Abstract
Adult T-cell leukemia/lymphoma (ATLL) is a malignancy caused by the human T-cell leukemia virus type 1. Aggressive ATLL is refractory to conventional chemotherapy and has a poor prognosis. Better therapeutic approaches, including cancer immunotherapy, are required to improve survival and prognosis. The genetic landscape of ATLL reveals frequent genetic alterations in genes associated with immune surveillance, including major histocompatibility complex (MHC) class I, CD58 antigen, and programmed cell death ligand 1. Clinicopathological investigations also revealed tumor immunity mechanisms in ATLL, including immune checkpoint molecules, MHC molecules, tumor-associated macrophages, and chemokines. However, the tumor microenvironment of ATLL remains complex because ATLL itself originates from T-cells, usually expressing regulatory T-cell markers. In this review, we discuss the recent literature describing the tumor microenvironment of ATLL.
Keywords: Lymphomas/Lymphoid Leukemias, Hematolymphoid Tissues, Reactive, Infectious, Lymphoproliferative Disorders
INTRODUCTION
Adult T-cell leukemia/lymphoma (ATLL) is a peripheral T-cell neoplasm caused by the human T-cell leukemia virus type 1 (HTLV-1).1 ATLL is classified into indolent ATLL (chronic and smoldering type) and aggressive ATLL (lymphomatous and acute type).2 Aggressive ATLL is refractory to conventional chemotherapy and has a poor prognosis, whereas indolent ATLL has a comparatively favorable clinical course. However, a long-term follow-up study of indolent ATLL revealed that 65.1% of patients with indolent ATLL died of acute ATLL, with 18.8 months as the median time of transformation to the aggressive type.3 Better therapeutic strategies, including cancer immunotherapy, are required to improve the prognosis of patients with ATLL. This review focuses on recent findings of the tumor microenvironment of ATLL.
Immune suppression by ATLL cells
Regulatory T-cells (Tregs) potently suppress the activation and proliferation of CD4+ T-cells and CD8+ T-cells, which prevent the development of autoimmunity. ATLL cells express CD4 and CD25, and are considered to originate from CD4+ T-cells with Treg phenotypes. ATLL cells exhibit strong immunosuppressive activity in vitro.4 Opportunistic infections, such as Pneumocystis carinii pneumonia, are observed in ATLL more frequently than in other lymphomas.5 Forkhead box P3 (FOXP3) is a representative marker of Tregs that converts naïve CD4+ T-cells into the Treg phenotype. We previously evaluated FOXP3 expression in ATLL cells and found that 36% of ATLLs have FOXP3 expression in lymphoma cells.6 The overall survival and clinical data were not significantly different between FOXP3+ and FOXP3- ATLL patients. However, FOXP3+ ATLL patients had severe infections and Epstein-Barr virus-infected cells more frequently than FOXP3- patients, which suggests immunosuppression caused by FOXP3-expressing ATLL cells.6
Immune escape from T-/NK cells
As HTLV-1-associated proteins and peptides are antigenic, immune escape from host immunity is necessary for HTLV-1 infected cells. ATLL gains numerous methods of immune escape from T-cells and/or natural killer (NK) cells. Major histocompatibility complex (MHC) class I is universally expressed on human cells, except for the central nervous system cells and erythrocytes. The MHC class I component is composed of human leukocyte antigen (HLA)-A, -B, and -C, and beta 2 microglobulin (β2M), and plays an important role in the immune response associated with CD8+ T-cells. Loss of MHC class I and/or β2M is frequently observed in tumors, which induces immune escape from CD8+ tumor-infiltrating lymphocytes (TILs).7,8 Kataoka et al. reported that 54% of all ATLL cases have gene alterations in the MHC class I complex (HLA-A, HLA-B, and β2M), revealing that immune escape by loss of MHC class I is also important for the pathogenesis of ATLL.9 Asano et al. conducted an immunohistochemical analysis, and found that MHC class I and β2M were expressed on the tumor cells in 74% and 72% of ATLL cases, respectively (Figure 1A-D).10 ATLL cells expressing both MHC class I and β2M accounted for 39% of the total cases, and these cases had better prognoses than others, confirming that loss of MHC class I and/or β2M is an adverse prognostic factor in ATLL.10
Fig. 1.
