Summary
Background
The hematopoietic stem cell disorders, myeloproliferative neoplasms (MPNs), are characterised by chronic low-grade inflammation (CLI). Recently, we showed that patients with MPNs have an increased prevalence of drusen and age-related macular degeneration (AMD), and drusen prevalence seemed associated with higher CLI. Studying MPNs may reveal more about drusen pathophysiology. This study investigated CLI further by measuring cytokine levels and complement system markers, comparing these between patients with MPNs and AMD.
Methods
This cross-sectional study, between July 2018 and November 2020 conducted at Zealand University Hospital (ZUH) – Roskilde, Denmark, included 29 patients with neovascular AMD (nAMD), 28 with intermediate-stage AMD (iAMD), 62 with MPNs (35 with drusen - MPNd and 27 with healthy retinas - MPNn). With flow cytometry, we measured complement-regulatory-proteins (Cregs). With immunoassays, we investigated cytokine levels combined into a summary-inflammation-score (SIS).
Findings
The MPNd and nAMD groups had similar SIS, significantly higher than the MPNn and iAMD groups. Additionally, we found SIS to increase over the MPN biological continuum from early cancer stage, essential thrombocytaemia (ET), over polycythaemia vera (PV) to the late-stage primary myelofibrosis (PMF). MPNs showed signs of complement dysregulation, with Cregs expression lower in PV than ET and PMF and even lower in PV patients with drusen.
Interpretation
This study suggests that MPNd have a higher CLI than MPNn and may indicate systemic CLI to play a greater part in, and even initiate drusen formation. We suggest using MPNs as a “Human Inflammation Model” of drusen development. The CLI in MPNs elicits drusen formation, triggering more CLI creating a vicious cycle, increasing the risk of developing AMD.
Funding
Fight for Sight, Denmark, and Region Zealand's research promotion fund.
Keywords: Myeloproliferative neoplasms, “Human inflammation model”, Biological continuum, Age-related macular degeneration, Chronic low-grade inflammation, Complement dysregulation, Drusen, Complement regulatory proteins, Cytokines, Growth factors, Anaphylatoxins
Research in context.
Evidence before this study
Evidence indicates a role of the immune system and chronic inflammation in the pathogenesis of the eye disease age-related macular degeneration (AMD). In a previous study, we found that patients with myeloproliferative neoplasms (MPNs), have a significantly higher drusen and AMD prevalence than the general population. MPN patients with drusen had a higher neutrophil-to-lymphocyte ratio than in MPNs with normal retinas, indicating an association between drusen prevalence and a higher degree of chronic low-grade inflammation (CLI).
Added value of this study
In this study we further investigated CLI, by measuring cytokine levels and complement system markers and we also investigated the presence of relevant single nucleotide polymorphisms (SNPs) previously shown to increase the risk of developing AMD. The results indicate a higher level of CLI in patients with MPNs and drusen than patients with MPNs and normal retinas, and also an inadequate regulation of the complement system in patients with MPNs. The SNPs investigated are not more common in patients with MPNs and cannot explain the higher prevalence of drusen.
Implications of all the available evidence
The study suggests that the inflammatory state present in MPN patients leads to drusen formation. We propose using the MPNs as a “Human Inflammation Model” on drusen development: The CLI in MPNs elicits drusen formation, triggering more CLI creating a vicious cycle with more drusen and, as a result, an increased risk of developing AMD. Further studying patients with MPNs has the potential to reveal more of the pathogenesis of AMD.
Alt-text: Unlabelled box
Introduction
Age-related macular degeneration (AMD) is a common retinal disease causing central vision loss. The early stages, which often progress slowly and asymptomatically, are characterized by extracellular deposits (drusen) between the retinal pigment epithelium (RPE) and Bruch's membrane (BM). When drusen reach a specific size and/or pigmentary abnormalities are present, the condition is termed intermediate-stage AMD. Late-stage AMD can be either neovascular or atrophic.1 The neovascular form (nAMD) shows abnormal blood vessel growth, with the formation of new and fragile blood vessels, leaking blood and proteins into the retina, often resulting in severe and rapid vision loss. The atrophic form, geographic atrophy (GA), often progresses more slowly and is characterized by areas of retinal atrophy.
The AMD pathogenesis is not entirely understood, but AMD is a multifactorial disease involving genetic and environmental risk factors, and changes in different immunological pathways have been implicated in the pathogenesis.2 Complement system dysregulation both within the eye and systemically is one such example of immunological changes that have been well-established as a contributor to AMD pathogenesis.3,4,13,14,5, 6, 7, 8, 9, 10, 11, 12
Besides constituting the first line of defence against infections, the complement system functions as an immunoregulatory system playing a part in adaptive immunity. Plasma complement-proteins circulate in the bloodstream in an inactive form and can be activated in several ways, interact with each other, and activate different complement pathways (the classical-, alternative-, and lectin pathway). All pathways lead to the activation of C3 convertase with C3a and C3b creation, and activation causes different effector activities: inflammation, phagocytosis, and membrane-attack-complex (MAC) formation. Complement regulatory proteins (Cregs) regulate complement activation to prevent the potentially dangerous and destructive effects of complement activation and thereby regulate inflammation.15,16 Studies show novel functions for Cregs in regulating the adaptive immune system and their involvement in the pathophysiology of several chronic inflammatory diseases.17, 18, 19 Our group and others have shown systemic Cregs dysregulation on monocytes in AMD patients compared to healthy controls.6,20 Several studies have shown elevated levels of complement components, and associations between AMD and single nucleotide polymorphisms (SNPs) in genes encoding complement proteins and regulators have been documented. Further, by-products of chronic inflammatory events, including the complement system, are thought to play a role in drusen formation since various inflammatory mediators, including complement products, have been identified in drusen.9,21, 22, 23, 24 We have recently shown that patients with Philadelphia-negative myeloproliferative neoplasms (MPNs) have a much higher prevalence of late AMD and an accelerated accumulation of drusen from an earlier age. The drusen accumulation was associated with a higher neutrophil-to-lymphocyte ratio (NLR), indicating a higher level of chronic low-grade inflammation (CLI).25,26 The MPNs are chronic hematopoietic stem cell disorders encompassing essential thrombocythaemia (ET), polycythaemia vera (PV), and primary myelofibrosis (PMF).27 It is suggested that the diseases evolve in a biological continuum from the early cancer stage (ET) to the late advanced myelofibrosis stage. Rising levels of inflammatory cytokines and growth factors are recorded in this continuum, accompanied by an increase in comorbidity burden. It has been proposed that MPNs should be considered inflammatory diseases, and a “Human inflammation Model” describing chronic inflammation as both an initiator and driver of the diseases has been developed.28, 29, 30, 31, 32 This model makes the MPNs unique as model diseases for elucidating mechanisms and associations between chronic inflammation and disease development. Most lately, the MPNs have been described as a human neuroinflammation model for the development of Alzheimer's disease.33 Accordingly, it is intriguing to use MPNs as a “Human inflammation model” on drusen/AMD development as well, implying CLI in MPNs to elicit drusen formation, which “triggers” more CLI, creating a vicious cycle.
This study aimed to study CLI and the complement system further in the MPNs with drusen (MPNd) and without drusen (MPNn), in MPN subtypes, and compare the results with patients having AMD. These comparative studies could potentially tell us more about drusen pathophysiology. We investigated if the inflammatory cytokine- and growth factor levels as combined summary-inflammation-scores (SIS) and summary-growth-factor-scores (SGS) are higher in MPNd than MPNn. We also explored if complement dysregulation is present in patients with MPNs as seen for AMD. This was done by exploring differences between MPNd and MPNn regarding complement activation, Cregs expression (CD35 and CD59), and the presence of relevant SNPs (CFH rs1061170, C3 rs2230199). The anaphylatoxins C3a and C5a were used as candidates for the evaluation of complement activation.
Methods
Study design and participants
This cross-sectional study was approved by the Ethics Committee, Region Zealand, Denmark. The study adhered to the tenets of the Declaration of Helsinki. We also adhered to the guidelines stated in the “STrengthening the Reporting of OBservational studies in Epidemiology” (STROBE).34 The study includes 63 patients with MPNs, a subgroup of patients from our previous work25 chosen because they did not receive immunomodulating treatment, which was one of the exclusion criteria. The participants included four patient types. According to the Beckman Classification,1 we included patients with nAMD (n = 30) and iAMD (n = 30). According to the WHO2016 criteria,35 we included patients with Philadelphia-negative MPNs: one group with drusen having early AMD or iAMD (MPNd, n = 35) and one group having normal retinas (MPNn, n = 28). We obtained written and oral informed consent from each participant. The study was conducted at Zealand University Hospital (ZUH) – Roskilde, Denmark. We consecutively included participants from the outpatient programs at the ophthalmology and haematology departments between July 2018 and November 2020.
Exclusion criteria were patients with other active cancer, inflammatory- or autoimmune diseases, patients receiving immunomodulating treatment (ruxolitinib, interferon-α), CRP levels >15, and anti-VEGF therapy injection within the last eight weeks.
