Abstract
Background
Implementation of sperm preparation techniques based on cellular and molecular characteristics can improve the clinical outcomes of couples with male factor infertility. These methods attempt to select better sperm compared to classical methods of preparation such as swim-up and density gradient centrifugation (DGC). In this view, the aim of this study was the comparison of clinical outcomes of magnetic-activated cell separation (MACS) followed by DGC or DGC alone in infertile men undergoing intracytoplasmic sperm injection (ICSI).
Materials and Methods
For this prospective single parallel blind clinical trial study, 206 infertile couples with male factor infertility and having abnormal sperm morphology higher than 96% were included. 106 and 100 couples were considered for the study (MACS-DGC) and control group (DGC), respectively. Clinical outcomes of ICSI; fertilization, embryo quality, and implantation, pregnancy rates were compared between two groups.
Materials and Methods
For this prospective single parallel blind clinical trial study, 206 infertile couples with male factor infertility and having abnormal sperm morphology higher than 96% were included. 106 and 100 couples were considered for the study (MACS-DGC) and control group (DGC), respectively. Clinical outcomes of ICSI; fertilization, embryo quality, and implantation, pregnancy rates were compared between two groups.
Results
Mean of fertilization (80.19 ± 1.88 vs. 75.63 ± 2.06, P=0.1), top embryo quality on the day 3 (30.22 ± 3.59 vs. 17.96 ± 2.9, P=0.009), clinical pregnancy (30.76% vs. 22.22%, P=0.19), and implantation rate (18.12% vs. 10.42%, P=0.04) were higher in the study group compared to the control group.
Conclusion
Sperm preparation by MACS followed by DGC in teratozoospermic men could improve the clinical outcomes after ICSI (Registration number: IRCT201610317223N8).
Keywords: Embryo, Fertilization, Intracytoplasmic Sperm Injection, Pregnancy
Introduction
The introduction of assisted reproduction techniques (ARTs) led to the development of a range of treatments for human infertility. It is however remaining unknown, whether the implementation of these techniques would improve the pregnancy rate (1). Density gradient centrifugation (DGC) and swim-up procedures are widely used for sperm preparation in ART. However, novel sperm selection procedures are being gradually introduced to this field, particularly for selecting sperm prior to intracytoplasmic sperm injection (ICSI) (2, 3).
Phosphatidylserine (PS), an anionic phospholipid, is one of the apoptosis or programmed cell death markers, actively translocates from the inner membrane to the outer surface of the sperm and is a signal for specific recognition cells for phagocyte the apoptotic cells (4, 5).
Externalization of PS allows Annexin V, a calcium-dependent phospholipid-binding protein with a high affinity for PS, to bind to apoptotic sperm and isolate them from the original population. To achieve the selection of sperm based on this natural principle, magnetic-activated cell separation (MACS) has been developed. MACS separate apoptotic from non-apoptotic sperm, through a column in presence of a magnetic field by applying Annexin V coated beads which bind to PS on the surface of apoptotic sperm (6-8). In this regard, previous studies demonstrated MACS have the ability to prepare a sperm population with better DNA integrity (9-14).
It is well known that serum-supplemented sperm washing media may facilitate the process of PS externalization as the physiological process during capacitation. Therefore, processing the sample through the DGC column in presence of serum in sperm washing media may result in the deselection of healthy capacitated sperm by MACS procedure (15, 16). Therefore, we previously showed that MACS followed by DGC (MACS/DGC) has higher efficiency than DGC followed by MACS (DGC/MACS) to deselect apoptotic sperm, especially in terms of DNA integrity (14, 17). It is of note that in DGC/MACS, capacitated sperm with normal chromatin integrity may be deselected. Based on the above finding, this clinical trial is designed to evaluate the clinical outcomes of MACS/ DGC and DGC in couples undergoing for ICSI with abnormal sperm morphology higher 96% (18).
