Summary
Background
Stimulation of immunity by vaccination may elicit adverse events. There is currently inconclusive evidence on the relationship between herpes zoster related hospitalization and COVID-19 vaccination. This study aimed to evaluate the effect of inactivated virus (CoronaVac, Sinovac) and mRNA (BNT162b2, BioNTech/Fosun Pharma) COVID-19 vaccine on the risk of herpes zoster related hospitalization.
Methods
Self-controlled case series (SCCS) analysis was conducted using the data from the electronic health records in Hospital Authority and COVID-19 vaccination records in the Department of Health in Hong Kong. We conducted the SCCS analysis including patients with a first primary diagnosis of herpes zoster in the hospital inpatient setting between February 23 and July 31, 2021. A confirmatory analysis by nested case-control method was also conducted. Each herpes zoster case was randomly matched with ten controls according to sex, age, Charlson comorbidity index, and date of hospital admission. Conditional Poisson regression and logistic regression models were used to assess the potential excess rates of herpes zoster after vaccination.
Findings
From February 23 to July 31, 2021, a total of 16 and 27 patients were identified with a first primary hospital diagnosis of herpes zoster within 28 days after CoronaVac and BNT162b2 vaccinations. The incidence of herpes zoster was 7.9 (95% Confidence interval [CI]: 5.2–11.5) for CoronaVac and 7.1 (95% CI: 4.1–11.5) for BNT162b2 per 1,000,000 doses administered. In SCCS analysis, CoronaVac vaccination was associated with significantly higher risk of herpes zoster within 14 days after first dose (adjusted incidence rate ratio [aIRR]=2.67, 95% CI: 1.08–6.59) but not in other periods afterwards compared to the baseline period. Regarding BNT162b2 vaccination, a significantly increased risk of herpes zoster was observed after first dose up to 14 days after second dose (0-13 days after first dose: aIRR=5.23, 95% CI: 1.61–17.03; 14–27 days after first dose: aIRR=5.82, 95% CI: 1.62–20.91; 0-13 days after second dose: aIRR=5.14, 95% CI: 1.29–20.47). Using these relative rates, we estimated that there has been an excess of approximately 5 and 7 cases of hospitalization as a result of herpes zoster after every 1,000,000 doses of CoronaVac and BNT162b2 vaccination, respectively. The findings in the nested case control analysis showed similar results.
Interpretation
We identified an increased risk of herpes zoster related hospitalization after CoronaVac and BNT162b2 vaccinations. However, the absolute risks of such adverse event after CoronaVac and BNT162b2 vaccinations were very low. In locations where COVID-19 is prevalent, the protective effects on COVID-19 from vaccinations will greatly outweigh the potential side effects of vaccination.
Funding
The project was funded by Research Grant from the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (Ref. No.COVID19F01). FTTL (Francisco Tsz Tsun Lai) and ICKW (Ian Chi Kei Wong)’s posts were partly funded by D24H; hence this work was partly supported by AIR@InnoHK administered by Innovation and Technology Commission.
Research in context.
Evidence before this study
We searched PubMed and Embase on October 11, 2021, for articles published in English using the search terms “herpes zoster”, “BNT162b2”, “vaccines”, “Comirnaty”, and “CoronaVac”, with no date restrictions. Most of the studies were either case reports or commentaries. There were three analytical studies in Israel to evaluate the association of herpes zoster with BNT162b2 (Pfizer–BioNTech). Only one of the three showed an increased risk of herpes zoster following BNT162b2 vaccinations, while the other two reported no significant increase. These studies limited to diagnosis of herpes zoster made in any settings, and suggested inconclusive evidence on the risk of herpes zoster following BNT162b2 vaccination. No analytical study on the risk of herpes zoster related hospitalization following CoronaVac vaccination was identified.
Added value of this study
This is the first population-based study in Hong Kong to investigate the association between COVID-19 vaccination and the risk of herpes zoster related hospitalization. Our study used self-controlled case series analysis as a main analysis and nested case control analysis as a confirmatory analysis to evaluate the association. Using both between- and within-individual comparison study designs, with different underlying assumptions, findings from this study show consistent association but with higher relative risk of herpes zoster related hospitalization after BNT162b2 vaccination.
Implications of all the available evidence
We found an increased risk of herpes zoster related hospitalization following CoronaVac and BNT162b2 vaccination. Our findings suggest that the absolute risks of herpes zoster related hospitalization after CoronaVac and BNT162b2 were very low. In locations where COVID-19 is prevalent, the protective effects on COVID-19 from vaccinations will greatly outweigh the potential side effects of vaccination. Continued surveillance is necessary to closely monitor COVID-19 vaccine safety and the risk of herpes zoster especially for the cases required hospitalization following COVID-19 vaccination.
Alt-text: Unlabelled box
Introduction
The emergency approval of COVID-19 vaccines requires close monitoring for adverse events following immunisation. Over 6000 and 2500 of herpes zoster cases have been reported through the Vaccine Adverse Event Reporting System in the United States and Yellow Card reports by the Medicines and Healthcare products Regulatory Agency in the United Kingdom, respectively, after the launch of mass vaccination.1,2 Some case studies reported patients developing herpes zoster shortly following mRNA and inactive vaccination.3, 4, 5, 6 Previous case reports showed the potential reactivation of herpes zoster after inactivated influenza, hepatitis A, and rabies and Japanese encephalitis vaccination.7 While herpes zoster may be a self-limiting dermatomal rash with pain,8 it is an acute neurologic disease that warrants immediate evaluation. About 20% of cases may develop postherpetic neuralgia, and around 2% of cases may cause various fatal complications including transient ischemic attacks, stroke and myocardial infarction.9,10 Hence, it is necessary to evaluate the risk of herpes zoster especially for the cases required hospitalization following COVID-19 vaccination.
