Highlights
-
•
Lignocellulosic substrates have been used for microbial pigment production.
-
•
Solid State Fermentation with agroindustrial side stream products is reported.
-
•
Submerged fermentation with hydrolysates yields biopigments.
Keywords: By-product, Colorant, Fermentation, Residue, Solid state fermentation, Sustainability
Abstract
The search for sustainable processes is constantly increasing in the last years, so reusing, recycling and adding value to residues and by-products from agroindustry is a consolidated area of research. Particularly in the field of fermentation technology, the lignocellulosic substrates have been used to produce a diversity of chemicals, fuels and food additives. These residues or by-products are rich sources of carbon, which may be used to yield fermentescible sugars upon hydrolysis, but are usually inaccessible to enzyme and microbial attack. Therefore, pre-treatments (e.g. hydrolysis, steam explosion, biological pretreatment or others) are required prior to microbial action. Biopigments are added-value compounds that can be produced biotechnologically, including fermentation processes employing lignocellulosic substrates. These molecules are important not only for their coloring properties, but also for their biological activities. Therefore, this paper discusses the most recent and relevant processes for biopigment production using lignocellulosic substrates (solid-state fermentation) or their hydrolysates.
Introduction
Sustainability is one of the key aspects to be achieved in industrial processes nowadays, since customers’ awareness about ecological challenges is constantly increasing. The idea of sustainability encompasses social, economical and environmental aspects and focuses on reducing waste production, among other aspects. One of the main factors to develop a sustainable process is reducing the volume of waste generation or using residues in other processes in order to add value to it (Woiciechowski et al., 2014). In this regard, a challenge yet to be fully addressed is the need to find a reasonable destination to lignocellulosic biomass produced as a by-product in several distinct agroindustrial processes.
Lignocellulosic biomass is generated as residue or by-product in many processes from the agri-food chain. This means it is underutilized or discarded in great amounts every year, while it could be employed to produce value-added products. For instance, lignocellulosic substrates can be used in fermentation processes (e.g. as part of culture media) to yield commercially relevant compounds such as ethanol (Robak & Balcerek, 2020), organic acids (Grewal and Khare, 2018, Jiménez-Quero et al., 2020), enzymes (Namnuch et al., 2021, Rodrigues et al., 2020), polymers (Kawaguchi, Ogino, & Kondo, 2017), and others, including processes based on an integrated biorefinery approach (de Corato et al., 2018, Philippini et al., 2020). Some of the challenges related to the use of lignocellulosic substrates are the recalcitrance of this feedstock and the pre-treatment costs required for the subsequent use in fermentations (Zoghlami & Paës, 2019). However, the integration of these side-stream products in biorefineries to develop more sustainable and profitable processes is still a major driving force to support many researches and developments in this field.
Pigments are molecules capable of absorbing light in different wavelengths, manifesting a given color. This is a key feature in different sectors, especially the food industry (Lopes & Ligabue-Braun, 2021). They can be used to confer, reinforce or restore a product’s color, which is one of the most important sensorial properties of foods. Therefore, the market for food colors has been constantly increasing, and it is expected to reach $5.4 billion by 2026 (Marketandmarkets, 2021). Although synthetic pigments still predominate over their natural counterparts, the demand for the latter has been constantly increasing. In 2025, roughly half of this market will be of “natural” compounds (Grand view research, 2017). Anthocyanins, for example, have an estimated market of $387.4 million in 2021 (Usmani, Sharma, Sudheer, Gupta, & Bhat, 2020). Besides being possibly used as natural colorants, these compounds, as for many other pigments, present interesting biological properties, such as antioxidant, antiproliferative, antimicrobial, among other possible activities (Lagashetti et al., 2019, Pailliè-Jiménez et al., 2020, Sen et al., 2019).
In this sense, biotechnologically-produced coloring molecules, the so-called “biopigments”, emerge as an attractive alternative to supply natural pigments for industrial applications. Although natural pigments can be also obtained from animals (i.e. insects, mollusks) and plants the biopigments may be a more attractive alternative, since it does not depend on seasonality, the process control is easier and enables the production of new unknown molecules, including the use of metabolic engineering or recombinant technology (Lopes and Ligabue-Braun, 2021, Usmani et al., 2020). These coloring substances may be found in different microorganisms: from bacteria and microalgae to yeasts and filamentous fungi (Pailliè-Jiménez et al., 2020). One interesting approach to screen for new pigment-producing strains is to look for microorganisms growing in extreme conditions, as these harsh conditions require a diverse metabolic arsenal and the production of protecting substances, such as pigments (Menezes, de Medeiros, & Lima, n.d).
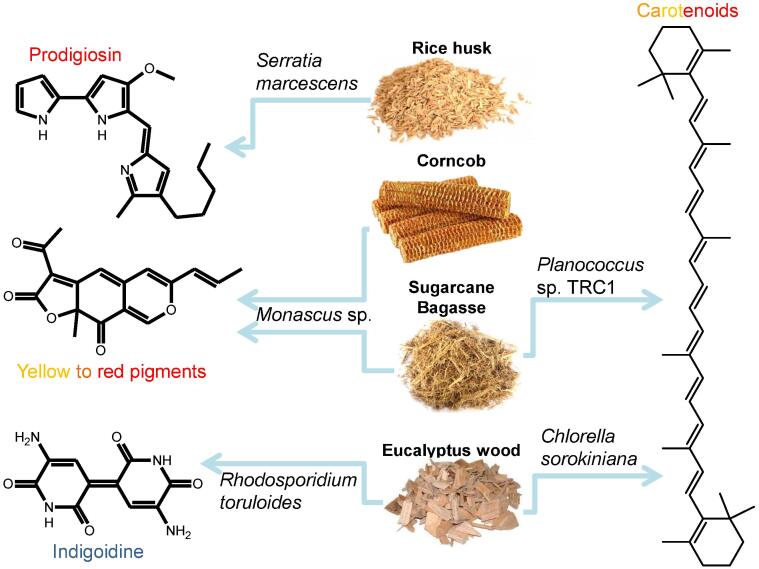
One of the main bottlenecks in biopigment large-scale production is related to the costs, which is mostly impacted by formulating the culture medium (Panesar, Kaur, & Panesar, 2015). In this sense, the use of side-stream industrial products can be a promising strategy to allow more commercially viable processes for supplying biopigments for the food market (Rana, Bhattacharyya, Patni, Arya, & Joshi, 2021) (Fig. 1). Therefore, this review aims to identify the main fermentative processes using lignocellulosic substrates to biopigments production, the parameters applied and the main challenges still faced in this field.
Fig. 1.
Coloring substances that may be produced by pigments using lignocellulosic substrates.
Lignocellulosic substrates
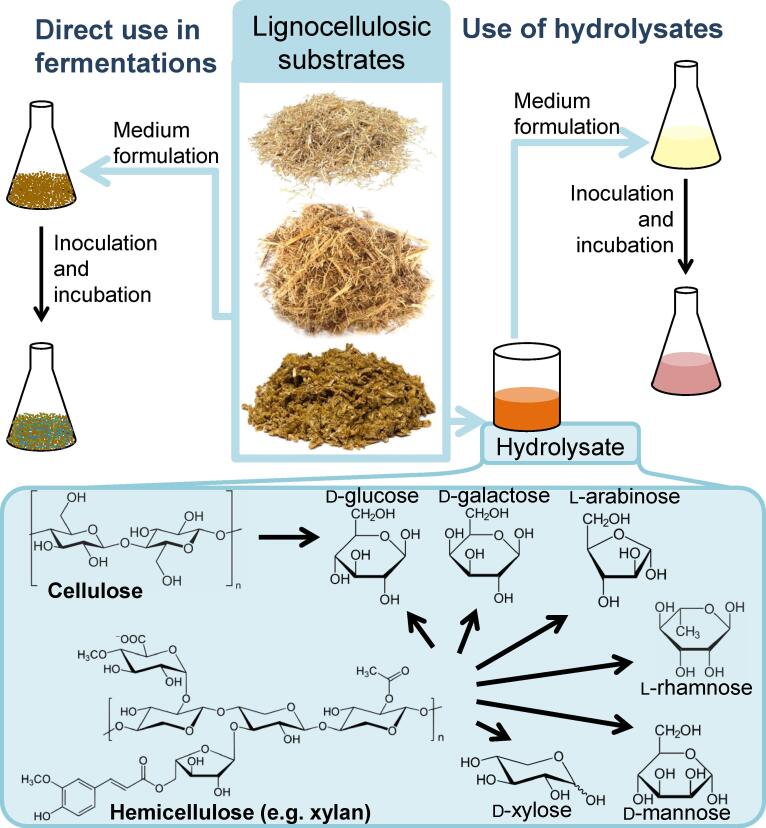
Lignocellulosic substrates correspond to the biomass composed of the main constituents of the outer layer of cell wall of vegetables, i.e. the carbohydrates cellulose and hemicellulose, and the phenolic-based lignin (Kumar et al., 2020, Pellera and Gidarakos, 2018). Cellulose, which is usually the main constituent of lignocellulosic substrates (around 35–55 %), refers to a linear homopolymer comprising of glucose residues linked by β-1,4 glycosidic bonds with a high degree of polymerization and a compact and highly crystalline structure not easily accessible to enzymes or chemical catalysts (Rajesh Banu & Preethi, 2021). Hemicellulose, in contrast, is a heterogenous group of small (DP ∼ 200) and non-crystalline heteropolymers made of varied monomers (xylose, arabinose, glucose, galactose, mannose and rhamnose), therefore its complete hydrolysis may require different enzymes, it constitutes 20–40 % of lignocellulosic substrate (Rajesh Banu & Preethi, 2021). Lignin is a non-carbohydrate polymer made of phenolic precursors, yielding a rigid material and highly resistant to biodegradation (da Silva, 2021, Huang et al., 2017, Reshmy et al., 2022) it accounts for 5 to 30 % of lignocellulosic biomass composition (Islam, Wang, Rehman, Dong, Hsu, Lin, & Leu, 2020).
