters after adjusting for other covariates.
Discussion
The study results will have an immediate impact, providing normative reference values for ABPM interpretation among South Asian children living in Canada. This will facilitate more accurate HTN diagnosis and monitoring among South Asian children, potentially leading to improvements in their cardiometabolic health. This study will seek to overcome several limitations of the existing normative data which was primarily established in white Caucasian children.
No previous studies have examined the relationship between BP and combined movement behaviors in South Asian children. Other modifiable risk factors for pediatric HTN are obesity, high salt intake, and low fruit/vegetable consumption.63,64 This study will give a better understanding of the relationship between ABPM and important modifiable lifestyle factors, identifying suitable intervention targets for at-risk and hypertensive South Asian children.
Undertaking a large multicenter Canadian study does pose significant challenges with regards to successful recruitment, as adequate patient enrollment is integral to the applicability of our findings. Based on 2016 census data, there are 87,770 South Asian children (5-19 years) in the Peel Region and 42,615 in the Vancouver central metropolitan area. The aim is to recruit 2746 children over 5 years, which amounts to 2% of this population, to achieve adequate sample size. Previous studies on this scale have shown us that random sampling is not feasible for this population.22,28,29 Instead, convenience sampling will be used. By obtaining approval from local school boards, a large student population will be more easily accessible, allowing for direct dissemination of information with prospective participants and their parents/guardians. In the Peel Region specifically, for another project, the study team were able to recruit approximately 32% of contacted participants and of those, over 99% were able to complete the study. 29 Similar recruitment rates are expected, using similar strategies as those outlined above. Sample representativeness will also be evaluated by determining the distribution of South Asian ethnic subgroups and comparing these against local 2016 census data (information will be collected on the country of origin, employment status, income, and education level) after recruitment of every 200 children.
In addition to ensuring high levels of enrollment, the potential selection bias must be addressed, which is introduced with an opt-in recruitment strategy. This will primarily be avoided by increasing the accessibility of study procedures and by performing in-person visits in convenient settings (ie, at home or school). In addition, selection bias will also be mitigated in the following ways: by inviting all eligible schools, places of worship, and community centers to participate; by contacting all parents/guardians of South Asian children at included schools; by providing study information in multiple languages to ensure accessibility and providing telephone interpretation services for study visits as required; and by providing flexible hours for study visits to accommodate schedules.
When running a large, multicenter study, it is imperative that data collection is standardized to allow for comparisons to be made between sites. To do this, all the same data gathering techniques (eg, office BP measurement) and type of equipment (ABPM and activity monitoring devices) will be used. Standardized training for research staff in all locations will be implemented. The safe storage and transfer of data is integral to this project, and a centralized secure database with required technological support will be used to ensure security of all data collected.
This study relies heavily on the use of ABPM and activity monitoring devices. Therefore, it is vital that these devices function correctly and that the team is equipped to troubleshoot problems. The research team will be trained specifically with the models used in this study and will be well equipped to troubleshoot common issues. Participants/caretakers will be given the contact information of research staff who they can contact in the event they run into technical issues during the data collection period. There may be participants who are not able to tolerate the devices and those who experience device failure or accidental removal, resulting in incomplete ABPM profiles. For these reasons, more participants than are statistically required will be enrolled, accounting for previously reported rates of participant drop-out or incomplete ABPM measures. In addition, specific procedures for addressing the problem of incomplete profiles have been developed. As outlined previously, profiles with short device disconnection periods (<1.5 h) will be accepted. For instances of prolonged disconnection, a second 24-h data gathering period will be offered, depending on the reason for device disconnection. To ensure that the activity data gathered by questionnaire is comparable to values obtained with activity monitoring devices, up to a 3-h discrepancy will be allowed. Activity guidance will also be provided to the family at the in-person ABPM fitting visit via a written handout, to attempt to minimize the likelihood of significant discrepancies in patient activity. It is also expected that younger participants may experience decreased tolerance for ABPM devices as they may be unaware of when to expect the readings. However, these ABPM devices and accelerometers have previously been used successfully in pediatric cohorts, including young children.41,43-46
To maximize the feasibility of this study, bloodwork was decided not to be included, nor the recruitment of a non-South Asian comparator cohort. However, the results from this study will form the basis of a research platform to address future questions, including metabolomic and genomic profiles related to BP classification, with the anticipated future collection of additional urine and DNA samples.