Materials and Methods
Patient selection and study design
Trail design
The prospective single parallel blind clinical trial was initially approved by the Ethical Committee of Royan Institute in 2017 (IR.ACECR.ROYAN.REC.1395.156) and registered in RCT in 2017 (IRCT201610317223N8) and, was performed in Isfahan Fertility and Infertility Center. 250 couples with abnormal sperm morphology higher or equal than 96% (WHO 5th edition) were informed regarding the MACS sperm selection procedure (18).
The couples were informed that based on literature, MACS has the potential to improve fertilization rate, embryo quality, implantation, and pregnancy rates. The selection of couples was not based on sperm analysis on the day of ICSI and was based on the last semen analysis performed before beginning of ovulation induction. Sperm morphology was not analyzed on the day of ICSI.
Couples that accepted to participate provided written informed consent for their participation and couples who refused to participate and allowed usage of their clinical information for this research study were included in the control group. Sperm processing for MACS followed by DGC or DGC was carried out in the andrology unit. Clinicians and embryologists performing ICSI and embryo scoring were blind to the study.
Inclusion criteria: Primary infertile couples with male factors and abnormal sperm morphology higher or equal to 96% were included in this study.
Female partners were considered normal if they had normal hysterosalpingography, regular cycle, and normal antral follicular count. Therefore, women with endometriosis, adenomyosis, polycystic ovary syndrome, hydrosalpinx, uterine malformations, and a poor response to ovarian stimulation protocols were not included in this study.
Exclusion criteria: Couples who had no oocyte available after recovery, a high number of immature oocytes, a high number of dysmorphic oocytes, and/or had no embryo (fresh or frozen) for transfer when the data last analysis were carried out, were also excluded from the study.
Semen analysis
Semen samples were obtained from infertile men candidates for ICSI, in a sterile sperm collection container by masturbation after 2 to 7 days of sexual abstinence. Samples were kept at room temperature for the liquefaction process (15-30 minutes). For analyzing sperm motility, semen samples were kept at 37°C, and then the percentage of sperm motility was evaluated by computer-assisted sperm analysis (CASA) using a LABOMED CxL optical microscope. For assessment of sperm concentration, a sperm counting chamber (Garkheda, Aurangabad, India) was used, and the results were expressed as million per milliliter. Since the result of sperm morphology assessment was considered as one of the inclusion criteria in this study, we assessed sperm morphology in our andrology unit on the cycle before ovulation induction by diffquick staining (Faradidpardazpars, Iran) according to WHO criteria (18).
Sperm preparation by DGC procedure (control group)
Briefly, semen samples were layered over discontinuous gradients (90% and 45%) of Pure Sperm (Nidacon, Sweden) followed by 1000 rpm centrifugation for 15 minutes). Subsequently, the sperm pellet was re-suspended and rinsed twice with VitaSperm (Incolone, Iran) supplemented with 10% human serum albumin (HSA). The sperm pellet was diluted in the same medium, and used for the ICSI procedure (19).
Sperm preparation by MACS-DGC procedure (MACS group)
The sperm preparation method was performed according to our previous protocol by Tavalaee et al. (17) and Ziarati et al. (14). In brief, the sperm samples were first rinsed in a serum-free medium (Incolone, Iran). MACS sperm preparation was performed in absence of serum. Briefly, semen samples were rinsed with VitaSperm media and then washed sperm were diluted to ten million per ml. After that, one hundred microliters of microbeads solution (Milteny Biotec, India) was added to the sperm sample and was incubated for 15 minutes in the room. Then, the sperm sample was loaded into the MACS column (Milteny Biotec, India). Annexin negative sperm were passed the column while sperm with annexin positive was retained into the column. After that, MACS isolated sperm were laid on DGC gradients (90 and 45%), and finally the pellet was re-suspended and rinsed twice with VitaSperm supplemented with 10% HSA. Lastly, prepared sperm was used for the ICSI procedure.
ICSI procedure
The media used for ICSI procedure and embryo culture were purchased from Vitrolife (Gothenburg, Sweden, G5 series plus). Briefly, oocytes were denuded with hyaluronidase enzyme [Hyase (90IU/ml) in G-MOPS medium] after oocyte retrieval. Then, oocytes were washed and transferred to G- MOPS droplets in ICSI prepared dishes and, processed sperm from either the DGC or MACS procedures were introduced into ICSI-100 (a viscous medium that reduces the motility of sperm to comfort sperm handling) in the prepared ICSI dish.