Retinal imaging and clinical data
We examined all participants at the ophthalmology department. Following pupil dilation with tropicamide 1%, we obtained a stereoscopic 45° colour fundus photograph centred on the macula (model TRG-NW8, Topcon). We used the digital fundus photographs and a simplified version of the Wisconsin age-related maculopathy grading system (WARMGS)25,36 to determine AMD status. Each participant was subjected to a simple questionnaire regarding their health status, medical conditions, medication, and lifestyle.
Blood sampling
We sampled venous blood from antecubital veins from each participant. We used ethylenediaminetetraacetic-acid-coated (EDTA) tubes for flow cytometric and SNP analyses, lithium-heparin-coated tubes for CRP analysis and isolation of plasma, and tubes with silica-act-clot-activator to isolate serum. Part of the blood, plasma, and serum was immediately stored frozen at −80 °C for later use (immunoassays and SNP analyses).
Flow cytometry
Flow cytometric analyses were done within 4 h. The EDTA-stabilized blood was analysed for white blood cell count on a Sysmex KX-21NTM (Sysmex Corporation). We prepared cells for flow cytometry by the following protocol: The white blood cell count was used to obtain 1·0 × 106 white blood cells in the test tube. Erythrocytes were lysed in a 1% lysis buffer (Nordic BioSite AB, Täby, Sweden), and the white blood cells were washed three times with isotonic buffer (BD FACSFlow; BD Biosciences, Franklin Lakes, NJ, USA), centrifuged for 5 min at 500 xg, and resuspended in isotonic buffer. We added monoclonal anti-human antibodies in the next step, and cells were incubated for 20 min in the dark at room temperature. From R&D Systems, Inc., Minneapolis, MN, USA, we used; Peridinin-chlorophyll-protein (PerCP) CD4 IgG2a (FAB3791C), and Allophycocyanin (APC) CD35 IgG1 (FAB5748A). From BioLegend, San Diego, CA, USA, we used; Brilliant Violet V510 CD8 IgG1 κ (301,048), Pacific Blue CD14 IgG1 (315,616), APC/Cyanine7 (Cy7) CD16 (302,018), and PE CD59 IgG2a (304,708). We used fluorochrome-matched negative isotype controls: from BioLegend, APC IgG1 (400,120); and from BD Biosciences; PE IgG2a (A09141). Finally, we washed the stained cells and resuspended them in the isotonic buffer. Immediately after that, we ran/analysed the cells on a flow cytometer (BD FACSCanto II; BD Biosciences) with a gating size of 100,000 singlet leukocytes. We analysed the flow data with Kaluza Analysis v. 2.1 (Beckman Coulter), blinded to the types and conditions of the participants. Cell height and area were used to gate singlets, size, and granularity. Forward scatter/side scatter were used to gate monocytes and lymphocytes. We further used markers for lymphocytes CD4 and CD8 to differentiate CD4+ T-cells and CD8+ T-cells and markers of monocytes, CD14 and CD16, to differentiate monocyte subsets in classical monocytes (CD14high/CD16low), intermediate monocytes (CD14high/CD16high), and non-classical monocytes (CD14low/CD16high). Finally, we investigated the expression of the markers CD35 and CD59 on the different subsets of cells. Negative isotype controls were used to eliminate nonspecific signalling and were set to a threshold of 1%. Results from flow cytometry are shown as the percentage of positive cells.
Immunoassays
Cytokines, anaphylatoxins, and growth factors were quantified with multiplex immunoassays (Meso Scale Discovery) at the Technical University of Denmark (DTU). The plates were prepared according to the manufacturer's instructions with prior identification of the dilution factor. The manufacturer's software was used to create a standard curve (plotting mean absorbence against protein concentration) from a standard added to each plate. This standard curve was used to determine target protein concentration. All tests were run in duplicates to determine the mean concentration, and the coefficient of variation (CV) was calculated as the ratio of standard deviation to the mean. The mean CV values were between 1·9–7·3 for all assays. Plate reading was done immediately after preparation on a QuickPlex SQ120 (Meso Scale Discovery).
Genotyping/SNP analyses
Genomic DNA extraction was done at the Kennedy Centre, Denmark, with Chemagic Magnetic Separation Module 1 (Chemagen). SNP genotyping was performed at BioXpedia, Denmark, with the Fluidigm 96.96 Dynamic Array Integrated Fluidic Circuit (Fluidigm Corp). The assays were performed according to the manufacturer's protocol. Data were imported to the Fluidigm SNP Genotyping Analysis software v.4.5.1 and analysed with the standard settings.
Statistics
We analysed data with RStudio version 4.1.1. Normally distributed data are shown as mean and 95% confidence interval (CI); non-normally distributed data as median and interquartile range (IQR). The distribution of continuous variables was assessed for normality with histograms and QQ-plots. For comparison between groups, we used the independent samples t-test, Wilcoxon's rank-sum test, One-way analysis of variance (ANOVA), the two-way ANOVA or Kruskal Wallis test for continuous variables, and the Chi-squared test or Fisher's Exact test for categorical variables. Levene's test was used to test the equality of variances for normally distributed data, where ANOVA was used. We used linear regression or robust linear regression where appropriate to test if the outcomes depended on age.
SIS and SGS were calculated to show an overall change in cytokine- and growth factor levels. We calculated a z-score for each marker and averaged the scores to obtain a summary score for each patient. To evaluate the size of the observed differences in the summary scores, we calculated Cohen's d, the ratio between the group difference and the standard deviation. The effect size was interpreted as small if 0·2, moderate if 0·5, and large if 0·8.
Statistical significance is defined as p<0·05. In all figures and tables, “n” represents the number of humans in the groups tested. The tests used are described in the footnotes of the tables.
Since the comparisons between patients with MPNs have not been made before, we based our power calculation on similar comparative immunologic studies on nAMD and previous levels of the outcomes. This exercise ended in a sample size of 26 in each group to detect a difference in variables between groups of at least 20%, with an alpha level of 0·05 and a power of 80%. We, therefore, aimed for 30 in each group.37, 38, 39
Role of the funding source
The funding sources had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The corresponding author confirms that she had full access to all the data in the study and was responsible for submitting it for publication.
Results
Study population
Table 1 shows patient characteristics. One-hundred-twenty-three patients were included in the study. One nAMD patient was excluded post hoc because of a high CRP (>15 mg/L), indicating an ongoing acute immune response. Two iAMD patients were excluded because they had atrophy lesions fulfilling GA criteria. One MPNn patient was excluded because the flow cytometric analyses failed, resulting in 119 patients: 29 nAMD, 28 iAMD, 35 MPNd, and 27 MPNn. The nAMD patients had a median age of 77 years, significantly older than 73 years in iAMD (p = 0·034), 72 years in MPNd (p = 0·0040), and 69 years in MPNn (p<0·001). We found no differences between the groups regarding sex, smoking habits, body mass index, and comorbidities, but the MPNd group had a significantly higher alcohol consumption in units per week than the other groups (nAMD: p<0·001·, iAMD: p = 0·019, MPNn: p = 0·021). Thirty-nine patients with MPNs had PV, 17 had ET, and six had PMF. Most patients with MPNs had the JAK2V617F-mutation, fewer mutations in the calreticulin (CALR) gene or the gene encoding the thrombopoietin receptor (MPL). There was no difference in allele burden between the two MPN groups (p = 0·62). The patients with MPNs were all receiving acetylsalicylic acid. Twenty-seven MPNd and 20 MPNn were receiving hydroxyurea (HU), and the distribution in HU treated versus non-HU was similar in the two groups (p = 0·98). Patients receiving statins were similar across all groups (p-value=0·58).
Table 1.