Therefore, they are mostly found as residues or by-products of agri-food industry, such as straws, bagasses, brans, husks etc., and represent a rich carbon source. The main examples of lignocellulosic substrates include barley straw, coconut hunk, corn stover, rice, sugarcane bagasse and wood (Rajesh Banu & Preethi, 2021). In parallel, the formulation of culture media represents an important part of the fermentation costs, hence using cheap and widely available lignocellulosic biomass can allow the development of more economically feasible bioprocesses (Lopes & Ligabue-Braun, 2021). These lignocellulosic substrates can be either used directly in fermentation processes (Solid State Fermentations) (Embaby, Hussein, & Hussein, 2018) or their components can be previously hydrolysated to yield fermentable sugars, which can be substrates to generate biofuels or other value-added products, such as the aforementioned coloring molecules. Either way, they usually must be treated before being used due to their recalcitrance and resistance to enzymatic hydrolysis. In fact, this remains one of the main challenges associated with the use of lignocellulosic substrates (Anu et al., 2020). The next section will briefly discuss the main pre-treatments employed to prepare lignocellulosic substrates for further fermentation.
Pre-treatments of lignocellulosic substrates
As previously mentioned, the use of agroindustrial residues and by-products might be an important strategy to reduce the fermentation costs and to improve the sustainability aspects of bioprocesses (Panesar et al., 2015). However, most of these side-stream products are lignocellulosic substrates which are not naturally prone to fermentation in their native form (Singh Nee Nigam and Pandey, 2009). Therefore, pre-treatments are usually required for homogenizing these materials, to separate their main components (cellulose, hemicellulose and lignin) and to disrupt their organized structure, exposing the chemical bonds to enzyme attack (de Bhowmick, Sarmah, & Sen, 2018) and also to reduce or remove toxic or recalcitrant components, allowing the microbial growth and metabolism. There are several physical, chemical or biological pre-treatments available, the most important being summarized in the next paragraphs. These pre-treatments may be combined to make the carbohydrates more accessible to the microorganisms in the following fermentation stage (Philippini et al., 2020).
The physical pre-treatments generally involve mechanical and/or thermal procedures and usually demand high energy input, impacting the processing costs. These treatments intend to yield a fine and decristalized structure, to increase pore size and hydrolyze hemicellulose and to alter the structure of these materials, favoring the separation of their components. The main examples of physical pre-treatments are mechanical extrusion, milling (and size classification), steam explosion (steam treatment), hydrothermal processing and irradiation (Ghoshal et al., 2012, Khan et al., 2021, Sadh et al., 2018).
Mechanical extrusion is used to pre-treat biomasses by continuous shear mixing under high temperature. Thus, crystalline cellulose is disrupted. This method requires high energy and cost; therefore, it is hard to use in large-scale industrial applications. However, it can be combined with other pretreatment methods for better efficiency. Combination of methods, of techniques is in fact strongly recommended. The continuous combination of an alkaline thermo-mechano-chemical pretreatment neutralization and enzymes into a twin-screw extruder has been used for some lignocellulosic co-products. In dry-milling, no chemicals are used, and thus, no inhibitors are produced, a true advantage. Wet-milling with alkali pretreatment of corn stover resulted in increased conversion of cellulose and hemicellulose to reduce-sugars. (Khan et al., 2021).
These pre-treatments may also apply chemicals to promote delignification of lignocellulosic substrates and the degradation of lignin or hemicellulose, but these procedures tend to require corrosion-resistant materials and may generate environmental liabilities. The main examples are acid (e.g. H2SO4, HCl, HNO3) or alkaline (e.g. NaOH or NH4OH) treatments, and the use of oxidizing agents (e.g. peroxides, ozone) or organosolvents (e.g. alcohols) (Chen et al., 2017, Zhang et al., 2020). Acid hydrolysis is a cost-effective strategy, but particularly in terms of bioprocesses, these treatments may result in an important disadvantage, since some of them may generate inhibitory substances to the microorganisms. Lignin degradation results in inhibitory compounds, such as phenolic compounds, while cellulose and hemicellulose degradation yield furfural, 5-hydroxymethylfurfural (HMF) and weak organic acids. Furfural and HMF interfere with cell replication and the enzymatic activity related to energy metabolism (glycolytic and TCA pathways), but their inhibitory effect is usually noticed in concentrations exceeding 1.0 g/L (Devi, Singh, Bajar, Pant, & Din, 2021). Phenolic compounds, in turn, interfere with the microbial growth by affecting the membrane integrity and the metabolic activities in microbial cells, usually presenting higher toxicity than furfural compounds (Devi et al., 2021, Kumar et al., 2020). Thus, these are undesirable compounds in fermentation procedures, so they must be removed (or their concentrations must be sharply reduced) to favour this bioprocess (Devi et al., 2021, Li et al., 2020). Strategies to counteract their inhibitory effect include removal strategies varying from physical (e.g active charcoal, membrane separation) to chemical (e.g neutralization and liquid–liquid extraction) and biological methods (e.g Membrane-assisted cell retention) (Kumar et al., 2020). Additionally, the search for tolerant strains or improving their resistance are possible approaches (Wang, Sun, & Yuan, 2018).
A novel, low-cost and easy-to-implement pretreatment method “Densifying Lignocellulosic biomass with acidic Chemicals (DLC)” was recently described by Yuan et al. (2022). In such procedure, sulfuric acid at high concentrations is able to hydrolyze biomass at low temperatures. However, concentrated acid hydrolysis techniques usually consume a lot of acid and cause severe corrosion to the reactor, as the reactions are typically conducted in aqueous solutions, which renders this treatment not promising. In the new process, the acid dosage used is similar to “Diluted Acid” pretreatments, in the range of 0.05–0.1 g/g biomass. However, as the biomass moisture is controlled at 20 %-35 %, the acid concentration in the biomass can be as high as 40 %. (Yuan et al., 2022).
Finally, microorganisms or enzymes might be used to selectively degrade lignin of hemicellulose in these lignocellulosic substrates. The most notable examples include the use of white-rot fungi and lignin-degrading enzymes (lignin peroxidase, manganese peroxidase or laccase) (da Silva, 2021, Reshmy et al., 2022). This strategy requires less energy input and operates at milder conditions, yielding fewer side-products. But they might require longer periods and present a lower hydrolysis rate. Thus, the use of this technique is better when combined with other treatments enhancing the results. More research is requested as described in the review written by Huang, Jiang, Shen, Hu, Tang, Wu, Ragauskas, Jameel, Meng, and Yong (2022). Lignin contributes significantly to biomass recalcitrance by physically limiting the access of enzymes to carbohydrates. To alleviate the negative effects of lignin on enzyme performance, a deep understanding of lignin structural transformation upon different types of pretreatments as well as how and where does lignin bind to enzymes are prerequisites. In the last decade, the progress toward a fundamental understanding of lignin-enzyme interaction, structural characterization of lignin during pretreatment and/or conformation change of enzyme during hydrolysis resulted in some advances in the development of methodologies to mitigate the negative effect of lignin, however there is still a lot of work to be done in the coming years and decades (Huang et al., 2022).
The next section will describe the main aspects of biopigment production in different lignocellulosic substrates, either used directly or as starting material for obtaining hydrolyzates rich in fermentable sugars.
Microbial production of pigments using lignocellulosic substrates
The production of biopigments using lignocellulosic substrates may be divided in two main groups: those fermentation processes using the lignocellulosic substrate itself as substrate to support microbial growth and the production of metabolites, and other processes which employ a hydrolysate (rich in fermentable sugars) of the lignocellulosic substrate for formulating the culture media (Fig. 2). As may be evidenced in the following sections, the latter is the most commonly used approach in recent studies.
Fig. 2.
Production of biopigments from lignocellulosic substrates by either directly fermenting them or their hydrolysates.
Direct use of lignocellulosic substrates
The direct use of lignocellulosic substrates in fermentation processes have been widely reported for the production of a diversity of microbial metabolites (Kumar et al., 2020). In this sense, as the substrate is solid or semisolid, the technology involved is referred to as Solid State Fermentation (SSF). Although not as widely employed as the submerged fermentations in commercial bioprocesses, SSF has been a prosperous research field given its possible advantages. In comparison to submerged fermentation, this technology consumes less energy, requires less initial investments, has lower operational costs, is less prone to contaminations, does not involve foam formation, and the structure of the substrate resembles the conditions usually found for the microbial growth (in case of molds) in their natural environment. However, heterogeneity, difficulty to control the operational variables and to scale-up these processes are still challenges to be overcome (Ghoshal et al., 2012, Sadh et al., 2018). In terms of biopigment production, SSF using lignocellulosic materials as substrates have been reported (Table 1), as the examples shown in the following paragraphs.
Table 1.
Examples of microbial pigments produced by solid-state fermentation using lignocellulosic substrates.
| Subsa | Compb | M.O.c | Condd | PPe | Reff |
|---|---|---|---|---|---|
| WB | n.a | Serratia marcescens UCP 1549 | 28 °C/120 h/Static | Prodigiosin119.8 g/kg dry substrate | 1 |
| SCB and PMS | n.a | Planococcussp. TRC1 | 30 °C/120 h/mixing each 12 h | PMS: 31.05 mg/g (36 h)SCB: 41.4 mg/g (48 h) | 2 |
| WB | Starch (15–25 %), Cellulose (∼10 %), Arabinoxylans Glucans (10–25 %) | Chitinophaga pinensisChromobacterium vacciniiGordonia alkanivorans | 22 °C/30 °C, 100–150 rpm | Flexirubin (0.15 mg/L)Violacein (1.47 mg/L)Carotenoids (0.07 mg/L) | 3 |
| Several | n.a | Mucor wosnessenskii | 23 °C/static/144 h | 261.5 mg β-carotene per kg fermented product | 4 |
n.a – not available.
Substrate: CC – Corncob; PMS – Paper mill sludge; SCB – Sugarcane Bagasse; WB – Wheat Bran.