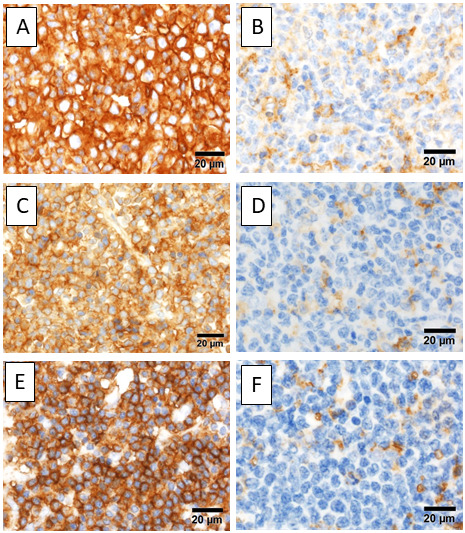
Immunohistochemistry of MHC class I and class II. A) ATLL cells expressing MHC class I. B) Loss of MHC class I. C) β2M is expressed on ATLL cells. D) Loss of β2M, E) ATLL cells, positive for MHC class II, F) Loss of MHC class II.
MHC, major histocompatibility complex; ATLL, adult T-cell leukemia/lymphoma; β2M, beta 2 microglobulin
On the other hand, MHC class II is mainly expressed on antigen-presenting cells (APCs), including B-cells, dendritic cells, and macrophages, and plays an important role in the immune response via CD4+ T-cells. MHC class II can be expressed on many types of tumor cells; however, MHC class II expression can influence both favorable and adverse prognostic factors depending on the cancer type.11-15 As tumor-associated antigens expressed on MHC class II are recognized by cytotoxic CD4+ T-cells, loss of MHC class II can lead to immune escape from the attack of CD4+ TILs.13 On the other hand, MHC class II expression on tumor cells can cause an adverse event by interaction with lymphocyte activation gene-3 on CD4+ TILs, preventing apoptosis of tumor cells in malignant melanoma.15 MHC class II expression is also observed in activated normal T-cells and some T-cell malignancies, and is regulated by the class II transactivator.16 Indolent ATLL cells exhibit higher MHC class II expression than aggressive ATLL cells, which may be associated with the transformation of indolent ATLL into aggressive ATLL.17 We previously examined MHC class II expression in ATLL cells using immunohistochemistry and found that 33.3% of ATLLs expressed MHC class II (Figure 1E-F).18 In our study, most patients had aggressive ATLL and MHC class II was detected as an independent favorable prognostic marker.18 Interaction between MHC class II on tumor cells and CD4+ TILs may be important for tumor immune escape in ATLL.
CD58 activates NK cells and cytotoxic T-cells. Loss and/or inactivation of CD58 can induce immune escape in tumors, including malignant lymphoma.19,20 Yoshida et al. previously reported the loss of CD58 in ATLL cells.21 Genetic alterations inactivating CD58 were more frequent in aggressive ATLL cells (29%) than in indolent ATLL cells (7%).21 This suggests that immune escape caused by CD58 genetic alterations is important for tumor immune escape and may be associated with transformation in ATLL.21
Immune checkpoint molecules in ATLL
Immune checkpoint molecules are also important in the tumor microenvironment of ATLL. Programmed cell death ligand 1 (PD-L1)/programmed cell death protein 1 (PD-1) is the most representative immune checkpoint pathway. PD-L1, which is expressed on tumor cells, binds with the PD-1 present on activated T-cells to suppress and inactivate the T-cells. In ATLL, PD-L1 can be overexpressed on tumor cells via numerous pathways, including gene amplification of PD-L1 and due to interferon-gamma.9 Kataoka et al. reported that 27% of ATLLs have a structural variation of the PD-L1 gene with disruption of the 3’-untranslated region (UTR).22 Disruption of the 3’-UTR of the PD-L1 gene leads to stabilization and increased PD-L1 transcripts, causing immune escape through PD-L1 overexpression. Miyoshi et al. evaluated PD-L1 expression in ATLL cells using immunohistochemistry and found that 7.4% of ATLLs had PD-L1 expression with a significantly worse clinical course than those without PD-L1 expression (Figure 2A).23 Stromal cells, including macrophages, can express PD-L1. PD-L1-expressing stromal cells have been identified as an independent favorable prognostic factor in ATLL, but the mechanism underlying this remains unclear (Figure 2B).23 However, it is known that PD-L1 expression can be induced by CD8+ cytotoxic T-cells and the upregulation of interferon-gamma, which suggests that PD-L1 expression on stromal cells reflects the activation of anti-tumor immunity in ATLL.
