Abstract
Background:
Despite the severity and frequency of streptococcal bloodstream infections (BSIs), the effectiveness of oral definitive therapy remains unknown. The objective of this study was to evaluate the clinical outcomes of step-down oral antibiotics for the treatment of uncomplicated streptococcal BSIs.
Methods:
In this retrospective cohort study, adult patients admitted with uncomplicated streptococcal BSI between June 2015 and June 2017 were included. Patients were excluded if they received <48 h of antibiotic therapy; therapy was started >48 h after first positive culture; had complicated infections of endocarditis, bone and joint infections, or central nervous system infections; Pitt bacteremia score (PBS) ⩾ 4; or failed to respond to effective therapy necessitating continued intravenous (IV) therapy. Patients were grouped by receipt of step-down oral antibiotic therapy (PO group) versus continued IV therapy (IV group). Outcomes included hospital length of stay (LOS), 30-day recurrence of BSI, 30-day readmission, 30-day all-cause mortality, and catheter-related or drug-related adverse events (AEs).
Results:
Of 244 patients included, 40% received step-down oral therapy (n = 98). Overall, the most common source of BSI was pneumonia (22%), followed by skin and soft tissue infections (SSTI) (18%). Severity of illness measured by intensive care unit (ICU) admission and PBS was similar. The IV group had significantly longer LOS [median 10 (interquartile range [IQR] = 5–21) versus 5 (4–6) days, p < 0.01] compared with the PO group. BSI recurrence, readmission, all-cause mortality within 30 days, and AEs were similar between the groups (p = ns).
Conclusion:
In uncomplicated streptococcal BSI, patients treated with step-down oral antibiotic therapy had significantly shorter LOS compared with continued IV therapy without compromise of clinical outcomes.
Keywords: oral definitive therapy, oral step-down therapy, uncomplicated streptococcal bloodstream infections
Introduction
Streptococcal bloodstream infections (BSIs) contribute to significant healthcare burden and are the third most frequent cause of BSIs in population-based studies, 1 with a case-fatality rate as high as 22%. 2 Prompt initiation of effective antimicrobial therapy for BSIs is known to be important for survival. 3 BSIs have been traditionally treated with intravenous (IV) antimicrobial therapy with durations of 1 to 6 weeks depending on the complexity. 4 The IV route has historically been favored over oral therapy due to possible variability in drug absorption from oral administration leading to subtherapeutic plasma concentrations, ultimately resulting in poor infection-related outcomes. Prolonged durations of IV therapy, however, can be economically and logistically challenging in the outpatient setting. Prior studies have shown those who need IV antibiotic therapy often require longer hospital stays, 5 which can lead to increased healthcare costs, higher risk for hospital-acquired complications, and suboptimal healthcare utilization. 6 In addition, patients report their preference for oral therapy over IV therapy in the outpatient setting. 7 Outpatient parenteral therapy (OPAT) carries risks, including venous thromboembolism and line-associated BSIs,8,9 which can ultimately result in therapy interruption, additional antibiotic use, patient inconvenience, and hospital readmissions. 10
Given these possible risks and negative outcomes, there has been increasing interest in using oral definitive therapy for the treatment of BSIs. Prior studies have demonstrated step-down oral therapy in comparison with continued IV therapy to be effective and well tolerated for patients with gram-negative rod BSI secondary to Enterobacterales 11 as well as gram-positive cocci BSI. 12 The outcomes of oral step-down therapy, however, have been mixed in Staphylococcus aureus BSI, with certain studies showing increased risk for treatment failure especially with severe methicillin resistant S. aureus infections.13 –15
Despite the aforementioned studies demonstrating the effectiveness of oral therapy for organism-specific BSIs, less is known on the outcomes of step-down oral therapy for streptococcal BSI. As uncomplicated BSI has a lower probability of treatment failure and infection relapse in comparison with complicated BSI, 16 the focus of this study was narrowed to uncomplicated BSI. We hypothesized that the use of step-down oral therapy in comparison with continued IV therapy for uncomplicated streptococcal BSI would have similar clinical outcomes without compromising safety.
Methods
Study design, setting, and patient population
This was a retrospective single-center cohort study conducted at a 600-bed county, teaching hospital located in Los Angeles, California. Approval of the study protocol was obtained from the institutional review board at the University of Southern California. Microbiology reports were screened between June 2015 and June 2017 for growth of Streptococcus in one or more blood cultures. Inclusion criteria were (1) age ⩾ 18 years old, (2) inpatient admission, and (3) receipt of ⩾ 48 h of effective IV or oral antibiotic therapy during the hospital admission. Blood cultures from the emergency department were reviewed and accounted for only if patients were admitted to the hospital. Polymicrobial cultures, defined as isolation of at least two different organisms from the same blood culture, were included. Patients were excluded if (1) there was ⩾48 h delay in initiating effective antimicrobial therapy, (2) the infection was deemed complicated, (3) patients failed to respond to the effective therapy necessitating the prolongation of IV therapy due to the lack of infection resolution based on the treating physician’s assessment, or (4) medical charts were unavailable for review.
