Key Points
Question
Does early use of moderate hypothermia (33-34 °C) compared with strict normothermia (36-37 °C) improve mortality in patients with cardiogenic shock receiving venoarterial extracorporeal membrane oxygenation (ECMO)?
Findings
In this randomized clinical trial that included 334 patients who had been endotracheally intubated and were treated with venoarterial ECMO for less than 6 hours for refractory cardiogenic shock, mortality at 30 days was 42% for patients in the moderate hypothermia group vs 51% in the normothermia group. This difference was not statistically significant.
Meaning
For patients with cardiogenic shock receiving venoarterial ECMO, moderate hypothermia for 24 hours compared with normothermia did not significantly decrease 30-day mortality.
Abstract
Importance
The optimal approach to the use of venoarterial extracorporeal membrane oxygenation (ECMO) during cardiogenic shock is uncertain.
Objective
To determine whether early use of moderate hypothermia (33-34 °C) compared with strict normothermia (36-37 °C) improves mortality in patients with cardiogenic shock receiving venoarterial ECMO.
Design, Setting, and Participants
Randomized clinical trial of patients (who were eligible if they had been endotracheally intubated and were receiving venoarterial ECMO for cardiogenic shock for <6 hours) conducted in the intensive care units at 20 French cardiac shock care centers between October 2016 and July 2019. Of 786 eligible patients, 374 were randomized. Final follow-up occurred in November 2019.
Interventions
Early moderate hypothermia (33-34 °C; n = 168) for 24 hours or strict normothermia (36-37 °C; n = 166).
Main Outcomes and Measures
The primary outcome was mortality at 30 days. There were 31 secondary outcomes including mortality at days 7, 60, and 180; a composite outcome of death, heart transplant, escalation to left ventricular assist device implantation, or stroke at days 30, 60, and 180; and days without requiring a ventilator or kidney replacement therapy at days 30, 60, and 180. Adverse events included rates of severe bleeding, sepsis, and number of units of packed red blood cells transfused during venoarterial ECMO.
Results
Among the 374 patients who were randomized, 334 completed the trial (mean age, 58 [SD, 12] years; 24% women) and were included in the primary analysis. At 30 days, 71 patients (42%) in the moderate hypothermia group had died vs 84 patients (51%) in the normothermia group (adjusted odds ratio, 0.71 [95% CI, 0.45 to 1.13], P = .15; risk difference, −8.3% [95% CI, −16.3% to −0.3%]). For the composite outcome of death, heart transplant, escalation to left ventricular assist device implantation, or stroke at day 30, the adjusted odds ratio was 0.61 (95% CI, 0.39 to 0.96; P = .03) for the moderate hypothermia group compared with the normothermia group and the risk difference was −11.5% (95% CI, −23.2% to 0.2%). Of the 31 secondary outcomes, 30 were inconclusive. The incidence of moderate or severe bleeding was 41% in the moderate hypothermia group vs 42% in the normothermia group. The incidence of infections was 52% in both groups. The incidence of bacteremia was 20% in the moderate hypothermia group vs 30% in the normothermia group.
Conclusions and Relevance
In this randomized clinical trial involving patients with refractory cardiogenic shock treated with venoarterial ECMO, early application of moderate hypothermia for 24 hours did not significantly increase survival compared with normothermia. However, because the 95% CI was wide and included a potentially important effect size, these findings should be considered inconclusive.
Trial Registration
ClinicalTrials.gov Identifier: NCT02754193
This randomized clinical trial compares the effect of early use of moderate hypothermia (33-34 °C) vs strict normothermia (36-37 °C) on mortality in patients with cardiogenic shock receiving venoarterial extracorporeal membrane oxygenation (ECMO).