The multidisciplinary team will launch several knowledge translation and advocacy projects based on our findings. To guide relevant integrated knowledge translation processes from study inception to data dissemination and evaluation, the Knowledge-to-Action (KTA) framework will be used. 65 To support best practices around relevant and impactful recruitment, data collection, and data dissemination activities, a diverse Research Advisory Committee (RAC) will be assembled with representatives from: youth, parents, teachers/educators, places of worship, city community centers, family physicians, Canadian Association of Pediatric Nephrologists (CAPN), Hypertension Canada, Canadian Pediatric Society (CPS), and Peel Region Public Health. The study’s results are most relevant to family physicians, general pediatricians, pediatric nephrologists, and cardiologists who care for children with HTN. An integrated KTA approach includes both knowledge creation and application. The primary goal of these initiatives will be to make this normative data widely accessible, easy to use, and well-integrated with available resources, in order to increase ABPM utilization among South Asian children and facilitate interpretation of this ABPM data with our reference values. To achieve this, we intend to develop a range of clinical tools and provide these tools at no cost on an open-access website. These tools will include ABPM reference value tables and centile curves, as well as a pocket reference guide with a simplified schematic for ABPM interpretation and classification. We will also design an online calculator where clinicians can input ABPM measures and generate an automated ABPM report, with classification based on our normative data. We plan to design a free mobile application featuring this calculator, for easy access in a clinic environment. We aim to include normative ABPM values from other pediatric populations (eg, Caucasian European, East Asian, and future ethnic group data) into this calculator, with potential integration with commonly used electronic medical record platforms.
The platforms of Canadian Association of Pediatric Nephrologists, Hypertension Canada, International Pediatric Nephrology Association, International Society of Nephrology, and Canadian Society of Nephrology will be used to widely disseminate our findings and advocate for the use of ethnic-specific ABPM normative data in South Asian children. We will develop short videos and infographics supplemented with educational resources on lifestyle measures to mitigate HTN and CVD. These will also be distributed to participating schools, places of worship, and community centers. Partnerships with regional and national public health agencies will allow us further distribute results to community-based primary-care providers.
To increase the generalizability of our results on an international level, data will subsequently be compared to ABPM normative data that is being simultaneously collected in India by a collaborative research team. This will provide insight into the impact of geographical location and environmental factors on ABPM measures among individuals of similar ethnic background. Normative data from these 2 populations could be subsequently combined to create a “global” pediatric South Asian normative dataset. Furthermore, this study will serve as a model for determining normative ABPM data in other pediatric populations, allowing for additional normative standards to be developed for clinical use.
Conclusions
Ambulatory BP monitoring is a useful tool for the diagnosis and management of childhood HTN. Establishing normative ABPM reference data for South Asian children may increase its clinical utilization in this population, resulting in more accurate HTN assessment and promoting improved BP control. Comparison against existing German and Hong Kong data will provide information on geographic, ethnic, and temporal changes in childhood BP. Furthermore, this study will serve as a model for determining normative ABPM data and allow for comprehensive standards to be developed for other pediatric populations.
Acknowledgments
The authors thank the Spacelabs company for loaning us ABPM monitors to collect pilot data for this study.
Footnotes
Author Note: Samina Nazarali, is now affiliated to Department of Medicine, Dalhousie University, Halifax, NS, Canada.
List of Abbreviations: HTN, hypertension; BP, blood pressure; ABPM, ambulatory blood pressure monitoring; CVD, cardiovascular disease; BC, British Columbia; BMI, body mass index; AAP, American Academy of Pediatrics; PDSB, Peel District School Board.
Ethics Approval and Consent to Participate: This study protocol has been approved by Hamilton Integrated Research Ethics Board.
Consent for Publication: All authors provided their consent for publication of the manuscript.