Currently in Hong Kong, there are two vaccines authorised for emergency use: CoronaVac from Sinovac Biotech (Hong Kong) Limited (equivalent to Sinovac Life Sciences Company Limited) and BNT162b2 from Fosun Pharma/BioNTech (equivalent to BioNTech vaccine marketed by Pfizer outside China). For both clinical trials of CoronaVac and BNT162b2, the number of cases of herpes zoster has not been reported.11,12 Three analytical studies in Israel assessed the risk of herpes zoster after BNT162b2 vaccinations were identified from literature.13, 14, 15 Two of these reported no increased risk, and the remaining one showed an increased risk of herpes zoster following BNT162b2 vaccinations.13, 14, 15 These conflicting findings warrant to further investigation. More importantly, there is no analytical study examining the risk of herpes zoster after CoronaVac vaccination to date. The current findings are limited to diagnosis of herpes zoster made in any settings. Hospitalized cases are usually those complicated or disseminated cases that require immediate medical attention. Those severe cases may increase risk of various complications including meningitis, encephalitis, bacterial superinfection, haemorrhagic disease and nephritis.16 As a consequence of these complications, the burden of healthcare utilization for herpes zoster is high.16 A previous study in the UK showed that the average yearly hospital cost (2013–2014) required was £13.0 million for herpes zoster related hospitalization.17 Focusing on herpes zoster related hospitalization will give a better understanding of clinical implication and measure on healthcare burden, which is important in the safety of vaccines to inform the public health guidance. Given the global use of CoronaVac and BNT162b2 vaccines, this study aimed to evaluate the risk of herpes zoster related hospitalization following CoronaVac and BNT162b2 vaccination using population-based electronic healthcare records in Hong Kong.
Methods
Study design and data sources
We conducted a population-based study using self-controlled case series (SCCS) as a main analysis and nested case-control as a confirmatory analysis to investigate the association between COVID-19 vaccination and the risk of hospitalization due to herpes zoster. Herpes zoster was defined using International Classification of Diseases, Ninth Revision, clinical modification (ICD-9-CM) codes of 053.x.
This study was conducted using the electronic health records in the clinical management system from the Hospital Authority (HA), which, as a statutory administrative body in Hong Kong Special Administrative Region (HKSAR), manages 43 public hospitals, 49 specialist outpatient clinics and 73 primary care clinics, more than 70% of hospitalization in Hong Kong and over 20 million attendances at these public healthcare facilities were recorded in the year 2018–2019.18,19 Each patient has a unique identifier using their Hong Kong Identity Card Number in the clinical management system, which links up with all public hospitals, ambulatory clinics, specialist clinics, general out-patient clinics, and emergency rooms in the HA. Real time updated clinical data, including patients’ demographic information, diagnoses, medication and laboratory tests, were provided from the clinical management system. This database was applied previously to analyse the association between COVID-19 vaccination and the risk of Bell's palsy, the risk of carditis following BNT162b2 vaccinations the risk of adverse events in patients with chronic diseases.20, 21, 22, 23, 24 It was also used in other pharmacovigilance studies on medication safety.25, 26, 27 Currently, the government of HKSAR has authorised two COVID-19 vaccines, BNT162b2 and CoronaVac, for emergency use starting from February 23, 2021. Supplementary Table 1 shows the rollout schedule of the vaccination program. Initially, people with high risk of COVID-19 inflection or complications after COVID-19 inflection including elderly population and healthcare workers have been prioritised for vaccination. The vaccination program has been made eligible for all people aged 30–59 and 16–29 (≥18 for CoronaVac) years on March 16, 2021 and April 15, 2021, respectively. Up to July 31, 2021, a total of 3,227,186 people were vaccinated in Hong Kong. This study obtained the vaccination data from the Department of Health (DH), the Government of the HKSAR, and then linked them with the data from the clinical management system with a de-identified unique identifier for each patient.
Self-controlled case series study
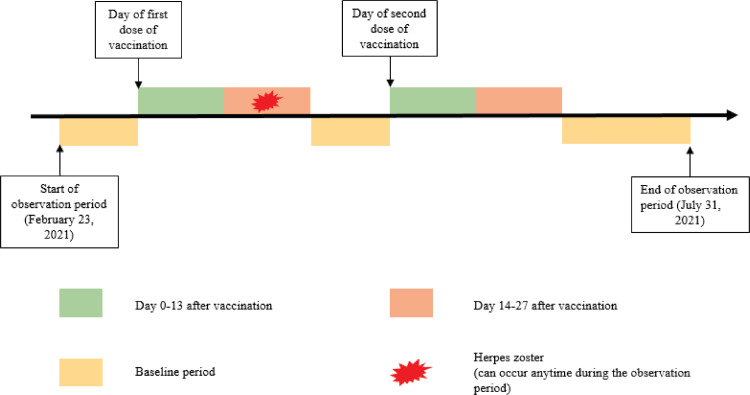
A SCCS design, which was specifically developed to assess vaccine safety,24,25 is able to compare the risks between exposure and non-exposure periods within the same individual with the outcome of interest. By using data from the same individual as the control, this study design can minimise both measured and unmeasured time-invariant confounding effects. Patients who were aged 16 or above, received at least one dose of BNT162b2 or CoronaVac vaccination in Hong Kong, and had their first primary inpatient diagnosis of herpes zoster between February 23 and July 31, 2021 were included in the SCCS analysis. Primary diagnosis means that the condition established after study to be primary medical diagnosis responsible for the patient's admission to the hospital. Patients with history of herpes zoster within 3 years before February 23, 2021 were excluded. The index date for all patients was the start date of observation period (February 23, 2021). The exposure periods in this study were defined as 0–13 days on or after each dose of vaccination. For people with single dose only, 14–27 days on or after first vaccination was considered as exposure period. For people with two doses of vaccination, day 14 to the day before the second dose but up to 27 days, and 14–27 days on or after second vaccination was considered as exposure period. All other periods were defined as baseline periods, over the observation period from February 23 to July 31, 2021, as illustrated in Fig. 1.