Introduction
The initiation of venoarterial extracorporeal membrane oxygenation (ECMO) has emerged as an increasingly common intervention in patients with cardiogenic shock, including after cardiac arrest that is refractory to standard therapies.1,2,3 The overall survival to hospital discharge using peripheral venoarterial ECMO in patients after cardiac arrest and with refractory cardiogenic shock is between 29% (extracorporeal cardiopulmonary resuscitation) and 41% (refractory cardiogenic shock).1,4
Severe cardiogenic shock, especially in patients with associated cardiac arrest, may be associated with ischemia reperfusion injury, a proinflammatory cytokine profile,5 and increased nitric oxide production,6 which may lead to intense vasoplegia, microcirculatory dysfunction,7 multiple organ failure, and death. Patients with refractory cardiogenic shock requiring venoarterial ECMO1,2,3 likely have the most severe ischemia reperfusion injury.
Moderate hypothermia may mitigate the deleterious effects of ischemia reperfusion injury4,5,6 and has been well tolerated in patients after cardiac arrest8 and in those with cardiogenic shock, although hypothermia was not associated with a significant change in hemodynamics or mortality.7 However, there is a lack of outcome data addressing this issue; therefore, the Extracorporeal Life Support Organization currently recommends normothermia for these patients (https://www.elso.org/resources/guidelines.aspx).
Despite the lack of a randomized clinical trial comparing standard therapy alone with standard therapy plus ECMO, use of ECMO during refractory cardiogenic shock management is increasing worldwide and it is important to determine the best approach to optimize this therapy. Therefore, the Hypothermia During ECMO (HYPO-ECMO) trial was designed to address the hypothesis that early use of moderate hypothermia (33-34 °C) compared with strict normothermia (36-37 °C) improves mortality in patients with cardiogenic shock receiving venoarterial ECMO.
Methods
Trial Design and Oversight
The trial was a pragmatic, multicenter, unblinded, parallel-group randomized clinical trial. The trial protocol was published9 and also appears in Supplement 1. The statistical analysis plan appears in Supplement 2. The trial followed the principles of the Declaration of Helsinki and was conducted in accordance with Good Clinical Practices and French regulations. The trial was supported by the international ECMO Network (ECMONet) and was approved by French health authorities (Agence Nationale de la Sécurité du Médicament et des Produits de Santé) and the appropriate ethics committee (Comité de Protection des Personnes).
In accordance with French law, the ethics committee waived the requirement for informed consent from patients because they were being treated in an emergency setting and were unable to provide informed consent. Before inclusion in the trial, investigators sought consent from the patient’s family, and if the family declined consent, the patients were not included (Figure 1). Written consent was requested from the patients as soon as they regained capacity. If they did not provide consent at this latter stage, their data could not be analyzed per French law.
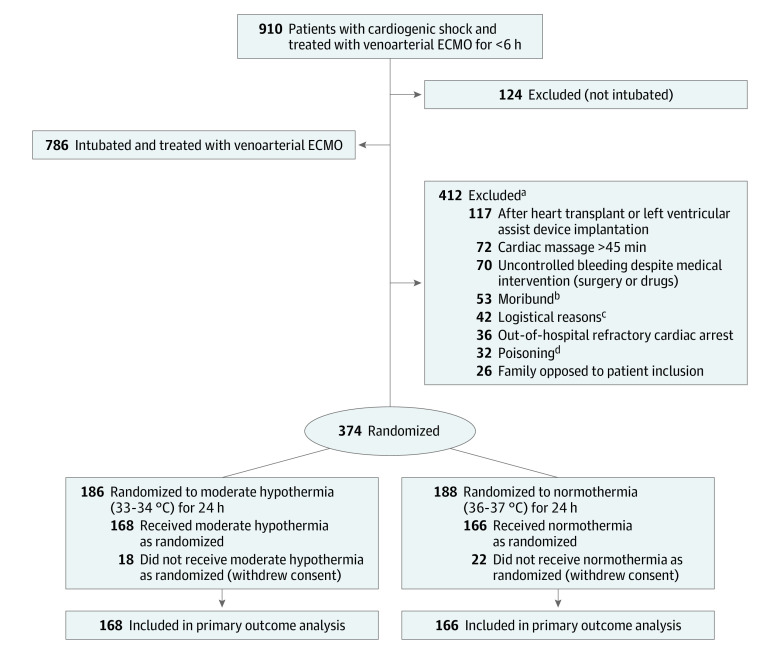
Figure 1. Enrollment, Randomization, and Follow-up of Patients in the HYPO-ECMO Trial.
