Abstract
Background
Persistence of Ebola virus (EBOV) in semen remains of deep concern, as sexual transmission of EBOV seems plausible up to 6 months after acute phase of Ebola virus disease (EVD). Favipiravir, a broad spectrum antiviral product, has been evaluated in reducing EVD mortality in Guinea in 2014–2015 in the JIKI trial, the pharmacokinetic results of which suggest that an increase of dose might be necessary to achieve a therapeutically relevant exposure. In FORCE trial, we aimed at evaluating the tolerance and activity of high doses of favipiravir in male EVD survivors with EBOV RNA detection in semen in Guinea.
Case
In 2016, we launched a phase IIa open-labeled multicenter dose escalation study. Male survivors of EVD with EBOV RT-PCR positive on semen received a loading dose of 2400 mg BID of favipiravir on day 1 then a maintenance dose of 1800 mg BID from day 2–14. The primary outcome was the tolerance, assessed daily during period treatment and up to day 90. Unfortunately only two participants were included and the trial was stopped for lack of recruitment. No clinical adverse event of grade 3/4 was reported for both patients. One patient experienced a grade 3 hypocalcemia at day 10 and 14.
Conclusions
High doses of favipiravir were well tolerated in these two participants. Better characterized tolerance and pharmacokinetics of high doses of favipiravir are of utmost importance considering that favipiravir is a candidate treatment for a variety of emerging severe viral diseases with poor prognosis.
Keywords: Favipiravir, Ebola survivors, QTc, Safety, Semen
Highlights
-
•
Ebola virus (EBOV) RNA in semen of survivors of EBOV disease up to 17 months after disease onset.
-
•
High doses of Favipiravir were well tolerated in EBOV disease survivors.
-
•
No QTc interval prolongation of grade 2, 3 or 4 with high doses of Favipiravir.
Introduction
Between December 2013 and June 2016, West Africa has experienced the largest outbreak of Ebola in history: 28,616 cases of patients with Ebola virus disease (EVD) have been reported in Guinea, Liberia and Sierra Leone. Besides, it has resulted in an unprecedented number of EVD survivors around 17,300 people. Whereas the dynamic of the epidemic slowed down by the week 30–2015 in Guinea, persistence of Ebola virus (EBOV) in bodily fluids including semen remains of deep concern. Moreover, as sexual transmission of Marburg virus another filovirus has been documented in 1968 [1], sexual transmission of EBOV seems definitely plausible.
Studies from the West Africa outbreak gave rise to two significant findings. First, sexual transmission of EBOV seems plausible up to 6 months after acute phase of the disease. Clusters with sexual transmission from men survivors have been identified in Liberia and in Guinea, months after the end of outbreak was declared in these countries [2]. Second, findings from Sierra Leone and Guinea survivors provide evidence of long-term persistence of EBOV RNA in semen, up to 531 days after disease onset (DO) [3]. Linear mixed-effect modeling of dynamics of virus persistence in seminal fluid over time led to the estimation that 50% of male survivors have RT-PCR-detectable EBOV RNA in seminal fluid 115 days after DO (90% prediction interval (PI) 72–160) and 10% have detectable virus 294 days after D0 (90%PI 212–399) [4].
Favipiravir (T-705) is a broad-spectrum direct antiviral targeting the RNA-dependant RNA polymerase. The product is approved in Japan for the treatment of non-complicated influenza infections. It has been evaluated in reducing EVD mortality in Guinea in 2014–2015 in the JIKI trial (NCT02329054). Favipiravir was administered to 126 patients at the dose of 6000 mg at day 1 (H0: 2400 mg, H8: 2400 mg, H16: 1200 mg) then 1200 mg BID from day 2 to day 10. JIKI results were inconclusive on mortality rate but the pharmacokinetic results showed that favipiravir residual concentrations in Ebola patients at day 2 and day 4 after treatment initiation were lower than those predicted from the favipiravir population pharmacokinetic model used to define the dosing regimen of the JIKI trial [5]. In addition, the diffusion of favipiravir from plasma to testicular tissue in monkey is limited, as indicated by the tissue/plasma ratio much lower than 1, i.e. 0.21 and 0.67 at 0.5 and 4 h after treatment initiation, respectively [6]. Taking into account the low tissue/plasma ratio and a possible binding of favipiravir to tissue protein compartment, the active residual concentration of favipiravir in the sperm would be much lower than that in the plasma, which may be already lower than produced in the in vivo IC50 [7]. Taken together, these results suggest that an increase of dose, especially of the maintenance dose, might be necessary to achieve a therapeutically relevant exposure. A two-fold increase of the maintenance dose could be necessary to achieve targeted concentrations. The loading dose aims to reach as fast as possible the targeted plasma concentration without reaching peak concentration that would expose patients to an increased toxicity risk (data from modeling).
