Abstract
Besides performing phagocytosis and degranulation, neutrophils are capable of eliminating microorganisms by releasing neutrophil extracellular traps (NETs). NET formation was found to be associated with increased mortality in sepsis. During sepsis levels of interleukin 1β (IL-1β), a cytokine, increases significantly and also was associated with increased mortality. Blocking of the interleukin 1 (IL-1) receptor by anakinra leads to less NET formation in gout patients. However, NET formation is crucial during infection by trapping pathogens and thereby slowing the process. Total or early blocking of cascades leading to NETs may lead to aggravation of infection in otherwise mild cases. The dose- and time-dependent effect of the IL-1 receptor antagonist anakinra was tested on spontaneous, lipopolysaccharide (LPS)-induced and phorbol-12-myristate 13-acetate (PMA)-induced formation of NETs in vitro. Quantitative detection of NETs was performed for NETspecific proteins and cell-free DNA. Immunostained microscopy imaging was used for visualization.
Our study shows a dose- and time-dependent inhibitory effect of anakinra that involves the change of intracellular calcium mobilization on the formation of NETs in vitro for PMA-stimulated neutrophils but not for LPS-stimulated neutrophils. It may be useful for treatment of sepsis as part of a multimodal treatment concept, but it seems that timing and dose need to be carefully chosen.
Keywords: neutrophil granulocytes, neutrophil extracellular traps, interleukin-1β receptor antagonist, anakinra, sepsis
Introduction
It has been demonstrated that neutrophils locate and eliminate microorganisms such as bacteria, protozoa, and fungi by releasing chromatin structures called neutrophil extracellular traps (NETs) [1-5]. NETs are composed of decondensed chromatin strands, histones, and over 30 peptides, including the granular-derived enzymes neutrophil elastase (NE) and myeloperoxidase (MPO) [6]. NET formation is activated through various cytokines, lipopolysaccharides (LPS) and phorbol 12-myristate 13-acetate (PMA). Currently, the complete mechanisms for NET formation are not fully understood [7, 8]. Nevertheless, two different ways of NETosis have been described: first, suicidal NETosis with cell membrane rupture and release of mature NETs resulting in cell death of the neutrophil induced by, among other factors, PMA; and second, vital NETosis where NETs are released by nuclear budding and vesicular release without cell death, induced by, among other factors, LPS [9]. After activation via PMA, release of calcium ions in the endoplasmic reticulum eventually leads to NET formation [2, 10]. Overwhelming NET formation has been identified in many diseases and inflammatory processes, including sepsis and SIRS. Furthermore, it is associated with increased mortality [11-16].
In sepsis interaction of neutrophils with activated platelets or activated endothelial cells enhances NET formation and causes endothelial cell damage, which results in organ damage [15, 16] and leads to increased mortality [11, 14, 17]. Furthermore, proteins and cell components released during NET formation such as MPO, histones or cf-DNA (circulating free DNA) are able to activate other immune cells and mediate the release of DAMPS (damage associated molecular patterns) from macrophages and endothelial cells, which also enhances the inflammation and causes cell death and organ failure [13]. With regard to this, Maruchi et al. found a correlation between severity of organ dysfunction and mortality rates with the level of MPO-DNA complexes in patients with septic shock [18].
Some studies have shown the undesirable effects of preventing NET formation in early infection stages. Treatment prior to or by the onset of infection frustrates the main role of NETs, namely trapping pathogens [19, 20]. Mancilla et al. observed higher mortality in vivo for septic rats treated with high doses of anakinra for long incubation times [21]. Consequently, it may be helpful to slow the process of NET formation without stopping it completely, in order to maintain basic antimicrobial function and prevent infection from advancing to more severe or septic stages [19].
In addition to elevated numbers of NETs, imbalance of produced interleukin 1 (IL-1) has been associated with higher mortality in sepsis [22, 23]. In particular, interleukin 1β (IL-1β) levels increase significantly in the acute phase of sepsis [24]. Anakinra, a recombinant IL-1 receptor antagonist (rIL1-ra), prevents binding of the proinflammatory cytokines IL-1α and IL-1β to the IL-1-receptor type 1, inhibiting downstream activation. Previous studies in gout patients have shown that blocking of the IL-1 receptor with anakinra decreases monosodium urate (MSU) crystal- induced NET formation, but without precise details regarding the timing and dosing of anakinra administration [25].