ICSI outcomes
About 16-18 hours after ICSI, fertilization was evaluated by assessing for the presence of male and female pronucleus. Fertilization rate was defined as the number of fertilized oocytes over the number of inseminated oocytes times 100. On day 3 after oocyte recovery, the embryos quality and also embryo score of transferred embryos on transfer day were evaluated based on the Giorgetti group. A high-quality embryo was defined as an embryo with 6-8 cells of equal blastomere size and fragmentation less than 25%. The percentage of high-quality embryos was calculated by dividing the number of high-quality embryos over the total number of embryos assessed on day 3 times 100 (20-22). Embryos were either freshly transferred on day 3 or vitrified. For fresh transfers, the patient received two 50 mg progesterone injections per day. For transfer of vitrified embryos, the endometrium was prepared with the use of oral estradiol valerate at a dose of 8 mg per day, starting on the second or third day of the menstrual cycle. Endometrial thickness was monitored to ensure that the endometrial thickness reached 8 mm and then the female received two 50 mg progesterone injection per day. On the 4th day of progesterone injection, day 3 embryos were thawed and transferred on the same day. At 5 weeks after the embryo transfer, clinical pregnancy was assessed by the aid of vaginal ultrasound with and at least one embryo with a fetal heart rate was considered as clinical pregnancy. The implantation rate was defined by dividing the number of fetal sacs over the number of transferred embryos.
Statistical analysis
The obtained data from this study were analyzed using the Statistical Package for the Social Sciences software (SPSS 18, Chicago, IL, USA). For comparison of variations between study and control groups, independent sample t test and Chi-square were used. When the P<0.05, the differences were considered significant. All the data in the text, tables, and figure were expressed as mean ± standard error of the mean (SEM).
Results
Out of 250 couples included in this study, 41 were excluded based on the exclusion criteria. Of the total of 206 remaining ICSI cycles, 106 and 100 were included in the study as a test and control group, respectively. Table 1 demonstrates characteristics of couples such as male and female age, total injected oocytes, number of previous ART, and also sperm parameters between study and control groups. All stated parameters including the age of couples, duration of infertility, number of previous ART cycles, semen characteristics, total oocytes were similar between study and control groups. Only, the mean number of previous ART courses was significantly higher in the study group.
Table 1.
Mean of semen parameters and couples’ characteristics in study and control groups
|
| |||
|---|---|---|---|
| Parameters | Study group106 cases(MACS-DGC) | Control group100 cases(DGC) | P value |
|
| |||
| Male age (Y) | 37.22 ± 00.64 | 37.58 ± 00.63 | 0.69 |
| Female age (Y) | 32.02 ± 00.55 | 31.55 ± 00.54 | 0.54 |
| Sperm concentration(106/ml) | 24.38 ± 01.52 | 25.04 ± 01.44 | 0.75 |
| Sperm motility (%) | 38.62 ± 01.36 | 35.71 ± 01.09 | 0.1 |
| Abnormal spermmorphology (%) | 98.31 ± 00.09 | 98.20 ± 00.07 | 0.34 |
| Total injected oocytes | 07.78 ± 00.53 | 07.92 ± 00.57 | 0.86 |
| Previous ART | 01.60 ± 00.09 | 00.90 ± 00.09 | 0.000 |
|
| |||
Data are presented as mean ± SD. DGC; Density gradient centrifugation, MACS; Magnetic-activated cell sorting, and ART; Assisted reproductive technology.