Fig. 1.
Observation timeline of a hypothetical patient in the self-controlled case series.
Three assumptions were required to be fulfilled to ensure an appropriate use of SCCS.30 Firstly, the event should be independently recurrent such that each occurrence does not affect subsequent events. However, it is possible for herpes zoster occur recurrently and thus increase the probability of future episodes, which might violate the assumption of event independence. Therefore, only the first episode of herpes zoster was treated as the outcome of interest in this study. Secondly, the event of interest should be independent of the exposure. Patients hospitalized with herpes zoster might be less likely to receive the vaccines, hence this assumption might be violated when applying the standard SCCS model, especially for the second dose vaccination. Therefore, we applied a modified SCCS model, which was designed for investigating outcomes that are associated with exposure.31 The modified SCCS model included unvaccinated people which hospitalized with herpes zoster to adjust the probability of receiving vaccination among patients with herpes zoster.31 Lastly, the event should not be censored during the observation period. We excluded individuals who died during the observation period to avoid any potential violation of this assumption.
Nested case-control study
Individuals with a first primary diagnosis of herpes zoster in the inpatient setting between February 23 and July 31, 2021 were selected as cases. Patients who were hospitalized during the same period but not included in cases were selected as controls. Patients who aged below 16 years or those with a history of herpes zoster within 3 years before index date were excluded from the analysis. Random matching was conducted for each case to assign ten controls with same sex, age, Charlson comorbidity index (within same group of 0, 1–2, 3–4, or ≥5), and date of attendance (within three calendar days). Only cases with vaccination within 28 days on or before the date of first diagnosis of herpes zoster and controls with vaccination within 28 days on or before the admission date of hospitalization were defined as vaccine recipients.
Statistical analysis
Self-controlled case series analysis
The R function "eventdepenexp" in the R-package “SCCS” was used to perform the modified SCCS for event dependent exposure.31 Season of the year was adjusted in monthly categories. Adjusted incidence rate ratio (aIRR) and 95% confidence intervals (CI) were estimated by comparing the incidence rates of herpes zoster in different risk periods with that in the baseline period using conditional Poisson regression. To evaluate the robustness of the results, three sensitivity analyses were conducted. First, although we treated the period between after the first dose and before the second dose as the baseline period, this may not fully explain the potential bias caused by the event dependent exposure subsequent to the first dose. Therefore, we conducted a sensitivity analysis where the standard SCCS model was applied with an observation period started on the date of the second dose of vaccination. Second, patients who were ever prescribed antiviral drugs any time before the index date may infer that the first diagnosis of herpes zoster in the database might not be the incident diagnosis of herpes zoster during patients’ course of life, and thus a sensitivity analysis was conducted by excluding those patients. Third, immune-compromised patients, who usually received immuno-suppressants, antibacterial drugs and systemic corticosteroids, may have higher risk of herpes zoster so we further excluded patients who received those medications within 90 days before the index date. In order to consolidate the analysis further, a negative control analysis using fractures, which has no known biological mechanisms associated with COVID-19 vaccines to-date, as an alternative outcome.
Nested case-control analysis
This study applied conditional logistic regression to evaluate the association between COVID-19 vaccines and risk of herpes zoster, with adjustment for patient characteristics, including smoking status, medical history (diabetes mellitus, hypertension, asthma, neoplasms, acute respiratory infections, viral infections, rheumatoid arthritis, stroke or systemic embolism, Guillain-Barré syndrome, migraine), and medications used in the past 90 days (antiviral drugs, systemic corticosteroids, antibacterial drugs, immune-suppressants, lipid lowering agents). To quantify the risk of herpes zoster, adjusted odds ratio (OR) and 95% CI were reported. The association in the risk periods 0–13 days and 14–27 days on or after first and second dose vaccination were evaluated individually.
Two-sided and p values of less than 0.05 were considered significant in all statistical tests. Statistical analysis was conducted using R version 4.0.3, by at least two investigators (EYFW, YW, VWSN and VKCY) independently for quality assurance. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) and Reporting of pharmacoepidemiological research undertaken using routinely collected data (RECORD-PE) statement checklists were followed in this study to guide transparent reporting of the case-control study.
Role of the funding source
The project was funded by Research Grant from the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (Ref. No.COVID19F01). FTTL (Francisco Tsz Tsun Lai) and ICKW (Ian Chi Kei Wong)’s posts were partly funded by D24H; hence this work was partly supported by AIR@InnoHK administered by Innovation and Technology Commission. The sponsor of this study was involved in the framework of study designs and data collection via the DH. The corresponding author had full access to all the data in the study and took final responsibility for the decision to submit for publication.
Ethical approval
Ethical approval for this study was granted by the Institutional Review Board of the University of HK/HA HK West Cluster (UW21-149 and UW21-138); and the DH Ethics Committee (LM21/2021).
Results
After excluding 45 people who received different vaccine types for their first and second doses of vaccinations, 1,655 people with second dose records but without first dose records, and 16 people with inconsistent duplicated records, there were 1,269,574 and 1,957,612 individuals receiving their first doses of CoronaVac and BNT162b2, respectively, between February 23, 2021 and July 31, 2021. Among these people, 982,255 (77.4%) and 1,451,858 (74.2%) individuals received the second doses of CoronaVac and BNT162b2, respectively. A total of 2,251,829 doses of CoronaVac, and 3,409,470 doses of BNT162b2 were administered. The number of first hospital diagnosis of herpes zoster within 28 days after first or second dose of CoronaVac and BNT162b2 was 16 and 27, respectively. The exact ICD-9-CM coding and other diagnosis other than herpes zoster recorded during the same hospital admission of 43 cases is showed in Supplementary Table 2. Therefore, the incidence of hospitalization due to herpes zoster was 7.9 (95% CI: 5.2–11.5) per 1,000,000 doses of BNT162b2 administrated and 7.1 (95% CI: 4.1–11.5) per 1,000,000 doses of CoronaVac administered. Supplementary Figure 1 shows the onset distribution of herpes zoster cases since the most recent dose of vaccination. For CoronaVac, 13 (81.3%) people hospitalised for herpes zoster were within 28 days after first dose and 12 (75%) cases were within 14 days after the most recent dose of vaccination. The average length of hospitalization was 4.06 (SD: 2.74) days, and only 1 (6.25%) patient had recurrent hospitalization due to herpes zoster. For BNT162b2, 18 (66.7%) people hospitalized for herpes zoster were within 28 days after first dose and 18 (66.7%) cases were within 14 days after the most recent dose of vaccination. The average length of hospitalization was 3.78 (SD: 3.04) days, and 2 (7.41%) patients had recurrent hospitalization due to herpes zoster.