Characteristics of the participants.
| nAMD (n = 29) | iAMD (n = 28) | MPNd (n = 35) | MPNn (n = 27) | p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years, median (IQR) | 77 (71–82) | 73 (68–76) | 72 (65–76) | 69 (62–74) | <0•001a |
| Sex | 0•28b | ||||
| Males, n (%) | 12 (41) | 10 (36) | 20 (57) | 10 (37) | |
| Females, n (%) | 17 (59) | 18 (64) | 15 (43) | 17 (63) | |
| Lifestyle factors | |||||
| Smoking, n (%) | 0•83c | ||||
| Never | 12 (41) | 12 (43) | 16 (46) | 11 (41) | |
| Former | 13 (45) | 13 (46) | 18 (51) | 14 (52) | |
| Current | 4 (14) | 3 (11) | 1 (3) | 2 (7) | |
| Body mass index, mean (95%CI) | 26 (24–27) | 25 (24–27) | 25 (24–27) | 27 (25–29) | 0•48d |
| Alcohol consumption, units per week, median (IQR) | 2 (0–7) | 3 (0–7) | 7 (2–14) | 2 (0–8) | 0•0036a |
| Comorbidities | |||||
| Cardiovascular disease, n (%) | 4 (14) | 5 (18) | 6 (17) | 6 (22) | 0•89c |
| hypertension, n (%) | 13 (45) | 8 (29) | 18 (51) | 17 (63) | 0•075b |
| hypercholesterolaemia, n (%) | 4 (14) | 2 (7) | 3 (9) | 2 (7) | 0•82c |
| Type 2 diabetes, n (%) | 2(7) | 1 (4) | 2 (6) | 0 (0) | 0•76c |
| MPN diagnosis (MPN patients only) | – | – | 0•082b | ||
| Essential thrombocythemia, n (%) | – | – | 6 (17) | 11 (41) | |
| Polycythemia vera, n (%) | – | – | 26 (74) | 13 (48) | |
| Pre-PMF, n (%) | – | – | 0 (0) | 1 (4) | |
| Primary myelofibrosis, n (%) | – | – | 3 (9) | 2 (7) | |
| Mutation status (MPN patients only) | – | – | 0•43c | ||
| JAK2V617F, n (%) | – | – | 31 (91) | 22 (82) | |
| CALR mutation, n (%) | – | – | 1 (3) | 3 (11) | |
| MPL mutation, n (%) | – | – | 1 (3) | 0 (0) | |
| Triple-Negative, n (%) | 1 (3) | 2 (7) | |||
| JAK2V617 allele burden | 0•61c | ||||
| 1–25%, n (%) | 15 (47) | 14 (67) | |||
| >25–50%, n (%) | 8 (25) | 3 (14) | |||
| >50–75%, n (%) | 7 (22) | 3 (14) | |||
| >75%, n (%) | 2 (6) | 1 (5) | |||
| Overall JAK2V617F allele burden%, median (IQR)(MPN patients only) | 33 (11–56) | 17 (6–28) | 0.089a |
P-values in bold are significant. Statistical comparisons between groups:
Kruskal Wallis test
Pearson's Chi-squared test
Fischer's exact test.
One-way ANOVA. AMD: age-related macular degeneration, nAMD: neovascular AMD, iAMD: intermediate AMD, MPN: myeloproliferative neoplasms, MPNd: Patients with MPN and drusen, MPNn: patients with MPN and normal retinas, IQR: interquartile range, PV: polycythaemia vera, ET: essential thrombocythemia, PreMF: pre-myelofibrosis, PMF: primary myelofibrosis, JAK2V617F: mutation in the JAK2 gene, CALR: calreticulin gene, MPL: MPL gene, the gene encoding the thrombopoietin receptor.
Inflammatory markers
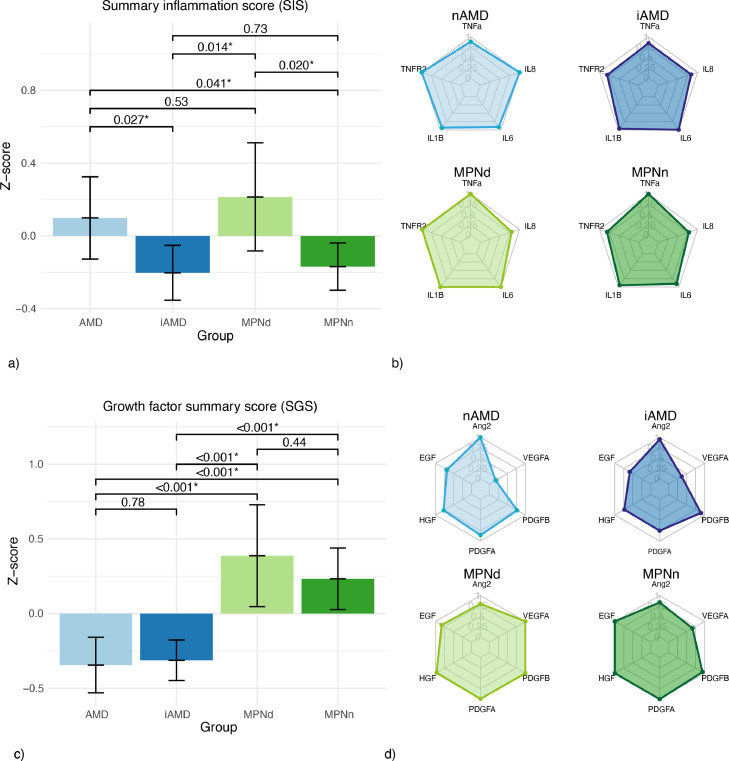
There was no statistically significant difference between the two MPN groups in levels of pro-inflammatory cytokines, growth factors, or anaphylatoxins, but there was a significant difference in SIS (p = 0·020) (Table 2/ Figure. 1a). MPNd SIS was 0·21 compared to −0·17 for MPNn; the difference had a moderate size (Cohen's d: 0·58). There was no difference between the MPN groups in the SGS (p = 0·44) (Table 2/Figure. 1c). For MPN subtypes, we found a PMF SIS of 0·39 and PV SIS of 0·05, both significantly higher than ET patients with −0·27 (p = 0·038, p = 0·014), table 4. The differences had a moderate size between PV-PMF and ET-PV (Cohen's d: 0·50 and 0·60) and a large size between ET-PMF (Cohen's d: 1·52)
Table 2.
Levels of pro-inflammatory markers and summary scores between patient groups.
| Pro-inflammatory markers | nAMD (n = 29) | iAMD (n = 28) | MPNd (n = 35) | MPNn (n = 27) | p-value 1 testing between MPN groups | P-value 2 testing between all groups |
|---|---|---|---|---|---|---|
| Pro-inflammatory cytokines (serum) | ||||||
| TNF-α pg/mL, median (IQR) | 1·4 (1·2–1·6) | 1·4 (1·2–1·7) | 1·6 (1·4–2·1) | 1·6 (1·5–1·9) | 0·60a | 0·0083b |
| TNF-RII ng/mL, median (IQR)f | 15·4 (13·1–18·5) | 12 (11·1–14) | 15 (12–18) | 13 (10–18) | 0·12a | 0·0054b |
| IL-1β pg/mL, median (IQR) | 0·59 (0·57–0·64) | 0·6 (0·5–0·7) | 0·63 (0·57–0·72) | 0·60 (0·55–0·65) | 0·17a | 0·41b |
| IL-6 pg/mL, median (IQR) | 1·2 (1·0–2·1) | 1·3 (1·1–2·0) | 1·4 (0·90–1·8) | 1·2 (1·0–1·5) | 0·70a | 0·62b |
| IL-8 pg/mL, median (IQR) | 12 (8·9–15) | 9·6 (7·5–12) | 9·5 (6·7–14) | 9·0 (7·5–14) | 0·92a | 0·17b |
| IL-11 pg/mL, median (IQR)e | – | – | – | – | – | |
| Anaphylatoxins (plasma) | ||||||
| C3a ng/mL, median (IQR) | 917 (752–1111) | 1031 (797–1238) | 972 (629–1227) | 855 (741–1063) | 0·82a | 0·78b |
| C5a ng/mL· median (IQR) | 4·0 (3·2–5·0) | 3·3 (2·8–3·2) | 3·4 (2·5–4·3) | 3·6 (3·0–4·1) | 0·78a | 0·16b |
| Growth Factors (serum) | ||||||
| Ang-2 ng/mL, median (IQR) | 1·4 (1·1–1·8) | 1·4 (1·1–1·8) | 1·2 (0·90–1·5) | 1·2 (1·1–1·5) | 0·35 | 0·098b |
| EGF ng/mL, median (IQR) | 0·33 (0·21–0·48) | 0·28 (0·25–0·42) | 0·40 (0·27–0·52) | 0·48 (0·36–0·59) | 0·16a | 0·021b |
| HGF ng/mL, median (IQR) | 0·63 (0·58–0·75) | 0·59 (0·48–0·70) | 0·79 (0·63–1·1) | 0·81 (0·72–0·96) | 0·76a | <0·001b |
| PDGF-A ng/mL, median (IQR) | 26 (20–31) | 22 (18–28) | 30 (24–40·) | 30 (26–39) | 0·99a | 0·0010b |
| PDGF-B ng/mL, median (IQR) | 3·2 (2·6–4·1) | 3·6 (2·9–4·4) | 4·0 (3·3–5·7) | 3·8 (2·3–5·4) | 0·18a | 0·049b |
| VEGF-A, ng/mL, median (IQR) | 0·08 (0·01–0·21) | 0·16 (0·11–0·28) | 0·45 (0·20–0·63) | 0·30 (0·18–0·46) | 0·26a | <0·001b |
| Chronic inflammation | ||||||
| Inflammation summary score, mean (95%CI) | 0·10 (−0·13–0·32) | −0·20 (−0·35:−0·05) | 0·21 (−0·08–0·51) | −0·17 (−0·30:−0·04) | 0·020c | 0·018d |
| Neutrophil-to-lymphocyte ratio (IQR) | 2·5 (1·9–3·6) | 2·1 (1·5–2·7) | 3·8 (2·8–5·1) | 2·4 (1·9–3·7) | 0·0090a | <0·001b |
| Growth factor summary score, mean (95%CI) | −0·34 (−0·53:−0·16) | −0·31 (−0·45:−0·18) | 0·39 (0·05–0·73) | 0·23 (0·03–0·44) | 0·44c | <0·001d |
P-values in bold are significant. Statistical test for comparisons: aWilcoxon rank-sum test.