The aim of the current study was to demonstrate that anakinra has a dose- and time-dependent inhibitory effect on NET formation. To accomplish this, neutrophils were pretreated with varying concentrations of anakinra in vitro at different timepoints before induction of PMA-induced and LPS-induced NET formation.
Material and methods
Isolation of neutrophils
Blood was obtained with informed, signed consent from healthy local donors following approval by the Ethics Committee of the Hamburg Medical Association (PV5921). All procedures used in the current work conformed to local rules and were in accordance with the 1964 Helsinki Declaration and its later amendments.
Neutrophil granulocytes were isolated from whole blood of several healthy donors using the MACSxpress Whole Blood Neutrophil Isolation Kit, human (Miltenyi Biotec, Bergisch Gladbach, Germany) as described in the manufacturer’s protocol. Erythrocyte lysis was assessed by resuspending the cell pellet with water, mixing thoroughly for 30 s prior to addition of 0.7 ml of 0.6 M potassium chloride (Sigma-Aldrich, Saint Louis, MO, USA) and 12 ml DPBS (Thermo Fischer, Waltham, MA, USA) followed by centrifugation at 1350 rpm for 7 minutes. Purity consisted of 98% neutrophils as shown by fluorescence-activated cell sorting (FACS) using anti-CD15-FITC (mAb HI98, IgM) and anti-CD16-PerCP (mAb 3G8, IgG1) antibody markers (BioLegend, San Diego, CA, USA). Cell morphology was analyzed by hematoxylin and eosin staining.
Immunostaining and imaging
0.5 × 106 neutrophils per well were seeded into 12-well plates containing coverslips (Glaswarenfabrik Karl Hecht GmbH & Co KG, Sondheim, Germany). After sedimentation for two hours at 37oC with 5% CO2, medium was removed and cells were treated with anakinra (see “Treatment of cells” below) before stimulation with 100 nM PMA. After three-hour incubation at 37oC with 5% CO2, cells were washed and fixed with 99% methanol until the staining had been done at –20oC, followed by additional washing. Subsequently, cover slides were blocked for 1 hour at room temperature (RT) in PBS containing 1% bovine serum albumin (BSA). Cells were incubated with primary antibodies in DPBS containing 0.5% BSA and 0.05% Tween (SERVA Electrophoresis GmbH, Heidelberg, Germany) for 1 hour at RT. Cells were then incubated with 1 : 200 anti-rabbit secondary antibody conjugated with Alexa Fluor 647 (ab150075, Abcam) and 1 : 1000 FITC-conjugated anti-mouse antibody (ab6785, Abcam). After washing, neutrophils were counterstained with DAPI prepared with mounting medium. Imaging was performed using an Apotome Microscope (Zeiss, Oberkochen, Germany) at a 40x magnification and processed using ImageJ software 1.51 (NIH, USA). Primary antibodies used were anti-neutrophil elastase antibody (ab68672, Abcam, UK) at 1 : 200 dilution and anti-myeloperoxidase antibody (ab25989, Abcam) at 1 : 50 dilution.
Five images (four extremes and center) per slide, for each condition performed in triplicate, were acquired by fluorescence microscopy. The fluorescence background readings were established with unstained cells for DAPI to analyze the mean gray value of signal per area for each color with the ImageJ software [26].
Treatment of cells
For all assays, neutrophils were resuspended in Gibco RPMI 1640 media (Thermo Fisher, Waltham, MA, USA) containing 1% BSA (Sigma-Aldrich, Saint Louis, MO, USA). For the MPO and calcium measurements, the cells were seeded into white flat-bottom 96-well assay plates (Corning Incorporated, New York, NY, USA) at a density of 2 × 105 cells per well. Supernatant sample generation for NE and cfDNA assays was done by seeding cells in a 24-well culture plate (Corning Incorporated, New York, NY, USA), at a concentration of 1 × 106 cells per well. Cells were treated with concentrations of 100 ng/ml, 100 µg/ml and 1 mg/ml anakinra (Swedish Orphan Biovitrum AB, Stockholm, Sweden) at 60, 30, and 10 minutes prior, at the time of and 30 and 60 minutes after stimulation with 100 nM PMA or 5 µg/ml LPS (E.coli O55:B5) as indicated by other studies [25, 27-29]. Stimulation was done for 3 hours.