Composition.
Microorganism.
Fermentation conditions.
Pigment production.
References: 1. dos Santos et al., 2021; 2. Majumdar et al., 2020; 3. Cassarini et al., 2021; 4. Klempová et al., 2020.
Prodigiosin is a red pigment produced by different microorganisms, especially the Gram-negative bacteria Serratia marcescens (Sen et al., 2019). Six agro-industrial by-products were tested to formulate culture media to produce this pigment: wheat bran (WB), sugarcane bagasse (SCB), instant noodle waste (INW), tangerine peels (TP), pineapple peels (PAP) and pineapple crown (PAC). The SSF was carried out for 120 h at 28 °C under static conditions, using Erlenmeyer flasks with 5 g of each dry solid agrosubstrate separately or in combination with 5 % of waste soybean oil (WSO) and saline solution. WB was the only substrate that yielded a red pigment production, reaching 119.8 mg/g dry substrate (dos Santos et al., 2021). Prodigiosin was also produced through SSF using S. marcescens grown on Paper Mill Sludge (PMS) as substrate, which was composed of 20 % lignin, 15 % hemicellulose and 62 % cellulose. In this case, a maximal production of 30.05 mg per g of PMS was obtained (Majumdar et al., 2020).
SCB and PMS were also evaluated for β-carotene production by the bacteria Planococcus sp. TRC1 using SSF. The highest yield for PMS was 31.05 mg/g after 36 h while the highest yield for SCB was 41.4 mg/g after 48 h. Therefore, PMS could be considered a suitable substrate for pigment production although better results were achieved with SCB, a more studied feedstock for solid state fermentation (Majumdar, Mandal, & Dasgupta Mandal, 2020).
WB is a low-cost lignocellulosic material, produced globally in high amounts, and it is mainly composed of starch (15–25 %), cellulose (∼10 %), arabinoxylans and glucans (10–25 %) and proteins (∼15 %) (Apprich et al., 2014). Due to its low lignin content, it has a low recalcitrance level and may be directly applied in formulating culture media for pigment production (Cassarini, Besaury, & Rémond, 2021). Four microorganisms were evaluated for producing pigments using WB as a carbon source: Gordonia alkanivorans, Chitinophaga pinensis, Chromobacterium vaccinii and Ashbya gossypii. These microorganisms were chosen as they possess genes related to fractionating lignocellulosic compounds or they were previously described as pigment producers. The culture media were prepared by supplementing 5 g/L of WB into Minimal Salt Medium. Compared to the optimized medium, the production on WB-based medium was much lower for all microorganisms, except C. pinensis, which produced approximately 50 % of flexirubin value in WB-based medium (0.15 mg/L) in comparison to the optimal medium (0.27 mg/L). The authors suggest that WB is a promising substrate for pigment production, but further studies on this material are encouraged to improve its use in such fermentation processes (Cassarini et al., 2021). Intending to increase pigment production with lignocellulosic substrates, some researchers have considered different approaches, as described below.
Combining other carbon sources and lignocellulosic substrates is one strategy to increase yield and pigment production by fermentation. Glucose, glycerol and starch, for example, have been added to several agroindustrial residues when formulating the media culture for the production of γ-linolenic acid and β-carotene by the strain Mucor wosnessenskii. After optimization of fermentation conditions, the production of γ -linolenic acid and β-carotene reached 10.7 g/kg and 261.5 mg/kg of fermented product, respectively (Klempová, Slaný, Šišmiš, Marcinčák, & Čertík, 2020). Indeed, since glycerol is also an important by-product resulting from biodiesel production, the use of this carbon source may be regarded as an attractive co-valorization strategy to use different by-products in a rational and optimized process for microbial pigment production. In this sense, compared to other raw agroindustrial products (rice grains, SCB, potato peel), corncob was the ideal substrate for the production of red and orange pigments from Monascus purpureus ATCC16436, especially when supplemented with glucose and glycerol, which increased color value in approximately 170–180 % compared to cultures lacking those supplements (Embaby et al., 2018). Glycerol was also used in combination with other agroindustrial residues, such as corn steep liquor and rice parboiling water, for the production of carotenoids by Sporidiobolus salmonicolor, but the carotenoid production obtained was only a few mg per liter (Colet et al., 2017, Colet et al., 2019) or even less (Valduga et al., 2014).
Bostrycin, an antimicrobial red pigment from Nigrospora sp. no.47 could be produced by submerged fermentation using cane molasses and SSF using SCB, RB, corn flour and soy meal. In the last case, SCB resulted in 10–40 folds higher pigment production than the other media. Comparing both fermentation modes, the antimicrobial activity from the culture medium extract was, at least, 30 % lower in SSF compared to submerged fermentation. On the other hand, less color contamination was noticed when using SSF, supposedly because other pigments were not concomitantly produced (Huang et al., 2017).
Despite the advantages already mentioned for SSF, these bioprocesses have the disadvantage of using highly heterogeneous and complex starting materials. This is a challenge in terms of downstream processing, since the recovery and purification of the bioproducts produced by SSF may be more complex as there is more diversity of interactions, increasing residual impurities which are undesirable for products requiring high purity (Banat, Carboué, Saucedo-Castañeda, & de Jesús Cázares-Marinero, 2021). In this sense, the use of submerged fermentation with hydrolysates of side-stream products might be more attractive from an industrial perspective since process parameters control and product recovery procedures are usually simpler in such conditions (Webb, 2017).
Fermentations using hydrolysates from lignocellulosic substrates
In contrast to the former section, focused on SSF, the present section presents the processes using hydrolysates as substrates, which are usually employed to formulate liquid culture media. Therefore, the resulting bioprocesses employing these hydrolysates are classified as submerged fermentations. In comparison to SSF, in submerged fermentations the control of the physico-chemical parameters is easier and higher productivity is expected, although the operational costs (mostly energy expenses) and foam formation are challenging features. In recent years, the use of submerged fermentation has been the first choice due to some advantages regarding parameters control, scale-up and processing capacity compared to solid state fermentation (Zhang et al., 2021). Thus, most commercial bioprocesses employ submerged fermentations as strategy (Lopes & Ligabue-Braun, 2021). In terms of biopigments production, the most recent examples are described in the next paragraphs and are also summarized in Table 2.
Table 2.
Examples of microbial pigments produced by submerged fermentation using hydrolysates of lignocellulosic substrates.
| Subsa | Pretreatment | Compb | M.O.c | Condd | PPe | Reff |
|---|---|---|---|---|---|---|
| BW | 3 % H2SO45 % solid concentration100 °C/ 60 min | Gly-1.2, Xyl – 4.7, Man – 0.26, Gal – 0.21, Ara- 0.26, Rha – 0.088, AA – 2.1, FF – 0.015, HMF – 0.050 | Chrorella sorokiniana | 32.5 h/25 °C Light irradiance:75 µE m−2/s (auto/mixotrofic)No light (heterotrophic) | 0.65 d.w Carotenoids | 1 |
| BSG | Immersion (1:6 w:w) in 2 % w/v H2SO4 at 120 °C/15 min. pH adjusted to 10, held at 55 °C/1h. Centrifugation and adjustment to pH 5.5 with 25 % (w/w) H2SO4 | n.a | M. purpureus CMU 001 | 30 °C/350 rpm/7 days | 22.25 UA500/mL red pigment | 2 |
| CC | 121 °C, 15 psi, solid:liquid ratio 1:10. 1.5 % v/v and 90 min. Filtration and detofixication | Cbi – 0.97, Gly-8.31, Xyl − 33.66, Ara − 6.39, AA −4.18 | Talaromyces atroroseus GH2 | 30 °C/200 rpm/8 days | 16.17 OD500nm | 3 |
| CC | Immersion (ratio 2:5) in 70 % w/w H2SO4 at 30 °C/1,5h. Filtration. 5 % w/w H2SO4, 2:5 at 120 °C/1.5 h. pH adjusted to 6.0. Detoxification for 1.5 h and centrifugation | Gly − 41.7, Xyl − 28.3, AA − 4.2, FF − 0.8, HMF − 0.8 | M. purpureus CICC5041 | Agitation varied from 100 to 350 rpm (days 1–5), aeration from 0.2 to 3 vvm | 25.8 UA500/mL, Monascus pigment | 4. |
| EUC | 200 °C/10 minSteam explosionSeparation solid – liquidLiquid underwent acid hydrolysis 4 % (w/w) sulfuric acid, 120 °C/ 60 min | Gly −14.02, Xyl – 58.06, Ara – 3.50, AA – 29.03, HMF – 1.28, FF – 4.82, FA – 6.96, TP – 4.29 | R. toruloides | 30 °C/300 rpm | n.a. | 5 |
| RH | Soaking in 2 % w/v H2SO4. 130 °C/2,5h.Neutralization with NaOH 1 mol/L until pH 5.0 | c.a. 30 g/L total sugars concentration | M. purpureus M630; M. purpureus M523 | 30 °C/150 rpm | 72.1 U/mL (submerged), 80.7 U/mL (immobilized) | 6 |
| RS | Soaking (ratio 1:10, w/v) into 2 % (w/v) H2SO4 125 °C/2,5h. Neutralization to pH 5.5. Decolorization and detoxification 30 °C/24 h/150 rpm | n.a | M. purpureus LQ-6 | 30 °C/10 days/150 rpm | 20.86 U/mL Monascus pigment | 7 |
| SCB | 0.3 M NaOH, 70 °C/4h. Enzymatic hydrolysis: 20 FPU.g−1, 50 °C/48 h. Filtration | Gly − 50.01, Xyl − 22.22, Cbi − 18.51, Ara − 2.55 | M. ruber Tieghem IOC 2225 | 30° 150 rpm 12 days | 18.71 UA490Monascus red pigment | 8 |
| SW | 200 °C/10 min Steam explosion. Acid hydrolysis (liquid fraction): 4 % (w/w) H2SO4 120 °C/ 60 min. pH adjusted to 4.6 | Gly −14,96, Xyl – 39.99, Ara – 3.47, AA – 18.44, HMF – 1.75, FF – 3.23, FA – 9.87, TP – 5.91 | R. toruloides | 30 °C/300 rpm | n.a. | 5 |
| SW + EUC | n.a | n.a | R. toruloides | n.a. | 0.67 g/L (mixture)1.51 g/L (EUC) Indigoidine | 9 |
| WS | Hot water: 195 °C/ 45 min. Acid hydrolysis (liquid fraction): 2 % (w/w) H2SO4 121 °C/20 min. Neutralization and centrifugation. Enzymatic hydrolysis (solid fraction): 50 °C/50 rpm/72 h | Gly-43.60, Xyl – 12.34, AA – 1.14, FF – 0.01, TP – 0.19 | R. toruloides NRRL Y-1091 | 30 °C/250 rpm/120 h | 24.58 mg/LCarotenoids | 10 |
n.a – not available
Substrate: BW – Beech Wood; BSG – Brewer’s spent grain; CC – Corncob; EUC – Eucalyptus; PMS – Paper mill sludge; RH – Rice Husk; RS – Rice Straw; SCB – Sugarcane Bagasse; SW – Switchgrass; WS – Wheat Straw.