In this context, we launched in 2016 the FORCE trial aiming at evaluating the tolerance and activity of high doses of favipiravir in male EVD survivors harboring EBOV RNA detection in semen in Guinea (clinicaltrials.gov registered NCT02739477). This trial was a phase IIa open-labeled multicenter dose escalation study based on tolerance, with three cohorts of six patients.
Unfortunately only two men were included in April 2016 and the trial was stopped for lack of recruitment as no other survivors could be found EBOV semen positive. Here we report clinical and biological assessments of two EVD survivors harboring EBOV RNA detection in semen who received high doses of favipiravir for 14 days.
The protocol was approved by the Guinean Comité National d′Éthique pour la Recherche en Santé on February 8th 2016, the IRB of the Institut National de la Santé et de la Recherche Médicale (Inserm, France) on February 9th 2016, and the Commission Recherche Ebola en Guinée on February 10th 2016. All patients provided written informed consent to participate in the study.
Case reports
The trial took place at two sites: Conakry and Nzerekore, Guinea. Patients were recruited from EVD survivors whose semen has already been tested positive for EBOV and who were still followed in the POSTEBOGUI cohort [19]. Male survivor of biologically confirmed EVD aged 18 years or older with EBOV RT-PCR on semen with cycle threshold [Ct] < 38 were enrolled. Main exclusion criteria were: EBOV RT-PCR on blood with CT < 38; biological abnormality higher than grade 2 according to CTCAE (v4.03) on following parameters: creatinine, AST, ALT, alkaline phosphatase, total bilirubin or any medical condition that could interfere with results interpretation or compromise participants' health; Fridericia corrected QT interval (QTc) > 450 ms; concomitant use of QT/QTc interval-prolonging drugs; previous gout attack or ongoing treatment for gout or hyperuricemia.
Follow-up visits were scheduled at day –7, day 1 (initiation of treatment), day 3, day 7, day 10, day 14, day 21 and day 90. Clinical assessment and favipiravir intake were monitored by daily phone call at days 2, 4–6, 8, 9, 11–13. A 12-lead electrocardiogram (ECG) were performed at each visit and sent to an independent centralized cardiac data analysis center in France for analysis by a board-certified cardiologist. At day 1 repeated ECG measurements were taken (3 measurements 2 min apart) before favipiravir initiation and 2 h after drug intake. Mean QTc value was recorded.
Blood samples were taken in EDTA and dry tubes at day –7, day 1, day 3, day 7, day 10, day 14, day 21 for hematological and biochemical testing; semen samples were collected by masturbation after a 3 days abstinence at day –7, day 1, day 7, day 14, day 21 and day 90 for virological assessment. Biochemical and hematological assays were performed using the Piccolo Xpress (Abaxis®) system. Corrected serum calcium (Cac) was computed as follow: Cac (mmol/L) = Calcium (mmol/L) + 0.025 × (40 − Albumine g/L). Viral RNA extraction was performed using QIAamp viral RNA isolation kit (Qiagen®) and detection of EBOV material in semen was made using a semi-quantitative RT-PCR assay (RealStar Ebolavirus Screen RT-PCR Kit 1.0, Altona Diagnostics®) according to manufacturer recommendations. The results were expressed in terms of Ct, whose value is inversely proportional to viral load. The Ct cutoff value for positivity was < 40.