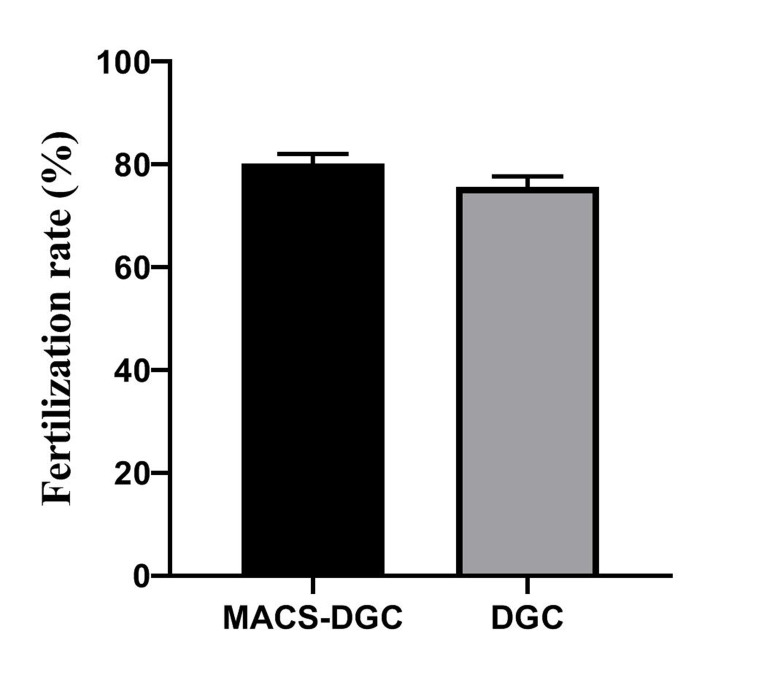
Fertilization rate of MACS-DGC and DGC were 80.19 ± 1.88 and 75.63 ± 2.06 (P=0.1), respectively. However, MACS-DGC (30.22 ± 3.59) resulted in a significantly (P=0.009) higher percentage for embryos with grade A quality in comparison with DGC (17.96 ± 2.9) groups, respectively (Fig .1).
Fig.1.
Comparison of fertilization rate (P=0.1) and percentage of top embryo quality (P=0.009) between study and control groups. DGC; Density gradient centrifugation and MACS; Magnetic-activated cell sorting.
In the study, group of 106 ICSI cycles, 54 and 37 cases had a fresh embryo or frozen embryo transfer, respectively, which resulted in the formation of 28 clinical pregnancies which ended up in the birth of 19 healthy singletons, 5 twins, and 4 abortions. While in the control group, 57 and 33 cases received fresh and frozen embryos respectively, which resulted in the formation of 20 clinical pregnancies consequently the birth of 15 singletons, 1 twin as well as 4 abortions. The mean percentage of clinical pregnancy showed an insignificantly (P=0.19) higher rate in the study group (30.76%) in comparison with the control group (22.22%). The implantation rates also indicated the rates of 18.12% and 10.42% for the study and control groups which were significantly different (P=0.04). More detail of the study is summarized in Table 2.
Table 2.
Assessment of clinical parameters in this study
|
| ||
|---|---|---|
| Study parameters | Study groupn=106 | Control groupn=100 |
| MACS-DGC(%) | DGC(%) | |
|
| ||
| Cancelled cycle | 13 | 8 |
| Frozen cycle with no ET | 2 | 2 |
| Number fresh of ET cycle | 54 | 57 |
| Number frozen of ET cycle | 37 | 33 |
| Total ET cycle | 91 | 90 |
| Mean number of ET/transfer | 1.75 | 1.81 |
| Mean embryo score of transferred embryos | 2.2 | 2.19 |
| Number of clinical pregnancy | 28/91 (30.76) | 20/90 (22.22) |
| Implantation rate(sac/number.ET) | (18.12) | (10.42) |
| Number of miscarriage | 4 | 4 |
| Number of singletons | 19 | 15 |
| Number of twins | 5 | 1 |
| Male/female | 17/12 | 6/11 |
| Sex ratio | 1.42 | 0.54 |
|
| ||
DGC; Density gradient centrifugation, MACS; Magnetic-activated cell sorting, and ET; Embryo transfer.