ECMO indicates extracorporeal membrane oxygenation; HYPO-ECMO, Hypothermia During ECMO.
aA patient may have been excluded based on 1 or more of the listed reasons.
bDefined as a state of imminent death without any medical therapeutic option.
cThe proper equipment was not available, a certified investigator was not on-site, or there was not enough time (>6 hours after ECMO).
dVoluntary or accidental with cardiotoxic drugs (eg, β-blockers, calcium channel blockers).
The trial was overseen by a blinded steering committee and an independent, unblinded data and safety monitoring board. The steering committee oversaw the conduct and decision-making during the trial and made recommendations to the trial’s sponsor. The data and safety monitoring board oversaw safety (additional details appear in the eMethods in Supplement 3).
Trial Sites and Patients
The trial was conducted in the intensive care units (ICUs) at 20 French cardiac shock care centers between October 2016 and July 2019 and final follow-up occurred in November 2019.10 All consecutive adult patients admitted in the ICUs and treated with venoarterial ECMO were screened for enrollment. Patients were eligible for enrollment if they had been endotracheally intubated and were receiving venoarterial ECMO for cardiogenic shock for less than 6 hours. Patients were excluded if they were (1) younger than 18 years of age, (2) pregnant, (3) receiving venoarterial ECMO after cardiac surgery for a heart transplant or both a lung and a heart transplant, (4) receiving left ventricular assist device (LVAD) implantation, (5) receiving venoarterial ECMO for acute poisoning with cardiotoxic drugs, (6) having uncontrolled bleeding (bleeding despite medical intervention with surgery or drugs), (7) receiving venoarterial ECMO after cardiopulmonary resuscitation for longer than 45 minutes, (8) having an out-of-hospital refractory cardiac arrest, (9) already participating in interventional research involving therapeutic interventions, (10) under a guardianship, (11) receiving a clinician-based assessment of irreversible neurological injury, or (2) no longer receiving life-sustaining therapies.
Randomization
Randomization was performed through a secured server using a predefined randomization list stratified by cardiac shock care center and using randomly alternating block sizes of 2 and 4. The randomization list was created by the trial’s data manager using computer-generated random numbers and verified by another scientist. These professionals had no interactions with the investigators, and the investigators were not aware of the block sizes and stratification because these details were not reported in the trial protocol.
Interventions
Venoarterial ECMO was initiated per local practice with blood flow settings set to ensure adequate tissue perfusion, which was determined by the clinicians at the bedside. Patients were then randomized to moderate hypothermia (intervention group) or normothermia (control group). Concealment of the randomized assignment was ensured by means of a centralized, secure, web-based system. Further ICU therapy was provided in accordance with generally accepted intensive care practices.
Core temperature had to be monitored through an esophageal or bladder probe, which was not possible outside the ICU; thus, the first measured temperature was collected after admission to the ICU. In the moderate hypothermia group, the target temperature of 33 °C to 34 °C was achieved just after randomization by adjusting the venoarterial ECMO circuit temperature controller for the initial 24 hours (± 1 hour; eFigure 1 in Supplement 3), followed by progressive rewarming (± 0.1-0.2 °C per hour) to 37 °C. During the subsequent 72 hours (± 4 hours) after reaching 37 °C, a temperature of 37 °C (± 0.3 °C) was maintained. Outside the ICU, temperature was intermittently measured (not with a tympanic thermometer).