Assays for measurement of NET formation
In order to quantify NET formation, we measured MPO and NE as markers of NET formation, together with cell-free DNA.
cfDNA assay: The amount of cfDNA was measured as described elsewhere [30], with replacement of SYTOX Green with SYTOX Orange (Thermo Fisher, Waltham, MA, USA). Fluorescence was measured at 544 nm for extinction and 590 nm for emission with a cutoff at 570 nm, using a microplate reader (Flex Station 3, Molecular Devices, San Jose, CA, USA).
NE assay: The amount of NE was measured according to the instructions of the NETosis Assay Kit from Cayman Chemical (Ann Arbor, MI, USA). Absorbance was measured at 405 nm with a microplate reader (Flex Station 3, Molecular Devices, San Jose, CA, USA).
MPO assay: The amount of MPO was measured according to the instructions of the Neutrophil Myeloperoxidase Activity Assay Kit from Cayman Chemical (Ann Arbor, MI, USA). Absorbance was measured at 650 nm with a microplate reader (Flex Station 3, Molecular Devices, San Jose, CA, USA).
Intracellular calcium mobilization
To detect intracellular calcium mobilization, the Fluo-8 No Wash Calcium Assay kit was used, following the manufacturer’s protocol (ab112129, Abcam). Briefly, 1 × 105 cells per well were seeded in black flat-bottom 96-well plates (Corning Incorporated, New York, NY, USA). Cells were treated as described before, with the final addition of Fluo-8 stock solution. Fluorescence was measured continuously for 3 hours every 10 minutes using a microplate reader (Flex Station 3, Molecular Devices, San Jose, CA, USA). Representative data for 160 minutes are presented.
Statistical analysis
All assays were performed at least three times in biological triplicate. All data were analyzed with SPSS Statistics 26 (IBM, Armonk, NY, USA) and GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). Differences between groups were calculated using ANOVA with Dunnett’s correction for repeated measurement. Data are presented as mean ± standard deviation (SD). The level of significance was set at 5%.
Results
IF staining shows inhibition of NET formation after treatment with anakinra
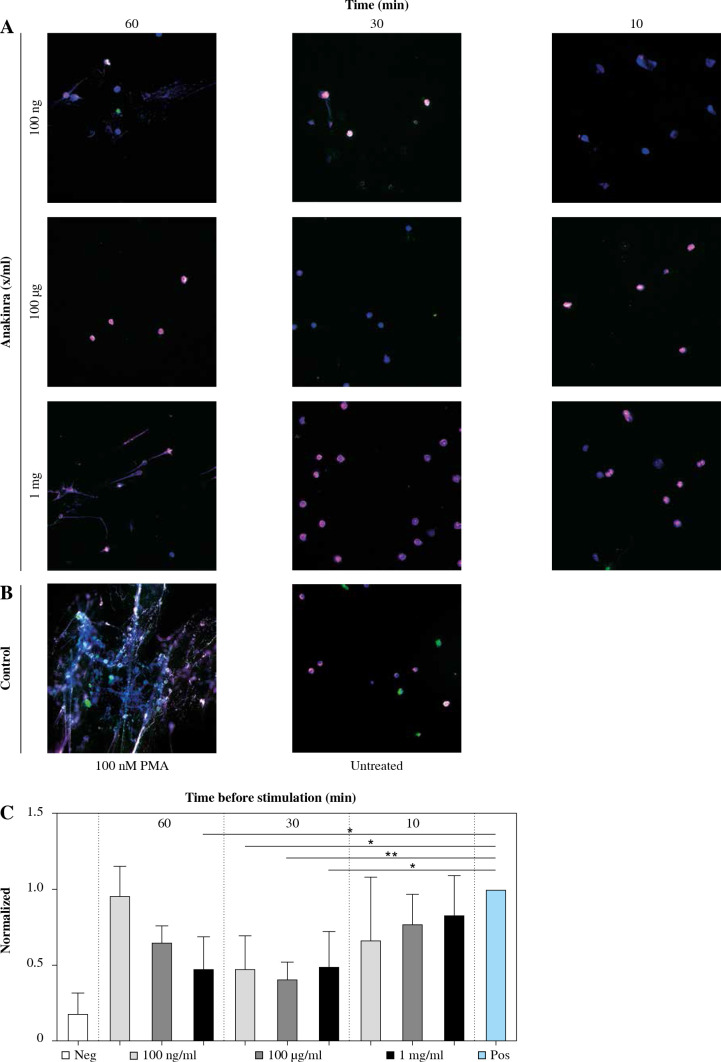
Immunofluorescence-stained (IF) images of the cover slips were taken to visualize the NETs and to evaluate the release of individual factors cfDNA, NE, and MPO. Concordant with the results for markers of NET formation, IF shows lower NET formation in neutrophils pretreated with anakinra compared to cells stimulated with PMA alone (Fig. 1).