Wilcoxon rank-sum test.
Kruskal Wallis test.
independent samples t-test.
One way ANOVA.
concentrations of IL-11 were below detection level.
TNF-RII is the TNF-α receptor and a more stable marker than TNF-α.
TNF-α: tumour necrosis factor alfa, TNF-RII: TNF−α receptor 2; IL: interleukins. Ang-2: Angiopoietin-2, EGF: epidermal growth factor, HGF: hepatocyte growth factor, PDGF-A: platelet-derived growth factor A, PDGF-B: platelet-derived growth factor B, VEGF-A: vascular endothelial growth factor A. AMD: age-related macular degeneration, iAMD: intermediate age-related macular degeneration, MPNd: patients with myeloproliferative neoplasms and signs of AMD, MPNn: patients with myeloproliferative neoplasms and normal retinas.
Figure 1.
Pro-inflammatory markers
Comparisons between patients with nAMD (n = 29), iAMD (n = 28), MPNd (n = 35), MPNn (n = 27)
a) Plot of summary inflammation score = [z score (IL6) + z score (IL8) + z score (TNF-R2) + z score (TNF-a) + z score (IL-1b)] - with 95% CIbars. b) Radar plots of standardized levels of pro-inflammatory markers. Patients with MPNn have a shape similar to patients with iAMD, where patients with AMD and MPNd look more alike. c) Plot of summary growth factor score = [z score (Ang2) + z score (VEGF-A) + z score (PDGF-B) + z score (PDGF-A) + z score (HGF) + z score (EGF)]) - with 95% CIbars. d) Radar plots of standardized levels of growth factors. Patients with MPNd and MPNn have a similar shape and higher levels of all growth factors than patients with AMD and iAMD.
AMD: age-related macular degeneration, nAMD: neovascular AMD, iAMD: intermediate age-related macular degeneration, MPNd: patients with myeloproliferative neoplasms and signs of AMD, MPNn: patients with MPNs and normal retinas.
TNF-a: tumour necrosis factor alfa, TNF-RII TNF-a receptor II; IL: interleukin, Ang-2: Angiopoietin-2, EGF: epidermal growth factor, HGF: hepatocyte growth factor, PDGF-A: platelet-derived growth factor A, PDGF-B: platelet-derived growth factor B, VEGF-A: vascular endothelial growth factor A.
Table 4.
Monocyte distribution and expression of CD35 and CD59 on monocytes and markers of inflammation in MPN subgroups regardless of signs of AMD.
| ET (n = 17) | PV (n = 39) | PMF (n = 6) | p-value ET vs PV | p-value PV vs PMF | p-value ET vs PMF | |
|---|---|---|---|---|---|---|
| Monocytes,%, median (IQR) | 3·0 (2·4–3·7) | 2·8 (2·3–3·8) | 2·7 (1·4–4·8) | 0·92a | 0·78a | 0·97a |
| CD35%, median (IQR) | 69 (66–84) | 78 (73–82) | 69 (65–74) | 0·38a | 0·11a | 0·66a |
| CD59%, median (IQR) | 70 (56–81) | 48 (32–67) | 77 (65–90) | 0·027a | 0·016a | 0·23a |
| Classical monocytes (CD14highCD16low),% median (IQR) | 78 (75–88) | 77 (71–85) | 69 (68–83) | 0·22a | 0·54a | 0·20a |
| CD35%, median (IQR) | 87 (75–94) | 91 (82–94) | 88 (80–92) | 0·29a | 0·55a | 1·0a |
| CD59%, median (IQR) | 72 (56–90) | 51 (34–73) | 91 (70–98) | 0·021a | 0·0070a | 0·13a |
| Intermediate monocytes (CD14highCD16high),% mean (95%CI) | 9·8 (6·6–14) | 13 (8·3–18) | 8·4 (6·2–15) | 0·21b | 0·46b | 0·97b |
| CD35%, median (IQR) | 46 (36·−55·) | 51 (36–64) | 42 (24–56) | 0·55a | 0·22a | 0·43a |
| CD59%, median (IQR) | 64 (51–83) | 46 (30–61) | 70 (68–85) | 0·010a | 0·029a | 0·35a |
| Non-classical monocytes (CD14lowCD16high)% mean (95%CI) | 7·0 (5·3–11) | 7·5 (5·9–10) | 12 (7·7–20) | 0·85b | 0·16b | 0·20b |
| CD35%, median (IQR) | 3·3 (1·4–4·8) | 3·1 (1·3–9·2) | 2·5 (1·7–3·4) | 0·67a | 0·38a | 0·52a |
| CD59%, median (IQR) | 30 (19–41) | 16 (13–32) | 36 (29–44) | 0·069a | 0·024a | 0·23a |
| Chronic inflammation | ||||||
| Inflammation summary score, mean (95%CI) | −0·27 (−0·38:−0·17) | 0·057 (−0·18–0·30) | 0·39 (−0·22–1·0) | 0·014b | 0·25b | 0·038b |
| Neutrophil-to-lymphocyte ratio (IQR) | 2·0 (1·8–2·6) | 3·6 (26–5·7) | 3·7 (2·8–4·4) | <0·001a | 0·23a | 0·049a |
| Growth factor summary score, mean (95%CI) | 0·033 (−0·23–0·29) | 0·023 (−0·24–0·28) | −0·045 (−0·29–0·20) | 0·95b | 0·69b | 0·62b |
P-values in bold are significant. Statistical comparisons between groups:
Wilcoxon rank-sum test.
independent samples t-test.
IQR: interquartile range; 95% CI: 95% confidence interval; ET: essential thrombocythemia; PV: polycythaemia vera, PMF: primary myelofibrosis.
To compare cytokine and growth factor levels in MPNs and AMD, we created radar plots (Figure. 1b+1d) to visually compare the groups' levels, and we also compared SIS and SGS (Figure. 1a+1c). The MPNd SIS resembled the SIS in nAMD of 0·10 and was significantly higher than in iAMD, −0·20 (p = 0·014) (Figure. 1a). The difference between MPNd and iAMD patients had a moderate size (Cohen's d: 0·62). Both the MPNd and MPNn had significantly higher SGS compared to nAMD and iAMD (all p-values <0·001) (Figure. 1c). The differences in SGS between MPNd-AMD, MPNd-iAMD, MPNn-AMD, and MPNn-iAMD were large (Cohen's d: 0·94, 0·94, 1·13, and 1·21).
The neutrophil-to-lymphocyte ratio (NLR), a marker of CLI, was published in our previous work for the two MPN groups.25 In this study, we observed that MPNd had a significantly higher NLR of 3·8 compared to all the other groups: nAMD 2·5 (p = 0·0070), iAMD 2·1 (<0·001), MPNn 2·4 (p = 0·0088). For MPN subtypes NLR was 2·0 for ET patients, significantly lower than in PV with 3·6 (p<0·001) and PMF 3·7 (p = 0·049), table 4.
Monocyte subsets and Cregs expression
Most monocytes in MPNd and MPNn were classical monocytes (77% and 82%), while 12% and 10% were intermediate, 8·7%, and 7·3% non-classical. The distributions did not differ significantly between the MPN groups (Table 3).
Table 3.
Distribution of monocytes and expression of CD35 and CD59 on monocytes and subsets.
| nAMD n = 29 | iAMD n = 28 | MPNd (n = 35) | MPNn (n = 27) | P-value 1 testing between MPN groups | P-value 2 testing between all groups | |
|---|---|---|---|---|---|---|
| Monocytes,%, median (IQR) | 3·7 (2·4–4·6) | 3·9 (2·7–4·9) | 2·8 (2·1–3·6) | 3·0 (2·1–3·4) | 0·67d | 0·073a |
| CD35%, median (IQR) | 72 (62–83) | 77 (68–82) | 76 (67–80) | 80 (67–85) | 0·30d | 0·58a |
| CD59%, median (IQR) | 72 (64–94) | 68 (54–78) | 50 (33–75) | 65 (49–80) | 0·13d | 0·0040a |
| Classical monocytes (CD14highCD16low),% median (IQR) | 83 (78–88) | 84 (80–86) | 77 (70–84) | 82 (75–88) | 0·10d | 0·016a |
| CD35%, median (IQR) | 83 (71–92) | 87 (79–93) | 91 (80–94) | 90 (80–93) | 0·73d | 0·45a |
| CD59%, median (IQR) | 82 (68–96) | 71 (56–83) | 53 (35–84) | 71 (53–82) | 0·20d | 0·0059a |
| Intermediate monocytes(CD14highCD16high),% mean (95%CI) | 6·8 (5·5–8·4) | 7·5 (6·1–9·1) | 12 (9·6–15) | 10 (8·3–13) | 0·31d | <0·001b |
| CD35%, median (IQR) | 40 (24–55) | 47 (27–61) | 45 (33–60) | 53 (37–60) | 0·49d | 0·22a |
| CD59%, median (IQR) | 69 (53–93) | 62 (50–81) | 49 (33–70) | 60 (44–81) | 0·16d | 0·013a |
| Non-classical monocytes (CD14lowCD16high)% mean (95%CI) | 7·6 (6·2–9·3) | 8·9 (7·7–10) | 8·4 (6·7–12) | 7·3 (5·8–9·2) | 0·48d | 0·13c |
| CD35%, median (IQR) | 2·2 (1·2–3·7) | 4·0 (1·8–6·2) | 3·4 (1·1–7·1) | 2·9 (1·5–5·2) | 0·92d | 0·34a |
| CD59%, median (IQR) | 26 (19–73) | 24 (19–36) | 18 (13–40) | 28 (18–42) | 0·16d | 0·062a |
P-values in bold are significant. Statistical comparisons between groups:
Kruskal Wallis test.