Self-controlled case series analysis
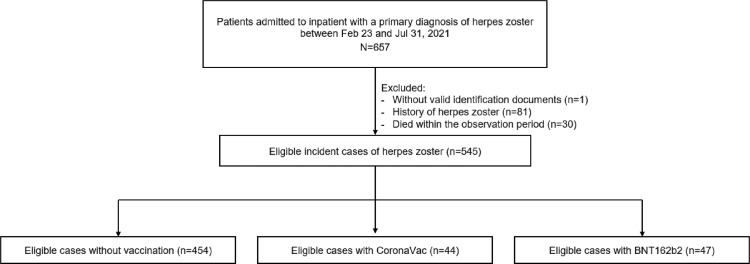
A total of 545 patients hospitalised with herpes zoster were included in the main analysis, including 91 vaccinated patients (CoronaVac: 44; BNT162b2: 47) and 454 unvaccinated patients (Fig. 2). Table 1 reported the patients’ demographics and baseline comorbidities. Table 2 showed the risk of herpes zoster following the COVID-19 vaccination. Regarding CoronaVac vaccination, a significantly increased risk of herpes zoster related hospitalization was observed within 14 days after first dose (aIRR=2.67, 95% CI 1.08–6.59) but not in other periods afterwards compared to the baseline period. Meanwhile, BNT162b2 vaccination was associated with significantly higher risk of herpes zoster related hospitalization after first dose until 14 days after second dose (0–13 days after first dose: aIRR=5.23, 95% CI 1.61–17.03; 14–27 days after first dose: aIRR=5.82, 95% CI 1.62–20.91; 0–13 days after second dose: aIRR=5.14, 95% CI 1.29–20.47). The sensitivity analyses found similar results as the main analysis (Supplementary Table 3). Supplementary Table 4 showed no association between fractures and COVID-19 vaccines in any risk periods in the negative control analysis. Given the relative risk of around 3 after CoronaVac and 5 after BNT162b2 vaccination, it was deduced that an additional 5 and 7 cases of herpes zoster related hospitalization may occur for every 1,000,000 doses of CoronaVac and BNT162b2, respectively, compared with no vaccination.
Fig. 2.
Flowchart of selection criteria in self-controlled case series analysis.
Table 1.
Baseline characteristics of patients in the self-controlled case series analysis.
| Characteristics | CoronaVac | BNT162b2 | Unvaccinated |
|---|---|---|---|
| (N = 44) | (N = 47) | (N = 454) | |
| Age, years (mean (SD)) | 58.89 (11.61) | 61.49 (15.30) | 70.48 (16.15) |
| Sex, male (%) | 19 (43.2) | 26 (55.3) | 205 (45.2) |
| Smoking (%) | 0 (0.0) | 0 (0.0) | 8 (1.8) |
| Charlson Comorbidity Index (mean (SD)) | 1.80 (1.15) | 2.19 (1.57) | 3.87 (2.24) |
| Diabetes (%) | 5 (11.4) | 3 (6.4) | 96 (21.1) |
| Hypertension (%) | 4 (9.1) | 14 (29.8) | 177 (39.0) |
| Asthma (%) | 0 (0.0) | 1 (2.1) | 7 (1.5) |
| Neoplasms (%) | 0 (0.0) | 1 (2.1) | 74 (16.3) |
| Acute respiratory infections (%) | 0 (0.0) | 2 (4.3) | 81 (17.8) |
| Viral infections (%) | 0 (0.0) | 0 (0.0) | 2 (0.4) |
| Rheumatoid Arthritis (%) | 1 (2.3) | 1 (2.1) | 16 (3.5) |
| Stroke or systemic embolism (%) | 0 (0.0) | 0 (0.0) | 15 (3.3) |
| Guillain–Barré syndrome (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Migraine (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antiviral drugs (%) | 1 (2.3) | 3 (6.4) | 41 (9.0) |
| Systemic corticosteroids (%) | 1 (2.3) | 5 (10.6) | 58 (12.8) |
| Antibacterial drugs (%) | 1 (2.3) | 4 (8.5) | 106 (23.3) |
| Immuno-suppressants (%) | 1 (2.3) | 5 (10.6) | 43 (9.5) |
| Lipid lowering agents (%) | 11 (25.0) | 13 (27.7) | 180 (39.6) |
SD=Standard deviation
Table 2.