Hydrolysate composition, in g/L: Glu-Glucose; Xyl – Xylose; Ara – Arabinose; FF- Furfural; HMF – Hidroxymetylfurfural; AA - Acetic Acid; Cbi – Cellobiose; Man – Mannose; Rha – Rhamnose; Gal – Galactose; FA – Formic Acid; TP – Total Phenolic.
Microorganism.
Fermentation conditions.
Pigment production.
References: 1. Miazek, 2017; 2. Silbir & Goksungur, 2019; 3. Morales-Oyervides et al., 2020; 4. Zhou et al., 2014; 5. Bonfiglio et al., 2021; 6. Zhang et al., 2021; 7. Liu et al., 2020; 8. Hilares et al., 2018; 9. Wehrs et al., 2019; 10. Liu et al. (2020).
Monascus pigments (MP) have been produced and applied in the food industry in Asian countries for more than 2,000 years. Currently, several side-stream products have been studied as sources of hydrolysates for MP production in submerged fermentation, especially those by-products from cereal processing, since they are produced in high amounts and present a reasonable similarity to the traditional rice matrix, even though nutrient supplementation might be required (Srianta et al., 2021). Rice husk (presenting 30–45 % cellulose and 19–34 % hemicellulose), for instance, was pretreated by soaking in 2 % (w/v) sulfuric acid followed by heat treatment in an autoclave at 130 °C/150 min and neutralization to pH 5.0 . The resulting rice husk hydrolysate (containing 30 g/L sugar) was supplemented with 60 g/L glucose and used as substrate in submerged fermentations. Productivities of 0.16 and 0.37 U/mL/h were achieved when the microorganism was used in free or immobilized (in calcium alginate) forms, respectively (Zhang et al., 2021). Rice straw was also pretreated by acid hydrolysis (2 % diluted sulfuric acid, 1:10 w/v ratio) followed by neutralization, discoloration and detoxification procedures before being used for preparing the fermentation medium. The resulting hydrolysate yielded a pigment production of 8.61 U/mL, which was further improved to 21.20 U/mL upon medium supplementation with glucose to reach an initial sugar concentration of 80 g/L. But the glucose-based fermentation medium, without hydrolysate, resulted in the best production, i.e. 33.96 U/mL (Liu et al., 2020). This example demonstrates how challenging it is to replace conventional culture media in terms of achieving equivalent production indicators. However, as the examples shown in the following paragraphs, it is possible to observe that some of these hydrolysates may result in equivalent or even higher pigment production. Additionally, statistical tools (e.g. Response Surface Methodology) might be a valuable approach to significantly increase pigment production. A recent example illustrates that extracellular MP was 2.5 times higher at optimized conditions compared to the original process (Liu et al., 2019).
SCB is a widely abundant residue obtained during sugarcane processing and it has been extensively studied for second-generation ethanol production (Alokika et al., 2021, Bezerra and Ragauskas, 2016). As a starting material for fermentation processes, it has been employed for red pigment production by Monascus ruber after an alkaline pre-treatment in a packed bed column reactor operating at 70 °C for 4 h with 0.3 M NaOH followed by an enzymatic hydrolysis using cellulase complex, yielding a mixture of glucose (50.01 g/L), xylose (22.22 g/L), cellobiose (18.51 g/L) and arabinose (2.55 g/L). The fermentation was carried out in 125 mL-erlenmeyers at 30 °C and 150 rpm for 12 days, at varied lighting conditions (dark condition, white light and orange light incidence). Under dark conditions, this hydrolysate yielded higher pigment production (18.71 UA 490 nm) compared to commercial glucose-based (7.45 AU490nm) (Hilares et al., 2018).
Corncob is the main by-product of maize processing, accounting for 30 % of the waste produced during the process (Embaby et al., 2018). Therefore, this is a highly abundant feedstock with a great potential to be used as substrate for fermentative processes, as it has high amounts of cellulose and hemicellulose. Several studies used corncob to substitute traditional carbon sources, such as glucose. For instance, corncob hydrolysate was used as a carbon source for Monascus pigment production, yielding 22.5 UA500/mL, after 120 h. This was quite similar to the production obtained with glucose-based medium (23.7 UA500/mL, after 109 h), hence, the use of corncob hydrolysate could be an efficient alternative for monascus pigment production (Zhou, Yin, & Hu, 2014). This was also approximately the same production achieved (22.25 UA500) when 2 % sulfuric acid was employed for the hydrolysis of brewer’s spent grain, the major side-stream product of beer production. Such production was achieved after testing the pre-treatment of this residue, composed of hemicellulose (53.1 %), cellulose (19.2 %) and lignin (8.5 %), at 120 °C for 15 min under different concentrations (from 1 to 6 %, w/v) of sulfuric acid (Silbir & Goksungur, 2019).
As may be noted in the previous paragraphs, most of the articles involving microbial pigment production in lignocellulosic substrates is related to MP. However, other examples have also been reported, as described next. The fungus Talaromyces atroroseus GH2, for instance, also produced pigments in a medium composed of corncob hydrolysate, which was obtained by acid hydrolysis at 121 °C and 15 psi. The sulfuric acid concentration and residence time were varied to assess the best conditions for preparing this hydrolysate. Xylose was the main component in all liquors generated. The best pigment production (16.17 OD500nm) was reached when using the dilute hydrolyzate, obtained with 1.5 % sulfuric acid (v/v) and 90 min, without nutrient supplementation. This result was comparable to the pigment production in the control medium (17.26 OD500nm). The pigments produced were most likely MP homologous (Morales-Oyervides et al., 2020).
Lignocellulosic hydrolysates from wood are rich in organic carbon sources and, therefore, they can also be used for cultivating microorganisms. In this case, the tree Fagus sylvatica treated by diluted acid (3 % H2SO4, 100 °C, 1 h), and its components isolated were used as a substrate for growing the microalgae Chlorella sorokiniana in mixotrophic conditions to produce carotenoids and fatty acids. The results revealed that such hydrolysate was composed mainly of acetate, xylose, glucose, mannose, galactose, arabinose, rhamnose, phenolic compounds, furfural and HMF. After neutralization, a 12 % v/v wood hydrolysate supported Chlorella growth and pigment production compared to photoautotrophic control, although the total pigment content was lower (0.65 × 0.86 % d.w, respectively) (Miazek, Remacle, Richel, & Goffin, 2017). Therefore, the authors suggested that this hydrolysate could be used as an attractive feedstock in both mixotrophic and heterotrophic modes, even though the results were best in mixotrophic conditions for all experiments carried out. Thus, light incidence in such processes is a key parameter to be evaluated, as light incidence has an important role in regulating metabolic pathways (Chen, Chen, Wu, & Li, 2021).
Switchgrass and eucalyptus hydrolysates were also mixed with synthetic defined media to produce biopigments. A mix of switchgrass and eucalyptus hydrolysates with synthetic media (9:1 ratio, v/v) was tested for indigoidine production by the engineered basidiomycete Rhodosporidium toruloides. The blue pigment production using lignocellulosic substrate was feasible, although the amount obtained (1.51 g/L in eucalyptus hydrolysate and 0.67 in a switchgrass and eucalyptus mixed hydrolysate) was smaller than obtained by using glucose-based (∼3.8 g/L) medium (Wehrs, Gladden, Liu, Platz, Prahl, Moon, Papa, Sundstrom, Geiselman, Tanjore, Keasling, Pray, Simmons, & Mukhopadhyay, 2019)).
Rhodotorula toruloides has also been applied for carotenoids production in hemicellulose hydrolysates produced by intensified steam explosion of switchgrass or Eucalyptus globulus chips as raw materials (Bonfiglio, Cagno, Yamakawa, & Mussatto, 2021). The pre-treatment procedure resulted in high contents of monosaccharides (14.96 g/L or 14.02 g/L glucose, 39.99 or 58.06 g/L xylose and 3.47 g/L or 3.50 g/L arabinose for switchgrass or E. globulus, respectively). The hydrolysates were detoxified to diminish the quantity of inhibitory compounds, such as phenolics and acetic acid, although they were still present in the media but R. toruloides was not able to grow in the detoxified hydrolysate (Bonfiglio et al., 2021). On the other hand, R. toruloides NRRL Y-1091 was able to produce carotenoids in a decolorized cellulosic hydrolysate produced from wheat straw,being 24.58 mg/L the highest concentration achieved (Liu, Feist, Dragone, & Mussatto, 2020).
Based on the information presented in this section, fermentation technology, particularly using hydrolysates from lignocellulosic substrates, may be regarded as a sustainable strategy for adding value to side-stream products from agro-industry. Moreover, the biopigments presented in this text are not only useful as coloring agents, but they may also be applied for pharmaceutical and nutritional purposes due to the increasing number of reports on their biological activities. Indeed, in some cases, these bioactivities may direct the primary application of such bioproducts in detriment to their ordinary coloring attributes, as also observed for other classical pigments. The next section will describe the main findings regarding the biological activities of biopigments and also suggest their potential applications beyond conferring color to food and other products.