Patients were grouped by those who were treated solely with IV antibiotics throughout the treatment duration (IV group) and those who were switched to the oral route after an initial course of IV antibiotic therapy (PO group). A minimal duration of IV therapy prior to switching to oral therapy was not required in the PO group. The two groups (IV and PO groups) were compared for demographics, clinical course, and treatment outcomes. All antibiotic doses were administered based on the institutional dosing guidelines and individualized to the patient’s renal function.
Streptococcal BSI was defined as the isolation of Streptococcus in one or more blood culture in association with the clinical features of BSI and/or sepsis. Uncomplicated BSI was defined as follows: having follow-up blood cultures that were negative by day 4 or if repeated blood cultures were considered unnecessary by the treating clinician; no evidence of metastatic foci or complicated infection, including endocarditis, bone and joint infections, and/or central nervous system infections; 13 and an initial Pitt bacteremia score (PBS) of less than 4. 17 Patients with myositis or necrotizing soft tissue infections as the source of BSI were included. Diagnosis of endocarditis was based on physician documentation in the medical chart. PBS cutoff of 4 was selected based on prior findings that the relative risk for mortality was approximately fivefold higher in patients with PBS of 4 or more compared with the patients with PBS of less than 4 for S. aureus BSI. 18
Nosocomial BSI was defined as having the first positive blood culture 48 h or more after the date of admission. 19 Otherwise, BSI was considered community acquired. The source of BSI was determined based on the isolation of Streptococcus from the site of entry or as documented in the medical chart by the treating physician. Co-infections were defined as concurrent infections with a different organism than the BSI requiring antibiotic therapy. Effective antimicrobial therapy was defined as demonstrating in vitro activity against the streptococcal isolate based on Clinical and Laboratory Standards Institute (CLSI) criteria and receiving an appropriate dose of the drug. The institutional microbiology lab routinely performs and reports the susceptibility testing for beta-hemolytic Streptococcus spp, viridian group Streptococci, and Streptococcus pneumoniae isolated from blood cultures in the electronic medical record.
Study outcomes
The outcome measures were hospital length of stay (LOS), 30-day recurrence of BSI, 30-day hospital readmission, 30-day all-cause mortality, and catheter-related and/or drug-related adverse events (AEs). Recurrence of BSI was defined as growth of the same organism as the initial BSI. Thirty-day recurrence of BSI, 30-day readmission, and 30-day all-cause mortality were assessed within 30 days after the end of therapy. Adverse events were evaluated by reviewing the electronic medical record for patient complaints, physical examination, and laboratory test abnormalities. Adverse events were considered serious if the event resulted in death, required hospitalization, or caused permanent disability.
Statistical analysis
Statistical analysis was performed utilizing chi-square test or Fisher’s exact test for categorical variables and Student’s t test or Mann–Whitney test for continuous variables. A logistic regression model was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for predicting risk factors associated with 30-day hospital readmission. For the logistic regression model, a likelihood ratio test was used to compare models with and without each variable to determine whether that variable significantly improved the model. 20 A partial least squares regression model was used for LOS to determine the factors that contributed the most. This model was chosen due to a high degree of skew and multicollinearity between the independent variables. The partial least squares method is based on a linear model and, therefore, a Box–Cox transformation was performed on the dependent variable to meet assumptions. For the partial least squares regression, the number of dimensions was chosen to produce the lowest cross validation error. Then, those variables were included showing the relative contributions as determined by R 2 . A two-sided p value < 0.05 was statistically significant. All statistics were performed using Stata v 15.1 (College Station, TX, USA) or The R Project software for statistical computing version 3.6.1 (R Foundation).
Results
Between 1 June 2015 and 1 June 2017, a total of 573 patients were screened and 244 patients met inclusion criteria. One hundred forty-six patients were treated with IV therapy only (IV group) and 98 patients were treated with step-down oral therapy after the initial course of IV therapy (PO group). The reasons for exclusion were as follows: receipt of effective therapy for <48 h (n = 120), PBS of 4 or more at the onset of BSI (n = 40), failure to respond to the effective therapy necessitating the prolongation of IV therapy (n = 39), bone and joint infections (n = 34), endocarditis (n = 33), <18 years old (n = 26), ⩾48 h delay in initiating effective antimicrobial therapy (n = 21), chart unavailable for review (n = 8), central nervous system (CNS) infection (n = 7), and receipt of only PO antibiotic without initial IV therapy (n = 1).
Baseline characteristics were similar between the two groups (Table 1). Overall, the mean age of the patients was 54 years with the majority of patients being male (66%), Hispanic (64%), and residing at home prior to admission (75%). The median Charlson Comorbidity Index (CCI) was 3 [interquartile range (IQR) = 1–5] and diabetes was the most common comorbid condition (29%). Notable differences between the groups included a significantly higher percentage of IV drug use (IV 8.9% versus PO 2.0%, p = 0.03) and co-infections (IV 21.2% versus PO 10.2%, p = 0.04) in the IV group. Clinical presentation at the onset of BSI included 40% requiring intensive unit care (ICU)-level care, of which 4% required both vasopressor and mechanical ventilation. Approximately three quarters of patients had PBS of 1 or less (73%). Overall, the clinical presentation between the two groups were similar.
Table 1.