Fig. 2.
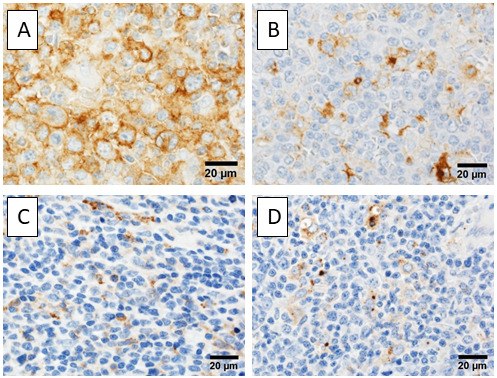
Immunohistochemistry of immune checkpoint molecules. A) ATLL cells expressing PD-L1. B) Stromal cells, positive for PD-L1. C) Stromal cells expressing Tim-3. D) OX40L+ stromal cells are observed.
ATLL, adult T-cell leukemia/lymphoma; PD-L1, programmed cell death ligand-1; Tim-3, T-cell immunoglobulin mucin-3; OX40L, a ligand for OX40
T-cell immunoglobulin mucin-3 (Tim-3) or hepatitis virus A cellular receptor 2 is expressed on T-cells, macrophages, and dendritic cells. Galectin-9 is one of the ligands of Tim-3 and can be expressed on some tumor cells. In addition to the PD-L1/PD-1 signaling pathway, the Galectin-9/Tim-3 signaling pathway works as an inhibitory immune checkpoint mechanism that promotes immune escape and exhaustion of T-cells in tumors.24 Horlad et al. reported that the interaction between Tim-3 expressed on ATLL cells and Galectin-9 expressed on macrophages promotes the proliferation of ATLLs.25 They reported that Tim-3+ ATLL patients are resistant to chemotherapy.25 In a previous study, we found that Tim-3 expression on stromal cells is a favorable prognostic factor in ATLL (Figure 2C).26 The precise function of Tim-3+ stromal cells remains unclear, as is that of PD-L1+ stromal cells. Although Galectin-9 is widely expressed on stromal cells in ATLL, Galectin-9 expression alone was not detected as a prognostic factor in ATLL.26
OX40, also called CD134 or tumor necrosis factor receptor superfamily member 4 (TNFRSF4), belongs to the tumor necrosis factor (TNF) co-stimulatory receptor superfamily and is expressed on activated T-cells. The ligand for OX40 (OX40L or TNFSF4) is expressed on APCs, including B-cells, dendritic cells, and macrophages. The OX40/OX40L interaction induces the proliferation and survival of T-cells and their cytokine production.27 OX40L is also expressed on non-lymphoid cells, including endothelial cells and mast cells, suggesting that the OX40/OX40L pathway has functions other than T-cell mediated immunity. ATLL cells often express OX40 because the HTLV-1-encoded oncoprotein Tax upregulates OX40 gene expression.28 We previously examined OX40L expression in ATLL cells using immunohistochemistry (Figure 2D). Many stromal cells expressed OX40L, which was found to be an independent favorable prognostic marker in ATLL.26 OX40L on stromal cells may activate OX40-expressing ATLL cells. However, the OX40/OX40L-mediated pathway also acts as an antagonist of Tregs. The OX40/OX40L pathway may suppress ATLL cells that usually demonstrate Treg phenotypes.