Bioactivities of microbial pigments
The interest for natural pigments has been rising, especially due to their sustainable appeal and also for the increasing demand for substituting synthetic additives for natural products, since the former have been related to health problems and hyperactivity in children (Sigurdson, Tang, & Giusti, 2017). In contrast, natural pigments have been related to health benefits and to positive biological activities, supporting their applications beyond the food industry. Carotenoids and anthocyanins, for instance, are the most classical and well studied natural pigments in this sense, presenting bioactivities with pharmacological and nutritional interest. The health properties of anthocyanins, involving their antioxidant, anticancer and antimicrobial effects, besides their benefits to cardiovascular disease, diabetes and visual health, is well documented (Khoo, Azlan, Tang, & Lim, 2017). Similarly, carotenoids have been widely reported as antioxidant agents presenting benefits to eye and heart health, cognitive function, cancer prevention, skin protection, among others (Eggersdorfer & Wyss, 2018). Therefore, the investigation of bioactivities of natural pigments have also expanded to the microbial-derived coloring compounds, such as described in this section.
Biopigments are secondary metabolites produced by microorganisms as a response to stressful conditions (e.g. lack of nutrient, extremes of temperature or pH, competition with other microorganisms) or for light harvesting purposes, in case of photosynthetic microorganisms (Ramesh, Vinithkumar, Kirubagaran, Venil, & Dufossé, 2019). This explains why the production of such metabolites is favored at low nitrogen content (Arikan, Canli, Caro, Dufossé, & Dizge, 2020) and high light incidence (W. W. Zhang et al., 2018), for example. This is also the origin of most of the biological activities associated with such molecules. An adaptation to the presence of competing microorganisms, for example, requires the production of antimicrobial compounds, while the oxidative stress might be overcome by the production of antioxidant compounds (Gmoser, Ferreira, Lennartsson, & Taherzadeh, 2017). Indeed, one of the mechanisms for antioxidant activity is related to the capacity of a molecule to stabilize radicals, which might be achieved by a system consisting of conjugated double bonds (Sandmann, 2019). This is also the basis of the chromophore of light-absorbing substances, so it is expected that pigments are also promising antioxidants (Marizcurrena, Cerdá, Alem, & Castro-Sowinski, 2019). Consequently, many biopigments have been reported as, for example, antiproliferative, antimicrobial, anti-inflammatory compounds, which have highly desirable properties for pharmaceutical and nutritional applications (Choksi, Vora, & Shrivastava, 2020). Some of these properties are already explored by the pharmaceutical industry, as may be evidenced by the example of Monascus pigments (MP).
MP are diverse in structure and also in biological activities. In addition to their use as colorants, they have been applied as medicinal agents for a long time and in fermented foods, specially in East Asia (Agboyibor, Kong, Chen, Zhang, & Niu, 2018). Several studies present their bioactivities as antimicrobial, antioxidant, anti-inflammatory and antitumor (Tan, Xing, Chen, Tian, & Wu, 2018). They also play a role in preventing obesity and acting as anti-diabetic, anti-osteoporosis and antidepressants (Agboyibor et al., 2018). Their capacity for positively modulated serum lipids, particularly lowering ldl-cholesterol, has been the basis for pharmaceutical products already available on the market presenting this health claim (Mohankumari, Naidu, Narasimhamurthy, & Vijayalakshmi, 2021).
Prodigiosin, another red pigment, has been widely studied in this regard (Lin, Chen, Tseng, & Weng, 2020) in a way that different bioactivities have been reported for this microbial metabolite, such as antimicrobial (Yip, Mahalingam, Wan, & Nathan, 2021) , antitumor (Nguyen et al., 2020) and immunomodulation properties (Cuevas et al., 2020). Prodigiosin produced from Serratia marcenscens using rice bran was assessed for antioxidant and antimicrobial activity. The results revealed its ability to scavenge free-radical, reaching 99 % scavenging activity (ABTS and DPPH methods) at a concentration of 10 µg/mL, and to inhibit the growth of Escherichia coli , Bacillus cereus, Staphylococcus. aureus, Clostridium botulinum, Vibrio vulnificus, and Salmonella Enteritidis in the disc diffusion assay. (Arivizhivendhan, 2018). Shrimp head powder was also used as a substrate for the production of prodigiosin, which was further purified and tested in terms of antioxidant capacity using DPPH method (97 % scavenging at 200 µg/mL and a IC50 of 77,4 µg/mL), anti-NO activity (99 % of reduction and a IC50 of 16.22 µg/mL), as well as the ability to inhibit acetylcholinesterase (maximum inhibition 91 % and a IC50 0.64 mg/mL), which may be useful for treating Alzheimer disease (Nguyen et al., 2021).
Violacein is a blue-to-purple pigment that has also been associated with bioactivities, such as antimicrobial, antiparasite, antitumoral properties (Durán et al., 2021, Sasidharan et al., 2015). This pigment presents. Its antimicrobial activity has been evidenced for Gram-positive microorganisms, such as Staphylococcus epidermidis, possibly acting by targeting the cytoplasm membrane (Cauz et al., 2019, Dodou et al., 2017). Therefore, the A combination of violacein and a Gram-negative bacterial predator Bdellovibrio bacteriovorus HD100, a predator of Gram-negative bacteria, was investigatedalso assessed as a controlling systemagents in polymicrobial pathogenic communities (Im, Choi, Son, & Mitchell, 2017). Moreover, similarly to prodigiosin, violacein presented immunomodulatory activity and the capacity to inhibit inflammation (Choi, Lim, & Yoon, 2021).
Apart from antimicrobial activity, bostrycin could inhibit in vitro proliferation of gastric, lung and prostate cancer cell lines (Y. H. Huang et al., 2017), as well as tongue squamous cell carcinoma (Jie, Shi, Yue, Wang, & Zhang, 2020). In such a study, its probable mechanism of action was the induction of mitochondrial apoptotic pathways. Finally, indigoidine is another blue pigment with scarce data on literature addressing its biological activities. One of these studies indicated that the antimicrobial and antioxidant properties, frequently reported for the other biopigments, was also noticed in such molecule (Celedón & Díaz, 2021).
To sum up, this section illustrated that the coloring molecules produced by microorganisms are usually biologically active, and can modulate some metabolic processes in the individuals who consume these biopigments. In terms of food applications, this characteristic might raise some concerns, since it is expected that food additives should be as biologically inert as possible. However, as previously mentioned, bioactive pigments are not automatically prohibited in foods (see the example of carotenoids), and may have an increased range of applications (e.g. pharmaceutical industry) instead (dos Santos & Bicas, 2021).
Perspectives and concluding remarks
Pigments are usually produced as a response mechanism to stressful conditions, which can explain why many biopigments are also bioactive compounds (e.g., antioxidant and antimicrobial activity), being useful for non-food applications as well. In this sense, the search for pigment-producing microorganisms using an ecological-niche approach is a wise strategy to identify novel strains of interest and to diversify the biotechnological tools for providing industrial pigments to the market. Thus, investigating extreme environments to find pigment-producing microorganisms (Rosa, 2019) may result in the discovery of new (unknown) molecules with rare colors or bioactivities.
In parallel, it is worth mentioning the intense advances in molecular biology and gene editing technology in the last decades. This biotechnological revolution has been influencing different research areas, including fermentation technology and biopigment production. For instance, Talaromyces atroroseus has been used as a platform for red biopigment production, but the color pallete can be much greater with the adoption of CRISPR technology and metabolomic networks. Indeed, such red (natural) biopigment production may also be useful to meet the rising demand for additives employed for elaborating plant-based “meat” recipes, another leading recent trend in Food Science (Gerit & Anders, 2020). Moreover, correlating this and the former paragraphs, heterologous expression might be an essential tool to allow the production of biopigments from extremophiles in microorganisms easier to cultivate and more adapted to the conventional fermentation conditions.
Finally, fermentation technology shall be increasingly influenced by the strong wave of computational sciences. The use of Machine Learning approach, for example, has already been considered for the enzymatic production of xylooligosaccharides from corncob xylan (Khangwal, Chhabra, & Shukla, 2021). This multidisciplinary strategy is expected to become more common in the field of biopigment production.
Regardless which of these strategies is adopted, the fermentation using lignocellulosic substrates may be also considered to decrease production costs and to make the target biopigments more economically viable. Therefore, this review is important to help to drive future research in this field.
Funding
This work was supported by the National Council of Technological and Scientific Development (CNPq) (scholarship for Tiago Daniel Madureira de Medeiros - grant 140902/2019-8) and Coordination for the Improvement of Higher Education Personnel (CAPES – Finance Code 001).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Laurent Dufossé, Email: laurent.dufosse@univ-reunion.fr.
Juliano Lemos Bicas, Email: jlbicas@gmail.com, bicas@unicamp.br.
References
- Agboyibor C., Kong W.B., Chen D., Zhang A.M., Niu S.Q. Vol. 16. Elsevier Ltd.; 2018. Monascus pigments production, composition, bioactivity and its application: A review; pp. 433–447. (Biocatalysis and Agricultural Biotechnology). [DOI] [Google Scholar]
- Alokika, Anu, Kumar, A., Kumar, V., & Singh, B. (2021). Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective. In International Journal of Biological Macromolecules (Vol. 169, pp. 564–582). Elsevier B.V. https://doi.org/10.1016/j.ijbiomac.2020.12.175. [DOI] [PubMed]
- Anu, Kumar, A., Rapoport, A., Kunze, G., Kumar, S., Singh, D., & Singh, B. (2020). Multifarious pretreatment strategies for the lignocellulosic substrates for the generation of renewable and sustainable biofuels: A review. In Renewable Energy (Vol. 160, pp. 1228–1252). Elsevier Ltd. https://doi.org/10.1016/j.renene.2020.07.031.