Discussion
Advance in our understanding of male genomic integrity is revealing light not only on conception, pregnancy, and live birth rates but also on the health of future progeny. Based on these understandings, researchers in the field of andrology have tried to introduce innovative sperm selection procedures in hope of reducing the possibility of inseminating oocytes with abnormal sperm chromatin and damaged DNA, despite selecting sperm based on viability and normal morphology for ICSI. In this regard, Avendan˜o et al. stated that in infertile population around 50% of sperm with normal morphology likely have DNA damage, thereby based on this result, one may conclude that in some cases there is a chance that 1 out of 2 oocytes being inseminated by DNA fragmented sperm (23, 24).
One of the processes that unhealthy sperm are being tagged for destruction is “apoptosis”. In the early stage of apoptosis, the PS is externalized from the inner member to the outer membrane before, the process of apoptosis is completely executed. Therefore, sperm in their early apoptotic stage are viable and motile and are likely to be selected for insemination during the ICSI procedure. The process of early apoptosis is likely to be concomitant with DNA oxidation which inevitably with the passage of time leads to DNA fragmentation (25). It is well known that the initiative enzyme required for DNA repair are provided by sperm while the remaining enzyme required to finalize the process of repair is presented in the oocyte (26, 27). Therefore, sperm and oocyte complement each other to repair damaged DNA which is highly dependent on oocyte age and quality. Consequently, the oocyte derived from a young female is believed to have a higher capacity to repaired sperm DNA damage. However, the developmental competency of an inseminated oocyte is related to the mutational load which is highly dependent on female age as well as the level of DNA fragmentation of inseminated sperm (27, 28). Thus, using the latest techniques for the preparation and selection of sperm with a low degree of DNA damage may improve the ICSI outcomes and overcome infertility.
PS externalization (PSE), in addition to being part of the early apoptosis process and can be induced by external factors including ROS stress, inflammation, and hypoxia (29, 30), also mediates cell to cell interaction, sperm-oocyte fusion (31), capacitation, acrosome reaction, and fertilization (31, 32). For instance, some researches showed the in vitro exposure of sperm samples to calcium ionophore A23187, an inducer of the acrosome reaction increases the number of living sperm with PSE (15). These findings indicated that the process of PSE could be independent of apoptosis.
Regardless of the role of PSE in processes of apoptosis and capacitation, two approaches have been proposed to isolate a healthier sperm population including MACS or MACS along with DGC (9-14). The advantage of using MACS is verified by different diagnostic techniques such as TUNEL assays and sperm chromatin dispersion test for assessment of DNA fragmentation as well as aniline blue, and CMA3 staining for assessment of chromatin maturity (13, 14, 17, 19, 33). In addition, a study by Grunewald et al. (10) showed that non-apoptotic sperm populations prepared by MACS may display high transmembrane mitochondrial potential, low activation of caspases-3, and high sperm chromatin decondensation. Unlike these studies, Cakar et al. (34) suggested sperm preparation with MACS could result in the reduction of sperm population with high total sperm motility and rapid progress. In addition, this method could not significantly separate the sperm with higher DNA integrity in comparison with swim-up or DGC. Considering these conflicting results, further studies are needed in a larger population of infertile men to confirm the usefulness of this sperm isolation method.
A meta-analysis from five prospective randomized trials studies suggested that unlike the rates of implantation and miscarriage which were similar between both DGC and MACS procedures, the rate of pregnancy significantly improved in MACS procedure compared to DGC or swimup techniques (11). A review paper (7) in this regard stated that the combination of DGC and MACS procedures could improve clinical pregnancy in infertile couples with male factor candidates for ICSI but there was no report on the live birth rate. Recently, Pacheco et al. (33) in a retrospective study demonstrated an improvement of clinical outcomes in terms of pregnancy, miscarriage, and live birth rates following ICSI by DGC-MACS in comparison with DGC in men with high DNA damage. In this regard, Hasanen et al. compared the clinical outcomes of ICSI between two sperm selection procedures; physiological ICSI (PICSI) based on hyaluronan binding, and MACS based on apoptosis surface marker in a prospective randomized trial. They demonstrated similar results in implantation and pregnancy rates between both procedures in men with SDF higher than 20.3% by TUNEL. Interesting, when couples were divided according to their wife's age, MACS was an effective method for females with ages lower than 30 years, while PICSI was suggested for females with ages higher than 30 years (35). Despite these conclusions, all these studies suggest further clinical trials are required to verify whether MACS has an advantage as a clinical complementary procedure for ICSI. One of the discrepancies that may account for different clinical outcomes between studies is likely related to the performance of DGC before or after MACS. It is of note that WHO (36) has also introduced a section on novel sperm selection procedures and also recommend the MACS/ DGC method instead of the DGC/MACS.