Co-stimulatory signals
Although T-cell receptors (TCRs) recognize an antigenic peptide present on the MHC complex, MHC-TCR signaling alone is not efficient for activating T-cells. Co-stimulatory signals induced by the binding of CD28 and B7-1 (CD80) or B7-2 (CD86) are necessary to activate T-cells.29 In normal T-cells, CD28 is downregulated after T-cell activation and is replaced by cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) or inducible co-stimulator (ICOS), which binds to CD80/CD86 more strongly than CD28.30 The CTLA-CD80/CD86 complex induces a co-inhibitory signal in T-cells. In peripheral T-cell lymphomas including ATLL, copy number variations and activating mutations in CD28 have been reported.9 Fusion of the C-terminal domains of CD28 (cytoplasmic side) and the N-terminal domains of CTLA-4 or ICOS (CD80/CD86 side) converts CTLA-4 or ICOS-mediated signal to a CD28-mediated co-stimulatory signal, which induces continuous or prolonged CD28 signaling (Figure 3A). Strong CD28 expression can be detected using immunohistochemistry in some ATLL cases (Figure 3B) (unpublished data). In addition, CTLA4-CD28 and ICOS-CD28 binding have been reported in some T-cell malignancies. The overall frequency of these fusions involving CD28 was 4.8% in T-cell lymphomas; however, the frequency depended on the disease entity: ATLL (17%), follicular T-cell-derived peripheral T-cell lymphoma (PTCL) (angioimmunoblastic lymphoma and PTCL with follicular helper phenotype) (6.5%), PTCL-not otherwise specified (3.1%), cutaneous T-cell lymphoma (3.8%), anaplastic large cell lymphoma (0%), and extranodal NK/T-cell lymphoma (0%).31 Of note, Yoshida et al. reported the unique biology of young patients with ATLL, 37.5% of whom had both CTLA-4-CD28 and ICOS-CD28 fusions.32 They also reported that CD80 and CD86 were expressed on ATLL and stromal cells, respectively, which suggests that the interaction between CD28+ ATLL cells and stromal cells expressing CD80 and/or CD86 is important. (Figure 3C-D).30 Sakamoto et al. also reported that ATLL patients with genetic alterations in CD28 were younger than those without CD28 abnormalities.33 Moreover, indolent ATLL patients with alterations activated by CD28 had poorer prognoses.33 According to the above reports, genetic alterations of CD28 may be important in the pathogenesis of ATLL, especially in monitoring disease progression.
Fig. 3.
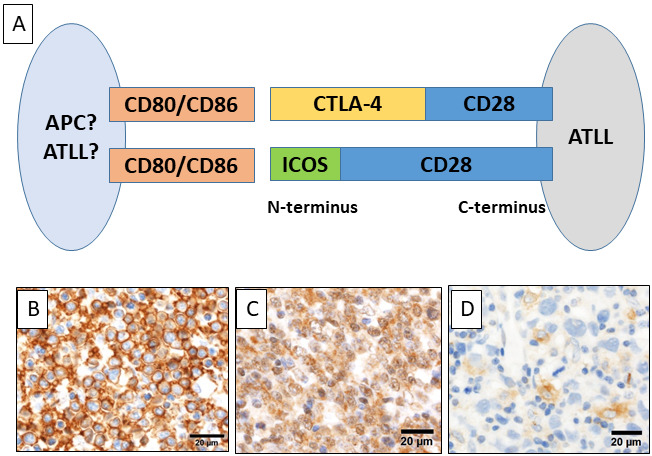
Fusions involving CD28. A) Scheme of CD28-CTLA4 and CD28-ICOS fusions. Fusion of the C-terminal domain of CD28 and the N-terminal domain of CTLA-4 or ICOS may interact with CD80 or CD86 on ATLL cells or stromal cells, and convert the CTLA-4- or ICOS-mediated signal to a CD28-mediated co-stimulatory signal. B) ATLL cells strongly expressing CD28. C) CD80 is expressed on ATLL cells. D) Stromal cells positive for CD86.
CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ICOS, inducible co-stimulator; ATLL, adult T-cell leukemia/lymphoma
Tumor-associated macrophages
Activated macrophages are classified into activated (M1) and alternatively activated (M2) macrophages. Tumor-associated macrophages (TAMs or tumor-infiltrating macrophages) exhibit an M2 phenotype and suppress anti-tumor immunity.34 TAMs also produce cytokines and chemokines to promote proliferation, angiogenesis, and metastasis of tumors. Komohara et al. clinicopathologically evaluated TAMs in ATLL and found that cases with increased M2 type TAMs have an unfavorable clinical course.35 Therefore, TAMs are important factors in the microenvironment of ATLL.