Fig. 1.
Immunofluorescence stain- ing of anakinra pretreated and PMA-stimulated neutrophils. Cells were pretreated with anakinra (100 ng/ml, 100 µg/ml, 1 mg/ml) for 60, 30 and 10 minutes before stimulation with 100 nM PMA for 3 hours (A). DNA was stained with DAPI (blue), NE (pink/AF 647) and MPO (green/FitC). Positive control: Cells were not pretreated with anakinra and stimulated with 100 nM PMA for 3 hours; negative control cells did not receive any treatment (B). DNA and markers were stained as described before and imaged at a magnification of 40×. Graphics show averages ±SD of mean gray value of signal per area of five images (four extremes and center) per slide performed in triplicate and analyzed with ImageJ software for DAPI-DNA (C)
For LPS-stimulated neutrophils we did not observe differences between anakinra-pretreated cells compared to cells stimulated with LPS alone (Supplementary Fig. 1). Treatment after stimulation with PMA or LPS had no effect on NET formation (Supplementary Figs. 4 and 7).
Anakinra prevents PMA-induced NET formation in a dose- and time-dependent manner as measured by key markers
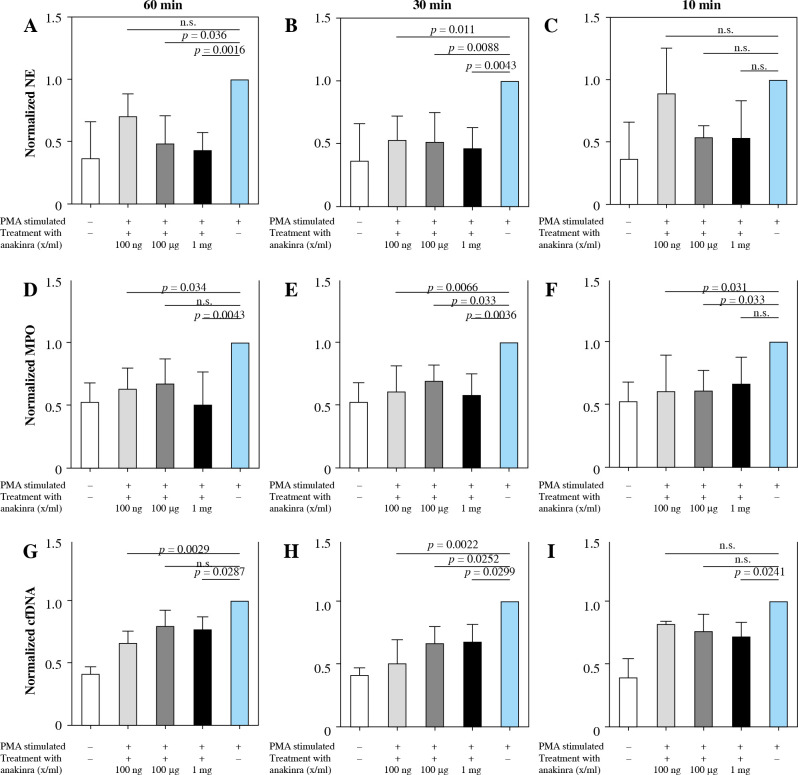
Anakinra was tested at concentrations of 100 ng/ml, 100 µg/ml and 1 mg/ml and incubation periods of 10, 30, and 60 minutes prior to stimulation of the neutrophils with PMA or LPS (Fig. 2 and Supplementary Fig. 2). Additionally, concentrations were tested at the same time as the stimulation, 30 and 60 minutes after stimulation. Amounts of NE, MPO and cell-free DNA were assessed as markers of NET formation. Alterations in all factors correlate with modulation of NET formation.
Fig. 2.