One-way ANOVA.
Two-way ANOVA.
Wilcoxon rank-sum test.
eindependent samples t-test
IQR: interquartile range; 95%CI: 95% confidence interval; MPN: myeloproliferative neoplasms; nAMD: neovascular age-related macular degeneration; iAMD: intermediate age-related macular degeneration.
We compared CD35 and CD59 expression on monocytes and subsets. We did not find statistically significant differences between the MPN groups (Table 3).
When evaluating MPN subtypes (Table 4), PV patients had a CD59 expression on monocytes of 48%, significantly lower than 70% in ET (p-value=0·027) and 77% in PMF (p = 0·016). There was no significant difference between MPN subtypes regarding CD35.
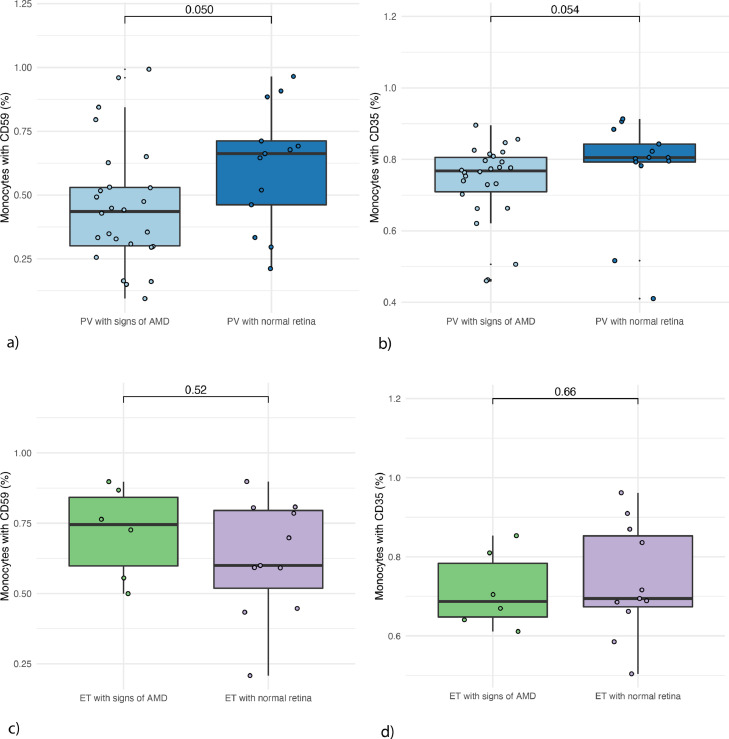
The PV group was large enough to subdivide into those with drusen (PVd, n = 26) and normal retinas (PVn, n = 13). PVd had a CD59 expression of 44%, significantly lower than PVn 66% (p-value=0·0500). There was a tendency for lower CD35 expression in PVd, 77%, than in PVn, 81% (p = 0·054). The SIS in PVd was 0·15 compared to PVn −0·27 (p = 0·024). The difference had a moderate size (Cohen's d: 0·70). We did not observe the same in ET patients (Figure. 2), but this group was small (17 patients).
Figure 2.
Expression of complement regulatory proteins CD35 and CD59 in patients with PV and ET (with and without AMD signs)
a) CD59 expression in patients with polycythaemia vera with drusen (n = 26) vs without drusen (n = 13) b) CD35 expression in patients with polycythaemia vera with drusen vs without drusen. c) CD59 expression in patients with essential thrombocythemia with drusen (n = 6) vs without drusen (n = 11). d) CD35 expression in patients with essential thrombocythemia with drusen vs without drusen.
PV: polycythaemia vera. ET: essential thrombocythemia. AMD: age-related macular degeneration.
Comparing MPNs with AMD, we found a statistically significant difference between the groups when we investigated monocyte subsets (Table 3). All p-values below are Bonferroni-corrected. The MPNd had a classical monocytes percentage of 77%, significantly lower than 89% in nAMD (p = 0·015) and 84% in iAMD (p = 0·071). Correspondingly MPNd had a higher intermediate monocytes percentage, 12%, compared to 6·8% in nAMD (p<0·001) and 7·5% in iAMD (0·0034). There was no difference between the groups in non-classical monocytes percentage. MPNd also expressed CD59 on monocytes of 50%, significantly lower than 72% in nAMD patients (p = 0·0040). The difference was also seen in classical- and intermediate monocytes (Table 3).
Single nucleotide polymorphisms
We found no statistical difference in CFH rs1061170 genotype distribution between MPN groups (p = 0·51). However, when comparing MPNs with AMD patients, we found significant differences. Both nAMD and iAMD had a significantly higher prevalence of CC and CT high-risk genotypes (Figure. 3). We found no differences in the SNPs in the C3 gene between any of the groups (Figure. 3).
Figure 3.
Distribution in genotypes in SNPs associated with AMD
Pie charts of single nucleotide polymorphisms (SNPs), with the distribution of genotypes including significance levels between groups in:
a) The CFH gene rs1061170. The genotypes CC and CT are associated with a 5•9- and 2•5-times higher risk of developing AMD, respectively. The TT genotype is associated with a 1.6 times lower risk of AMD. Patients with nAMD and iAMD show a higher prevalence of the high-risk genotypes compared to patients with MPNs.
b) The C3 gene rs2230199. The genotypes GG and CG are associated with a 2•5- and 1•6-times higher risk of AMD. We observed no differences in the distribution between groups.
Differences between groups were tested with Fischer's exact test.
nAMD: neovascular age-related macular degeneration, iAMD: intermediate AMD, MPN: myeloproliferative neoplasms, MPNd: MPN with drusen, MPNn: MPN with normal retinas.
Further analyses
HU has an anti-inflammatory effect.40 Despite the small numbers of patients not treated with HU (MPNd=8, MPNn=7), we investigated differences between the HU-treated and the non-HU group regarding monocytes, subset, Cregs, NLR, SIS, and SGS. We also investigated differences between JAK2V617F-negative patients (n = 8) and JAK2V617F-positive (n = 53), and differences between JAK2V617F-positive patients with an allele burden <50% (n = 43) and >50% (n = 13) regarding the same variables as above. Finally, we evaluated Cregs on lymphocytes, CD4+ and CD8+ t-cells. The above analyses were not statistically significant (data not shown).
Discussion
In this study, we found that MPNd compared to MPNn had a higher CLI level, indicated by a higher NLR, a higher SIS, and they also showed signs of inadequate systemic complement system regulation. For Cregs, the numbers gave us an impression of a much lower Cregs expression in the MPNd group than the MPNn, and when investigating MPN subtypes, we observed that PV patients had a significantly lower CD59 expression than ET and PMF patients.
The SIS and NLR were rising from ET over PV to PMF. This data supports the concept of the MPNs as a biological continuum with increasing inflammation, including rising cytokine levels. Most of the patients with MPNs were suffering from PV, and the group was large enough to divide into those with and without drusen. The PVd had a lower CD59 expression and a near significant lower CD35 expression than PVn. The SIS was higher in the PVd than PVn.
The rationale for investigating CLI and the complement system in MPNs is that we have recently shown that drusen and late-stage AMD prevalence are higher in these patients than in the general population and that drusen develop at a younger age.25 Since drusen are believed to be by-products of chronic inflammatory events,9,21,41 and drusen components, such as oxidized apolipoprotein, can activate the complement system,9,42 it is plausible that CLI in MPN patients leads to drusen formation by local and systemic inflammation-mediated complement activation, which is steadily being enhanced in a vicious cycle. We do not know if the drusen seen in patients with MPNs have the same composition as those seen in patients with AMD, but since patients with MPNs also have a higher prevalence of late AMD, it seems likely that the drusen are similar.
An overactivated or dysregulated complement system is involved in a broad spectrum of disorders such as thrombotic and autoimmune disorders and cancer,43, 44, 45, 46, 47, 48 but studies on MPNs and the complement system are sparse,49, 50, 51, 52 and has never been systematically studied in larger series of MPN patients. To the best of our knowledge, no studies have previously performed concurrent studies of Cregs and circulating biomarkers of CLI in MPNs or comparative studies with another chronic inflammatory disease, herein AMD.