Signal-regulatory protein alpha (SIRPα) is expressed on macrophages and CD47 is ubiquitously expressed on human cells. Binding of CD47/SIRPα is termed the “Don’t eat me” signal, which prevents phagocytosis. Apoptotic cells usually lose CD47 and are processed by macrophages; however, some tumor cells overexpress CD47 and/or SIRPα. The “Don’t eat me” signal promotes immune escape and tumor invasion, and is associated with a poor prognosis in some malignancies.36,37 Yanagida et al. clinicopathologically evaluated the expression of CD47 and SIRPα in ATLL patients, and found that CD47 expression was not associated with prognosis (Figure 4A-D).33 On the other hand, stromal expression of SIRPα was detected as a good prognostic factor.38 Although the precise mechanism remains unclear, those with SIRPα-expressing stromal cells had significantly higher expression of MHC class I and/or MHC class II, and also had PD-L1 expressing stromal cells, suggesting that SIRPα-expressing stromal cells are associated with activated anti-tumor immunity.38
Fig. 4.
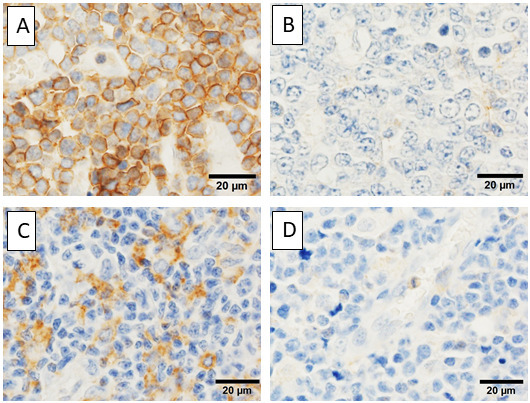
Immunohistochemistry of CD47 and SIRPα. A) ATLL cells expressing CD47. B) CD47- ATLL cells. C) Stromal cells positive for SIRPα. D) SIRPα is negative in stromal cells.
SIRPα, signal-regulatory protein alpha; ATLL, adult T-cell leukemia/lymphoma
Chemokines in ATLL
Chemokines are a family of small, secreted proteins that regulate the migration, trafficking, and homing of immune cells. CC (one of the four subfamilies of chemokines) chemokine receptor 4 (CCR4) is expressed on ATLL cells, which is a target of anti-CCR4 monoclonal antibody (mogamulizumab) treatment. Hasegawa et al. reported that CCR7 expression correlates with lymphoid organ involvement in ATLL.39 We previously analyzed chemokines and their receptors in ATLL using DNA chips, and found that CC-ligand 18 (CCL18) is the most upregulated and CX3C (another subfamily of chemokines) chemokine receptor 1 (CX3CR1) is the most downregulated.40 Immunohistochemistry revealed that both CCL18 and CX3CR1 are not expressed on ATLL cells, but on stromal cells.40 In general, CCL18 is mainly expressed on monocytes, macrophages, and dendritic cells and is associated with the initiation of the immune response. CCL18 is a marker of M2 macrophages, and the production of CCL18 by TAMs promotes immunosuppression and immune escape in the tumor microenvironment.41 Upregulated CCL18 suggests immune evasion of ATLL associated with TAMs. CX3CR1 and its ligand CX3C-motif ligand 1 mediate cell adhesion and migration. CX3CR1 is expressed on cytotoxic effector lymphocytes, including NK cells, γδ T-cells, and CD8+ T-cells, and plays an important role in the elimination of pathogens and cancer cells.42 Downregulated CX3CR1 suggests the suppression of cytotoxic T-cell function in ATLL.
Cancer immunotherapy in ATLL
As described above, immune escape is important in the pathogenesis of ATLL and in other malignancies. Overexpression of PD-L1 caused by the deletion of the 3’-UTR, and gene amplification was detected as an adverse prognostic factor in ATLL, which supports the efficacy of PD-1 inhibitor therapy in ATLL. In contrast to this, Ratner et al. performed a clinical trial of PD-1 inhibitory therapy in a small number of ATLL patients and found that PD-1 inhibitor was not effective and induced rapid disease progression.43 However, Ishitsuka et al. performed other clinical trials of PD-1 blockade therapy in ATLL patients and observed no rapid disease progression.44 The patients enrolled in the former trial included those with indolent ATLL with a mild clinical course, whereas those enrolled in the latter trial had refractory/recurrent aggressive ATLL, which suggests that the clinical stage is associated with the response to PD-1 inhibitor therapy in ATLL patients. Expression and genetic alterations of PD-L1, PD-1, and MHC class I should be evaluated to clarify this point. MHC class II expression on tumor cells was reported to predict the efficacy of PD-1 blockade therapy in classical Hodgkin lymphoma and malignant melanoma.45,46 MHC class II expression may also be associated with the efficacy of PD-1 blockade in ATLL.