Changes of markers of NET formation in anakinra pretreated and PMA-stimulated neutrophils. Cells were pre- treated with anakinra in concentrations of 100 ng/ml, 100 µg/ml and 1 mg/ml for 60, 30 and 10 minutes. Afterwards, cells were stimulated with PMA to induce NET formation. All data are values of absorbance/fluorescence normalized to PMA-stimulated controls. Normalized data for NE activity show significant results for pretreatment 60 and 30 min prior to PMA stimulation at different concentrations (A, B), while 10 min pretreatment was not significant for any con- centration (C). Normalized data for MPO are shown in (D-F) with no significant results for 60 min pretreatment with 100 µg/ml and 10 min pretreatment with 1 mg/ml. Results for cfDNA are shown in (G-I)
Treatment at the same time and 30 and 60 min after stimulation showed no alterations in marker activity and cfDNA amount (Supplementary Figs. 5 and 8).
Neutrophil elastase activity
As shown in Figure 2A, NE activity is inversely correlated with the concentration of anakinra in 60 minute pretreated and PMA-stimulated neutrophils. Amounts of NE are significantly decreased for all concentrations with 30-minute pretreatment and PMA-stimulated neutrophils (Fig. 2B). Our results demonstrated no effect on NE activity levels after 10-minute anakinra incubation (Fig. 2C).
We did not observe significant changes in NE activity of LPS-stimulated neutrophils whether they were pretreated with anakinra or not (Supplementary Fig. 2A).
Myeloperoxidase activity
Interestingly, MPO significantly decreased for pretreatment dosage of 100 ng/ml anakinra at all timepoints (Fig. 2D-F) whereas at concentration of 100 µg/ml anakinra it only decreased with pretreatment 30 and 10 min prior to treatment related to stimulation with PMA. The highest dosage of anakinra after 60- and 30-minute pretreatment produced a significant decrease in MPO regarding PMA-stimulated neutrophils.
Significant changes in MPO activity of LPS-stimulated neutrophils versus anakinra pretreated neutrophils were not observed (Supplementary Fig. 2B).
Cell-free DNA
cfDNA amounts were reduced in neutrophils stimulated with PMA and pretreated with anakinra. Whereas treatment with 1 mg/ml anakinra led to a decrease in cfDNA levels for all incubation timepoints, 0.1 mg/ml anakinra caused a cfDNA reduction exclusively at the 30 minute pretreatment timepoint. Our results show a significant cfDNA reduction in cells treated for 60 and 30 minutes with 100 ng/ml anakinra (Fig. 2G-I) for NETosis induction via PMA.
cfDNA amounts did not change significantly in neutrophils stimulated with LPS independent of pretreatment with anakinra (Supplementary Fig. 2C).
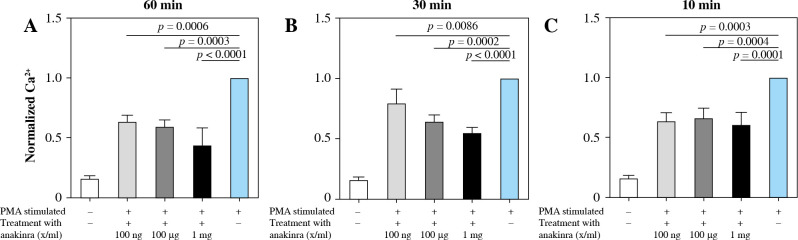
Anakinra significantly reduces intracellular calcium
Blocking the IL-1 receptor with anakinra led to significantly decreased intracellular calcium concentrations at all pretreatment timepoints for PMA-stimulated neutrophils compared to stimulated controls. The most significant results were observed at pretreatment concentrations of 1 mg/ml at all pretreatment timepoints (Fig. 3).
Fig. 3.
Calcium influx in anakinra pretreated neutrophils. Cells were treated with anakinra in concentrations of 100 ng/ml, 100 µg/ml and 1 mg/ml for 60 (A), 30 (B) and 10 minutes (C). Afterwards, cells were stimulated with 100 nM PMA to induce NET formation. After stimulation with PMA, calcium influx was measured. PMA stimulation was performed for a total of 3 hours. All data are values of fluorescence normalized to PMA-stimulated controls. Calcium influx significantly changed for all concentrations with 60, 30 and 10 (A-C) min pretreatment
LPS-stimulated neutrophils compared to anakinra pretreated and LPS-stimulated neutrophils did not show a significant change in intracellular calcium mobilization (Supplementary Fig. 3). Treatment at the same time or after stimulation had no effect on calcium mobilization (Supplementary Figs. 6 and 9).