Baseline characteristics and clinical presentations of study participants.
| IV group (n = 146) | PO group (n = 98) | p value | |
|---|---|---|---|
| Age, years, mean ± SD | 54 ± 16.3 | 53 ± 15.5 | 0.53 |
| Male, n (%) | 95 (65.1) | 66 (66.3) | 0.78 |
| Ethnicity/race, n (%) | 0.54 | ||
| White | 14 (9.6) | 6 (6.1) | |
| African American | 12 (8.2) | 7 (7.1) | |
| Hispanic | 89 (61.0) | 68 (69.4) | |
| Asian | 8 (5.5) | 2 (2.0) | |
| Unknown | 23 (15.8) | 15 (15.3) | |
| Residence prior to admission, n (%) | 0.44 | ||
| Home | 112 (76.7) | 72 (73.5) | |
| LTCF/SNF | 3 (2.1) | 1 (1.0) | |
| Community housing/Shelter | 2 (1.4) | 4 (4.1) | |
| Homeless | 21 (14.4) | 13 (13.4) | |
| Jail | 8 (5.5) | 6 (6.1) | |
| Outside hospital | 0 (0.0) | 2 (2.0) | |
| Prior hospitalization, n (%) | 39 (26.7) | 18 (18.4) | 0.16 |
| IV drug use history, n (%) | 13 (8.9) | 2 (2.0) | 0.03 |
| Immunocompromised, n (%) | 31 (21.2) | 22 (22.4) | 0.87 |
| Chemotherapy w/n 6 mo | 12 (8.2) | 7 (7.1) | 0.81 |
| Absolute neutrophil count ⩽ 100 | 0 (0.0) | 0 (0.0) | >0.99 |
| Immunomodulatory therapy or corticosteroids ⩾ 14 days | 10 (6.8) | 5 (5.1) | 0.79 |
| Other comorbidities, n (%) | |||
| End-stage renal disease | 7 (4.8) | 2 (2.0) | 0.32 |
| Liver dysfunction | 27 (18.5) | 19 (19.4) | 0.87 |
| Diabetes mellitus | 45 (30.8) | 26 (26.5) | 0.57 |
| Congestive heart failure | 7 (4.8) | 6 (6.1) | 0.77 |
| CCI, median (IQR) | 3 (1–5) | 2 (1–5) | 0.07 |
| Co-infections, n (%) | 31 (21.2) | 10 (10.2) | 0.04 |
| ICU admission, n (%) | 61 (41.8) | 38 (38.8) | 0.69 |
| Use of vasopressor and mechanical ventilation, n (%) | |||
| Use of vasopressor | 10 (6.8) | 5 (5.1) | 0.68 |
| Mechanical ventilation | 8 (5.5) | 5 (5.1) | 0.98 |
| Both | 7 (4.8) | 2 (2.0) | 0.48 |
| Lactate, median (IQR) | 2.0 (1.4–2.95) | 1.7 (1.3–3.2) | 0.66 |
| Pitt bacteremia score | |||
| 0–1 | 105 (71.9) | 73 (75.5) | 0.69 |
| 2–3 | 41 (28.1) | 25 (25.5) | |
| Median (IQR) | 1 (0–2) | 0 (0–2) | 0.97 |
CCI, Charlson Comorbidity Index; ICU, intensive care unit; IQR, interquartile range; IV, intravenous; LTCF, long-term care facility; PO, oral; SD, standard deviation; SNF, skilled nursing facility.
Majority of patients had community-onset BSI (89.8%) (Table 2). The most common source of BSI in the IV group was skin and soft tissue infection (SSTI) (21.2%), intra-abdominal infection (IAI) (19.9%), and pneumonia (17.8%). For the PO group, pneumonia (28.6%) was the most common source of BSI, followed by SSTI (14.3%) and IAI (11.2%). Viridans group streptococcus was the most common cause of BSI (IV 25.3% versus PO 22.4%, p = 0.65), followed by S. pneumoniae (IV 15.1% versus PO 25.5%, p = 0.05) and Streptococcus agalactiae (IV 18.5% versus PO 13.3%, p = 0.30). The rate of polymicrobial organisms in the blood cultures was similar between the two groups (IV 16.4% versus PO 11.2%, p = 0.25).
Table 2.
Source of bloodstream infection and microbiology.