Both clinical trials involved a small number of patients; thus, the efficacy of PD-1 blockade therapy remains controversial. However, the microenvironment of ATLL may be more complicated than that of the other cancers that respond well to PD-L1/PD-1 blockade therapy (Figure 5 and Table 1). ATLL originates from CD4+ T-cells, usually with a Treg phenotype, and some ATLL cells can express immune checkpoint molecules, including PD-1.28,31 In addition, stromal cells expressing immune checkpoint molecules have been reported as favorable prognostic factors.31 Immune checkpoint-targeted therapy, including PD-1 blockade, may affect not only TILs, but also ATLL and/or stromal cells. Further studies on the microenvironment of ATLL are necessary for the development of new, effective therapeutic strategies.
Fig. 5.
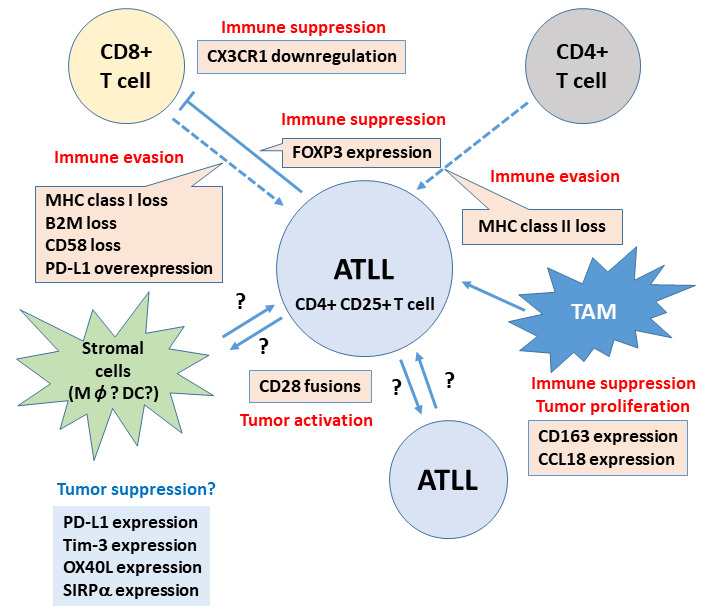
The microenvironment of ATLL. Scheme summarizing recent findings of the tumor microenvironment of ATLL. Immune evasion from CD8+ T-cells by loss of MHC class I and class II, loss of CD58, and overexpression of PD-L1 has been reported. Loss of MHC class II may be important for immune evasion from CD4+ T-cells. Immune suppression by TAMs, chemokines, and Treg function of ATLL itself was also reported. Interaction between CD80/CD86 and CD28 fusion proteins can mediate continuous CD28-mediated tumor activation. However, some stromal cells express immune checkpoint molecules and/or SIRPα, which play a role in the better prognosis of ATLL patients through an unknown mechanism.
ATLL, adult T-cell leukemia/lymphoma; MHC, major histocompatibility complex; PD-L1, programmed cell death ligand-1; TAM, tumor-associated macrophage; Treg, regulatory T-cell; SIRPα, signal-regulatory protein alpha
Table 1. Prognostic significance of markers in the tumor microenvironment of ATLL.