Availability of Data and Materials: No additional data and materials are available.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an Early Career Award from Hamilton Health Sciences (award no: ECA-2020-Chanchlani) and a Project Grant from Canadian Institute of Health Research (award no.: PJT 178408).
ORCID iDs: Cal H. Robinson  https://orcid.org/0000-0002-2223-0646
https://orcid.org/0000-0002-2223-0646
Allison Dart  https://orcid.org/0000-0002-4718-9408
https://orcid.org/0000-0002-4718-9408
References
- 1. Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taksler GB, Rothberg MB. Assessing years of life lost versus number of deaths in the United States, 1995-2015. Am J Public Health. 2017;107(10):1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine study. Pediatrics. 1989;84(4):633-641. [PubMed] [Google Scholar]
- 4. Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa heart study. Am J Hypertens. 1995;8(7):657-665. [DOI] [PubMed] [Google Scholar]
- 5. Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338(23):1650-1656. [DOI] [PubMed] [Google Scholar]
- 6. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117(25):3171-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song P, Zhang Y, Yu J, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 2019;173(12):1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rao DP, Kropac E, Do MT, Roberts KC, Jayaraman GC. Childhood overweight and obesity trends in Canada. Health Promot Chronic Dis Prev Can. 2016;36(9):194-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 10. Weaver CG, Clement FM, Campbell NRC, et al. Healthcare costs attributable to hypertension: Canadian population-based cohort study. Hypertension. 2015;66(3):502-508. [DOI] [PubMed] [Google Scholar]
- 11. Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34(5):506-525. [DOI] [PubMed] [Google Scholar]
- 12. Whelton Paul K, Carey Robert M, Aronow Wilbert S, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13-e115. [DOI] [PubMed] [Google Scholar]
- 13. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. [DOI] [PubMed] [Google Scholar]
- 14. Flynn JT, Urbina EM. Pediatric ambulatory blood pressure monitoring: indications and interpretations. J Clin Hypertens (Greenwich). 2012;14(6):372-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaughan CJ, Murphy MB. The use of ambulatory blood pressure monitoring in the evaluation of racial differences in blood pressure. J Cardiovasc Risk. 1994;1(2):132-135. [DOI] [PubMed] [Google Scholar]
- 16. Kaneda T, Dupuis G. 2017 World Population Data Sheet With a Special Focus on Youth. Washington, DC: Population Reference Bureau; 2017. [Google Scholar]
- 17. Xi B, Zong Xn, Kelishadi R, Hong YM, et al. Establishing international blood pressure references among nonoverweight children and adolescents aged 6 to 17 years. Circulation. 2016;133(4):398-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiu M, Austin PC, Manuel DG, Tu JV. Cardiovascular risk factor profiles of recent immigrants vs long-term residents of Ontario: a multi-ethnic study. Can J Cardiol. 2012;28(1):20-26. [DOI] [PubMed] [Google Scholar]
- 19. Anand SS, Yusuf S, Vuksan V, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet. 2000;356(9226):279-284. [DOI] [PubMed] [Google Scholar]
- 20. Samaan Z, Schulze KM, Middleton C, et al. South Asian Heart Risk Assessment (SAHARA): randomized controlled trial design and pilot study. JMIR Res Protoc. 2013;2(2):e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta M, Doobay AV, Singh N, et al. Risk factors, hospital management and outcomes after acute myocardial infarction in South Asian Canadians and matched control subjects. CMAJ. 2002;166(6):717-722. [PMC free article] [PubMed] [Google Scholar]