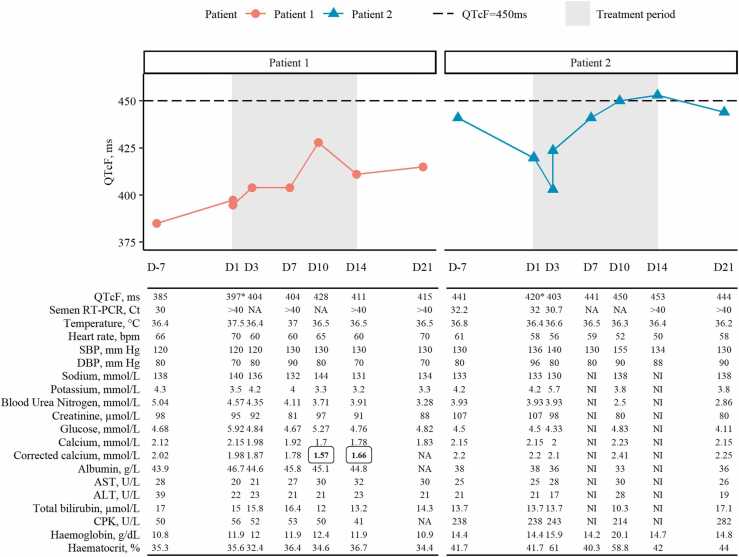
The main clinical characteristics of the two participants at inclusion are presented in Table 1. Both participants received a loading dose of 4800 mg of favipiravir on day 1 then a dose of 1800 mg BID from day 2 to 14. Longitudinal data on QTc with Fridericia’s correction, heart rate, blood pressure, biochemistry, and RT-PCR in semen for both patients are presented in Fig. 1. No clinical adverse event of grade 3 or 4 was reported for Patient 1 and 2.
Table 1.
Baseline characteristics of participants.
| Patient 1 | Patient 2 | |
|---|---|---|
| Age, years | 29 | 56 |
| BMI, kg/m2 | 22.1 | 24.6 |
| Antecedent (Ebola virus disease) | ||
| Time from Ebola treatment centers admission, months | 14.1 | 17.7 |
Fig. 1.
Individual clinical and biological results during follow-up. Shaded frames represent treatment period (D1-D14). Dashed line represents threshold of QTcF duration of 450 ms. Abbreviation: ‘D’ indicates day; ‘NA’ indicates non-available data, ‘NI’ indicates non-interpretable data. Values in bold boxed text were grade 3 observations according to CTCAE v4.03. QTcF values at D1 for both patients (*) were mean of three measurements (before the start of treatment). Corrected calcium was calculated with formula: calciummmol/L + 0.025(40-Albuming/L) and was rounded to 10−1.
Both participants experienced a slight QTc interval prolongation during treatment period. Patient 1 had a QTc interval of less than 450 ms during treatment and follow-up period. In Patient 2, the QTc interval increased from 420 ms at day 1–441, 450 and 453 ms (grade 1 adverse event) at days 7, 10 and 14, respectively, and decreased to 444 ms at day 21. Resulting maximum ∆QTc was of 31 ms between day 1 (397 ms) and day 10 (428 ms) and of 33 ms between day 1 (420 ms) and day 14 (453 ms) for patient 1 and 2, respectively.
Notably, transaminases remain within laboratory normal values for both participants during treatment period. Patient 1 experienced a grade 3 hypocalcemia at day 10 and day 14 with a Cac at 1.57 mmol/L and 1.66, respectively (< 1.75–1.5 mmol/L, CTCAE v4.03). Cac could not be computed at day 21 as albuminemia was not available at this visit. Of note is that patient 1 had low Cac at day –7 (corresponding to a grade 1 adverse event) and at days 1, 3 and 7 (grade 2 adverse event).
No biological adverse event of grade 3 or 4 was reported for Patient 2. Patient 2 experienced following biological grade 2 adverse events according to CTCAE v4.03: hyperkalemia at day 3, hemoglobin increased at day 10 and high blood pressure at day 10 (155/90 mmHg with vertigo) and day 37 (150/90 mmHg). This adverse event was not related to favipiravir according to the investigator.
The RT-PCR in semen was negative from day 1 and day 14 for patient 1 and 2, respectively, and remains negative until the end of follow-up at day 90.
Discussion
Recently a case report underlines the concomitant prescription of favipiravir and other drugs with QTc prolongation potential [8]. However, from this specific report, we think that favipiravir definitely cannot be considered as the unique causative factor for the observed QTc prolongation [9]. Otherwise, a randomized controlled double-blind study (4 groups, 4 periods in cross-over with placebo and moxifloxacin) was conducted in 56 Japanese participants to analyze the effect of favipiravir on the QT/QTc interval and showed no particular warning signs in a single oral administration at doses of 1200 mg and 2400 mg [10]. In any case, close cardiac monitoring should be planned when evaluating high doses of favipiravir, and particular care would be taken not to combine favipiravir with other QTc-lengthening drugs.