During sperm processing methods, sperm are washed and exposed to serum-containing media, which facilitated the process of capacitation and also PSE. Therefore, DGC in presence of serum followed by MACS can result in the removal of healthy sperm which have gone through the process of capacitation rather than removal of early apoptotic sperm, which has been verified previously by our group (17). The degree of removal of true apoptotic sperm from capacitated sperm depends highly on the duration that sperm are exposed to the serum which may vary within and between studies. Therefore, this clinical trial is designed on teratozoospermic men candidates for ICSI, and the MACS procedure was carried out in absence of serum. Then, the processed sample was exposed to DGC in hope of removing "true apoptotic sperm" and not "capacitated sperm". The results of this study demonstrated an insignificant improvement in the fertilization and clinical pregnancy rates in addition to significant improvements in embryo quality and implantation rates following MACS followed by DGC carried out in serum-free media. One of the limitations in this study was the small sample size, and un-unified ET protocol (fresh and frozen ET). Another limitation, we did not have a chance to culture these embryos to the blastocyst stage to see what is the blastocyst development rate in each arm. Also, the sperm DNA fragmentation status of semen samples in the control group was not known which might be a confounding factor for this study. Therefore, further studies were needed to verify the efficiency of this sperm preparation method. Assessment of confounding factors between the two groups revealed a higher number of ART cycles in the study group compared to control groups. Considering as the number of previous ART cycles increases the likelihood of ICSI success decrease, where the number of previous cycles in the control group would have been against our finding but as the number of previous studies, ART cycles are higher in our study group further verifies our finding. Although age as the main influential factor on fertility outcomes was similar between the two groups, we, unfortunately, did not account for other factors like body mass index (BMI).
Conclusion
MACS followed by DGC improves embryo quality and implantation rates, but higher sample size is required to confirm the effect of this procedure on the clinical pregnancy rate
Acknowledgements
This study was supported by Royan Institute and we would like to express our gratitude to the staff of Isfahan Fertility and Infertility for their full support. There is no conflict of interest in this study.
Authors’ Contributions
M.H.N.-E., M.T.; Conception, design, data analysis, interpretation, manuscript writing and final approval of the manuscript. M.N.-H.; Semen analysis, prepared samples, carried out experimental, collected data, and manuscript writing. F.Sh.; Data collection, manuscript writing and final approval of the manuscript. M.A.; Semen analysis. M.H.N.-E.; Carried out ICSI and assessment of fertilization and embryo quality. All authors read and approved the final manuscript.