Although rare, potential physiological effects of hypothermia were managed according to current recommendations.11 Hypothermia was discontinued if uncontrolled bleeding occurred despite medical intervention (surgery or drugs). Hypothermia was resumed when bleeding was controlled and was continued until the patient had reached therapeutic hypothermia for a total of 24 hours. During hypothermia, deep sedation and neuromuscular blocking agents were used to prevent shivering.
In the normothermia group, temperature was maintained between 36 °C and 37 °C during the first 24 hours and at 37 °C (± 0.3 °C) during the subsequent 72 hours. Among the patients who had a cardiac arrest, core temperature was strictly maintained at 36 °C (eFigure 1 in Supplement 3).
Data Collection and Monitoring
The investigators or staff reported any serious adverse events to the trial’s sponsor and entered the baseline characteristics, process variables, and outcome data from the patient files into web-based case report forms for days 1 to 7 and for days 30, 60, and 180. Patient medical files were used to obtain follow-up data; however, patients, their surrogates, or their primary care physicians were contacted if additional data were needed. Trial data were monitored on-site (including consent and source data verification) by independent monitors and the data were monitored centrally by staff from the coordinating center according to a prespecified monitoring plan.
Outcomes
The primary outcome was mortality at 30 days. There were 31 secondary outcomes including mortality at 48 hours and at days 7, 60, and 180; venoarterial ECMO duration; a composite outcome of death, heart transplant, escalation to LVAD implantation, or stroke at days 30, 60, and 180; 7-day net intake of intravenous fluids; length of use of vasopressors in the ICU; time to normalization of lactate level; the number of days alive without failure of an organ or organs at day 7 (defined using the sequential organ failure assessment [SOFA] score and its components of respiratory, liver, coagulation, and kidney); duration of mechanical ventilation and number of ventilator-free days at 30, 60, and 180 days; kidney replacement therapy–free days at days 30, 60, and 180; and duration of ICU stay and hospital stay. Adverse events included rates of severe and moderate bleeding, sepsis (pulmonary, bloodstream, and cannula-related ECMO), and the number of units of packed red blood cells transfused during venoarterial ECMO.
Sample Size Calculation
Based on the literature, we estimated that mortality is 50% in patients with cardiogenic shock supported by venoarterial ECMO.12 We calculated that 334 patients were required for the trial to have 80% power to show a relative reduction of 15% in 30-day mortality using a χ2 test with 80% power and a 2-sided global α level of 5% using the Lan-DeMets method with the O’Brien-Fleming boundary for 1 interim analysis after inclusion of two-thirds of the patients. The 15% threshold was chosen based both on the effect size reported in previous critical care trials13 and on an earlier trial testing the use of hypothermia in the setting of cardiac arrest.14
Statistical Analysis
Baseline characteristics are expressed as mean (SD) for continuous variables, median (IQR) for data with skewed distributions, and frequency (percentage) for categorical variables. The data were analyzed according to randomization group and every randomized patient (who did not withdraw consent) was analyzed for the primary and secondary outcomes. For all the mortality end points, patients who were lost to follow-up were analyzed with the last observation carried forward method (ie, these patients were considered to be alive after being lost to follow-up).
For the primary outcome and the binary secondary outcomes, the 2 groups were compared using logistic regression adjusted for the following variables known to be associated with mortality or morbidity in patients with cardiogenic shock: age, prior myocardial infarction, prior cardiac arrest, after cardiac surgery, vasopressor dose (patients who did not receive vasopressor therapy were coded as having a dose of 0 μg/kg/min), lactate level, and SOFA score at baseline. Because all continuous secondary outcomes had highly skewed distributions, the 2 groups were compared using the adjusted proportional odds model, which is a generalization of the Wilcoxon test.
