Abstract
Clostridium subterminale is an anaerobic spore-forming bacterium rarely isolated in human infections. This case study presents a necrotizing C. subterminale infection stemming from a dental abscess that progressed into sepsis, a small pericardial effusion, moderate bilateral pulmonary effusions, and multiple organ dysfunction syndrome. The management of the infection, along with other relevant cases is discussed.
Keywords: Clostridium subterminale, Clostridium, Odontogenic infection, Oral infection, Necrotizing fasciitis, Anaerobic Resistance
Introduction
Clostridia are a frequently isolated family of bacteria found in environments ranging from soil to the GI tract of humans and other animals. Clostridia species are known to cause several serious diseases including botulism, tetanus, and necrotizing soft tissue infections. However, within this family, Clostridium subterminale is a very infrequently isolated bacteria as a human pathogen with fewer than 15 cases reported in the literature. These examples include soft tissue infections due to injury [9], injection drug needle wound infections [1], [2], and an odontogenic infection [3]. We present the second known infection by C. subterminale with an odontogenic origin and the second successful odontogenic infection treatment with piperacillin-tazobactam.
Case report
A 40-year-old woman without significant medical history presented to an outside emergency department (ED) with a chief complaint of facial and neck swelling and abdominal discomfort. Two days prior she was started on clindamycin for treatment of a presumed tooth abscess. Despite treatment, swelling of her face and neck had progressively worsened by the time of ED presentation. Due to concerns for patency of her airway, she was endotracheally intubated and placed on mechanical ventilation. CT (Computed Tomography) scan of her neck showed signs of necrotizing fasciitis and soft tissue edema with gas formation extending from the neck inferiorly to the superior mediastinum and a retropharyngeal abscess. She was tachycardic, tachypneic and exhibited a blood pressure of 89/41 mmHg with a MAP of 69 mmHg. Other significant findings included a WBC count of 6.7, H&H of 12.5/37.2, creatinine of 1.8 and a lactic acid level of 2.9. A central venous line was placed and 3 liters of IV fluids were infused. Following consultation with ENT and Pulmonology, the patient was transferred from the outside ED to our ICU for treatment of necrotizing fasciitis, soft tissue abscess and progressing mediastinitis.
Upon arrival at the ICU, the patient was fluid resuscitated and sedated on Versed. Physical examination demonstrated marked swelling of the neck and rhonchorous breath sounds. It was decided that she had severe sepsis and multiple organ dysfunction syndrome due to a soft tissue infection that had advanced to involve the deep soft tissue of the neck with extension towards the superior mediastinum. Intravenous Fentanyl was initiated and she received an additional 1 liter of.9% Sodium Chloride. Clindamycin, piperacillin-tazobactam, vancomycin, heparin, and pantoprazole were started. Later that day it was necessary to start a Levophed infusion in order to maintain a MAP> 65 mmHg. ENT surgically debrided the neck region, packed the submandibular and anterior neck incisions, and removed two teeth. A right radial arterial line was placed.
On hospital day 2, a CT chest showed a small pericardial effusion, moderate bilateral pleural effusions with loculation on the left side as well as ground glass opacities and nodular consolidation in the upper and lower lobes consistent with acute lung injury/ARDS secondary to sepsis and or aspiration. Based on these findings, bilateral chest tubes were placed. A tracheostomy was performed on hospital day 4 to assure airway stability throughout the treatment and recovery period. A percutaneous enterogastrostomy (PEG) tube was placed for nutritional support. Pericardiocentesis was performed on hospital day 10 to evacuate the pericardial space. A PICC line was placed on hospital day 11 for long term antibiotic therapy and intravenous access.
On hospital day 11, aerobic and anaerobic culture results of the fluid from the neck wound returned Clostridium subterminale. Results from the pleural fluid culture also returned C. subterminale on hospital day 14. Culture results were obtained using a Remel™ Rapid Anaerobic ID Kit at Emory University Hospital. A sample of the pleural fluid specimen was also used to determine sensitivities at Emory University Hospital. The results indicated sensitivity to ampicillin-sulbactam, cefoxitin, meropenem, metronidazole, and penicillin. Infectious disease consultation determined that the patient should complete a full course of piperacillin-tazobactam followed by maintenance enteral antibiotic therapy. Chest tubes were removed by hospital day 26. The patient did well and was discharged to subacute rehab on hospital day 37.