Discussion
High levels of IL-1 (especially IL-1β), as well as high levels of NETs, are associated with increased mortality in sepsis [11, 14, 17, 22]. On the other hand, NET formation is crucial during infection by trapping pathogens and thereby slowing the process.
This study aimed to elucidate for the first time the dynamics of inhibition of NET formation by the recombinant rIL1-ra anakinra dependent on timing and dosing of the antagonist. The results of this study suggest that IL-1 receptor antagonism influences PMA-induced NET formation in a time- and dose-dependent manner but not LPS-induced NET formation.
It has been shown that several mechanisms of NET formation exist, which differ in regard to stimulus and executed pathway [9]. Our group shows the first evidence for inhibition of PMA-induced NET formation in vitro by anakinra-mediated IL-1 receptor blockade. Other studies used monosodium urate crystals (MUS) that successfully demonstrated the inhibition of NET formation by anakinra in neutrophils [25]. The fact that all components, NE activity, MPO activity, and amount of cf-DNA are reduced indicates a reduction in NET formation [7, 31, 32].
We did not detect inhibition of LPS-induced NET formation in vitro by anakinra-mediated IL-1 receptor blockade. This was apparent from the lack of significant changes in the amount of cf-DNA, NE and MPO activity, which was also confirmed by IF (supplemental Figs. 1 and 2). Considering that the levels of these NET-associated components have been shown to be much lower in LPS-stimulated neutrophils than in PMA-stimulated neutrophils [7, 33], the effect of a potential NETosis-inhibitor would probably be very small. Furthermore, it has been shown that LPS-stimulated neutrophils show lower interleukin signaling than PMA-stimulated neutrophils [33]. Similarly, we did not detect any significant differences in intracellular calcium mobilization between anakinra treated and untreated cells stimulated with LPS. These data suggest that the calcium influx may be independent from IL-1 signaling, whereas a connection seems to exist between IL-1 signaling and calcium influx in PMA-induced NETosis.
Immunofluorescence imaging showed little NET formation in anakinra pretreated and PMA-stimulated neutrophils, even though the assays showed decreased markers of NETs for every timepoint, sometimes with no significant change from the controls. Ten minutes of pretreatment with anakinra could not prevent neutrophils from accomplishing the NET formation evidenced by marker detection. This is reasonable, considering the binding affinity of the recombinant IL-1 receptor antagonist, which is 20 to 100 times lower than that of the interleukin-1 receptor antagonist [34]. Pretreatment with anakinra for 60 minutes before stimulation led to non-significant changes as well, whereas high doses of anakinra may overcome the time effect seen in our study, and lead to a decrease in markers of NET formation. Considering that interleukin-1 production is promoted in an autocrine manner, loss of binding over time and thus the release of the receptor binding site may be the reason for these results [35]. Only with the intermediate pretreatment time of 30 min did we observe that medium and even small doses of anakinra can decrease all markers of NET formation. From a clinical perspective, this suggests that small and intermediate doses would be preferable in light of the adverse events that have been reported for anakinra [36]. We did observe a significant reduction of released cf-DNA for anakinra pretreated and PMA-stimulated cells in comparison to neutrophils without anakinra pretreatment and stimulation. In contrast to NE and MPO activity, the reduction of cf-DNA was not dose dependent. The mechanisms of IL-1 signaling in NETosis are not fully understood and hardly examined. The IL-1 pathway might have an effect on NE and MPO processing other than on DNA decondensation and DNA release, which needs to be investigated further. Moreover, even though measurements of cf-DNA are highly objective and highly quantitative and were often used to detect the occurrence of NETosis [37, 38], parts of the measured cfDNA may be caused by other processes such as cell death unaffected by anakinra pretreatment [37; 39].
We observed a reduction of intracellular calcium levels. Although the role of calcium influx in the pathway of NET formation has not yet been determined in detail, previous research has shown that changes in intracellular calcium concentration occur during the process of NET formation [7]. Combining previous studies with our findings, we postulate that blocking the IL1 pathway reduces NET formation in a calcium-dependent manner in PMA-stimulated neutrophils. Some compounds used to induce formation of NETs promote PKC activation with consecutive elevation of cytoplasmatic calcium levels in suicidal NETosis similar to our results [2, 40].