| IV group (n = 146) | PO group (n = 98) | p value | |
|---|---|---|---|
| Community-acquired, n (%) | 127 (87.0) | 92 (93.9) | 0.08 |
| Polymicrobial Blood Culture | 24 (16.4) | 11 (11.2) | 0.25 |
| Source of bloodstream infection, n (%) | |||
| Pneumonia | 26 (17.8) | 28 (28.6) | 0.06 |
| Skin and soft tissue infections | 31 (21.2) | 14 (14.3) | 0.18 |
| Intra-abdominal infections | 29 (19.9) | 11 (11.2) | 0.08 |
| Urinary | 6 (4.0) | 7 (7.1) | 0.39 |
| Ear–nose–throat infections | 5 (3.4) | 2 (2.0) | 0.71 |
| Surgical manipulation | 2 (1.4) | 0 (0) | 0.52 |
| Catheter-associated | 1 (0.7) | 1 (1.0) | >0.99 |
| Other | 4 (2.7) | 14 (14.3) | <0.01 |
| Unknown | 42 (28.8) | 21 (21.4) | 0.23 |
| Microbiology, n (%) | |||
| Viridans group streptococcus | 37 (25.3) | 22 (22.4) | 0.65 |
| Streptococcus pneumoniae | 22 (15.1) | 25 (25.5) | 0.05 |
| Streptococcus agalactiae (group B) | 27 (18.5) | 13 (13.3) | 0.30 |
| Streptococcus pyogenes (group A) | 23 (15.8) | 17 (17.3) | 0.86 |
| Streptococcus anginosus | 8 (5.5) | 9 (9.2) | 0.31 |
| Streptococcus mitis/oralis | 10 (6.8) | 1 (1.0) | 0.05 |
| Streptococcus gallolyticus | 6 (4.1) | 2 (2.0) | 0.48 |
| Streptococcus constellatus | 2 (1.4) | 5 (5.1) | 0.12 |
| Streptococcus, beta hemolytic (not group A, B, or D) | 4 (2.7) | 0 (0) | 0.15 |
IV, intravenous; PO, oral.
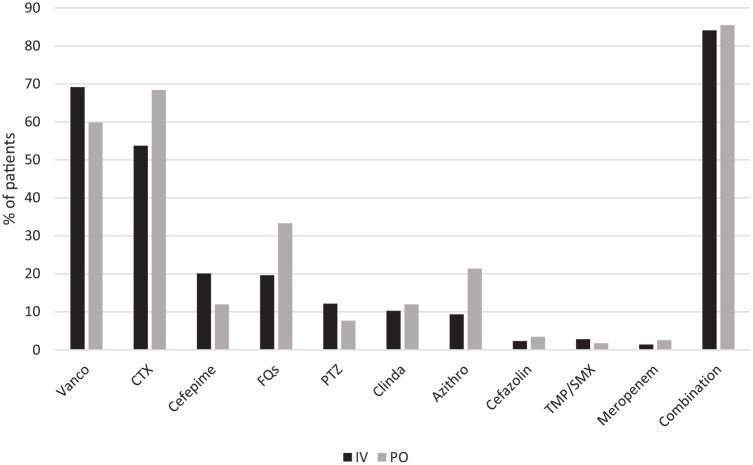
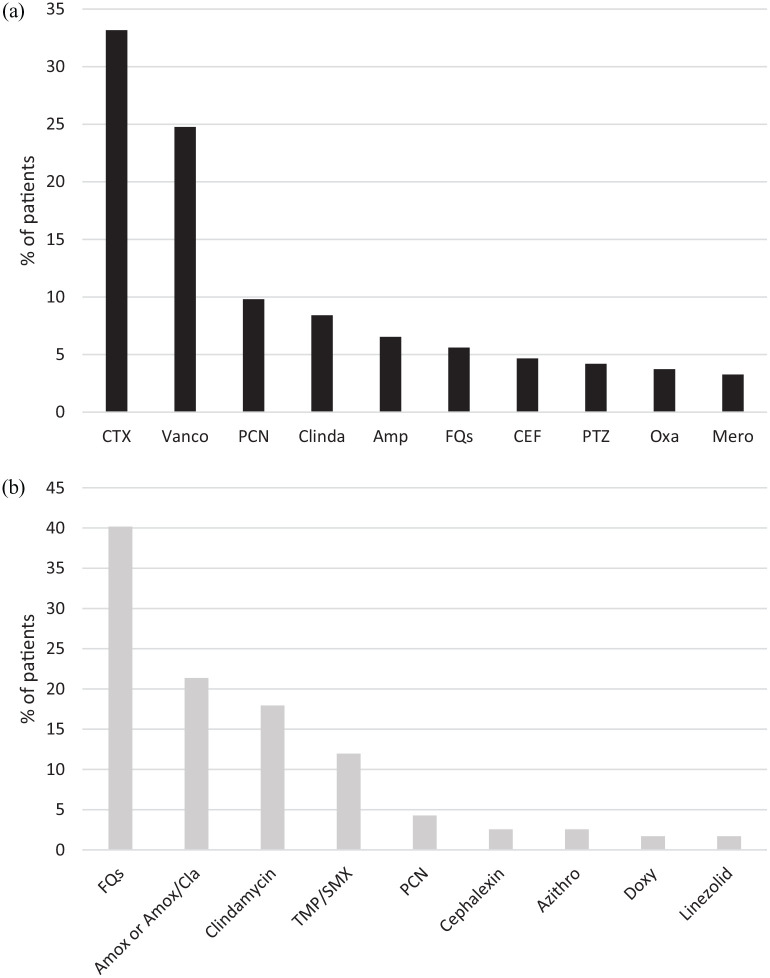
All patients except for one patient in the IV group received effective empiric antibiotic therapy (Table 3, Figure 1). Ceftriaxone (49%) and fluoroquinolones (41%) were the most common definitive antibiotics prescribed in the IV and PO group, respectively (Figure 2). Median duration of IV therapy prior to PO therapy was 4 days and this duration was similar regardless of BSI source or infecting pathogen. The total duration of therapy of inpatient and planned outpatient therapy was significantly longer in the PO group (PO 14 days (11–18) versus IV 14 days (7–17), p < 0.01).
