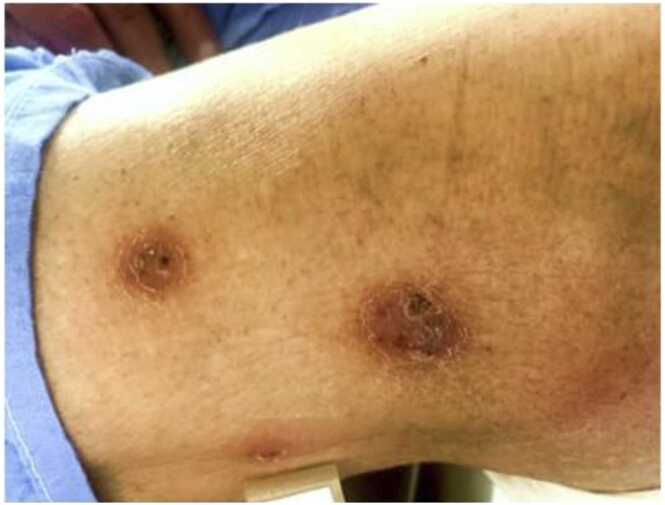
A 66 year-old male heart transplant recipient was hospitalized with heart failure. He incidentally reported multiple nodular, painless skin lesions on his right thigh (Fig. 1). He denied fever, chills, cough, shortness of breath, or recent trauma. He had undergone heart transplantation four months prior and home medications included mycophenolate mofetil, sirolimus, prednisone, and prophylactic trimethoprim-sulfamethoxazole. Skin biopsy was performed and histopathology revealed suppurative dermatitis and slightly beaded, filamentous bacilli which were positive on Gram and Fite stains. Ziehl-Neelsen stain was positive for acid fast bacilli (Fig. 2; arrow). No fungal organisms on Periodic-acid Schiff or Grocott’s methamine silver stains were identified. Blood cultures were negative. Computed Tomography (CT) of chest revealed interlobular septal thickening and scattered tree-in-bud opacities. CT abdomen and pelvis did not reveal any acute process. MRI brain was negative for mass-effect, midline shift, or space-occupying lesion. A broad differential diagnosis was considered, including nocardiosis, non-tuberculous mycobacteria infection, and invasive fungal infection). After six days of incubation, right thigh tissue culture grew gram-positive beaded bacilli which was identified as Mycobacterium chelonae by MALDI-TOF mass spectrometry. The patient was treated with imipenem, azithromycin and moxifloxacin and the skin lesions improved after several weeks of treatment.
Fig. 1.
Erythematous, nodular skin lesions on the right thigh of the patient.
Fig. 2.
Ziehl-Nelson staining of the skin biopsy specimen (arrow showing acid-fast bacilli).
Mycobacterium chelonae is an environmental, rapidly growing non-tuberculous mycobacteria (NTM) that may cause infection in both immunocompetent and immunocompromised hosts [1], [2]. M chelonae infection in immunocompetent patients commonly manifests as a localized, cutaneous disease as a result of direct trauma such as contact with contaminated water or material after cosmetic procedures. Patients with immunosuppressive conditions such as chronic steroid use, transplantation, hematologic malignancy and biologic therapy are particularly susceptible to NTM infections including M. chelonae [3]. In immunosuppressed patients, cutaneous lesions are often associated with disseminated disease and a history of direct skin trauma is commonly absent. Biochemical tests may be inadequate to identify NTM to the species level. Molecular techniques such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) are often utilized [4]. Culture and susceptibility testing is recommended for all isolates. Treatment of Mycobacterium chelonae infection in solid organ transplant recipients can be challenging due to variation in susceptibility pattern and drug-drug interactions. The treatment regimen should be based on in vitro susceptibilities. M. chelonae is generally susceptible to tobramycin (l00%), clarithromycin (l00%), linezolid (90%), imipenem (60%), amikacin (50%), clofazimine, doxycycline (25%), and ciprofloxacin (20%) [5]. Imipenem is preferred over cefoxitin because M. chelonae isolates are uniformly resistant to cefoxitin [5]. A combination therapy with 2 or 3 antimicrobials is recommended as failure with clarithromycin monotherapy due to the development of drug resistance has been reported [6]. In addition to antimicrobials, reduction of immunosuppression may be warranted.
Author contribution
Both authors contributed equally.
Funding
None.
Consent
A written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of written consent is available for review by the Editor.
Conflicts of interest
None.
References
- 1.Gonzalez-Santiago T.M., Drage L.A. Nontuberculous mycobacteria: skin and soft tissue infection. Dermatol Clin. 2015;33:563–577. doi: 10.1016/j.det.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Henkle E., Winthrop K.L. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36(1):91–99. doi: 10.1016/j.ccm.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stelzmueller I., Dunst K.M., Wiesmayr S., Zangerie R., Hengster P., Bonatti H. Mycobacterium chelonae skin infection in kidney-pancreas recipient. Emerg Infect Dis. 2005;11(2):352–354. doi: 10.3201/eid1102.040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocchetti T.T., Silbert S., Gostnell A., Kubasek C., Campos Pignatari A.C., Widen R. Detection of Mycobacterium chelonae, Mycobacterium abscessus Group, and Mycobacterium fortuitum Complex by a Multiplex Real-Time PCR directly from clinical samples using the BD MAX system. J Mol Diagn. 2017;19(2):295–302. doi: 10.1016/j.jmoldx.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F., et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. Erratum in: Am J Respir Crit Care Med. 2007 Apr 1;175(7):744-5. [DOI] [PubMed] [Google Scholar]
- 6.Vemulapalli R.K., Cantey J.R., Steed L.L., Knapp T.L., Thielman N.M. Emergence of resistance to clarithromycin during treatment of disseminated cutaneous Mycobacterium chelonae infection: case report and literature review. J Infect. 2001;43(3):163–168. doi: 10.1053/jinf.2001.0880. [DOI] [PubMed] [Google Scholar]