Discussion
Clostridia is a family of bacteria found in soil and the GI tracts of many organisms and, due to multiple distinct presentations, is widely studied. Clostridia may present in several unique human diseases including gas gangrene, tetanus, botulism, and food poisoning. All the pathogenic Clostridia are anaerobic, spore-forming, gram-positive bacilli [9] that produce a variety of proteolytic exotoxins [11]. Within the Clostridia family, several species are known to cause systemic disease including C. perfringens, C. histolyticum, and C. septicum, whilst others, such as C. Tetani, tend to remain isolated to the necrotic tissue [11]. C. subterminale, the focus of this case report, is a rare Clostridia having only 12 previous isolates recorded in the literature.
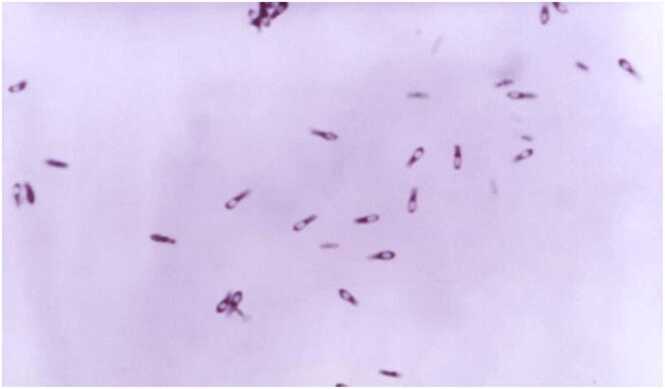
C. subterminale, which takes its name from its subterminally located spores (Fig. 1), was isolated as the pathogenic organism in our patient. Previously reported cases of C. subterminale include an odontogenic multi-fascial plane infection [3], two cases of a Botulism-like syndrome [1], [2], sepsis in an immunocompromised patient undergoing chemotherapy for gastric adenocarcinoma [10], sepsis in a patient with esophageal cancer [8], a case of sepsis in a recipient of an allogeneic cord blood transplant [6], sepsis in an adult patient with acute lymphoblastic leukemia [5], a pleuropulmonary infection [4], two soft tissue infections, and one infection due to soil contamination of an open fracture [9].
Fig. 1.
Clostridium subterminale bacteria from the CDC Public Health Image Library. Note the terminally located spores. [Holdeman. ID# 12055 Clostridium Subterminale [website]. Centers for Disease Control, Public Health Image Library; 1965.
Our case is the second C. subterminale case of an infection stemming from an odontogenic source and the 13th case that we know of to be recorded in the literature. Unfortunately, several of the patients in the other studies did not survive their infections. This was most often a result of being immunocompromised at the time of the infection or possibly due to a prolonged period before organism isolation, identification, and initiation of definitive therapy. In some instances, the time until culture positivity was between 11 and 13 days [3], [8], [9], [10].
Notably, a number Clostridia are resistant to cephalosporins, clindamycin, quinolones, and aminoglycosides [6], [9]. The duration of C. subterminale isolation, as noted above, can delay the start of effective therapy. Initial treatment with clindamycin and vancomycin, in some reported cases, may have led to worsening of the patient's condition before C. subterminale identification [3], [7], [9], [10]. Clindamycin has been included in some treatment regimens as a toxin-mediating agent [1]. One case of botulism-like infection due to C. subterminale reported significant improvement upon addition of clindamycin to the treatment regimen [1]. Resistance based on penicillinase production has also been reported, making penicillin-based antibiotic choices in this category unsuccessful [9], [11]. Fortunately, C. subterminale has remained susceptible to Meropenem, Doripenem, and Metronidazole [3]. Successful treatment in this case included Vancomycin and Piperacillin-tazobactam, substitution with ampicillin-sulbactam, followed by oral amoxicillin-clavulanate and sulfamethoxazole-trimethoprim.
In conclusion, Clostridium subterminale is a rare cause of infection in humans. It most commonly presents in immunocompromised patients or in wounds exposed to the bacteria. C. subterminale has been found to be most susceptible to combination treatment with piperacillin-tazobactam based antibiotic regimens that may also include vancomycin [1], [2], [3], [10]. Though uncommon, C. subterminale infection requires early bacterial isolation and definitive therapy to prevent soft tissue necrosis and a decline in condition, especially when the patient is immunocompromised or has severe comorbidities. In clinical presentations similar to those exemplified in this case, suspicion for C. subterminale should be present and specific antibiotic coverage should be afforded. In addition, surgical debridement plays an important role in removal of necrotic tissues contributing to infection source control.
CRediT authorship contribution statement
Charles J. Grodzin (MD): Head physician for case study, in charge of patient care/record acquisition, main editor and writing contributor.