Results of self-controlled case series analysis.
| Risk periods | Number of events | Patient-years | Adjusted Incidence Rate Ratio(95% CI) |
|---|---|---|---|
| Extension for the SCCS model: Event-dependent exposure | |||
| CoronaVac (n=498) After first dose |
|||
| 0 to 13 days | 9 | 1.61 | 2.67 (1.08–6.59) |
| 14 to 27 days | 4 | 1.40 | 1.17 (0.34–3.97) |
| After second dose 0 to 13 days |
3 | 0.89 | 1.67 (0.54–5.23) |
| 14 to 27 days | 0 | 0.67 | NA |
| Baseline | 482 | 212.21 | 1.00 |
| BNT162b2 (n=501) After first dose |
|||
| 0 to 13 days | 11 | 1.65 | 5.23 (1.61–17.03) |
| 14 to 27 days | 7 | 1.08 | 5.82 (1.62–20.91) |
| After second dose 0 to 13 days |
7 | 0.94 | 5.14 (1.29–20.47) |
| 14 to 27 days | 2 | 0.81 | 1.56 (0.36–6.81) |
| Baseline | 474 | 213.61 | 1.00 |
Adjusted for seasonality by month, potential individual-level confounders such as age and sex are already accounted for by the self-controlled design; CI=Confidence interval; NA= Not applicable
Nested case-control analysis
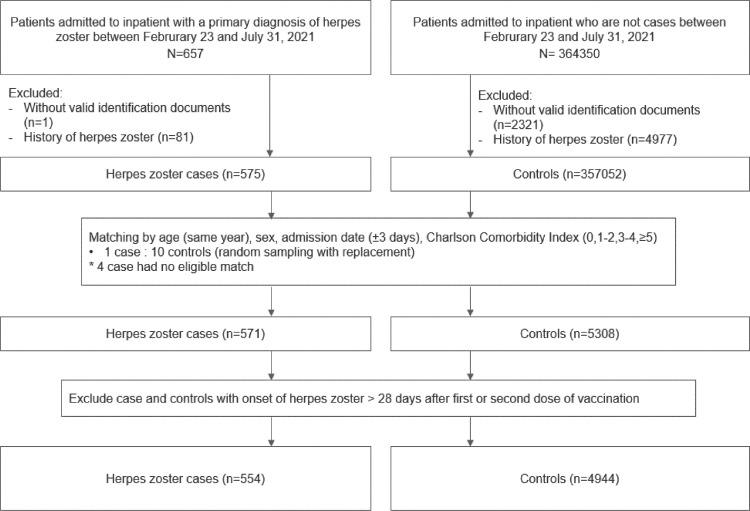
Fig. 3 shows the selection flow of cases and controls. A total of 554 cases and 4944 matched controls were identified. Table 3 and Supplementary Table 5 summarized the characteristics of cases and controls by vaccine exposure. 16 cases and 138 controls were CoronaVac vaccine recipient, while 27 cases and 126 controls were BNT162b2 vaccine recipient. Table 4 showed similar findings on the risk of hospitalization for herpes zoster with the SCCS analysis. CoronaVac vaccination was significantly associated with higher risk of herpes zoster related hospitalization within 14 days after first dose (adjusted OR: 3.44, 95% CI: 1.56–7.56) but not in other periods afterwards compared to people without vaccination. Besides, BNT162b2 vaccination was significantly associated with higher risk of herpes zoster related hospitalization after first dose (adjusted OR in 0–13 days: 2.60, 95% CI: 1.29–5.25; in 14–27 days: 3.93, 95% CI:1.50– 10.26) but not after second dose (0–13 days: adjusted OR: 2.01, 95% CI: 0.84–4.77; 14–27 days: adjusted OR: 0.70, 95% CI: 0.16–3.14).
Fig. 3.
Flowchart of inclusion and exclusion criteria in the nested case-control study.
Table 3.
Baseline characteristics of patients in the nested case-control study.
| Characteristics | Case | Controls |
|---|---|---|
| (N = 554) | (N = 4944) | |
| Age, years (mean (SD)) | 69.78 (16.24) | 70.23 (15.94) |
| Sex, male (%) | 249 (44.9) | 2196 (44.4) |
| Smoking (%) | 9 (1.6) | 82 (1.7) |
| Charlson Comorbidity Index (mean (SD)) | 3.76 (2.34) | 3.56 (2.16) |
| Diabetes (%) | 105 (19.0) | 1115 (22.6) |
| Hypertension (%) | 205 (37.0) | 2065 (41.8) |
| Asthma (%) | 8 (1.4) | 96 (1.9) |
| Neoplasms (%) | 85 (15.3) | 506 (10.2) |
| Acute respiratory infections (%) | 94 (17.0) | 520 (10.5) |
| Viral infections (%) | 2 (0.4) | 2 (0.0) |
| Rheumatoid Arthritis (%) | 19 (3.4) | 57 (1.2) |
| Stroke or systemic embolism (%) | 17 (3.1) | 192 (3.9) |
| Guillain–Barré syndrome (%) | 0 (0.0) | 8 (0.2) |
| Migraine (%) | 0 (0.0) | 4 (0.1) |
| Antiviral drugs (%) | 93 (16.8) | 150 (3.0) |
| Systemic corticosteroids (%) | 80 (14.4) | 260 (5.3) |
| Antibacterial drugs (%) | 146 (26.4) | 686 (13.9) |
| Immuno-suppressants (%) | 48 (8.7) | 70 (1.4) |
| Lipid lowering agents (%) | 214 (38.6) | 1959 (39.6) |
SD=Standard deviation
Table 4.
Risk of herpes zoster events among participants in the nested case-control study.