- Apprich S., Tirpanalan Ö., Hell J., Reisinger M., Böhmdorfer S., Siebenhandl-Ehn S.…Kneifel W. Wheat bran-based biorefinery 2: Valorization of products. LWT - Food Science and Technology. 2014;56(2):222–231. doi: 10.1016/j.lwt.2013.12.003. [DOI] [Google Scholar]
- Arikan E.B., Canli O., Caro Y., Dufossé L., Dizge N. Production of bio-based pigments from food processing industry by-products (apple, pomegranate, black carrot, red beet pulps) using aspergillus carbonarius. Journal of Fungi. 2020;6(4):1–18. doi: 10.3390/jof6040240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arivizhivendhan, K. v., Mahesh, M., Boopathy, R., Swarnalatha, S., Regina Mary, R., & Sekaran, G. (2018). Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate. Journal of Food Science and Technology, 55(7), 2661–2670. https://doi.org/10.1007/s13197-018-3188-9. [DOI] [PMC free article] [PubMed]
- Banat, I. M., Carboué, Q., Saucedo-Castañeda, G., & de Jesús Cázares-Marinero, J. (2021). Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. In Bioresource Technology (Vol. 320). Elsevier Ltd. https://doi.org/10.1016/j.biortech.2020.124222. [DOI] [PubMed]
- Bezerra, T. L., & Ragauskas, A. J. (2016). A review of sugarcane bagasse for second-generation bioethanol and biopower production. In Biofuels, Bioproducts and Biorefining (Vol. 10, Issue 5, pp. 634–647). John Wiley and Sons Ltd. https://doi.org/10.1002/bbb.1662.
- Bonfiglio F., Cagno M., Yamakawa C.K., Mussatto S.I. Production of xylitol and carotenoids from switchgrass and Eucalyptus globulus hydrolysates obtained by intensified steam explosion pretreatment. Industrial Crops and Products. 2021;170 doi: 10.1016/j.indcrop.2021.113800. [DOI] [Google Scholar]
- Cassarini M., Besaury L., Rémond C. Valorisation of wheat bran to produce natural pigments using selected microorganisms. Journal of Biotechnology. 2021;339:81–92. doi: 10.1016/j.jbiotec.2021.08.003. [DOI] [PubMed] [Google Scholar]
- Cauz A.C.G., Carretero G.P.B., Saraiva G.K.V., Park P., Mortara L., Cuccovia I.M.…Gueiros-Filho F.J. Violacein targets the cytoplasmic membrane of bacteria. ACS Infectious Diseases. 2019;5(4):539–549. doi: 10.1021/acsinfecdis.8b00245. [DOI] [PubMed] [Google Scholar]
- Celedón, R. S., & Díaz, L. B. (2021). Natural pigments of bacterial origin and their possible biomedical applications. In Microorganisms (Vol. 9, Issue 4). MDPI AG. https://doi.org/10.3390/microorganisms9040739. [DOI] [PMC free article] [PubMed]
- Chen, H., Liu, J., Chang, X., Chen, D., Xue, Y., Liu, P., Lin, H., & Han, S. (2017). A review on the pretreatment of lignocellulose for high-value chemicals. In Fuel Processing Technology (Vol. 160, pp. 196–206). Elsevier B.V. https://doi.org/10.1016/j.fuproc.2016.12.007.
- Chen X., Chen M., Wu X., Li X. Cost-effective process for the production of Monascus pigments using potato pomace as carbon source by fed-batch submerged fermentation. Food Science and Nutrition. 2021;9(10):5415–5427. doi: 10.1002/fsn3.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S. Y., Lim, S., Yoon, K. hye, Lee, J. I., & Mitchell, R. J. (2021). Biotechnological Activities and Applications of Bacterial Pigments Violacein and Prodigiosin. In Journal of Biological Engineering (Vol. 15, Issue 1). BioMed Central Ltd. https://doi.org/10.1186/s13036-021-00262-9. [DOI] [PMC free article] [PubMed]
- Choksi J., Vora J., Shrivastava N. Bioactive pigments from isolated bacteria and its antibacterial, antioxidant and sun protective application useful for cosmetic products. Indian Journal of Microbiology. 2020;60(3):379–382. doi: 10.1007/s12088-020-00870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colet R., Urnau L., Bampi J., Zeni J., Dias B.B., Rodrigues E.…Valduga E. Use of low-cost agro products as substrate in semi-continuous process to obtain carotenoids by Sporidiobolus salmonicolor. Biocatalysis and Agricultural Biotechnology. 2017;11:268–274. doi: 10.1016/j.bcab.2017.07.015. [DOI] [Google Scholar]
- Colet R., Urnau L., de Souza Hassemer G., Dias B.B., Zeni J., Rodrigues E.…Valduga E. Kinetic Parameters of fed-batch production of carotenoids by sporidiobolus salmonicolor using low-cost agro-industrial substrates. Industrial Biotechnology. 2019;15(5):311–321. doi: 10.1089/ind.2019.0015. [DOI] [Google Scholar]
- Cuevas A., Saavedra N., Salazar L.A., Cavalcante M.F., Silva J.C., Abdalla D.S.P. Prodigiosin modulates the immune response and could promote a stable atherosclerotic lession in c57bl/6 ldlr-/-mice. International Journal of Molecular Sciences. 2020;21(17):1–18. doi: 10.3390/ijms21176417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bhowmick, G., Sarmah, A. K., & Sen, R. (2018). Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. In Bioresource Technology (Vol. 247, pp. 1144–1154). Elsevier Ltd. https://doi.org/10.1016/j.biortech.2017.09.163. [DOI] [PubMed]
- de Corato, U., de Bari, I., Viola, E., & Pugliese, M. (2018). Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review. In Renewable and Sustainable Energy Reviews (Vol. 88, pp. 326–346). Elsevier Ltd. https://doi.org/10.1016/j.rser.2018.02.041.
- Devi A., Singh A., Bajar S., Pant D., Din Z.U. Ethanol from lignocellulosic biomass: An in-depth analysis of pre-treatment methods, fermentation approaches and detoxification processes. Journal of Environmental Chemical Engineering. 2021;9(5) doi: 10.1016/j.jece.2021.105798. [DOI] [Google Scholar]
- Dodou, H. v., de Morais Batista, A. H., Sales, G. W. P., de Medeiros, S. C., Rodrigues, M. L., Nogueira, P. C. N., Silveira, E. R., & Nogueira, N. A. P. (2017). Violacein antimicrobial activity on Staphylococcus epidermidis and synergistic effect on commercially available antibiotics. Journal of Applied Microbiology, 123(4), 853–860. https://doi.org/10.1111/jam.13547. [DOI] [PubMed]
- dos Santos R.A., Rodríguez D.M., da Silva L.A.R., de Almeida S.M., de Campos-Takaki G.M., de Lima M.A.B. Enhanced production of prodigiosin by Serratia marcescens UCP 1549 using agrosubstrates in solid-state fermentation. Archives of Microbiology. 2021;203(7):4091–4100. doi: 10.1007/s00203-021-02399-z. [DOI] [PubMed] [Google Scholar]
- Durán, N., Nakazato, G., Durán, M., Berti, I. R., Castro, G. R., Stanisic, D., Brocchi, M., Fávaro, W. J., Ferreira-Halder, C. v., Justo, G. Z., & Tasic, L. (2021). Multi-target drug with potential applications: violacein in the spotlight. In World Journal of Microbiology and Biotechnology (Vol. 37, Issue 9). Springer Science and Business Media B.V. https://doi.org/10.1007/s11274-021-03120-4. [DOI] [PubMed]
- Eggersdorfer, M., & Wyss, A. (2018). Carotenoids in human nutrition and health. In Archives of Biochemistry and Biophysics (Vol. 652, pp. 18–26). Academic Press Inc. https://doi.org/10.1016/j.abb.2018.06.001. [DOI] [PubMed]
- Embaby A.M., Hussein M.N., Hussein A. Monascus orange and red pigments production by Monascus purpureus ATCC16436 through co-solid state fermentation of corn cob and glycerol: An eco-friendly environmental low cost approach. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0207755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva V.G. Laccases and ionic liquids as an alternative method for lignin depolymerization: A review. Bioresource Technology Reports. 2021;16 doi: 10.1016/j.biteb.2021.100824. [DOI] [Google Scholar]
- Ghoshal G., Basu S., Shivhare U.S. Solid state fermentation in food processing. International Journal of Food Engineering. 2012;8(3) doi: 10.1515/1556-3758.1246. [DOI] [Google Scholar]
- Gmoser, R., Ferreira, J. A., Lennartsson, P. R., & Taherzadeh, M. J. (2017). Filamentous ascomycetes fungi as a source of natural pigments. In Fungal Biology and Biotechnology (Vol. 4, Issue 1, pp. 1–25). BioMed Central Ltd. https://doi.org/10.1186/s40694-017-0033-2. [DOI] [PMC free article] [PubMed]
- Grand view research. (2017). Natural Food Colors Market Size Worth $2.50 Billion By 2025. Https://Www.Grandviewresearch.Com/Press-Release/Global-Natural-Food-Colors-Market.
- Grewal J., Khare S.K. One-pot bioprocess for lactic acid production from lignocellulosic agro-wastes by using ionic liquid stable Lactobacillus brevis. Bioresource Technology. 2018;251:268–273. doi: 10.1016/j.biortech.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Huang, C., Jiang, X., Shen, X., Hu, J., Tang, W., Wu, X., Ragauskas, A., Jameel, H., Meng, X., & Yong, Q. (2022). Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. In Renewable and Sustainable Energy Reviews (Vol. 154). Elsevier Ltd. https://doi.org/10.1016/j.rser.2021.111822.