One of the scientific arguments supporting the need for better characterization of tolerance and pharmacokinetics profile of high doses of favipiravir is the definition of the dosage of favipiravir to be administered in the event of a new outbreak of EVD, Marburg virus disease (MVM) or Lassa fever. Indeed, although trials conducted during the Ebola epidemic (EBOV) in 2014–2015 failed to demonstrate the efficacy of WHO-prioritized treatment candidates (including favipiravir), two independent studies conducted by the REACTION! consortium and USAMRIID, respectively, demonstrated the effect of prophylactic administration of high-dose favipiravir in non-human primate models of Ebola and Marburg disease [11], [12]. Hence, favipiravir remains of particular interest for the management of patients with MVE, MVM or Lassa fever, potentially in combination with other promising compounds identified in non-human primate models, or in contact individuals for post-exposure prophylaxis. Preclinical studies have identified effective plasma concentrations of the order of 70–80 µg/mL for MVE. But the complexity of the pharmacokinetics of thi molecule that is characterized with dose- and time-dependent non-linearity [13] makes difficult to identify the dose necessary to achieve these above concentrations in humans. Hence, this calls for characterization of the pharmacokinetics profile of favipiravir for doses likely to be used in humans, in order to be able to achieve these target concentrations.
Moreover, it becomes of growing interest considering that favipiravir is a candidate treatment for a variety of emerging infectious viral diseases with poor prognosis and for which there is little or no documented curative counter-measure or vaccine. This is particularly the case for Nipah virus infection [14], and Crimean-Congo viral hemorrhagic fever [15] of which two cases have recently been reported in Spain. Of note in Belgium, an imported case of Junin fever was recently managed with the drug as specific targeted therapy [16]. More recently, favipiravir has shown antiviral activity against SARS-CoV-2 both in vitro and in animal studies [17], [18] and is currently undergoing several clinical trials in various countries worldwide in the treatment of coronavirus disease 2019 (COVID-19) [19], even though the doses used are unlikely to achieve target drug concentrations against SARS-CoV-2 [20].
In addition, another area of indication to be considered refers to the ability of favipiravir to cross the blood-meningeal barrier and reach the anatomically or immunologically preserved sanctuaries (central nervous system, male genital tract) constituting the reservoir from which the chronic disease (relapse, prolonged carriage in fluids) of emerging viral infections such as – although not exclusively – Ebola virus infection is expressed.
We are convinced of the necessity of performing dose-ranging studies with high doses of favipiravir in healthy volunteers to inform any further development of favipiravir for treatment or prophylaxis of various virus infections.
CRediT authorship contribution statement
All authors contributed to study design, data collection, data analysis and writing.
Ethical approval
The protocol was approved by the Guinean Comité National d’Éthique pour la Recherche en Santé on February 8th 2016, the IRB of the Institut National de la Santé et de la Recherche Médicale (Inserm, France) on February 9th 2016, and the Commission Recherche Ebola en Guinée on February 10th 2016.
Consent
All patients provided written informed consent to participate in the study.
Sources of funding
This work was supported by the REACTing (REsearch & ACtion emergING infectious diseases) Consortium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information
The study included a scientific advisory board composed of Brigitte BAZIN, Rodolphe GARRAFFO, Bruno LINA, Raphaël PORCHER, Pierre TATTEVIN, Marie-Blanche VALNET-RABIER. Jérémie GUEDJ, Xavier de LAMBALLERIE, Hervé RAOUL, Anne-Marie TABURET were members of the scientific committee.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Acknowledgements
PostEbogui cohort study group, Yves LEVY.
References
- 1.Martini G.A., Schmidt H.A. [Spermatogenic transmission of the “Marburg virus”. (Causes of “Marburg simian disease”)] Klin Wochenschr. 1968;46(7):398–400. doi: 10.1007/BF01734141. [DOI] [PubMed] [Google Scholar]
- 2.Mate S.E., Kugelman J.R., Nyenswah T.G., Ladner J.T., Wiley M.R., Cordier-Lassalle T., et al. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med. 2015;373(25):2448–2454. doi: 10.1056/NEJMoa1509773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subtil F., Delaunay C., Keita A.K., Sow M.S., Touré A., Leroy S., et al. Dynamics of Ebola RNA persistence in semen: a report from the postebogui cohort in Guinea. Clin Infect Dis. 2017;64(12):1788–1790. doi: 10.1093/cid/cix210. [DOI] [PubMed] [Google Scholar]
- 4.Sissoko D., Duraffour S., Kerber R., Kolie J.S., Beavogui A.H., Camara A.-M., et al. Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Health. 2017;5(1):e80–e88. doi: 10.1016/S2214-109X(16)30243-1. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen T.H., Guedj J., Anglaret X., Laouénan C., Madelain V., Taburet A.-M., et al. Favipiravir pharmacokinetics in Ebola-infected patients of the JIKI trial reveals concentrations lower than targeted. Horby PW, éditeur. PLoS Negl Trop Dis. 2017;11(2) doi: 10.1371/journal.pntd.0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Japanese Pharmaceuticals and Medical Devices Agency (PMDA). Report on the Deliberation Results (English version) [Internet]; 2014. Available: 〈https://www.pmda.go.jp/files/000210319.pdf〉.