To preserve completeness of the data in the adjusted analyses, multiple imputation with chained equations was used to impute missing values. Among the variables used for adjustment, 4 had missing data: prior myocardial infarction, vasopressor dose, lactate level, and SOFA score. We performed multiple imputation with chained equations and generated 100 imputed data sets. The following variables were used as predictors: age, sex, body mass index (calculated as weight in kilograms divided by height in meters squared), history of cardiovascular disease (hypertension, heart failure, myocardial infarction, ischemic cardiomyopathy, valvular cardiomyopathy), prior cardiac arrest, cardiogenic shock etiologies (acute coronary syndrome, ischemic cardiomyopathy, after cardiac surgery, arrhythmia-induced cardiomyopathy, other etiologies), vasopressor dose, dobutamine dose, pH, lactate level, and SOFA score. After imputation, we calculated the SOFA score within each imputed data set. For the adjusted analyses (adjusted logistic regression or adjusted proportional odds model), the adjusted model in each imputed data set was used and then the results were pooled by applying the rules of Rubin.
One interim analysis for efficacy or futility was performed after inclusion of two-thirds of the patients. To maintain an overall type I error rate of 5% (2-sided), a bilateral P value less than .049 was considered significant for the final analysis. The secondary outcomes were analyzed using a hierarchical testing procedure until the P value exceeded .049.15
Unadjusted Kaplan-Meier survival curves were created for mortality at days 30, 60, and 180 and were compared using a log-rank test. Crude estimates of odds ratios (ORs) and hazard ratios were obtained from Cox regression models and computed for illustration.
A subgroup analysis was performed for the primary outcome of mortality at 30 days using all the aforementioned adjustment variables. For each continuous adjustment variable, subgroups were defined by dichotomizing the variable according to the median. Interactions between the moderate hypothermia group and the subgroups were assessed by introducing an interaction term within the adjusted logistic regression models. The adjusted ORs in each subgroup and the P values for interaction were assessed using the Wald test.
All analyses were performed using R software version 4.0.3 (R Foundation for Statistical Computing).
Post Hoc Analyses
To quantify the treatment effect on an absolute scale, we calculated risk differences using a binomial generalized estimating equation model with an identity link for the binary outcomes, accounting for intracenter correlation.16 Similarly, we calculated mean differences using a gaussian generalized estimating equation model with an identity link for continuous outcomes that accounted for intracenter correlation.
A post hoc bayesian analysis also was performed to analyze the primary outcome of mortality at 30 days. The prior distributions were not definitively known given the absence of published trials investigating hypothermia in patients with cardiogenic shock. Therefore, we assumed a wide range of prior assumptions (from minimally informative to strongly enthusiastic to skeptical) modeled after a previous bayesian analysis.17 Separate bayesian logistic regression models were run for each of the prior distributions for moderate hypothermia on the logarithm of the OR by placing a uniform prior on the intercept. Markov-chain Monte Carlo modeling (with 3 chains, 20 000 warm-up iterations, and 20 000 sampling iterations per chain) was used to derive effect estimates and 95% credible intervals for moderate hypothermia from the median, 2.5th and 97.5th percentiles of the posterior distribution, and to estimate the posterior probabilities of exceeding certain thresholds. The Gelman-Rubin statistic was used to assess convergence of all models. The bayesian analysis was performed using version 2.21.1 of the rstanarm package in R version 4.0.3 (R Foundation for Statistical Computing).
Results
Patients
The trial was conducted between October 2016 and July 2019 and final follow-up occurred in November 2019. Of the 786 eligible patients, 374 (48%) were randomized. There were 40 patients who withdrew consent, leaving 334 to be included in the primary analysis (168 in the moderate hypothermia group and 166 in the normothermia group; Figure 1). The baseline characteristics of the patients were similar in both groups (Table 1). Cardiogenic shock was due to ischemic cardiac disease in 58% of the patients (acute myocardial infarction in 36% and decompensated ischemic heart failure in 22%) and occurred after cardiac surgery in 15%. Forty-eight percent of patients experienced cardiac arrest prior to trial inclusion. Norepinephrine or epinephrine was used in 80% of the patients with a median vasopressor dose of 0.40 μg/kg/min and a median lactate level of 4.8 mmol/L or greater in both groups.