References
- 1.Haddad M, Stewart J, Xie P, Cheung S, Trout A, Keating D, et al. Thoughts on the popularity of ICSI. J Assist Reprod Genet. 2021;38(1):101–123. doi: 10.1007/s10815-020-01987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughan DA, Sakkas D. Sperm selection methods in the 21st century. Biol Reprod. 2019;101(6):1076–1082. doi: 10.1093/biolre/ioz032. [DOI] [PubMed] [Google Scholar]
- 3.Marzano G, Chiriacò MS, Primiceri E, Dell'Aquila ME, RamalhoSantos J, Zara V, et al. Sperm selection in assisted reproduction: a review of established methods and cutting-edge possibilities. Biotechnol Adv. 2020;40:107498–107498. doi: 10.1016/j.biotechadv.2019.107498. [DOI] [PubMed] [Google Scholar]
- 4.Ammar O, Mehdi M, Muratori M. Teratozoospermia: its association with sperm DNA defects, apoptotic alterations, and oxidative stress. Andrology. 2020;8(5):1095–1106. doi: 10.1111/andr.12778. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S, Segawa K. Sensing and clearance of apoptotic cells. Curr Opin Immunol. 2021;68:1–8. doi: 10.1016/j.coi.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Lestari SW, Lestari SH, Margiana R. An update of sperm preparation: a review of supplementation substances to improve sperm quality. Biomed Pharmacol J. 2018;11(1):1–2. [Google Scholar]
- 7.Oseguera-López I, Ruiz-Díaz S, Ramos-Ibeas P, Pérez-Cerezales S. Novel techniques of sperm selection for improving IVF and ICSI outcomes. Front Cell Dev Biol. 2019;7:298–298. doi: 10.3389/fcell.2019.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simopoulou M, Gkoles L, Bakas P, Giannelou P, Kalampokas T, Pantos K, et al. Improving ICSI: a review from the spermatozoon perspective. Syst Biol Reprod Med. 2016;62(6):359–371. doi: 10.1080/19396368.2016.1229365. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Martín P, Dorado-Silva M, Sánchez-Martín F, González Martínez M, Johnston SD, Gosálvez J. Magnetic cell sorting of semen containing spermatozoa with high DNA fragmentation in ICSI cycles decreases miscarriage rate. Reprod Biomed Online. 2017;34(5):506–512. doi: 10.1016/j.rbmo.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Grunewald S, Reinhardt M, Blumenauer V, Said TM, Agarwal A, Abu Hmeidan F, et al. Increased sperm chromatin decondensation in selected nonapoptotic spermatozoa of patients with male infertility. Fertil Steril. 2009;92(2):572–577. doi: 10.1016/j.fertnstert.2008.07.1705. [DOI] [PubMed] [Google Scholar]
- 11.Gil M, Sar-Shalom V, Melendez Sivira Y, Carreras R, Checa MA. Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: a systematic review and meta-analysis. J Assist Reprod Genet. 2013;30(4):479–485. doi: 10.1007/s10815-013-9962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stimpfel M, Verdenik I, Zorn B, Virant-Klun I. Magnetic-activated cell sorting of non-apoptotic spermatozoa improves the quality of embryos according to female age: a prospective sibling oocyte study. J Assist Reprod Genet. 2018;35(9):1665–1674. doi: 10.1007/s10815-018-1242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hozyen M, Hasanen E, Elqusi K, ElTanbouly S, Gamal S, Hussin AG, et al. Reproductive outcomes of different sperm selection techniques for ICSI patients with abnormal sperm DNA fragmentation: a randomized controlled trial. Reprod Sci. 2022;29(1):220–228. doi: 10.1007/s43032-021-00642-y. [DOI] [PubMed] [Google Scholar]
- 14.Ziarati N, Tavalaee M, Bahadorani M, Nasr Esfahani MH. Clinical outcomes of magnetic activated sperm sorting in infertile men candidate for ICSI. Hum Fertil (Camb) 2019;22(2):118–125. doi: 10.1080/14647273.2018.1424354. [DOI] [PubMed] [Google Scholar]
- 15.Martin G, Sabido O, Durand P, Levy R. Phosphatidylserine externalization in human sperm induced by calcium ionophore A23187: relationship with apoptosis, membrane scrambling and the acrosome reaction. Hum Reprod. 2005;20(12):3459–3468. doi: 10.1093/humrep/dei245. [DOI] [PubMed] [Google Scholar]
- 16.Shin HW, Takatsu H. Phosphatidylserine exposure in living cells. Crit Rev Biochem Mol Biol. 2020;55(2):166–178. doi: 10.1080/10409238.2020.1758624. [DOI] [PubMed] [Google Scholar]