| Case(N = 554) | Control (N = 4944) | Crude odds ratio (95% CI) | Adjusted odds ratio (95% CI) | ||
|---|---|---|---|---|---|
| Events after first dose and before second dose | |||||
| Not vaccinated | 511 | 4490 | |||
| CoronaVac (0–13 days) | 9 | 30 | 2.78 (1.30–5.91) | 3.44 (1.56–7.56) | |
| BNT162b2 (0–13 days) | 11 | 39 | 2.27 (1.14–4.51) | 2.60 (1.29–5.25) | |
| Not vaccinated | 511 | 4417 | |||
| CoronaVac (14–27 days) | 4 | 34 | 1.06 (0.37–3.00) | 1.20 (0.41–3.47) | |
| BNT162b2 (14–27 days) | 7 | 16 | 3.46 (1.42–8.46) | 3.93 (1.50–10.26) | |
| Events after second dose | |||||
| Not vaccinated | 511 | 4416 | |||
| CoronaVac (0–13 days) | 3 | 32 | 0.78 (0.24–2.58) | 0.88 (0.26–2.96) | |
| BNT162b2 (0–13 days) | 7 | 36 | 1.63 (0.70–3.79) | 2.01 (0.84–4.77) | |
| Not vaccinated | 511 | 4347 | |||
| CoronaVac (14–27 days) | 0 | 29 | NA | NA | |
| BNT162b2 (14–27 days) | 2 | 21 | 0.71 (0.16–3.04) | 0.70 (0.16–3.14) | |
Cases and controls were matched according to age, sex, admission date and Charlson Comorbidity index. Odds ratios for herpes zoster events were estimated by conditional logistic regression adjusted for smoking status, medical history (diabetes, hypertension, asthma, malignancy, respiratory infections, viral infections, rheumatoid arthritis and other inflammatory polyarthropathies, stroke or systemic embolism, Guillain-Barré Syndrome, migraine), and medication use in the past 90 days (antiviral drugs, glucocorticoids, antibacterial drugs, immune-suppressants, lipid lowering agents). CI =confidence interval. NA = not applicable.
Discussion
Our population-based study in Hong Kong was the first to identify an increased risk of herpes zoster related hospitalization following CoronaVac and BNT162b2 vaccination using both between- and within-individual comparison study designs. The risk increased up to 14 days following the first dose of CoronaVac vaccination only. Meanwhile, the BNT162b2 vaccination increased the risk within 28 days after first dose and within 14 days after second dose. Given the relative risk of around 3 after CoronaVac and 5 after BNT162b2 vaccination, it was deduced that an additional 5 and 7 cases of herpes zoster related hospitalization may occur for every 1,000,000 doses of CoronaVac and BNT162b2, respectively, compared with no vaccination. While our findings showed the absolute risks of herpes zoster related hospitalization after CoronaVac and BNT162b2 were very low, close monitoring for the risk of herpes zoster will be warranted for the potential need of a booster dose of COVID-19 vaccine in the future.
The inconclusive evidence from three analytical studies in Israel was identified from the literature. One study reported no difference in prevalence of herpes zoster between before vaccination and a week after BNT162b2 vaccination among 103 people.14 Another retrospective cohort study with 233,159 people receiving BNT162b2 vaccine and the same number of matched unvaccinated controls also obtained no increased risk after BNT162b2 vaccination (Odd ratio: 1.07, 95% CI: 42 0.85–1.35).15 However, a study with a much larger cohort size including 884,828 participants in each BNT162b2 vaccinated and matched unvaccinated control group reported higher risk of herpes zoster after BNT162b2 vaccination (Risk ratio: 1.43, 95% CI: 1.20–1.73).13 Our findings showed the consistent association using two different study designs but with higher relative risk than the latter study. Our results were adjusted for not only demographics and morbidities but also medication history, and thus should be more robust. However, such discrepancy may be attributed to our outcome focusing on the hospitalized cases, and thus direct comparison between studies may not be appropriate. More importantly, no analytical study on the investigation of the risk of herpes zoster following CoronaVac vaccination has yet been conducted. Our study extended the current evidence that the increased risk of herpes zoster related hospitalization after not only BNT162b2 but also CoronaVac vaccination using different study designs. Moreover, similar to previous published case series studies, most of the herpes zoster incidents happened after the first rather than second dose of mRNA vaccination.6 However, the findings in our analysis also showed that the increased risk was observed within 14 days after second dose of BNT162b2 vaccination. Hence, attention on the risk of herpes zoster should be considered after not only the first dose but also second dose of BNT162b2 vaccination.
It is worthwhile to mention that the relative risk in the SCCS analysis was slightly higher than that in nested case-control analysis, although the confidence intervals overlapped. This potential difference may be related to unmeasured confounders in the nested case-control analysis. Higher proportion of patients of patients with drugs used such as lipid lowering agents in unvaccinated group compared to those in vaccinated group was observed. This observation may be attributed to health-seeking behavior. People who decided to get vaccinated were those who had better health condition as people with poor health condition may concern about their health condition, which may increase the probability of side effect of vaccine. Similar to other previous studies,28,29 the health conditions in vaccinated patients were more likely healthier than the unvaccinated individuals in our study. Specifically, the SCCS method was developed to evaluate vaccine safety with the advantage of controlling unmeasured time-invariant confounding through the comparisons within individuals.24,25 While slight difference in magnitude of relative risk between study designs, both inter-subjects and within-subject comparison analysis were convergent and indicated the increased risk of hospitalization for herpes zoster following COVID-19 vaccination.
Several potential reasons for the incidence of herpes zoster after COVID-19 vaccination were proposed. One suggestion for the trigger of herpes zoster could be associated with autoimmune phenomenon, which is hypothesized to occur via either mimicry of host molecules by the vicinal antigen or bystander activation of dormant autoreactive T-cells.32 Other possible explanations may be related to toll-like receptors signalling, which is often implicated in the reactivation process of herpes viruses as a maintenance mechanism in the host.33,34 Particularly, administration of mRNA vaccines stimulates induction of type I interferon and potent inflammatory cytokines, which instigate T and B cells immune responses but may enhance antigen presentation possibly leading to reactivation of varicella zoster virus.3 Another potential reason for the incidence of herpes zoster after COVID-19 vaccination is attributed to COVID-19 virus induced by COVID-19 vaccines. COVID-19 vaccines contain the materials from the virus that causes COVID-19, which leads to activation of immune system in theory. A previous study proposed that COVID-19 infection could potentially suppress the immune system, leading to a decrease in T lymphocytes including CD4+ T cells and CD8+ T cells, and thus lymphocyte exhaustion may contribute to reactivation of varicella zoster virus.35 However, another study reported an increase in CD4+ T cells and CD8+ T cells shortly after mRNA vaccination.33 A population cohort study in Israel also demonstrated an increased risk of herpes zoster after BNT162b2 vaccination but not after COVID-19 infection. Hence, the causality between herpes zoster and COVID-19 infection remain inconclusive. Further study will be warranted to confirm the mechanism between COVID-19 vaccines and herpes zoster.