| Expression | Prognosis | References | |
|---|---|---|---|
| MHC class I | tumor cells | favorable | 10 |
| β2M | tumor cells | favorable | 10 |
| MHC class II | tumor cells | favorable | 18 |
| CD58 | tumor cells | favorable | 21 |
| CD28 | tumor cells | unfavorable | 32,33 |
| PD-L1 | tumor cells | unfavorable | 23 |
| PD-L1 | stromal cells | favorable | 23 |
| OX40L | stromal cells | favorable | 26 |
| Galectin-9 | stromal cells | not associated | 26 |
| Tim-3 | stromal cells | favorable | 26 |
| CD163 | macrophage | unfavorable | 35 |
| CD47 | tumor cells | not associated | 38 |
| SIRPa | macrophage | favorable | 38 |
Representative markers of the tumor microenvironment and their association with prognosis in ATLL. ATLL, adult T-cell leukemia/lymphoma; MHC, major histocompatibility complex; β2M, beta 2 microglobulin; PD-L1, programmed cell death ligand-1; Tim-3, T-cell immunoglobulin mucin-3; OX40L, a ligand for OX40; SIRPα, signal-regulatory protein alpha
CONCLUSION
The microenvironment of ATLL is still being investigated and the intercellular interactions remain unclear. Further studies, including single-cell analyses, are required to understand the exact mechanisms underlying tumor immunity in ATLL.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127: 2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991; 79: 428-437. [DOI] [PubMed] [Google Scholar]
- 3.Takasaki Y, Iwanaga M, Imaizumi Y, et al. Long-term study of indolent adult T-cell leukemia-lymphoma. Blood. 2010; 115: 4337-4343. [DOI] [PubMed] [Google Scholar]
- 4.Yamada Y. Phenotypic and functional analysis of leukemic cells from 16 patients with adult T-cell leukemia/lymphoma. Blood. 1983; 61: 192-199. [PubMed] [Google Scholar]
- 5.Takatsuki K, Yamaguchi K, Watanabe T. Adult T-cell leukemia and HTLV-I related diseases. Gann Mono Can Res. 1992; 39: 1-15. [Google Scholar]
- 6.Karube K, Ohshima K, Tsuchiya T, et al. Expression of FoxP3, a key molecule in CD4 + CD25 + regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol. 2004; 126: 81-84. [DOI] [PubMed] [Google Scholar]
- 7.Hicklin DJ, Wang Z, Arienti F, et al. beta2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J Clin Invest. 1998; 101: 2720-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Momburg F, Möller P, Moldenhauer G, Hämmerling GJ. Loss of HLA-A,B,C in colorectal carcinoma is related to the degree of de-differentiation. J Immunogenet. 1986; 13: 195-199. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015; 47: 1304-1315. [DOI] [PubMed] [Google Scholar]
- 10.Asano N, Miyoshi H, Kato T, et al. Expression pattern of immunosurveillance-related antigen in adult T cell leukaemia/lymphoma. Histopathology. 2018; 72: 945-954. [DOI] [PubMed] [Google Scholar]
- 11.Sconocchia G, Eppenberger-Castori S, Zlobec I, et al. HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia. 2014; 16: 31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteban F, Ruiz-Cabello F, Concha A, et al. HLA-DR expression is associated with excellent prognosis in squamous cell carcinoma of the larynx. Clin Exp Metastasis. 1990; 8: 319-328. [DOI] [PubMed] [Google Scholar]
- 13.Rimsza LM, Roberts RA, Miller TP, et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood. 2004; 103: 4251-4258. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Rozeboom L, Rivard CJ, et al. MHC class II expression in lung cancer. Lung Cancer. 2017; 112: 75-80. [DOI] [PubMed] [Google Scholar]
- 15.Hemon P, Jean-Louis F, Ramgolam K, et al. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol. 2011; 186: 5173-5183. [DOI] [PubMed] [Google Scholar]
- 16.Holling TM, Schooten E, Langerak AW, van den Elsen PJ. Regulation of MHC class II expression in human T-cell malignancies. Blood. 2004; 103: 1438-1444. [DOI] [PubMed] [Google Scholar]
- 17.Shirono K, Hattori T, Hata H, Nishimura H, Takatsuki K. Profiles of expression of activated cell antigens on peripheral blood and lymph node cells from different clinical stages of adult T-cell leukemia. Blood. 1989; 73: 1664-1671. [PubMed] [Google Scholar]
- 18.Takeuchi M, Miyoshi H, Asano N, et al. Human leukocyte antigen class II expression is a good prognostic factor in adult T-cell leukemia/lymphoma. Haematologica. 2019; 104: 1626-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of β2-Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011; 20: 728-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palomero T, Couronné L, Khiabanian H, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014; 46: 166-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida N, Karube K, Utsunomiya A, et al. Molecular characterization of chronic-type adult T-cell leukemia/lymphoma. Cancer Res. 2014; 74: 6129-6138. [DOI] [PubMed] [Google Scholar]