Earlier clinical studies using anakinra for treatment of sepsis were not successful [35, 41, 42]. Reasons might be the subcutaneous administration that may reduce final concentrations to levels which are no longer sufficient to influence neutrophils involved in NET formation in vivo. Furthermore, the initial level of naturally occurring plasma IL-1ra and the interactions between this endogenous IL-1ra and IL-1β in septic patients before treatment with IL-1ra seems to be a decisive factor in determining whether IL-1ra therapy is effective or not [43]. Analysis of the dynamics of NET formation in relation to IL-1β levels and disease stage is an important area of future study in order to develop optimal therapy approaches based on timing of intervention in vivo.
Various studies did not show a beneficial outcome in animal models that completely prevented the process of NETosis in sepsis [19, 20]. Considering the negative effect of total blocking, it might be useful only to prevent or reduce the formation of new NETs being produced by migrating neutrophils during sepsis. The reasoning is that it is probably not NETosis per se that worsens the outcome in sepsis, but the overshooting of this process [19]. While Mai et al. examined the destruction of existing NETs, we considered that preventing the formation of NETs by migrating neutrophils may be a possible therapeutic target. In terms of severe sepsis, it might be helpful not just to destroy existing NETs but also to slow down the process by inhibiting the formation of new NETs at the same moment.
Since it is almost impossible to simulate the migration and interaction of neutrophils in vitro, we decided to apply the anakinra treatment before the stimulus. This most closely corresponds to the situation of the new recruitment of neutrophils in the case of sepsis.
In summary, it has been shown that therapy combination of antibiotics and NETs, degrading DNase at the optimal timepoint, increased the survival of septic mice [19, 20] by combining the antimicrobial effect of antibiotics and the destruction of existing NETs. However, the formation of new NETs is not prevented by any of these reagents. Consequently, we hypothesize that a triple-drug treatment of antibiotics, NET-degrading DNase and anakinra may further improve the outcome, considering that new formation of NETs is a risk factor in sepsis pathogenesis. This may be reduced by treatment with anakinra in the adequate dosage. Nevertheless, whether synergistic or antagonistic effects exist has to be verified in animal experiments due the limitation of cell culture.
Supplementary Figures are available on the journal’s website.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bruns S, Kniemeyer O, Hasenberg M, et al. (2010): Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog 6: e1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delgado-Rizo V, Martinez-Guzman MA, Iniguez-Gutierrez L, et al. (2017): Neutrophil extracellular traps and its implications in inflammation: an overview. Front Immunol 8: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, et al. (2004): Neutrophil extracellular traps kill bacteria. Science 303: 1532-1535. [DOI] [PubMed] [Google Scholar]
- 4.Imbert S, Bresler P, Boissonnas A, et al. (2016): Calcineurin inhibitors impair neutrophil activity against Aspergillus fumigatus in allogeneic hematopoietic stem cell transplant recipients. J Allergy Clin Immunol 138: 860-868. [DOI] [PubMed] [Google Scholar]
- 5.McCormick A, Heesemann L, Wagener J, et al. (2010): NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect 12: 928-936. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann V (2018): Neutrophil extracellular traps in the second decade. J Innate Immun 10: 414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bont CM, Koopman WJH, Boelens WC, et al. (2018): Stimulus-dependent chromatin dynamics, citrullination, calcium signalling and ROS production during NET formation. Biochim Biophys Acta Mol Cell Res 1865 (11 Pt A): 1621-1629. [DOI] [PubMed] [Google Scholar]
- 8.Douda DN, Khan MA, Grasemann H, et al. (2015): SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A 112: 2817-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yipp BG, Kubes P (2013): NETosis: how vital is it? Blood 122: 2784-2794. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MJ, Radic M (2012): Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 189: 2689-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camicia G, Pozner R, de Larranaga G (2014): Neutrophil extracellular traps in sepsis. Shock 42: 286-294. [DOI] [PubMed] [Google Scholar]
- 12.Colon DF, Wanderley CW, Franchin M, et al. (2019): Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit Care 23: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denning NL, Aziz M, Gurien SD, et al. (2019): DAMPs and NETs in sepsis. Front Immunol 10: 2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipinska-Gediga M (2017): Neutrophils, NETs, NETosis–old or new factors in sepsis and septic shock? Anaesthesiol Intensive Ther 49: 235-240. [DOI] [PubMed] [Google Scholar]