Limitations
This study has several limitations. Firstly, only information of people who used public healthcare services in Hong Kong was recorded in the clinical management system, thus information of patients who used private medical practitioners were not captured in the database and this study. However, HA covers over 70% of hospitalization in Hong Kong.36 Moreover, due to highly subsidised public health service from the government, the majority of patients would intend to use public healthcare services, and thus most of the cases were likely to be included in our study. Additionally, SCCS analysis would not be affected by completeness of data as it is based on comparison within individual instead of comparison among different individuals. Secondly, diagnosis coding was used to identify outcome of interest, thus this study might induce bias due to misclassified or under-reported events. However, our database is for the routine clinical practices used in the hospital. It is unlike insurance claim databases. The diagnosis coding in current database was made by the hospital clinicians for clinical management. Previous studies have validated for the diagnoses of various medical diseases include cardiovascular, gastroenterology, osteoporosis and cancer.25,27,37,38 Therefore the diagnosis coding of herpes zoster was likely to be high accuracy. Thirdly, the current electronic health record dataset allowed to check the history of herpes zoster based on the diagnosis coding of ICD-9-CM for patients up to 2018. 3-year period for checking history may not be sufficiently long. Besides, the detailed descriptions of diagnosis of the cases was not available in the current study, and thus the severity of the cases cannot be distinguished. Moreover, there are some unmeasured factors (e.g. education, income level and the record of herpes zoster vaccination) which might confound the association between exposure and outcome, and thus bias the estimation. However, the SCCS analysis in this study is free of any measured or unmeasured time-invariant confounding effect. Moreover, it can also ensure the validity of the nested case-control analysis with consistent results. Fourthly, the outcome in the current study was limited to herpes zoster related hospitalization. Other cases that do not require hospitalization were not covered in the study. These less severe cases may be self-limiting or require symptomatic treatment only,16 but further studies should be conducted to confirm the risk of non-severe cases herpes zoster following the COVID-19 vaccination. Fifthly, under the current pandemic situation, clinicians might have increased vigilance and admit to hospital those vaccinated even for the mild cases, and thus the findings might be overestimated. Lastly, this study is restricted to patients who were newly diagnosed with herpes zoster at the inpatient setting in Hong Kong. Our results may not be generalized to other countries or population due to different ethnicities, which we only covered Chinese population in our study. Thus, further studies in other population or different ethnicities, and on patients with history of herpes zoster would be valuable to confirm the finding.
Conclusion
Our findings showed an increased risk of herpes zoster related hospitalization after CoronaVac and BNT162b2 vaccinations. However, the absolute risks of such adverse event after CoronaVac and BNT162b2 vaccinations were very low. The protective effects of the vaccinations in reducing the risk of severe COVID-19 greatly outweigh the potential side effects of vaccination including a slight increase in risk of herpes zoster.
Declaration of interests
EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, and the Hong Kong Research Grants Council, outside the submitted work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; personal fee from Primevigilance Ltd.; outside the submitted work. FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from Food and Health Bureau of the Government of the Hong Kong SAR, outside the submitted work. EWYC reports honorarium from Hospital Authority, grants from Research Grants Council (RGC, Hong Kong), grants from Research Fund Secretariat of the Food and Health Bureau, grants from National Natural Science Fund of China, grants from Wellcome Trust, grants from Bayer, grants from Bristol-Myers Squibb, grants from Pfizer, grants from Janssen, grants from Amgen, grants from Takeda, grants from Narcotics Division of the Security Bureau of HKSAR, outside the submitted work. XL has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, research and educational grants from Janssen and Pfizer; internal funding from University of Hong Kong; consultancy fee from Merck Sharp & Dohme, unrelated to this work. CKHW reports the receipt of General Research Fund, Research Grant Council, Government of Hong Kong SAR; EuroQol Research Foundation, all outside the submitted work. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, and also received speaker fees from Janssen and Medice in the previous 3 years. He is also an independent non-executive director of Jacobson Medical in Hong Kong.
Acknowledgments
Contributors
EYFW, CSLC and ICKW had the original idea for the study, contributed to the development of the study, extracted data from the source database, constructed the study design and the statistical model, reviewed the literature, and act as guarantors for the study. EYFW, YW, VWSN and VKCY undertook the statistical analysis. EYFW, CSLC and ICKW wrote the first draft of the manuscript. ICKW is the principal investigator and provided oversight for all aspects of this project. FTTL, XL. CKHW, EWYC, CSMW, KSML, MYN, SAV, JSMP, JTKW, BJC, DMA, IFNH and GML provided critical input to the analyses, design and discussion. All authors contributed to the interpretation of the analysis, critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
Acknowledgment
Research Grant from the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (Ref. No. COVID19F01). Members of the Expert Committee on Clinical Events Assessment Following COVID-19 Immunization for case assessment. Colleagues from the Drug Office of the Department of Health, and Hospital Authority for providing vaccination and clinical data. We also thank Ms Cheyenne Chan for administrative assistance.
Data sharing statement
Data will not be available for others as the data custodians have not given permission.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100393.
Appendix. Supplementary materials
References
- 1.United States Department of Health and Human Services (DHHS), Public Health Service (PHS), Centers for Disease Control (CDC) /Food and Drug Administration (FDA). Vaccine Adverse Event Reporting System (VAERS) 1990 - 09/10/2021, CDC WONDER On-line Database; 2021. http://wonder.cdc.gov/vaers.html. Accessed 20 September 2021.
- 2.Medicines & Healthcare Products Regulatory Agency. Coronavirus vaccine weekly summary of yellow card reporting; 2021. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. Accessed 3 October 2021.