- 22.Kataoka K, Shiraishi Y, Takeda Y, et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 2016; 534: 402-406. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi H, Kiyasu J, Kato T, et al. PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood. 2016; 128: 1374-1381. [DOI] [PubMed] [Google Scholar]
- 24.Yasinska IM, Sakhnevych SS, Pavlova L, et al. The Tim-3-Galectin-9 pathway and its regulatory mechanisms in human breast cancer. Front Immunol. 2019; 10: 1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horlad H, Ohnishi K, Ma C, et al. TIM-3 expression in lymphoma cells predicts chemoresistance in patients with adult T-cell leukemia/lymphoma. Oncol Lett. 2016; 12: 1519-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi M, Miyoshi H, Nakashima K, et al. Comprehensive immunohistochemical analysis of immune checkpoint molecules in adult T cell leukemia/lymphoma. Ann Hematol. 2020; 99: 1093-1098. [DOI] [PubMed] [Google Scholar]
- 27.Fu Y, Lin Q, Zhang Z, Zhang L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm Sin B. 2020; 10: 414-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito M, Tanaka R, Arishima S, et al. Increased expression of OX40 is associated with progressive disease in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Retrovirology. 2013; 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004; 21: 401-413. [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013; 13: 227-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallois D, Dupuy A, Lemonnier F, et al. RNA fusions involving CD28 are rare in peripheral T-cell lymphomas and concentrate mainly in those derived from follicular helper T cells. Haematologica. 2018; 103: e360-e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida N, Shigemori K, Donaldson N, et al. Genomic landscape of young ATLL patients identifies frequent targetable CD28 fusions. Blood. 2020; 135: 1467-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakamoto Y, Ishida T, Masaki A, et al. Clinical significance of CD28 gene-related activating alterations in adult T-cell leukaemia/lymphoma. Br J Haematol. 2021; 192: 281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao NB, Lü MH, Fan YH, et al. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012; 2012: 948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komohara Y, Niino D, Saito Y, et al. Clinical significance of CD 163 + tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci. 2013; 104: 945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Lu S, Xu Y, et al. Overexpression of CD47 predicts poor prognosis and promotes cancer cell invasion in high-grade serous ovarian carcinoma. Am J Transl Res. 2017; 9: 2901-2910. [PMC free article] [PubMed] [Google Scholar]
- 37.Barrera L, Montes-Servín E, Hernandez-Martinez JM, et al. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer. 2017; 117: 385-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagida E, Miyoshi H, Takeuchi M, et al. Clinicopathological analysis of immunohistochemical expression of CD47 and SIRPα in adult T-cell leukemia/lymphoma. Hematol Oncol. 2020; 38: 680-688. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa H, Nomura T, Kohno M, et al. Increased chemokine receptor CCR7/EBI1 expression enhances the infiltration of lymphoid organs by adult T-cell leukemia cells. Blood. 2000; 95: 30-38. [PubMed] [Google Scholar]
- 40.Shimizu K, Karube K, Arakawa F, et al. Upregulation of CC chemokine ligand 18 and downregulation of CX3C chemokine receptor 1 expression in human T-cell leukemia virus type 1-associated lymph node lesions: Results of chemokine and chemokine receptor DNA chip analysis. Cancer Sci. 2007; 98: 1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korbecki J, Olbromski M, Dzięgiel P. CCL18 in the progression of cancer. Int J Mol Sci. 2020; 21: 7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura M, Umehara H, Nakayama T, et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002; 168: 6173-6180. [DOI] [PubMed] [Google Scholar]
- 43.Ratner L, Waldmann TA, Janakiram M, Brammer JE. Rapid progression of adult T-cell leukemia–lymphoma after PD-1 inhibitor therapy. N Engl J Med. 2018; 378: 1947-1948. [DOI] [PubMed] [Google Scholar]
- 44.Ishitsuka K, Utsunomiya A, Ishida T. PD-1 inhibitor therapy in adult T-cell leukemia–lymphoma. N Engl J Med. 2018; 379: 695-697. [DOI] [PubMed] [Google Scholar]
- 45.Roemer MGM, Redd RA, Cader FZ, et al. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. J Clin Oncol. 2018; 36: 942-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson DB, Estrada MV, Salgado R, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun. 2016; 7: 10582. [DOI] [PMC free article] [PubMed] [Google Scholar]