- Huang Y.H., Yang W.J., Cheng C.Y., Sung H.M., Lin S.F. Bostrycin production by agro-industrial residues and its potential for food processing. Food Science and Biotechnology. 2017;26(3):715–721. doi: 10.1007/s10068-017-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im H., Choi S.Y., Son S., Mitchell R.J. Combined application of bacterial predation and violacein to kill polymicrobial pathogenic communities. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-14567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, M. K., Wang, H., Rehman, S., Dong, C., Hsu, H. Y., Lin, C. S. K., & Leu, S. Y. (2020). Sustainability metrics of pretreatment processes in a waste derived lignocellulosic biomass biorefinery. In Bioresource Technology (Vol. 298). Elsevier Ltd. https://doi.org/10.1016/j.biortech.2019.122558. [DOI] [PubMed]
- Jie J., Shi L., Yue S., Wang M., Zhang J. Bostrycin inhibits growth of tongue squamous cell carcinoma cells by inducing mitochondrial apoptosis. Translational Cancer Research. 2020;9(6):3926–3936. doi: 10.21037/tcr-19-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Quero A., Pollet E., Avérous L., Phalip V. Optimized bioproduction of itaconic and fumaric acids based on solid-state fermentation of lignocellulosic biomass. Molecules. 2020;25(5) doi: 10.3390/molecules25051070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi, H., Ogino, C., & Kondo, A. (2017). Microbial conversion of biomass into bio-based polymers. In Bioresource Technology (Vol. 245, pp. 1664–1673). Elsevier Ltd. https://doi.org/10.1016/j.biortech.2017.06.135. [DOI] [PubMed]
- Khangwal I., Chhabra D., Shukla P. Multi-objective optimization through machine learning modeling for production of xylooligosaccharides from alkali-pretreated corn-cob xylan via enzymatic hydrolysis. Indian Journal of Microbiology. 2021;61(4):458–466. doi: 10.1007/s12088-021-00970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. F. S., Akbar, M., Xu, Z., & Wang, H. (2021). A review on the role of pretreatment technologies in the hydrolysis of lignocellulosic biomass of corn stover. In Biomass and Bioenergy (Vol. 155). Elsevier Ltd. https://doi.org/10.1016/j.biombioe.2021.106276.
- Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M. (2017). Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. In Food and Nutrition Research (Vol. 61). Swedish Nutrition Foundation. https://doi.org/10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed]
- Klempová T., Slaný O., Šišmiš M., Marcinčák S., Čertík M. Dual production of polyunsaturated fatty acids and beta-carotene with Mucor wosnessenskii by the process of solid-state fermentation using agro-industrial waste. Journal of Biotechnology. 2020;311:1–11. doi: 10.1016/j.jbiotec.2020.02.006. [DOI] [PubMed] [Google Scholar]
- Kumar, V., Yadav, S. K., Kumar, J., & Ahluwalia, V. (2020). A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. In Bioresource Technology (Vol. 299). Elsevier Ltd. https://doi.org/10.1016/j.biortech.2019.122633. [DOI] [PubMed]
- Lagashetti, A. C., Dufossé, L., Singh, S. K., & Singh, P. N. (2019). Fungal pigments and their prospects in different industries. In Microorganisms (Vol. 7, Issue 12). MDPI AG. https://doi.org/10.3390/microorganisms7120604. [DOI] [PMC free article] [PubMed]
- Lin, S. R., Chen, Y. H., Tseng, F. J., & Weng, C. F. (2020). The production and bioactivity of prodigiosin: quo vadis? In Drug Discovery Today (Vol. 25, Issue 5, pp. 828–836). Elsevier Ltd. https://doi.org/10.1016/j.drudis.2020.03.017. [DOI] [PubMed]
- Liu J., Guo T., Luo Y., Chai X., Wu J., Zhao W.…Lin Q. Enhancement of Monascus pigment productivity via a simultaneous fermentation process and separation system using immobilized-cell fermentation. Bioresource Technology. 2019;272:552–560. doi: 10.1016/j.biortech.2018.10.072. [DOI] [PubMed] [Google Scholar]
- Liu J., Luo Y., Guo T., Tang C., Chai X., Zhao W.…Lin Q. Cost-effective pigment production by Monascus purpureus using rice straw hydrolysate as substrate in submerged fermentation. Journal of Bioscience and Bioengineering. 2020;129(2):229–236. doi: 10.1016/j.jbiosc.2019.08.007. [DOI] [PubMed] [Google Scholar]
- Liu Z., Feist A.M., Dragone G., Mussatto S.I. Lipid and carotenoid production from wheat straw hydrolysates by different oleaginous yeasts. Journal of Cleaner Production. 2020;249 doi: 10.1016/j.jclepro.2019.119308. [DOI] [Google Scholar]
- Li Y., Qi B., Wan Y. Separation of monosaccharides from pretreatment inhibitors by nanofiltration in lignocellulosic hydrolysate: Fouling mitigation by activated carbon adsorption. Biomass and Bioenergy. 2020;136 doi: 10.1016/j.biombioe.2020.105527. [DOI] [Google Scholar]
- Lopes, F. C., & Ligabue-Braun, R. (2021). Agro-Industrial Residues: Eco-Friendly and Inexpensive Substrates for Microbial Pigments Production. In Frontiers in Sustainable Food Systems (Vol. 5). Frontiers Media S.A. https://doi.org/10.3389/fsufs.2021.589414.
- Majumdar S., Mandal T., Dasgupta Mandal D. Production kinetics of β-carotene from Planococcus sp. TRC1 with concomitant bioconversion of industrial solid waste into crystalline cellulose rich biomass. Process Biochemistry. 2020;92:202–213. doi: 10.1016/j.procbio.2020.01.012. [DOI] [Google Scholar]
- Majumdar S., Paul I., Dey S., Dutta S., Mandal T., Mandal D.D. Biotransformation of paper mill sludge by Serratia marcescens NITDPER1 for prodigiosin and cellulose nanocrystals: A strategic valorization approach. Biochemical Engineering Journal. 2020;164 doi: 10.1016/j.bej.2020.107766. [DOI] [Google Scholar]
- Marizcurrena, J. J., Cerdá, M. F., Diego Alem, & Castro-Sowinski, S. (2019). Living with Pigments: The Colour Palette of Antarctic Life. In The Ecological Role of Micro-organisms in the Antarctic Environment (pp. 65–82). http://www.springer.com/series/15180.
- Marketandmarkets. (2021). Food Colors Market by Type (Natural, synthetic, nature-identical), Application (Food products, and beverages), Form (Liquid, powder and gel), Solubility (Dyes and lakes) & Region - Global Forecast to 2026. Https://Www.Marketsandmarkets.Com/Market-Reports/Food-Colors-Market-36725323.Html.
- Menezes, G. C. de A., de Medeiros, T. D. M., Lima, I. G. de O., da Silva, M. B., de Queiroz, A. C., Duarte, A. W. F., de Oliveira, V. M., Rosa, L. H., & Bicas, J. L. (n.d.). Pigments produced by fungi and bacteria from extremophes environments. In Microbes in the Food Industry.
- Miazek K., Remacle C., Richel A., Goffin D. Beech wood Fagus sylvatica dilute-acid hydrolysate as a feedstock to support Chlorella sorokiniana biomass, fatty acid and pigment production. Bioresource Technology. 2017;230:122–131. doi: 10.1016/j.biortech.2017.01.034. [DOI] [PubMed] [Google Scholar]
- Mohankumari H.P., Naidu K.A., Narasimhamurthy K., Vijayalakshmi G. Bioactive Pigments of Monascus purpureus Attributed to Antioxidant, HMG-CoA Reductase Inhibition and Anti-atherogenic Functions. Frontiers in Sustainable Food Systems. 2021;5 doi: 10.3389/fsufs.2021.590427. [DOI] [Google Scholar]
- Morales-Oyervides L., Ruiz-Sánchez J.P., Oliveira J.C., Sousa-Gallagher M.J., Morales-Martínez T.K., Albergamo A.…Montañez J. Medium design from corncob hydrolyzate for pigment production by Talaromyces atroroseus GH2: Kinetics modeling and pigments characterization. Biochemical Engineering Journal. 2020;161 doi: 10.1016/j.bej.2020.107698. [DOI] [Google Scholar]
- Namnuch N., Thammasittirong A., Thammasittirong S.N.R. Lignocellulose hydrolytic enzymes production by Aspergillus flavus KUB2 using submerged fermentation of sugarcane bagasse waste. Mycology. 2021;12(2):119–127. doi: 10.1080/21501203.2020.1806938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.B., Chen S.P., Nguyen T.H., Nguyen M.T., Tran T.T.T., Doan C.T.…Wang S.L. Novel efficient bioprocessing of marine chitins into active anticancer prodigiosin. Marine Drugs. 2020;18(1) doi: 10.3390/md18010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.B., Wang S.L., Nguyen A.D., Phan T.Q., Techato K., Pradit S. Bioproduction of prodigiosin from fishery processing waste shrimp heads and evaluation of its potential bioactivities. Fishes. 2021;6(3) doi: 10.3390/fishes6030030. [DOI] [Google Scholar]
- Pailliè-Jiménez, M. E., Stincone, P., & Brandelli, A. (2020). Natural Pigments of Microbial Origin. In Frontiers in Sustainable Food Systems (Vol. 4). Frontiers Media S.A. https://doi.org/10.3389/fsufs.2020.590439.
- Panesar R., Kaur S., Panesar P.S. Vol. 1. Elsevier Ltd.; 2015. Production of microbial pigments utilizing agro-industrial waste: A review; pp. 70–76. (Current Opinion in Food Science). [DOI] [Google Scholar]
- Pellera F.M., Gidarakos E. Chemical pretreatment of lignocellulosic agroindustrial waste for methane production. Waste Management. 2018;71:689–703. doi: 10.1016/j.wasman.2017.04.038. [DOI] [PubMed] [Google Scholar]
- Philippini, R. R., Martiniano, S. E., Ingle, A. P., Franco Marcelino, P. R., Silva, G. M., Barbosa, F. G., dos Santos, J. C., & da Silva, S. S. (2020). Agroindustrial Byproducts for the Generation of Biobased Products: Alternatives for Sustainable Biorefineries. In Frontiers in Energy Research (Vol. 8). Frontiers Media S.A. https://doi.org/10.3389/fenrg.2020.00152.