- 7.Smither S.J., Eastaugh L.S., Steward J.A., Nelson M., Lenk R.P., Lever M.S. Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model. Antivir Res. 2014;104:153–155. doi: 10.1016/j.antiviral.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Chinello P., Petrosillo N., Pittalis S., Biava G., Ippolito G., Nicastri E. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. Schibler M, éditeur. PLoS Negl Trop Dis. 2017;11(12) doi: 10.1371/journal.pntd.0006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malvy D., Taburet A.-M., de Lamballerie X., Mentre F., Extramiana F. The safety profile of favipiravir should not be the first argument to suspend its evaluation in viral hemorrhagic fevers. PLoS Negl Trop Dis. 2020;14(6) doi: 10.1371/journal.pntd.0008259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumagai Y., Murakawa Y., Hasunuma T., Aso M., Yuji W., Sakurai T., et al. Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adults. Int J Clin Pharm Ther. 2015;53(10):866–874. doi: 10.5414/CP202388. [DOI] [PubMed] [Google Scholar]
- 11.Bixler S.L., Bocan T.M., Wells J., Wetzel K.S., Van Tongeren S.A., Dong L., et al. Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus. Antivir Res. 2018;151:97–104. doi: 10.1016/j.antiviral.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Guedj J., Piorkowski G., Jacquot F., Madelain V., Nguyen T.H.T., Rodallec A., et al. Antiviral efficacy of favipiravir against Ebola virus: a translational study in cynomolgus macaques. PLoS Med. 2018;15(3) doi: 10.1371/journal.pmed.1002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madelain V., Nguyen T.H., Olivo A., de Lamballerie X., Guedj J., Taburet A.-M., et al. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet. 2016;55(8):907–923. doi: 10.1007/s40262-015-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawes B.E., Kalveram B., Ikegami T., Juelich T., Smith J.K., Zhang L., et al. Favipiravir (T-705) protects against Nipah virus infection in the hamster model. Sci Rep. 2018;8(1):7604. doi: 10.1038/s41598-018-25780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oestereich L., Rieger T., Neumann M., Bernreuther C., Lehmann M., Krasemann S., et al. Evaluation of antiviral efficacy of ribavirin, arbidol, and T-705 (Favipiravir) in a mouse model for Crimean-Congo hemorrhagic fever. Singh SK, éditeur. PLoS Negl Trop Dis. 2014;8(5) doi: 10.1371/journal.pntd.0002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veliziotis I., Roman A., Martiny D., Schuldt G., Claus M., Dauby N., et al. Clinical management of argentine hemorrhagic fever using ribavirin and favipiravir, Belgium, 2020. Emerg Infect Dis. 2020;26(7):1562–1566. doi: 10.3201/eid2607.200275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driouich J.-S., Cochin M., Lingas G., Moureau G., Touret F., Petit P.-R., et al. Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat Commun. 2021;12(1):1735. doi: 10.1038/s41467-021-21992-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelnabi R., Foo C.S., De Jonghe S., Maes P., Weynand B., Neyts J. Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a hamster infection model. J Infect Dis. 2021;224(5):749–753. doi: 10.1093/infdis/jiab361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fragkou P.C., Belhadi D., Peiffer-Smadja N., Moschopoulos C.D., Lescure F.-X., Janocha H., et al. Review of trials currently testing treatment and prevention of COVID-19. Clin Microbiol Infect. 2020;26(8):988–998. doi: 10.1016/j.cmi.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eloy P., Solas C., Touret F., Mentré F., Malvy D., de Lamballerie X., et al. Dose rationale for favipiravir use in patients infected with SARS-CoV-2. Clin Pharm Ther. 2020;108(2):188. doi: 10.1002/cpt.1877. [DOI] [PubMed] [Google Scholar]