Table 3.
Therapeutic management.
| IV group (n = 146) | PO group (n = 98) | p value | |
|---|---|---|---|
| Ineffective empiric antibiotic, n (%) | 1 (0.7) | 0 (0) | 0.41 |
| Days until directed therapy initiated, median (IQR) | 3 (2–4) | 2 (2–4) | 0.47 |
| Days of IV therapy until PO antibiotics, median (IQR) | – | 4 (3–6) | – |
| Total duration of inpatient and outpatient therapy, median (IQR) | 14 (7–17) | 14 (11–18) | <0.01 |
IQR, interquartile range; IV, intravenous; PO, oral.
Figure 1.
Empiric therapy.
Azithro, azithromycin; Clinda, clindamycin; CTX, ceftriaxone; FQs, fluoroquinolones; IV, intravenous; PTZ, piperacillin/tazobactam; TMP/SMX, trimethoprim/sulfamethoxazole; Vanco, vancomycin.
Some patients received more than one antibiotic.
Figure 2.
Directed therapy: (a) IV-directed therapy. (b) PO-directed therapy.
Amox, amoxicillin; Amox/Cla, amoxicillin/clavulanate; Amp, ampicillin; Azithro, azithromycin; CEF, cefepime; Clinda, clindamycin; CTX, ceftriaxone; Doxy, doxycycline; FQs, fluoroquinolones; IV, intravenous; Oxa, oxacillin; Mero, meropenem; PCN, penicillin; PO, oral; PTZ, piperacillin/tazobactam; TMP/SMX, trimethoprim/sulfamethoxazole; Vanco, vancomycin.
Some patients received more than one antibiotic.
The IV group had significantly longer hospital LOS compared with the PO group by a median of 5 days (Table 4). By partial least squares regression analysis, the most significant determinants for prolonging hospital LOS were having a co-infection, ICU admission, end-stage renal disease (ESRD) on dialysis, diabetes, and prior hospitalization within 3 months. Thirty-day recurrence of BSI (IV 2.1% versus PO 1.0%, p = 0.65) and adverse reactions (IV 1.4% versus PO 3.1%, p = 0.39) were low in both groups. Of note, all three adverse reactions in the PO group occurred during the IV antibiotic administration prior to switching to the oral formulation, which were nausea/vomiting, localized rash, and catheter-related thrombophlebitis. In the IV group, one patient had vomiting and one patient had a localized rash. None of these adverse reactions were considered serious. There were no treatment failures identified in the PO group requiring escalation of oral therapy to IV therapy. A subanalysis of clinical outcomes evaluated by infecting pathogen as well as the presence/absence of co-infections resulted in a similar trend for hospital LOS and no difference in 30-day readmission, 30-day mortality, or 30-day recurrence rates.
Table 4.
Clinical Outcomes.
| IV (n = 146) | PO (n = 98) | p value | |
|---|---|---|---|
| LOS, days, median (IQR) | 10 (5–21) | 5 (4–6) | <0.01 |
| 30-day recurrence, n (%) | 3 (2.1) | 1 (1.0) | 0.65 |
| 30-day readmission, n (%) | 29 (19.9) | 15 (15.3) | 0.40 |
| 30-day all-cause mortality, n (%) | 6 (4.1) | 1 (1.0) | 0.25 |
| Adverse reactions, n (%) | 2 (1.4) | 3 (3.1) | 0.39 |
IQR, interquartile range; IV, intravenous; LOS, length of stay; PO, oral.
By multivariable logistic regression analysis, diabetes mellitus (adjusted OR = 2.4, 95% CI = 1.02–5.58) and prior admission within 3 months (adjusted OR = 3.2, 95% CI = 1.43–7.10) were the most significant factors associated with 30-day readmission. As the choice of IV or PO therapy was not significant by univariate analysis, it was not included in the multivariable model. Overall, 30-day all-cause mortality was 7 (3%) (IV 4% versus PO 1%, p = 0.16). Of the patients who died, four were immunocompromised, four were admitted to the ICU, four had a presumed or identified source of pneumonia, and five had hospital-acquired BSI. Descriptive analysis of clinical outcomes based on PO antibiotic class is provided in Table 5.
Table 5.