- 22. Fowokan AO, Punthakee Z, Waddell C, et al. Adiposity measures and their validity in estimating risk of hypertension in South Asian children: a cross-sectional study. BMJ Open. 2019;9(2):e024087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fowokan A, Vincent K, Punthakee Z, et al. Exploring knowledge and perspectives of South Asian children and their parents regarding healthy cardiovascular behaviors: a qualitative analysis. Glob Pediatr Health. 2020;7:2333794X20924505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rana A, de Souza RJ, Kandasamy S, Lear SA, Anand SS. Cardiovascular risk among South Asians living in Canada: a systematic review and meta-analysis. CMAJ Open. 2014;2(3):E183-E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Banerjee AT, Flora PK, Stone M, Faulkner G. Differences in the prevalence of overweight between 10-12-year-old South Asian and non-South Asian children in Toronto, Ontario: findings from project BEAT. J Racial Ethn Health Disparities. 2015;2(2):184-191. [DOI] [PubMed] [Google Scholar]
- 26. Hudda MT, Nightingale CM, Donin AS, et al. Patterns of childhood body mass index (BMI), overweight and obesity in South Asian and black participants in the English National child measurement programme: effect of applying BMI adjustments standardising for ethnic differences in BMI-body fatness associations. Int J Obes (Lond). 2018;42(4):662-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balakrishnan R, Webster P, Sinclair D. Trends in overweight and obesity among 5-7-year-old White and South Asian children born between 1991 and 1999. J Public Health (Oxf). 2008;30(2):139-44. [DOI] [PubMed] [Google Scholar]
- 28. Fowokan A, Punthakee Z, Waddell C, et al. Multifactorial correlates of blood pressure in South Asian children in Canada: a cross-sectional study. BMJ Open. 2019;9(4):e027844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noor S, Dehghan M, Lear SA, et al. Relationship between diet and acculturation among South Asian children living in Canada. Appetite. 2020;147:104524. [DOI] [PubMed] [Google Scholar]
- 30. Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 31. Nowak M, Papiernik M, Mikulska A, Czarkowska-Paczek B. Smoking, alcohol consumption, and illicit substances use among adolescents in Poland. Subst Abuse Treat Prev Policy. 2018;13(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anand SS, Gupta M, Teo KK, et al. Causes and consequences of gestational diabetes in South Asians living in Canada: results from a prospective cohort study. CMAJ Open. 2017;5(3):e604-e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anand SS, Razak F, Davis AD, et al. Social disadvantage and cardiovascular disease: development of an index and analysis of age, sex, and ethnicity effects. Int J Epidemiol. 2006;35(5):1239-1245. [DOI] [PubMed] [Google Scholar]
- 34. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396. [PubMed] [Google Scholar]
- 35. Kurnianto A, Kurniadi Sunjaya D, Ruluwedrata Rinawan F, Hilmanto D. Prevalence of Hypertension and Its Associated Factors among Indonesian Adolescents. Int J Hypertens. 2020;2020:4262034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sardinha LB, Santos DA, Silva AM, Grøntved A, Andersen LB, Ekelund U. A comparison between BMI, waist circumference, and waist-to-height ratio for identifying cardio-metabolic risk in children and adolescents. PLoS One. 2016;11(2):e0149351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Teo KK, Rafiq T, Anand SS, et al. Associations of cardiometabolic outcomes with indices of obesity in children aged 5 years and younger. PLoS One. 2019;14(7):e0218816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dieticians of Canada and Canadian Paediatric Society. A Health Professional’s Guide for Using the WHO Growth Charts for Canada. Toronto, ON: Dieticians of Canada and Canadian Paediatric Society; 2014. [Google Scholar]
- 39. McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. Body fat reference curves for children. Int J Obes (Lond). 2006;30(4):598-602. [DOI] [PubMed] [Google Scholar]
- 40. Flynn JT, Daniels SR, Hayman LL, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63(5):1116-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Redwine KM, James LP, O’Riordan M, Sullivan JE, Blumer JL, Network of Pediatric Pharmacology Research Units. Accuracy of the Spacelabs 90217 ambulatory blood pressure monitor in a pediatric population. Blood Press Monit. 2015;20(5):295-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reichert M, Giurgiu M, Koch E, et al. Ambulatory assessment for physical activity research: state of the science, best practices and future directions. Psychol Sport Exerc. 2020;50:101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26(14):1557-1565. [DOI] [PubMed] [Google Scholar]