Table 1. Baseline Characteristics of the Patients.
| Characteristic | Moderate hypothermia (n = 168) |
Normothermia (n = 166) |
|---|---|---|
| Demographics | ||
| Age, mean (SD), y | 57 (12) | 59 (12) |
| Sex, No. (%) | ||
| Male | 128 (76) | 125 (75) |
| Female | 40 (24) | 41 (25) |
| Body mass index, mean (SD)a | (n = 163) 26.6 (5.7) |
(n = 160) 27.1 (5.4) |
| Cardiovascular history, No. (%) b | ||
| Cardiac arrest | 81 (48) | 78 (47) |
| Hypertension, No./total (%) | 61/164 (37) | 60/162 (37) |
| Heart failure, No./total (%) | 32/160 (20) | 33/161 (20) |
| Ischemic cardiomyopathy, No./total (%) | 31/161 (19) | 41/160 (26) |
| Myocardial infarction, No./total (%) | 25/160 (16) | 22/159 (14) |
| Valvular cardiomyopathy, No./total (%) | 21/160 (13) | 22/160 (14) |
| Etiologies of cardiogenic shockc | ||
| Acute coronary syndrome | 59 (35) | 62 (37) |
| Ischemic cardiomyopathy | 38 (23) | 36 (22) |
| Rhythmic cardiomyopathy | 28 (17) | 15 (9) |
| After cardiac surgery | 23 (14) | 27 (16) |
| Acute myocarditis | 17 (10) | 14 (8) |
| Dilated cardiomyopathy | 15 (9) | 11 (7) |
| Pulmonary embolism | 9 (5) | 8 (5) |
| Takotsubo syndrome | 4 (2) | 7 (4) |
| Echocardiogram and laboratory results | ||
| LVEF before venoarterial ECMO, median (IQR), % | (n = 96) 20 (15-30) |
(n = 117) 20 (15-35) |
| pH, mean (SD)d | (n = 165) 7.32 (0.14) |
(n = 160) 7.31 (0.16) |
| Arterial lactate level, median (IQR), mmol/Le | (n = 160) 4.8 (2.5-8.0) |
(n = 158) 4.9 (2.7-8.6) |
| Sequential organ failure assessment score, median (IQR)f | (n = 136) 10 (8-12) |
(n = 126) 10 (7-13) |
| Medications | ||
| Any vasopressor | ||
| Use, No./total (%) | 135/163 (83) | 134/162 (83) |
| Dose, median (IQR), μg/kg/min | (n = 156)g 0.38 (0.10-0.95) |
(n = 155)g 0.41 (0.08-1.15) |
| Norepinephrine | ||
| Use, No./total (%) | 123/163 (75) | 123/162 (76) |
| Dose, median (IQR), μg/kg/min | (n = 118)g 0.47 (0.22-1.00) |
(n = 117)g 0.53 (0.20-1.50) |
| Dobutamine | ||
| Use, No./total (%) | 96/163 (59) | 106/162 (65) |
| Dose, median (IQR), μg/kg/min | (n = 92)g 7.54 (4.62-10.85) |
(n = 101)g 6.00 (4.68-10.00) |
| Epinephrine | ||
| Use, No./total (%) | 37/163 (23) | 44/162 (27) |
| Dose, median (IQR), μg/kg/min | (n = 33)g 0.27 (0.15-0.72) |
(n = 41)g 0.24 (0.13-0.70) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; LVEF, left ventricular ejection fraction.
SI conversion factor: To convert lactate to mg/dL, divide by 0.111.
Calculated as weight in kilograms divided by height in meters squared.
Determined by the clinician in charge of the patient and based on chart review.
May have 1 or more of the cardiogenic shock etiologies.
Normal range is 7.35 to 7.45.
A normal level is less than 2 mmol/L.
Mortality prediction score based on the degree of dysfunction in 6 organ systems. Scores range from 0 to 24. The score was quantified by the clinicians in charge of the patients during the trial.
Only considered in treated patients with available data.