Clinical outcomes of those treated with step-down oral therapy (n = 98). a
| Fluoroquinolone, b n = 39 | Beta-lactams, c n = 31 | Clindamycin, n = 16 | TMP-SMX, n = 13 | Azithromycin, n = 3 | Doxycycline, n = 2 | Linezolid, n = 2 | |
|---|---|---|---|---|---|---|---|
| Infection source, n (%) | |||||||
| PNA | 18 (46.2) | 6 (19.4) | 0 (0) | 3 (23.1) | 2 (66.7) | 0 (0) | 0 (0) |
| SSTI | 3 (7.7) | 6 (19.4) | 5 (31.3) | 6 (46.2) | 0 (0) | 1 (50.5) | 1 (50.0) |
| IAI | 3 (7.7) | 9 (29.0) | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) |
| Urinary | 2 (5.1) | 3 (9.7) | 1 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ENT | 0 (0) | 0 (0) | 2 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Catheter-associated | 1 (2.6) | 0 (0) | 1 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 4 (10.3) | 2 (6.5) | 1 (6.3) | 0 (0) | 0 (0) | 1 (50.5) | 1 (50.0) |
| Unknown | 8 (20.5) | 5 (16.1) | 6 (37.5) | 3 (23.1) | 1 (33.3) | 0 (0) | 0 (0) |
| 30-day all-cause mortality, n (%) | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 30-day hospital readmission, n (%) | 4 (10) | 4 (13) | 3 (19) | 1 (8) | 1 (33) | 0 (0) | 1 (50) |
| 30-day bacteremia recurrence, n (%) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
ENT: ear–nose–throat infections; IAI: intra-abdominal infections; PNA: pneumonia; SSTI: skin and soft tissue infection; TMP/SMX: trimethoprim/sulfamethoxazole.
Some patients received more than one antibiotic.
Levofloxacin = 26, ciprofloxacin = 13.
Amoxicillin = 8, amoxicillin/clavulanate = 12, ampicillin = 3, cefdinir = 1, cephalexin = 2, penicillin = 5.
Discussion
The primary objective of this study was to evaluate the effectiveness and safety of step-down oral antibiotic therapy for uncomplicated streptococcal BSIs. We found that the oral group had significantly shorter hospital LOS compared with the IV group, and similar incidence of 30-day recurrence, 30-day mortality, 30-day readmission, and adverse reactions for patients with uncomplicated streptococcal BSI. The convenience of prescribing oral formulations may have aided in earlier discharge from the hospital, although it cannot be concluded this was the contributing factor. The route of discharge antibiotic was not found to be a significant factor to influence hospital LOS by univariate analysis. Potential benefits of discharging patients early from the hospital include decreased risk of hospital-acquired infections and pressure sores, reduced healthcare costs, enhanced utilization of hospital occupancy, and enriched self-dignity. 21
IV therapy has previously been the mainstay for BSIs to ensure adequate drug concentrations for culture sterilization and source eradication. Recently, oral therapy has been drawing more attention in light of recent randomized controlled trials in deep-seated infections of bone and joint infections and endocarditis.12,22 In these two trials, outcomes of patients treated with oral therapy were noninferior to continued IV therapy for bone and joint infections and uncomplicated left-sided endocarditis caused by streptococcus, Enterococcus faecalis, S. aureus, or coagulase-negative staphylococci.12,22 As these two disease states require prolonged durations of therapy, an option of oral step-down therapy can be particularly helpful. Specifically in the endocarditis study, combination oral therapy of two drugs was used for Streptococcus spp; thus, the applicability of these regimens will need to be more clearly defined for BSIs without endocarditis.
In BSIs, S. aureus BSI has shown conflicting findings in the efficacy of oral therapy,13,15,23,24 while clinical outcomes were comparable between continued IV therapy and step-down oral therapy in Enterobacterales BSI.11,25,26 Specifically in streptococcal BSI, there are limited studies investigating step-down oral therapy for patients compared with IV therapy in an uncomplicated BSI population. Prior studies have focused on species-specific BSI or the choice of oral antibiotic therapy on outcomes. One small retrospective study (N = 36) investigating patients with bacteremic S. pneumoniae pneumonia suggested that it would be safe to initiate step-down oral therapy within 7 days of hospitalization once patient reaches clinical stability. The mean duration of IV therapy prior to oral therapy was 3.5 days. 27 Similarly, we found that duration of IV therapy prior to oral therapy was relatively short with a median of 4 days, suggesting that early switch to oral therapy may be safe in uncomplicated BSI without a prolonged initial IV course.
From a pharmacokinetic/pharmacodynamic standpoint, Streptococcus spp is unique with lower minimum inhibitory concentrations (MICs) compared with other organisms, which makes the oral option very attractive, even for some of the beta-lactam agents that may not have high bioavailability. Recent studies have evaluated oral antibiotics of high (fluoroquinolones, clindamycin, and doxycycline) versus low bioavailability (beta-lactam) 28 and fluoroquinolones versus oral beta-lactams 29 and found comparable outcomes in the beta-lactam group. In our study, beta-lactam agents were the second most commonly utilized oral agents (32%) after fluoroquinolones (40%), although we could not make meaningful comparisons based on oral antibiotic choice on its effect on outcomes due to the small sample size. As the use of fluoroquinolones has increased safety concerns, including Black box warnings and other collateral damage, oral beta-lactams may be a more attractive option in certain populations.
We found no difference in the clinical outcomes in a subgroup analysis of patients by infecting streptococcal pathogen, except for significantly shorter hospital LOS associated with the PO group. This, however, may have been due to inadequate power to detect such a difference. The selection of oral therapy should be individualized based on patient-specific parameters such as organism MICs, comorbidities, allergy/intolerance, site(s) of infection, ease of administration as well as drug-specific parameters such as pharmacokinetic/dynamics of antibiotics and associated adverse reactions.