- 3.Furer V., Zisman D., Kibari A., Rimar D., Paran Y., Elkayam O. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology. 2021 doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Jiménez P., Chicharro P., Cabrera L.M., et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: Report of 5 cases. JAAD Case Rep. 2021;12:58–59. doi: 10.1016/j.jdcr.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Channa L., Torre K., Rothe M. Herpes zoster reactivation after mRNA-1273 (Moderna) SARS-CoV-2 vaccination. JAAD Case Rep. 2021;15:60–61. doi: 10.1016/j.jdcr.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lladó I., Fernández-Bernáldez A., Rodríguez-Jiménez P. Varicella zoster virus reactivation and mRNA vaccines as a trigger. JAAD Case Rep. 2021;15:62–63. doi: 10.1016/j.jdcr.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter R., Hartmann K., Fleisch F., Reinhart W.H., Kuhn M. Reactivation of herpesvirus infections after vaccinations? Lancet. 1999;353(9155):810. doi: 10.1016/S0140-6736(99)00623-6. [DOI] [PubMed] [Google Scholar]
- 8.Helgason S., Petursson G., Gudmundsson S., Sigurdsson J.A. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ (Clinical research ed) 2000;321(7264):794–796. doi: 10.1136/bmj.321.7264.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang J.H., Ho J.D., Chen Y.H., Lin H.C. Increased risk of stroke after a herpes zoster attack. Stroke. 2009;40(11):3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 10.Patterson B.J., Rausch D.A., Irwin D.E., Liang M., Yan S., Yawn B.P. Analysis of vascular event risk after herpes zoster from 2007 to 2014 US insurance claims data. Mayo Clin Proc. 2019;94(5):763–775. doi: 10.1016/j.mayocp.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanriover M.D., Doğanay H.L., Akova M., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barda N., Dagan N., Ben-Shlomo Y., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brosh-Nissimov T., Sorek N., Yeshayahu M., et al. Oropharyngeal shedding of herpesviruses before and after BNT162b2 mRNA vaccination against COVID-19. Vaccine. 2021;39(40):5729–5731. doi: 10.1016/j.vaccine.2021.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shasha D., Bareket R., Sikron F.H., et al. Real-world safety data for the Pfizer BNT162b2 SARS-CoV-2 vaccine, historical cohort study. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azzari C., Baldo V., Giuffrida S., et al. The cost-effectiveness of universal varicella vaccination in Italy: a model-based assessment of vaccination strategies. Clinicoecon Outcomes Res. 2020;12:273. doi: 10.2147/CEOR.S229685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobbelen P.H., Stowe J., Amirthalingam G., Miller L., van Hoek A.J. The burden of hospitalisation for varicella and herpes zoster in England from 2004 to 2013. J Infect. 2016;73(3):241–253. doi: 10.1016/j.jinf.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Singhal A., Ross J., Seminog O., Hawton K., Goldacre M.J. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194–204. doi: 10.1177/0141076814522033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Census and Statistics Department. Thematic household survey report no. 58. Hong Kong, 2015.
- 20.Wan E.Y.F., Chui C.S.L., Lai F.T.T., et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua G.T., Kwan M.Y.W., Chui C.S.L., et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Tong X., Yeung W.W.Y., et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-221571. annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai F, Huang L, Chui CSL, et al. Multimorbidity and adverse events of special interest associated with CoronaVac (Sinovac) and Comirnaty (Pfizer-BioNTech). 2021.
- 24.Lai F.T.T., Li X., Peng K., et al. Carditis following Covid-19 vaccination with messenger RNA vaccine (BNT162b2) and inactivated virus vaccine (CoronaVac): a case-control study. Annu Intern Med. 2022;175(3):362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau W.C., Chan E.W., Cheung C.L., et al. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA. 2017;317(11):1151–1158. doi: 10.1001/jama.2017.1363. [DOI] [PubMed] [Google Scholar]
- 26.Lau W.C.Y., Cheung C.L., Man K.K.C., et al. Association between treatment with apixaban, dabigatran, rivaroxaban, or warfarin and risk for osteoporotic fractures among patients with atrial fibrillation: a population-based cohort study. Ann Intern Med. 2020;173(1):1–9. doi: 10.7326/M19-3671. [DOI] [PubMed] [Google Scholar]
- 27.Wong A.Y., Root A., Douglas I.J., et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926. doi: 10.1136/bmj.h6926. [DOI] [PubMed] [Google Scholar]
- 28.Whitaker H.J., Paddy Farrington C., Spiessens B., Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 29.Weldeselassie Y.G., Whitaker H.J., Farrington C.P. Use of the self-controlled case-series method in vaccine safety studies: review and recommendations for best practice. Epidemiol Infect. 2011;139(12):1805–1817. doi: 10.1017/S0950268811001531. [DOI] [PubMed] [Google Scholar]
- 30.Petersen I., Douglas I., Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 31.Ghebremichael-Weldeselassie Y. The self-controlled case series method.
- 32.Principi N., Esposito S. Do vaccines have a role as a cause of autoimmune neurological syndromes? Front Public Health. 2020;8:361. doi: 10.3389/fpubh.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C., Maruggi G., Shan H., Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West J.A., Gregory S.M., Damania B. Toll-like receptor sensing of human herpesvirus infection. Front Cell Infect Microbiol. 2012;2:122. doi: 10.3389/fcimb.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bo X., Fan C.Y., Wang A.L., et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Census and Statistics Department. Thematic household survey report no. 58. 2015.
- 37.Chan E.W., Lau W.C.Y., Leung W.K., et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149(3):586–595. doi: 10.1053/j.gastro.2015.05.002. e3. [DOI] [PubMed] [Google Scholar]
- 38.Cheung K.S., Chan E.W., Wong A.Y.S., Chen L., Wong I.C.K., Leung W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2018;67(1):28–35. doi: 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.