The complement system has been extensively studied in AMD.4,7, 8, 9, 10, 11 Several complement proteins, regulators, and components have been found in drusen and are produced by the RPE.22,23,53 Associations between AMD and variants in genes encoding complement proteins and regulators have been found.8,9,22,54, 55, 56, 57, 58, 59 In addition, major risk factors for AMD development, ageing, smoking, and oxidative stress have been linked to overactivation of the complement system.60,61 Current evidence shows that the complement system's alternative pathway (AP) is particularly important in relation to the pathogenesis.10,12,13,62 Initially, the AP is activated by constant low-level hydrolysis of C3, and in later steps of the pathway, an amplification loop for the classical and lectin pathway initiates more C3 cleavage, which uncontrolled would lead to tissue damage. Thus, the AP is continuously active and therefore requires continuous control, partly by Cregs. Data show that AMD patients have increased levels of peripheral blood complement proteins13,14,63,64 and decreased CD59 levels on monocytes compared to healthy controls.20 Another study found increased CD35 levels on monocytes, lymphocytes, and granulocytes.6
CFH is an AP regulator, and genetic variants in the CFH gene are associated with an increased risk of developing AMD. In mice, the absence of the gene for CFH leads to reduced CD59 expression with age in the RPE, resulting in increased MAC formation.65, 66, 67 RPE CD59 expression is considered critical to AMD development, and several complement-targeted therapeutics, including gene-therapy with CD59 in GA and nAMD, are ongoing, with others on the way (NCT04358471, NCT03144999, NCT03585556).
CD35 can both bind C3b and C4b, keeping them from convertases inhibiting the complement pathway, and it also acts as a cofactor for factor I-mediated inactivation of C3b and C4b. Data has shown that CD35 can further limit C6 deposition on RPE cells and could therefore be a possible inhibitor of MAC formation, and RPE cell protection could be important for AMD patients.68 Another function of CD35 is binding to immune complexes (IC), thus contributing to the clearance of these. This clearance of IC is suggested to be implicated in drusen formation and AMD development.21,69 It is intriguing to consider if decreased CD35 expression might lead to defective IC clearance in MPNs since elevated circulating IC levels have been recorded in several studies.70, 71, 72, 73 Systemic Cregs dysregulation could contribute to AMD pathogenesis in patients with MPNs. Data show evidence of damage to the choriocapillaris as a primary event in AMD pathophysiology and a higher degree of MAC on the choriocapillaris than the RPE in AMD patients.21,74 MAC is even detectable in the human choriocapillaris in healthy eyes, and the presence increases with age.75 Damage to the choriocapillaris could impact the RPE since the choriocapillaris serves multiple functions, including oxygen- and nutrients supply and waste products filtering for the RPE and outer retina.
The subretinal space is usually immune privileged, and in healthy individuals, it is devoid of mononuclear phagocytes (MP).76, 77, 78, 79 A feature of AMD development is an MP accumulation in the subretinal space, and both animal models and investigation of donor's eyes from AMD patients show blood-derived-monocyte-derived-inflammatory-macrophages amongst these cells.76,80,81 Therefore, in AMD, the blood-derived monocytes can enter the subretinal space and possibly influence the inflammatory milieu, making the Cregs expression on these monocytes interesting.
The comparative MPN and AMD studies, in which CLI and complement activation are paramount in disease pathogenesis, have elucidated and unravelled novel insights on common pathogenic mechanisms for disease pathobiology in these diseases. Thus, when we compared findings in MPNs to AMD patients, we first noticed that the higher SIS in MPNd resembled the SIS in nAMD and the lower SIS in MPNn resembled the SIS in iAMD. Although the retinal appearance in MPNd is closer to the appearance in iAMD, the inflammatory state in these patients resembles the state seen in nAMD. Likewise, MPNn, despite not having drusen resemble the inflammatory state in iAMD. This could suggest a possible role for systemic CLI in drusen development. The higher inflammatory milieu in MPNd is seen even though many patients with MPNs receive HU treatment, which has an anti-inflammatory effect.40 Another observation regarding inflammation in this study was a lower percentage of classical- and a higher percentage of intermediate monocytes in MPNs, especially in MPNd. This further underlines the higher inflammatory milieu in patients with MPNs since classical monocytes are considered the more reparative monocyte and intermediate the more inflammatory type.82 Secondly, we noticed similar (high) levels of the anaphylatoxins across all groups. We do not have a healthy control group to compare with, but anaphylatoxins are found to be elevated in AMD,64,83,84 and we find similar levels in patients with MPNs.48 Thirdly, we observed a more substantial CD59 downregulation on monocytes in MPNs than AMD patients, further supporting current evidence on a role for CD59 in drusen formation. Interestingly, case reports have shown a possible link between paroxysmal nocturnal haemoglobinuria (PNH) and MPNs. PNH is partly characterized by chronic intravascular haemolysis and venous thrombosis apparently due to an unregulated complement activation following the reduction or absence of different Cregs (including CD59).49 To our knowledge, no previous systematic studies on Cregs expression in MPNs are available. Finally, the SGS was similar in the MPN groups but significantly higher than in AMD groups, so this is a characteristic of the MPNs that is not present to the same extent in AMD patients.
We also evaluated the presence of SNPs associated with a higher risk of AMD to investigate if the high-risk genotypes were present in patients with MPNs. From a meta-analysis,85 we calculated the prevalence of the CFH genotypes in studies of nAMD patients and controls (Caucasian ethnicity). We found 14 studies including 3001 patients with nAMD (not including GA). The mean prevalence of CC, CT, and TT was 33%, 50%, and 17%, respectively. The corresponding numbers for controls were 13%, 43%, and 44% (40 studies including 24,338 controls). In our study, prevalence rates in nAMD and iAMD resembled the prevalence found in the meta-analysis, and we found lower rates of the high-risk CC and CT genotypes in the MPN groups. Another meta-analysis of the C3 gene (15 studies including 7903 AMD patients and 6478 controls),86 reported mean prevalence of high-risk genotypes CG and GG of 38% and 9% in AMD and 30% and 4% in controls.87 All groups in our study had similar or lower rates of CG than the controls and lower rates of GG. Accordingly, our results did not indicate a higher prevalence of high-risk genotypes in MPNs, explaining the higher drusen prevalence. The MPNd group seemed to have a higher prevalence of the high-risk genotype CC in the CFH gene than MPNn, but the difference was not significant, and the prevalence was much lower than in AMD patients. These results suggest that polymorphisms cannot, at least alone, explain the higher drusen prevalence in patients with MPNd.
Important limitations should be kept in mind when interpreting the results. The study is observational, and we can only speculate on causality. Further, the distribution of included patients with different MPN subtypes is a limitation since we only had six PMF patients. The ET and PMF patient groups were too small to divide into groups with and without drusen.
In conclusion, our study indicates a higher degree of CLI in patients with MPNd and inadequate complement system regulation. It is intriguing to apply the MPNs as a “Human Inflammation model” to AMD pathogenesis with CLI “triggering” drusen formation, leading to more CLI creating a vicious cycle, and with this, an increased risk of developing AMD. In MPNs, a treatment concept is to dampen the CLI since it has an essential role in initiating and driving the diseases. This concept may be a treatment option for patients with AMD, decreasing the possible inflammation-driven drusen formation.
Contributors
Conceptualization, C.L., T.L.S., H.C.H., V.S., and L.S.; Methodology, C.L., T.L.S., and H.C.H.; Investigation, C.L. with help from V.S., L.S. (mutant allele burden data), BioXpedia (SNP genotyping) and DTU (bioassays); Data access and responsibility, C.L.; Formal Analysis, C.L.; Visualization, C.L.; Writing – Original Draft: C.L.; Writing – Review & Editing, C.L., T.L.S., H.C.H., V.S., and L.S.; Funding Acquisition, C.L. with help from T.L.S.; Resources, C.L.; Supervision, T.L.S. and H.C.H.; Decision to submit manuscript for publication, C.L., T.L.S., H.C.H., V.S.
Declaration of interests
C.L. reports grants from “Fight for Sight, Denmark” and Region Zealand's Research Promotion Fund. H.C.H reports grants from Novartis outside the submitted work and is a part of the advisory board of AOP Orphan. All other authors declare no competing interests.
Acknowledgments
Acknowledgements
The first author C.L. is a PhD candidate at the University of Copenhagen (UCPH) and would like to thank “Fight for Sight, Denmark” and Region Zealand's Research Promotion Fund for funding her PhD. This work submitted is a partial requirement for a PhD at UCPH.
Further, CL would like to thank BioXpedia for help with SNP genotyping and the Technical University of Denmark (DTU) for help with immunoassays. A special thanks to the helpful employees at BioXpedia Hans-Christian Ingerslev, Charlotte Busch Ahler, and DTU Sanne Schou Berger and Natascha Morton.
Data sharing statement
De-identified data are available from the corresponding author (C.L.), upon request.”
Funding
Fight for Sight, Denmark, and Region Zealand's research promotion fund.