- Rajesh Banu, J., Preethi, Kavitha, S., Tyagi, V. K., Gunasekaran, M., Karthikeyan, O. P., & Kumar, G. (2021). Lignocellulosic biomass based biorefinery: A successful platform towards circular bioeconomy. Fuel, 302. https://doi.org/10.1016/j.fuel.2021.121086.
- Ramesh, C., Vinithkumar, N. V., Kirubagaran, R., Venil, C. K., & Dufossé, L. (2019). Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. In Microorganisms (Vol. 7, Issue 7). MDPI AG. https://doi.org/10.3390/microorganisms7070186. [DOI] [PMC free article] [PubMed]
- Rana, B., Bhattacharyya, M., Patni, B., Arya, M., & Joshi, G. K. (2021). The Realm of Microbial Pigments in the Food Color Market. In Frontiers in Sustainable Food Systems (Vol. 5). Frontiers Media S.A. https://doi.org/10.3389/fsufs.2021.603892.
- Reshmy, R., Athiyaman Balakumaran, P., Divakar, K., Philip, E., Madhavan, A., Pugazhendhi, A., Sirohi, R., Binod, P., Kumar Awasthi, M., & Sindhu, R. (2022). Microbial valorization of lignin: Prospects and challenges. In Bioresource Technology (Vol. 344). Elsevier Ltd. https://doi.org/10.1016/j.biortech.2021.126240. [DOI] [PubMed]
- Robak, K., & Balcerek, M. (2020). Current state-of-the-art in ethanol production from lignocellulosic feedstocks. In Microbiological Research (Vol. 240). Elsevier GmbH. https://doi.org/10.1016/j.micres.2020.126534. [DOI] [PubMed]
- Rodrigues, P. de O., Gurgel, L. V. A., Pasquini, D., Badotti, F., Góes-Neto, A., & Baffi, M. A. (2020). Lignocellulose-degrading enzymes production by solid-state fermentation through fungal consortium among Ascomycetes and Basidiomycetes. Renewable Energy, 145, 2683–2693. https://doi.org/10.1016/j.renene.2019.08.041.
- Rosa L.H. Fungi of Antarctica. Fungi of Antarctica. 2019 doi: 10.1007/978-3-030-18367-7. [DOI] [Google Scholar]
- Sadh, P. K., Duhan, S., & Duhan, J. S. (2018). Agro-industrial wastes and their utilization using solid state fermentation: a review. In Bioresources and Bioprocessing (Vol. 5, Issue 1). Springer. https://doi.org/10.1186/s40643-017-0187-z.
- Sandmann, G. (2019). Antioxidant protection from UV-and light-stress related to carotenoid structures. In Antioxidants (Vol. 8, Issue 7). MDPI. https://doi.org/10.3390/antiox8070219. [DOI] [PMC free article] [PubMed]
- Santos, M. C. dos, & Bicas, J. L. (2021). Natural blue pigments and bikaverin. In Microbiological Research (Vol. 244). Elsevier GmbH. https://doi.org/10.1016/j.micres.2020.126653. [DOI] [PubMed]
- Sasidharan A., Sasidharan N.K., Amma D.B.N.S., Vasu R.K., Nataraja A.V., Bhaskaran K. Antifungal activity of violacein purified from a novel strain of Chromobacterium sp. NIIST (MTCC 5522) Journal of Microbiology. 2015;53(10):694–701. doi: 10.1007/s12275-015-5173-6. [DOI] [PubMed] [Google Scholar]
- Sen T., Barrow C.J., Deshmukh S.K. Microbial pigments in the food industry—challenges and the way forward. Frontiers in Nutrition. 2019;6(March):1–14. doi: 10.3389/fnut.2019.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson, G. T., Tang, P., & Giusti, M. M. (2017). Natural Colorants: Food Colorants from Natural Sources. In Annual Review of Food Science and Technology (Vol. 8, pp. 261–280). Annual Reviews Inc. https://doi.org/10.1146/annurev-food-030216-025923. [DOI] [PubMed]
- Silbir S., Goksungur Y. Natural red pigment production by monascus purpureus in submerged fermentation systems using a food industry waste: Brewer’s spent grain. Foods. 2019;8(5) doi: 10.3390/foods8050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Nee Nigam, P., & Pandey, A. (2009). Biotechnology for agro-industrial residues utilisation: Utilisation of agro-residues. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues. Springer Netherlands. https://doi.org/10.1007/978-1-4020-9942-7.
- Srianta I., Kusdiyantini E., Zubaidah E., Ristiarini S., Nugerahani I., Alvin A.…Zhang B.-B. Utilization of agro-industrial by-products in Monascus fermentation: A review. Bioresources and Bioprocessing. 2021;8(1):129. doi: 10.1186/s40643-021-00473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H., Xing Z., Chen G., Tian X., Wu Z. Evaluating antitumor and antioxidant activities of yellow monascus pigments from monascus ruber fermentation. Molecules. 2018;23(12) doi: 10.3390/molecules23123242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilares R.T., de Souza R.A., Marcelino P.F., da Silva S.S., Dragone G., Mussatto S.I., Santos J.C. Sugarcane bagasse hydrolysate as a potential feedstock for red pigment production by Monascus ruber. Food Chemistry. 2018;245:786–791. doi: 10.1016/j.foodchem.2017.11.111. [DOI] [PubMed] [Google Scholar]
- Gerit T., Anders Ø. Sustainable food colours. FST Journal. 2020;34(4):50–53. [Google Scholar]
- Usmani Z., Sharma M., Sudheer S., Gupta V.K., Bhat R. Engineered microbes for pigment production using waste biomass. Current Genomics. 2020;21(2):80–95. doi: 10.2174/1389202921999200330152007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valduga E., Rausch Ribeiro A.H., Cence K., Colet R., Tiggemann L., Zeni J., Toniazzo G. Carotenoids production from a newly isolated Sporidiobolus pararoseus strain using agroindustrial substrates. Biocatalysis and Agricultural Biotechnology. 2014;3(2):207–213. doi: 10.1016/j.bcab.2013.10.001. [DOI] [Google Scholar]
- Wang S., Sun X., Yuan Q. Vol. 258. Elsevier Ltd.; 2018. Strategies for enhancing microbial tolerance to inhibitors for biofuel production: A review; pp. 302–309. (Bioresource Technology). [DOI] [PubMed] [Google Scholar]
- Webb C. Design aspects of solid state fermentation as applied to microbial bioprocessing. Journal of Applied Biotechnology & Bioengineering. 2017;4(1) doi: 10.15406/jabb.2017.04.00094. [DOI] [Google Scholar]
- Wehrs, M., Gladden, J. M., Liu, Y., Platz, L., Prahl, J. P., Moon, J., Papa, G., Sundstrom, E., Geiselman, G. M., Tanjore, D., Keasling, J. D., Pray, T. R., Simmons, B. A., & Mukhopadhyay, A. (2019). Sustainable bioproduction of the blue pigment indigoidine: Expanding the range of heterologous products in: R. toruloides to include non-ribosomal peptides. Green Chemistry, 21(12), 3394–3406. https://doi.org/10.1039/c9gc00920e.
- Woiciechowski A.L., Karp S.G., Sobral K., de Carvalho J.C., Letti L.A.J., Soccol V.T., Soccol C.R. Pretreatment strategies to enhance value addition of agro-industrial wastes. 2014;Vol. 9781461480051:29–49. doi: 10.1007/978-1-4614-8005-1_2. [DOI] [Google Scholar]
- Yip, C. H., Mahalingam, S., Wan, K. L., & Nathan, S. (2021). Prodigiosin inhibits bacterial growth and virulence factors as a potential physiological response to interspecies competition. PLoS One, 16(6 June). https://doi.org/10.1371/journal.pone.0253445. [DOI] [PMC free article] [PubMed]
- Yuan X., Chen X., Shen G., Chen S., Yu J., Zhai R.…Jin M. Densifying lignocellulosic biomass with sulfuric acid provides a durable feedstock with high digestibility and high fermentability for cellulosic ethanol production. Renewable Energy. 2022;182:377–389. doi: 10.1016/j.renene.2021.10.015. [DOI] [Google Scholar]
- Zhang, J., Cai, D., Qin, Y., Liu, D., & Zhao, X. (2020). High value-added monomer chemicals and functional bio-based materials derived from polymeric components of lignocellulose by organosolv fractionation. In Biofuels, Bioproducts and Biorefining (Vol. 14, Issue 2, pp. 371–401). John Wiley and Sons Ltd. https://doi.org/10.1002/bbb.2057.
- Zhang S., Zhao W., Nkechi O., Lu P., Bai J., Lin Q., Liu J. Utilization of low-cost agricultural by-product rice husk for Monascus pigments production via submerged batch-fermentation. Journal of the Science of Food and Agriculture. 2021 doi: 10.1002/jsfa.11585. [DOI] [PubMed] [Google Scholar]
- Zhang W.W., Zhou X.F., Zhang Y.L., Cheng P.F., Ma R., Cheng W.L., Chu H.Q. Enhancing astaxanthin accumulation in haematococcus pluvialis by coupled light intensity and nitrogen starvation in column photobioreactors. Journal of Microbiology and Biotechnology. 2018;28(12):2019–2028. doi: 10.4014/jmb.1807.07008. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Yin Z., Hu X. Corncob hydrolysate, an efficient substrate for Monascus pigment production through submerged fermentation. Biotechnology and Applied Biochemistry. 2014;61(6):716–723. doi: 10.1002/bab.1225. [DOI] [PubMed] [Google Scholar]
- Zoghlami, A., & Paës, G. (2019). Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. In Frontiers in Chemistry (Vol. 7). Frontiers Media S.A. https://doi.org/10.3389/fchem.2019.00874. [DOI] [PMC free article] [PubMed]