Edward B. Henderson: Large writing contributor and editor, data/information collector, related literature analysis.
Alvaro Velasquez (MD): Contributor in patient care and treatment, data collection.
Soraya Smith-Farmer: Contributor in patient care and treatment, data collection.
Samuel Gebreyonas: Contributor in patient care and treatment, data collection
Funding
This case report did not receive any funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of Interest
The authors report no conflicts of interest.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors and Treatment Team
Dr. Charles J. Grodzin is a board-certified Pulmonologist and Critical Care physician and an Assistant professor of medicine in the Department of Pulmonary, Critical care, Allergy and sleep Medicine at Emory University in Atlanta, Georgia. CV available upon request.
Edward Henderson completed his Bachelor of Arts degree at Davidson College in Davidson, North Carolina, and is currently a Research Specialist at Emory University’s Winship Cancer Institute in Atlanta, Georgia.
Dr. Alvaro Velasquez is a board-certified Pulmonologist and Critical Care physician and an Assistant professor of medicine in the Department of Pulmonary, Critical care, Allergy and sleep Medicine at Emory University in Atlanta, Georgia. CV available upon request.
Soraya Smith-Farmer Ph.D., APRN, ACNP-BC, CCRN is an advanced practice provider in the Emory University Midtown medical intensive care unit.
Samuel Gebreyonas ACNP-BC. Cardiac and Pulmonary Critical Care is an advanced practice provider in the Emory University Midtown medical intensive care unit.
References
- 1.Carrasquillo M., Dever L.L., Sonyey A. Botulism-like symptoms in an immunocompetent patient with Clostridium subterminale bacteremia. IDCases. 2018;11:80–82. doi: 10.1016/j.idcr.2018.01.014. PMID: 29619329; PMCID: PMC5881439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook P.A., Mishler A., Quan D., Parrish-Garcia A. Wound botulism caused by Clostridium subterminale after a heroin injection. Infect Dis Rep. 2018;10(2):7654. doi: 10.4081/idr.2018.7654. PMID: 30344967; PMCID: PMC6176474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis K., Gill D., Mouton C.P., Southerland J., Halpern L. An unusual odontogenic infection due to Clostridium subterminale in an immunocompetent patient: a case report and review of the literature. IDCases. 2018;12:34–40. doi: 10.1016/j.idcr.2018.03.009. PMID: 29942744; PMCID: PMC6011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubler J.G.H., Wuest J., Hany A. Pleuropulmonary infection due to Clostridium subterminale. J Infect. 1989;19(3):277–280. doi: 10.1016/S0163-4453(89)90849-9. [ISSN 0163-4453] [DOI] [PubMed] [Google Scholar]
- 5.Haussen D.C., Macedo F.Y., Caperton C.V., Zuckerman D.C. Clostridium subterminale sepsis in adult acute lymphoblastic leukemia. Leuk Lymphoma. 2011;52(6):1137–1138. doi: 10.3109/10428194.2011.555895. PMID: 21599594. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki K., Mori T., Takayama N., Tsukada Y., Ikeda Y., Okamoto S. Clostridium subterminale septicemia in a recipient of allogeneic cord blood transplantation. Intern Med. 2003;42(4):374–375. doi: 10.2169/internalmedicine.42.374. [DOI] [PubMed] [Google Scholar]
- 7.Patel K., Cao K. Clostridium subterminale sepsis in an immunocompetent patient without other risk factors: a case report. Chest. 2016;150(4):421A. doi: 10.1016/j.chest.2016.08.434. [Supplement] [DOI] [Google Scholar]
- 8.Thind S.K., Preis J.I. Clostridium subterminale septicemia in a patient with esophageal cancer. IDCases. 2014;1(I3):47–49. doi: 10.1016/j.idcr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tappe D., Valenza G., Duwe T., Koebe H., Frosch M., Abele-Horn M. Clostridium subterminale infection secondary to an open fracture. Infect Med. 2009;26:28–30. [Google Scholar]
- 10.Trapani K.M., Boghossian L.J., Caskey E. Clostridium subterminale septicemia in a patient with metastatic gastrointestinal adenocarcinoma. Case Rep Infect Dis. 2018;2018 doi: 10.1155/2018/6031510. PMID: 29951328; PMCID: PMC5987309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells C.L., Wilkins T.D. In: Medical microbiology. 4th edition. Baron S., editor. University of Texas Medical Branch at Galveston; Galveston (TX): 1996. Clostridia: spore forming anaerobic Bacilli. [Chapter 18] [Google Scholar]