References
- 1.Ferris F.L., Wilkinson C.P., Bird A., et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P., Liew G., Gopinath B., Wong T.Y. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 3.Charbel Issa P., Victor Chong N., Scholl H.P.N. The significance of the complement system for the pathogenesis of age-related macular degeneration — Current evidence and translation into clinical application. Graefe's Arch Clin Exp Ophthalmol. 2011;249:163–174. doi: 10.1007/s00417-010-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maugeri A., Barchitta M., Mazzone M.G., Giuliano F., Agodi A. Complement System and Age-Related Macular Degeneration: implications of Gene-Environment Interaction for Preventive and Personalized Medicine. Biomed Res Int. 2018;2018:1–13. doi: 10.1155/2018/7532507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozing M.P., Durhuus J.A., Krogh Nielsen M., et al. Age-related macular degeneration: a two-level model hypothesis. Prog Retin Eye Res. 2020;76 doi: 10.1016/j.preteyeres.2019.100825. [DOI] [PubMed] [Google Scholar]
- 6.Haas P., Aggermann T., Nagl M., Steindl-Kuscher K., Krugluger W., Binder S. Implication of CD21, CD35, and CD55 in the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2011;152 doi: 10.1016/j.ajo.2011.02.017. 396-399.e1. [DOI] [PubMed] [Google Scholar]
- 7.Wu J., Sun X. Complement system and age-related macular degeneration: drugs and challenges. Drug Des Devel Ther. 2019;13:2413–2425. doi: 10.2147/DDDT.S206355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geerlings M.J., de Jong E.K., den Hollander A.I. The complement system in age-related macular degeneration: a review of rare genetic variants and implications for personalized treatment. Mol Immunol. 2017;84:65–76. doi: 10.1016/j.molimm.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson D.H., Radeke M.J., Gallo N.B., et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mcharg S., Clark S.J., Day A.J., Bishop P.N. Age-related macular degeneration and the role of the complement system. Mol Immunol. 2015;67:43–50. doi: 10.1016/j.molimm.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Khandhadia S., Cipriani V., Yates J.R.W., Lotery A.J. Age-related macular degeneration and the complement system. Immunobiology. 2012;217:127–146. doi: 10.1016/j.imbio.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Loyet K.M., DeForge L.E., Katschke K.J., et al. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Investig Opthalmology Vis Sci. 2012;53:6628. doi: 10.1167/iovs.12-9587. [DOI] [PubMed] [Google Scholar]
- 13.Scholl H.P.N., Issa P.C., Walier M., et al. Systemic complement activation in age-related macular degeneration. PLoS ONE. 2008;3:e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds R., Hartnett M.E., Atkinson J.P., Giclas P.C., Rosner B., Seddon J.M. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Investig Opthalmology Vis Sci. 2009;50:5818. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling M., Murali M. Analysis of the complement system in the clinical immunology laboratory. Clin Lab Med. 2019;39:579–590. doi: 10.1016/j.cll.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Blom A.M. The role of complement inhibitors beyond controlling inflammation. J Intern Med. 2017;282:116–128. doi: 10.1111/joim.12606. [DOI] [PubMed] [Google Scholar]
- 17.Astier A.L., Meiffren G., Freeman S., Hafler D.A. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Astier A.L. T-cell regulation by CD46 and its relevance in multiple sclerosis. Immunology. 2008;124:149–154. doi: 10.1111/j.1365-2567.2008.02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellinghaus U., Cortini A., Pinder C.L., Le Friec G., Kemper C., Vyse T.J. Dysregulated CD46 shedding interferes with Th1-contraction in systemic lupus erythematosus. Eur J Immunol. 2017;47:1200–1210. doi: 10.1002/eji.201646822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A., Faber C., Falk M., Nissen M.H., Hviid T.V.F., Sørensen T.L. Altered expression of CD46 and CD59 on leukocytes in neo-vascular age-related macular degeneration. Am J Ophthalmol. 2012;154:193–199. doi: 10.1016/j.ajo.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 21.Anderson D.H., Mullins R.F., Hageman G.S., Johnson L.V. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 22.Hageman G.S., Anderson D.H., Johnson L.V., et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullins R.F., Russell S.R., Anderson D.H., Hageman G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 24.Perkins S.J., Nan R., Okemefuna A.I., Li K., Khan S., Miller A. Multiple interactions of complement factor H with its ligands in solution: a progress report. Adv Exp Med Biol. 2010;703:25–47. doi: 10.1007/978-1-4419-5635-4_3. [DOI] [PubMed] [Google Scholar]
- 25.Liisborg C., Nielsen M.K., Hasselbalch H.C., Sørensen T.L. Patients with myeloproliferative neoplasms and high levels of systemic inflammation develop age-related macular degeneration. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bak M., Sørensen T.L., Flachs E.M., et al. Age-related macular degeneration in patients with chronic myeloproliferative neoplasms. JAMA Ophthalmol. 2017;135:835–843. doi: 10.1001/jamaophthalmol.2017.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spivak J.L. Myeloproliferative neoplasms. N Engl J Med. 2017;376:2168–2181. doi: 10.1056/NEJMra1406186. [DOI] [PubMed] [Google Scholar]
- 28.Hasselbalch H.C., Bjørn M.E. MPNs as inflammatory diseases: the evidence, consequences, and perspectives. Mediators Inflamm. 2015;2015:1–16. doi: 10.1155/2015/102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasselbalch H.C. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk Res. 2013;37:214–220. doi: 10.1016/j.leukres.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 30.Hasselbalch H.C. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev. 2013;24:133–145. doi: 10.1016/j.cytogfr.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Andersen M., Sajid Z., Pedersen R.K., et al. Mathematical modelling as a proof of concept for MPNs as a human inflammation model for cancer development. PLoS ONE. 2017;12:1–18. doi: 10.1371/journal.pone.0183620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasselbalch H.C. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119:3219–3225. doi: 10.1182/blood-2011-11-394775. [DOI] [PubMed] [Google Scholar]
- 33.Hasselbalch H.C., Skov V., Kjær L., Sørensen T.L., Ellervik C., Wienecke T. Myeloproliferative blood cancers as a human neuroinflammation model for development of Alzheimer's disease: evidences and perspectives. J Neuroinflammation. 2020;17:248. doi: 10.1186/s12974-020-01877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenbroucke J.P., von Elm E., Altman D.G., et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Barbui T., Thiele J., Gisslinger H., et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8:15. doi: 10.1038/s41408-018-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein R., Davis M.D., Magli Y.L., Segal P., Klein B.E., Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 37.Subhi Y., Krogh Nielsen M., Molbech C.R., Sørensen T.L. Altered proportion of CCR2 + and CX3CR1 + circulating monocytes in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Clin Experiment Ophthalmol. 2018;46:661–669. doi: 10.1111/ceo.13152. [DOI] [PubMed] [Google Scholar]
- 38.Subhi Y., Krogh Nielsen M., Molbech C.R., et al. CD11b and CD200 on circulating monocytes differentiate two angiographic subtypes of polypoidal choroidal vasculopathy. Investig Ophthalmol Vis Sci. 2017;58:5242–5250. doi: 10.1167/iovs.17-22479. [DOI] [PubMed] [Google Scholar]
- 39.Krogh Nielsen M., Subhi Y., Molbech C.R., et al. Patients with a fast progression profile in geographic atrophy have increased CD200 expression on circulating monocytes. Clin Experiment Ophthalmol. 2019;47:69–78. doi: 10.1111/ceo.13362. [DOI] [PubMed] [Google Scholar]
- 40.Guarda C.C., Silveira-Mattos P.S.M., Yahouédéhou S.C.M.A., et al. Hydroxyurea alters circulating monocyte subsets and dampens its inflammatory potential in sickle cell anemia patients. Sci Rep. 2019;9:14829. doi: 10.1038/s41598-019-51339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson L.V., Ozaki S., Staples M.K., Erickson P.A., Anderson D.H. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- 42.Zhou J., Jang Y.P., Kim S.R., Sparrow J.R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dijkstra D.J., Joeloemsingh J.V., Bajema I.M., Trouw L.A. Complement activation and regulation in rheumatic disease. Semin Immunol. 2019;45 doi: 10.1016/j.smim.2019.101339. [DOI] [PubMed] [Google Scholar]
- 44.Ajjan R.A., Schroeder V. Role of complement in diabetes. Mol Immunol. 2019;114:270–277. doi: 10.1016/j.molimm.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 45.Leffler J., Bengtsson A.A., Blom A.M. The complement system in systemic lupus erythematosus: an update. Ann Rheum Dis. 2014;73:1601–1606. doi: 10.1136/annrheumdis-2014-205287. [DOI] [PubMed] [Google Scholar]