Preventing hospital readmission after an index BSI is important for patients and hospitals as this can contribute to significant healthcare and economic burden. 30 The overall rate of 30-day readmission in our study was 18%. By logistic regression analysis, having diabetes and history of prior admission within the last 3 months were identified as significant factors associated with 30-day readmission; the route of antibiotic therapy, however, was not. Previous studies also identified prior hospitalizations31 –33 and diabetes34,35 to significantly increase patient risk for hospital readmission in various patient populations. Thus, optimizing comorbidity management in conjunction with antimicrobial treatment is important to prevent rehospitalizations.
Adverse reactions due to antibiotic therapy were low in our study in both groups. There were no complications such as line infections or thrombosis in the IV group that was found after discharge from the hospital. This is similar to a study by Iversen et al., 12 in which complications from the IV therapy were low. Low complication rates may be due to the relatively short durations of therapy in our study (median 14 days total). Although the rate of complication was low in the IV group, patients likely prefer oral antibiotic therapy for the flexibility of administration and lack of need of coordination with home health services, as well as the decreased costs overall. 7
Our study has several limitations worth mentioning. This was a single-center, retrospective study with a relatively small sample size; thus, there may be potential confounding factors we could not control for that may have influenced the choice for IV versus PO therapy or patient management. To overcome this bias, we applied strict inclusion and exclusion criteria, as well as conducting a logistic regression and partial least square regression analysis. Socioeconomic characteristics, including payer status, were not assessed, which may have affected outpatient therapy selection. Adverse effects secondary to antibiotic therapy were based on physician or nursing documentation in the electronic medical record; thus, there may have been some adverse effects that were missed. In addition, we were unable to assess adherence of oral antibiotics after discharge, although the rate of recurrence or readmission was low in the PO group. Patients may have been lost to follow-up after hospital discharge if they presented to a hospital outside of the hospital network. It is, however, noteworthy that majority of patients (66%) had outpatient or inpatient follow-up assessments after the index infection. In addition, the hospital is a county facility serving a medically underserved population, where most patients are likely to return to the same hospital if complications were to occur. The overall low 30-day all-cause mortality rate can be attributed to the preselected population with low risk of failure. As this study only included uncomplicated BSI, our findings may not be generalizable for patients with complicated BSIs or those with a more severe initial clinical presentation.
In conclusion, in uncomplicated streptococcal BSI, safety was not compromised by step-down oral antibiotic therapy compared with patient treated with IV therapy. Transition to oral therapy was early in the treatment course at a median of 4 days; the optimal time to transition as well as the optimal choice of oral antibiotic therapy for streptococcal BSI, however, warrant further investigation in a prospective trial.
Acknowledgments
We would like to thank Jaimie Chen, Michelle Gandawidjaja, and Cynthia Bor for their assistance with data collection.
Footnotes
Author contributions: Amy Kang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing – original draft; Writing – review & editing.
Richard Beuttler: Conceptualization; Data curation; Formal analysis; Methodology; Resources; Software; Validation; Writing – original draft.
Emi Minejima: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Supervision; Validation; Visualization; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical statements: Approval of the study protocol was obtained from the Institutional Review Board at the University of Southern California (Approval No. HS-18-40028). Our study did not require consent due to the retrospective nature of this study as medical record review would not change the existing results or care the individuals already received. This study poses no more than minimal risk as the only foreseeable risk is breachable confidentiality. To minimize the risks associated with confidentiality breach, patient data were collected and stored in a secure manner and any patient identifiers were destroyed promptly upon the completion of data collection and analysis.
ORCID iD: Amy Kang  https://orcid.org/0000-0003-3619-8904
https://orcid.org/0000-0003-3619-8904
Contributor Information
Amy Kang, Department of Pharmacy Practice, School of Pharmacy, Chapman University, Irvine, CA, USA; Department of Pharmacy, Harbor-UCLA Medical Center, Torrance, CA, USA; The Lundquist Institute, Torrance, CA, USA.
Richard Beuttler, Department of Pharmacy Practice, School of Pharmacy, Chapman University, Irvine, CA, USA.
Emi Minejima, Department of Clinical Pharmacy, School of Pharmacy, University of Southern California, Los Angeles, CA, USA; Department of Pharmacy, LAC + USC Medical Center, PSC B15-B, Health Sciences Campus, 90089-9121, Los Angeles, CA, USA.