- 44. Quante M, Kaplan ER, Cailler M, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. 2018;10:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith C, Galland B, Taylor R, Meredith-Jones K. ActiGraph GT3X+ and actical wrist and hip worn accelerometers for sleep and wake indices in young children using an automated algorithm: validation with polysomnography. Front Psychiatry. 2019;10:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Evenson KR, Wen F. Performance of the ActiGraph accelerometer using a national population-based sample of youth and adults. BMC Res Notes. 2015;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trost SG, Pate RR, Freedson PS, Sallis JF, Taylor WC. Using objective physical activity measures with youth: how many days of monitoring are needed. Med Sci Sports Exerc. 2000;32(2):426-431. [DOI] [PubMed] [Google Scholar]
- 48. Baquet G, Stratton G, Van Praagh E, Berthoin S. Improving physical activity assessment in prepubertal children with high-frequency accelerometry monitoring: a methodological issue. Prev Med. 2007;44(2):143-147. [DOI] [PubMed] [Google Scholar]
- 49. Rich C, Geraci M, Griffiths L, Sera F, Dezateux C, Cortina-Borja M. Quality control methods in accelerometer data processing: defining minimum wear time. PLoS One. 2013;8(6):e67206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tremblay MS, Carson V, Chaput JP, et al. Canadian 24-Hour movement guidelines for children and youth: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2016;41(6 suppl 3):S311-S327. [DOI] [PubMed] [Google Scholar]
- 51. Barreira TV, Schuna JM, Jr, Mire EF, et al. Identifying children’s nocturnal sleep using 24-h waist accelerometry. Med Sci Sports Exerc. 2015;47(5):937-943. [DOI] [PubMed] [Google Scholar]
- 52. Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201-207. [DOI] [PubMed] [Google Scholar]
- 53. Tudor-Locke C, Barreira TV, Schuna JM, Mire EF, Katzmarzyk PT. Fully automated waist-worn accelerometer algorithm for detecting children’s sleep-period time separate from 24-h physical activity or sedentary behaviors. Appl Physiol Nutr Metab. 2014;39(1):53-57. [DOI] [PubMed] [Google Scholar]
- 54. Wühl E, Witte K, Soergel M, Mehls O, Schaefer F. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens. 2002;20(10):1995-2007. [DOI] [PubMed] [Google Scholar]
- 55. Soergel M, Kirschstein M, Busch C, et al. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents. J Pediatr. 1997;130(2):178-184. [DOI] [PubMed] [Google Scholar]
- 56. Yip GWK, Li AM, So H-K, et al. Oscillometric 24-h ambulatory blood pressure reference values in Hong Kong Chinese children and adolescents. J Hypertens. 2014;32(3):606-619. [DOI] [PubMed] [Google Scholar]
- 57. Khan IA, Gajaria M, Stephens D, Balfe JW. Ambulatory blood pressure monitoring in children: a large center’s experience. Pediatr Nephrol. 2000;14(8-9):802-805. [DOI] [PubMed] [Google Scholar]
- 58. Krull F, Buck T, Offner G, Brodehl J. Twenty-four hour blood pressure monitoring in healthy children. Eur J Pediatr. 1993;152(7):555-558. [DOI] [PubMed] [Google Scholar]
- 59. Cole TJ. The international growth standard for preadolescent and adolescent children: statistical considerations. Food Nutr Bull. 2006;27(4 Suppl Growth Standard):S237-S243. [DOI] [PubMed] [Google Scholar]
- 60. Canada PHAo. Tackling obesity in Canada: childhood obesity and excess weight rates in Canada 2018. https://www.canada.ca/en/public-health/services/publications/healthy-living/obesity-excess-weight-rates-canadian-children.html. Accessed December 30, 2021.
- 61. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44(1):45-60. [PubMed] [Google Scholar]
- 62. Chastin SF, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS One. 2015;10(10):e0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40(4):441-447. [DOI] [PubMed] [Google Scholar]
- 64. Bucher BS, Ferrarini A, Weber N, Bullo M, Bianchetti MG, Simonetti GD. Primary hypertension in childhood. Curr Hypertens Rep. 2013;15(5):444-452. [DOI] [PubMed] [Google Scholar]
- 65. Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map. J Contin Educ Health Prof. 2006;26(1):13-24. [DOI] [PubMed] [Google Scholar]