- 46.Hovland A., Jonasson L., Garred P., et al. The complement system and toll-like receptors as integrated players in the pathophysiology of atherosclerosis. Atherosclerosis. 2015;241:480–494. doi: 10.1016/j.atherosclerosis.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 47.Afshar-Kharghan V. The role of the complement system in cancer. J Clin Invest. 2017;127:780–789. doi: 10.1172/JCI90962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ajona D., Ortiz-Espinosa S., Pio R. Complement anaphylatoxins C3a and C5a: emerging roles in cancer progression and treatment. Semin Cell Dev Biol. 2019;85:153–163. doi: 10.1016/j.semcdb.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 49.Chatzidavid S., Giannakopoulou N., Diamantopoulos P.T., et al. JAK2V617F positive polycythemia vera with paroxysmal nocturnal hemoglobinuria and visceral thromboses: a case report and review of the literature. Thromb J. 2021;19:16. doi: 10.1186/s12959-021-00269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guc D., Canpınar H., Kucukaksu C., Kansu E. Expression of complement regulatory proteins CR1, DAF, MCP and CD59in haematological malignancies. Eur J Haematol. 2000;64:3–9. doi: 10.1034/j.1600-0609.2000.80097.x. [DOI] [PubMed] [Google Scholar]
- 51.Gordon B.R., Coleman M., Kohen P., Day N.K. Immunologic abnormalities in myelofibrosis with activation of the complement system. Blood. 1981;58:904–910. [PubMed] [Google Scholar]
- 52.Cedzyński M., Świerzko A.S. Components of the lectin pathway of complement in haematologic malignancies. Cancers (Basel) 2020;12 doi: 10.3390/cancers12071792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang P., Tyrrell J., Han I., Jaffe G.J. Expression and modulation of RPE cell membrane complement regulatory proteins. Investig Opthalmology Vis Sci. 2009;50:3473. doi: 10.1167/iovs.08-3202. [DOI] [PubMed] [Google Scholar]
- 54.Haines J.L., Hauser M.A., Schmidt S., et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 55.Edwards A.O., Ritter R., Abel K.J., Manning A., Panhuysen C., Farrer L.A. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 56.Klein R.J., Zeiss C., Chew E.Y., et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li M., Atmaca-Sonmez P., Othman M., et al. CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat Genet. 2006;38:1049–1054. doi: 10.1038/ng1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan P.L., Garrett M.E., Willer J.R., et al. Systematic functional testing of rare variants: contributions of CFI to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:1570–1576. doi: 10.1167/iovs.16-20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhan X., Larson D.E., Wang C., et al. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nat Genet. 2013;45:1375–1379. doi: 10.1038/ng.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang A.L., Lukas T.J., Yuan M., Du N., Handa J.T., Neufeld A.H. Changes in retinal pigment epithelium related to cigarette smoke: possible relevance to smoking as a risk factor for age-related macular degeneration. PLoS ONE. 2009;4:e5304. doi: 10.1371/journal.pone.0005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thurman J.M., Renner B., Kunchithapautham K., et al. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009;284:16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rohrer B., Coughlin B., Kunchithapautham K., et al. The alternative pathway is required, but not alone sufficient, for retinal pathology in mouse laser-induced choroidal neovascularization. Mol Immunol. 2011;48:1–8. doi: 10.1016/j.molimm.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sivaprasad S., Adewoyin T., Bailey T.A., et al. Estimation of systemic complement C3 activity in age-related macular degeneration. Arch Ophthalmol. 2007;125:515–519. doi: 10.1001/archopht.125.4.515. [DOI] [PubMed] [Google Scholar]
- 64.Machalińska A., Dziedziejko V., Mozolewska-Piotrowska K., Karczewicz D., Wiszniewska B., Machaliński B. Elevated plasma levels of C3a complement compound in the exudative form of age-related macular degeneration. Ophthalmic Res. 2009;42:54–59. doi: 10.1159/000219686. [DOI] [PubMed] [Google Scholar]
- 65.Faber C., Williams J., Juel H.B., Greenwood J., Nissen M.H., Moss S.E. Complement factor H deficiency results in decreased neuroretinal expression of Cd59a in aged mice. Investig Opthalmology Vis Sci. 2012;53:6324. doi: 10.1167/iovs.12-10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bora N.S., Kaliappan S., Jha P., et al. CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J Immunol. 2007;178:1783–1790. doi: 10.4049/jimmunol.178.3.1783. [DOI] [PubMed] [Google Scholar]
- 67.Bora P.S., Sohn J.-.H., Cruz J.M.C., et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174:491–497. doi: 10.4049/jimmunol.174.1.491. [DOI] [PubMed] [Google Scholar]
- 68.Simmons K.T., Mazzilli J.L., Mueller-Ortiz S.L., et al. Complement receptor 1 (CR1/CD35)-expressing retinal pigment epithelial cells as a potential therapy for age-related macular degeneration. Mol Immunol. 2020;118:91–98. doi: 10.1016/j.molimm.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Murinello S., Mullins R.F., Lotery A.J., Perry V.H., Teeling J.L. Fcγ receptor upregulation is associated with immune complex inflammation in the mouse retina and early age-related macular degeneration. Investig Opthalmology Vis Sci. 2014;55:247. doi: 10.1167/iovs.13-11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cappio F.C., Vigliani R., Novarino A., Camussi G., Campana D., Gavosto F. Idiopathic myelofibrosis: a possible role for immune-complexes in the pathogenesis of bone marrow fibrosis. Br J Haematol. 1981;49:17–21. doi: 10.1111/j.1365-2141.1981.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 71.Lewis C.M., Pegrum G.D. Immune complexes in myelofibrosis: a possible guide to management. Br J Haematol. 1978;39:233–239. doi: 10.1111/j.1365-2141.1978.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 72.Hasselbalch H., Nielsen H., Berild D., Kappelgaard E. Circulating immune complexes in myelofibrosis. Scand J Haematol. 1985;34:177–180. doi: 10.1111/j.1600-0609.1985.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 73.Vellenga E., Mulder N.H., The T.H., Nieweg H.O. A study of the cellular and humoral immune response in patients with myelofibrosis. Clin Lab Haematol. 1982;4:239–246. doi: 10.1111/j.1365-2257.1982.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 74.Mullins R.F., Dewald A.D., Streb L.M., Wang K., Kuehn M.H., Stone E.M. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Exp Eye Res. 2011;93:565–567. doi: 10.1016/j.exer.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mullins R.F., Schoo D.P., Sohn E.H., et al. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014;184:3142–3153. doi: 10.1016/j.ajpath.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta N., Brown K.E., Milam A.H. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003;76:463–471. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 77.Combadière C., Feumi C., Raoul W., et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sennlaub F., Auvynet C., Calippe B., et al. CCR 2 + monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med. 2013;5:1775–1793. doi: 10.1002/emmm.201302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levy O., Calippe B., Lavalette S., et al. Apolipoprotein E promotes subretinal mononuclear phagocyte survival and chronic inflammation in age-related macular degeneration. EMBO Mol Med. 2015;7:211–226. doi: 10.15252/emmm.201404524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guillonneau X., Eandi C.M., Paques M., Sahel J.-.A., Sapieha P., Sennlaub F. On phagocytes and macular degeneration. Prog Retin Eye Res. 2017;61:98–128. doi: 10.1016/j.preteyeres.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Lad E.M., Cousins S.W., Van Arnam J.S., Proia A.D. Abundance of infiltrating CD163+ cells in the retina of postmortem eyes with dry and neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2015;253:1941–1945. doi: 10.1007/s00417-015-3094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong K.L., Yeap W.H., Tai J.J.Y., Ong S.M., Dang T.M., Wong S.C. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 83.Lynch A.M., Mandava N., Patnaik J.L., et al. Systemic activation of the complement system in patients with advanced age-related macular degeneration. Eur J Ophthalmol. 2020;30:1061–1068. doi: 10.1177/1120672119857896. [DOI] [PubMed] [Google Scholar]
- 84.Rohrer B., Frazer-Abel A., Leonard A., et al. Association of age-related macular degeneration with complement activation products, smoking, and single nucleotide polymorphisms in South Carolinians of European and African descent. Mol Vis. 2019;25:79–92. [PMC free article] [PubMed] [Google Scholar]
- 85.Maugeri A., Barchitta M., Agodi A. The association between complement factor H rs1061170 polymorphism and age-related macular degeneration: a comprehensive meta-analysis stratified by stage of disease and ethnicity. Acta Ophthalmol. 2019;97:e8–21. doi: 10.1111/aos.13849. [DOI] [PubMed] [Google Scholar]
- 86.Thakkinstian A., McKay G.J., McEvoy M., et al. Systematic review and meta-analysis of the association between complement component 3 and age-related macular degeneration: a HuGE review and meta-analysis. Am J Epidemiol. 2011;173:1365–1379. doi: 10.1093/aje/kwr025. [DOI] [PubMed] [Google Scholar]
- 87.Park K.H., Fridley B.L., Ryu E., Tosakulwong N., Edwards A.O. Complement component 3 (C3) haplotypes and risk of advanced age-related macular degeneration. Investig Opthalmol Vis Sci. 2009;50:3386. doi: 10.1167/iovs.08-3231. [DOI] [PubMed] [Google Scholar]