- 15.Clark SR, Ma AC, Tavener SA, et al. (2007): Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13: 463-469. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K, Koike Y, Shimura T, et al. (2014): In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS One 9: e111888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papayannopoulos V (2018): Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18: 134-147. [DOI] [PubMed] [Google Scholar]
- 18.Maruchi Y, Tsuda M, Mori H, et al. (2018): Plasma myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit Care 22: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mai SH, Khan M, Dwivedi DJ, et al. (2015): Delayed but not early treatment with DNase reduces organ damage and improves outcome in a murine model of sepsis. Shock 44: 166-172. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien XM, Biron BM, Reichner JS (2017): Consequences of extracellular trap formation in sepsis. Curr Opin Hematol 24: 66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancilla J, García P, Dinarello CA (1993): The interleukin-1 receptor antagonist can either reduce or enhance the lethality of Klebsiella pneumoniae sepsis in newborn rats. Infect Immun 61: 926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynn JL, Wilson CS, Hawiger J, et al. (2016): Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proc Natl Acad Sci U S A 113: E2627-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruitt JH, Copeland EM 3rd, Moldawer LL (1995): Interleukin-1 and interleukin-1 antagonism in sepsis, systemic inflammatory response syndrome, and septic shock. Shock 3: 235-251. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto H, Ogura H, Shimizu K, et al. (2018): The clinical importance of a cytokine network in the acute phase of sepsis. Sci Rep 8: 13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitroulis I, Kambas K, Chrysanthopoulou A, et al. (2011): Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One 6: e29318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sosa-Luis SA, Ríos-Ríos W de J, Gómez-Bustamante ÁE, et al. (2021): Structural differences of neutrophil extracellular traps induced by biochemical and microbiologic stimuli under healthy and autoimmune milieus. Immunol Res 69: 264-274. [DOI] [PubMed] [Google Scholar]
- 27.Iannitti RG, Napolioni V, Oikonomou V, et al. (2016): IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Commun 7: 10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mistry P, Carmona-Rivera C, Ombrello AK, et al. (2018): Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome. Ann Rheum Dis 77: 1825-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sil P, Wicklum H, Surell C, et al. (2017): Macrophage-derived IL-1beta enhances monosodium urate crystal-triggered NET formation. Inflamm Res 66: 227-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs TA, Kremer Hovinga JA, Schatzberg D, et al. (2012): Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood 120: 1157-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lapponi MJ, Carestia A, Landoni VI, et al. (2013): Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Ther 345: 430-437. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs TA, Abed U, Goosmann C, et al. (2007): Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176: 231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petretto A, Bruschi M, Pratesi F, et al. (2019): Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS One 14: e0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svenson M, Hansen MB, Heegaard P, et al. (1993): Specific binding of interleukin 1 (IL-1) beta and IL-1 receptor antagonist (IL-1ra) to human serum. High-affinity binding of IL-1ra to soluble IL-1 receptor type I. Cytokine 5: 427-435. [DOI] [PubMed] [Google Scholar]
- 35.Cavalli G, Dinarello CA (2018): Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol 9: 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumaine C, Bekkar S, Belot A, et al. (2020): Infectious adverse events in children with juvenile idiopathic arthritis treated with biological agents in a real-life setting: data from the JIRcohorte. Joint Bone Spine 87: 49-55. [DOI] [PubMed] [Google Scholar]
- 37.Masuda S, Nakazawa D, Shida H, et al. (2016): NETosis markers: Quest for specific, objective, and quantitative markers. Clin Chim Acta 459: 89-93. [DOI] [PubMed] [Google Scholar]
- 38.Garley M, Jablonska E, Surazynski A, et al. (2017): Cytokine network & NETs. Folia Biol (Praha) 63: 182-189. [PubMed] [Google Scholar]
- 39.Aucamp J, Bronkhorst AJ, Badenhorst CPS, et al. (2018): The diverse origins of circulating cell-free DNA in the human body: a critical re-evaluation of the literature. Biol Rev Camb Philos Soc 93: 1649-1683. [DOI] [PubMed] [Google Scholar]
- 40.Neeli I, Radic M (2013): Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front Immunol 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteagudo LA, Boothby A, Gertner E (2020): Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol 2: 276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinarello CA (2011): Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117: 3720-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer NJ, Reilly JP, Anderson BJ, et al. (2018): Mortality benefit of recombinant human interleukin-1 receptor antagonist for sepsis varies by initial interleukin-1 receptor antagonist plasma concentration. Crit Care Med 46: 21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]