References
- 1. Laupland KB. Incidence of bloodstream infection: a review of population-based studies. Clin Microbiol Infect 2013; 19: 492–500. [DOI] [PubMed] [Google Scholar]
- 2. Rantala S, Vuopio-Varkila J, Vuento R, et al. Predictors of mortality in beta-hemolytic streptococcal bacteremia: a population-based study. J Infect 2009; 58: 266–272. [DOI] [PubMed] [Google Scholar]
- 3. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 4. Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: a systematic review and meta-analysis. Crit Care Lond Engl 2011; 15: R267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laing RBS, Mackenzie AR, Shaw H, et al. Duration of intravenous therapy and hospital stay according to choice of empirical antimicrobial treatment for community-acquired respiratory infection. Int J Antimicrob Agents 1999; 13: 53–56. [DOI] [PubMed] [Google Scholar]
- 6. Hauck K, Zhao X. How dangerous is a day in hospital? A model of adverse events and length of stay for medical inpatients. Med Care 2011; 49: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 7. Krohe M, Eek D, Mazar I, et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adherence 2016; 10: 1609–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chopra V, Anand S, Krein SL, et al. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med 2012; 125: 733–741. [DOI] [PubMed] [Google Scholar]
- 9. Chopra V, O’Horo JC, Rogers MAM, et al. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2013; 34: 908–918. [DOI] [PubMed] [Google Scholar]
- 10. Baumgarten K, Hale Y, Messonnier M, et al. Bridging the gap: a collaborative to reduce peripherally inserted central catheter infections in the home care environment. Ochsner J 2013; 13: 352–358. [PMC free article] [PubMed] [Google Scholar]
- 11. Tamma PD, Conley AT, Cosgrove SE, et al. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med 2019; 179: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019; 380: 415–424. [DOI] [PubMed] [Google Scholar]
- 13. Willekens R, Puig-Asensio M, Ruiz-Camps I, et al. Early oral switch to linezolid for low-risk patients with Staphylococcus aureus bloodstream infections: a propensity-matched cohort study. Clin Infect Dis 2019; 69: 381–387. [DOI] [PubMed] [Google Scholar]
- 14. Daver NG, Shelburne SA, Atmar RL, et al. Oral step-down therapy is comparable to intravenous therapy for Staphylococcus aureus osteomyelitis. J Infect 2007; 54: 539–544. [DOI] [PubMed] [Google Scholar]
- 15. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ 2015; 350: h2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holland TL, Arnold C, Fowler VG. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312: 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 1999; 11: 7–12. [DOI] [PubMed] [Google Scholar]
- 18. Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J 2001; 31: 97–103. [PubMed] [Google Scholar]
- 19. Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988; 16: 128–140. [DOI] [PubMed] [Google Scholar]
- 20. Fox J, Weisberg S. An R companion to applied regression. 3rd ed. Los Angeles, CA: SAGE, 2019. [Google Scholar]
- 21. Dryden M, Saeed K, Townsend R, et al. Antibiotic stewardship and early discharge from hospital: impact of a structured approach to antimicrobial management. J Antimicrob Chemother 2012; 67: 2289–2296. [DOI] [PubMed] [Google Scholar]
- 22. Li H-K, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380: 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Itoh N, Hadano Y, Saito S, et al. Intravenous to oral switch therapy in cancer patients with catheter-related bloodstream infection due to methicillin-sensitive Staphylococcus aureus: a single-center retrospective observational study. PLoS One 2018; 13: e0207413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heldman AW, Hartert TV, Ray SC, et al. Oral antibiotic treatment of right-sided staphylococcal endocarditis in injection drug users: prospective randomized comparison with parenteral therapy. Am J Med 1996; 101: 68–76. [DOI] [PubMed] [Google Scholar]
- 25. Rieger KL, Bosso JA, MacVane SH, et al. Intravenous-only or intravenous transitioned to oral antimicrobials for Enterobacteriaceae-associated bacteremic urinary tract infection. Pharmacotherapy 2017; 37: 1479–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kutob LF, Justo JA, Bookstaver PB, et al. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents 2016; 48: 498–503. [DOI] [PubMed] [Google Scholar]
- 27. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med 2001; 161: 848–850. [DOI] [PubMed] [Google Scholar]
- 28. Quinn NJ, Sebaaly JC, Patel BA, et al. Effectiveness of oral antibiotics for definitive therapy of non-Staphylococcal Gram-positive bacterial bloodstream infections. Ther Adv Infect Dis 2019; 6: 2049936119863013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arensman K, Shields M, Beganovic M, et al. Fluoroquinolone versus beta-lactam oral step-down therapy for uncomplicated streptococcal bloodstream infections. Antimicrob Agents Chemother 2020; 64: e01515–e01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang DW, Tseng C-H, Shapiro MF. Rehospitalizations following sepsis: common and costly. Crit Care Med 2015; 43: 2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toledo D, Soldevila N, Torner N, et al. Factors associated with 30-day readmission after hospitalisation for community-acquired pneumonia in older patients: a cross-sectional study in seven Spanish regions. BMJ Open 2018; 8: e020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alqahtani JS, Njoku CM, Bereznicki B, et al. Risk factors for all-cause hospital readmission following exacerbation of COPD: a systematic review and meta-analysis. Eur Respir Rev 2020; 29: 190166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shorr AF, Zilberberg MD, Reichley R, et al. Readmission following hospitalization for pneumonia: the impact of pneumonia type and its implication for hospitals. Clin Infect Dis 2013; 57: 362–367. [DOI] [PubMed] [Google Scholar]
- 34. Dobrzynski DM, Ndi DN, Zhu Y, et al. Hospital readmissions after laboratory-confirmed influenza hospitalization. J Infect Dis 2020; 222: 583–589. [DOI] [PubMed] [Google Scholar]
- 35. Ostling S, Wyckoff J, Ciarkowski SL, et al. The relationship between diabetes mellitus and 30-day readmission rates. Clin Diabetes Endocrinol 2017; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]