- 17.Tavalaee M, Deemeh MR, Arbabian M, Nasr-Esfahani MH. Density gradient centrifugation before or after magnetic-activated cell sorting: which technique is more useful for clinical sperm selection? J Assist Reprod Genet. 2012;29(1):31–38. doi: 10.1007/s10815-011-9686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Cambridge: Cambridge University Press; 2010. 271 [Google Scholar]
- 19.Zahedi A, Tavalaee M, Deemeh MR, Azadi L, Fazilati M, NasrEsfahani MH. Zeta potential vs apoptotic marker: which is more suitable for ICSI sperm selection? J Assist Reprod Genet. 2013;30(9):1181–1186. doi: 10.1007/s10815-013-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, et al. Embryo score to predict implantation after in-vitro fertilization: based on 957 single embryo transfers. Hum Reprod. 1995;10(9):2427–2431. doi: 10.1093/oxfordjournals.humrep.a136312. [DOI] [PubMed] [Google Scholar]
- 21.Terriou P, Giorgetti C, Hans E, Salzmann J, Charles O, Cignetti L, et al. Relationship between even early cleavage and day 2 embryo score and assessment of their predictive value for pregnancy. Reprod Biomed Online. 2007;14(3):294–299. doi: 10.1016/s1472-6483(10)60870-x. [DOI] [PubMed] [Google Scholar]
- 22.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32(9):1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avendaño C, Franchi A, Taylor S, Morshedi M, Bocca S, Oehninger S. Fragmentation of DNA in morphologically normal human spermatozoa. Fertil Steril. 2009;91(4):1077–1084. doi: 10.1016/j.fertnstert.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Avendaño C, Franchi A, Duran H, Oehninger S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplas mic sperm injection outcome. Fertil Steril. 2010;94(2):549–557. doi: 10.1016/j.fertnstert.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 25.Aitken RJ, Baker MA, Nixon B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J Androl. 2015;17(4):633–639. doi: 10.4103/1008-682X.153850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Marín C, Gosálvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13(11):14026–14052. doi: 10.3390/ijms131114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Rodríguez A, Gosálvez J, Agarwal A, Roy R, Johnston S. DNA damage and repair in human reproductive cells. Int J Mol Sci. 2018;20(1):31–31. doi: 10.3390/ijms20010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dada R. Sperm DNA damage diagnostics: when and why. Transl Androl Urol. 2017;6(Suppl 4):S691–S694. doi: 10.21037/tau.2017.05.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim BK, Park SK. Phosphatidylserine modulates response to oxidative stress through hormesis and increases lifespan via DAF-16 in Caenorhabditis elegans. Biogerontology. 2020;21(2):231–244. doi: 10.1007/s10522-020-09856-0. [DOI] [PubMed] [Google Scholar]
- 30.Renes J. Phosphatidylserine, another player in macrophage recruitment in white adipose tissue? Adipobiology. 2010;2:23–32. [Google Scholar]
- 31.Rival CM, Xu W, Shankman LS, Morioka S, Arandjelovic S, Lee CS, et al. Phosphatidylserine on viable sperm and phagocytic machinery in oocytes regulate mammalian fertilization. Nat Commun. 2019;10(1):4456–4456. doi: 10.1038/s41467-019-12406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, et al. Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl. 2007;65:245–259. [PubMed] [Google Scholar]
- 33.Pacheco A, Blanco A, Bronet F, Cruz M, García-Fernández J, García-Velasco JA. Magnetic-activated cell sorting (MACS): a useful sperm-selection technique in cases of high levels of sperm DNA fragmentation. J Clin Med. 2020;9(12):3976–3976. doi: 10.3390/jcm9123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cakar Z, Cetinkaya B, Aras D, Koca B, Ozkavukcu S, Kaplanoglu I, et al. Does combining magnetic-activated cell sorting with density gradient or swim-up improve sperm selection? J Assist Reprod Genet. 2016;33(8):1059–1065. doi: 10.1007/s10815-016-0742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasanen E, Elqusi K, ElTanbouly S, Hussin AE, AlKhadr H, Zaki H, et al. PICSI vs.MACS for abnormal sperm DNA fragmentation ICSI cases: a prospective randomized trial. J Assist Reprod Genet. 2020;37(10):2605–2613. doi: 10.1007/s10815-020-01913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO laboratory manual for the examination and processing of human semen. Sixth edition. [ July 27; 2021 ]. Avialabale from: https:// wwwwhoint/publications/i/item/9789240030787. (15 Jun 2021) [Google Scholar]