Temperature Management
The target temperature was reached in all patients. After admission to the ICU, the median temperature achieved was 34.7 °C (IQR, 33.9 °C-36.2 °C) in the moderate hypothermia group and 36.4 °C (IQR, 35.9 °C-36.8 °C) in the normothermia group. Because it was not possible to monitor core body temperature outside the ICU, it was measured after admission to the ICU, which explains the between-group difference for the first measured core body temperature (Figure 2). The median time from starting venoarterial ECMO to randomization was 3.0 hours (IQR, 1.7-4.5 hours). The median time from randomization to the first measured core body temperature was 30 minutes (IQR, 8-82 minutes).
Figure 2. Core Body Temperatures Measured in the Intensive Care Unit During the First 96 Hours After Randomization.
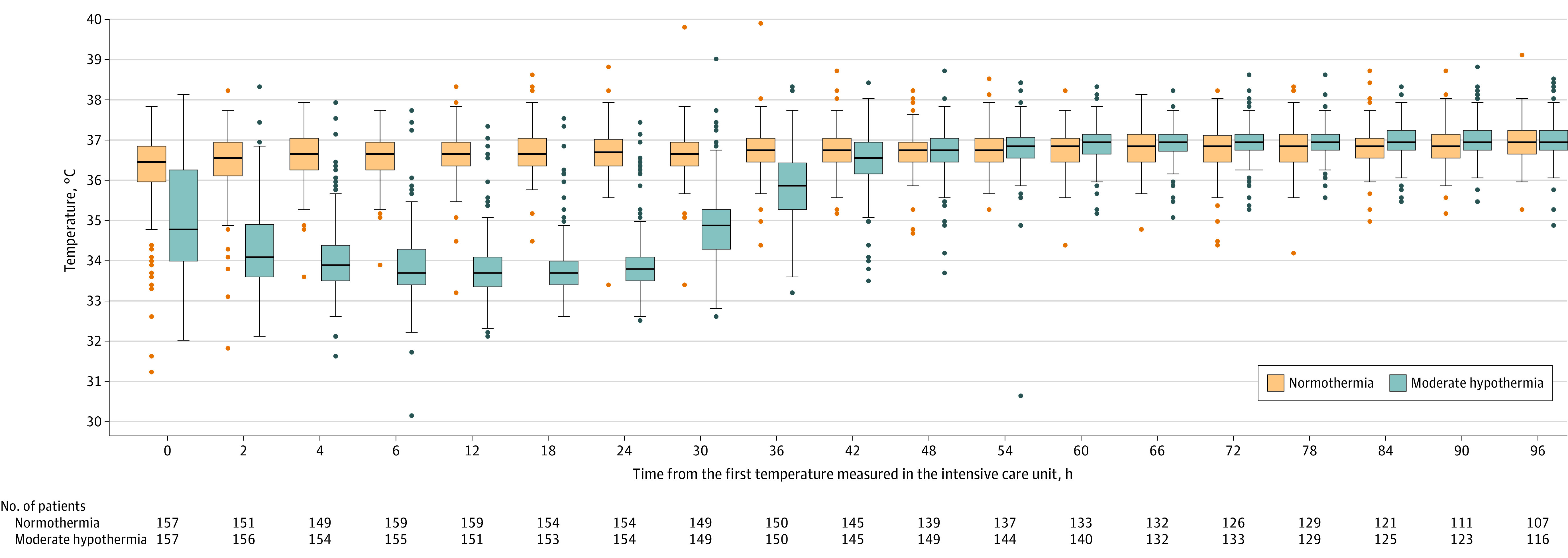
The middle line in the box plot represents the median observed esophageal core temperatures in the moderate hypothermia group (33-34 °C) and in the normothermia group (36-37 °C). The boxes represent the IQR. The whiskers extend to the most extreme observed values with 1.5 × IQR of the nearer quartile. The dots represent the observed values outside that range. Temperature control was initiated just after randomization but before admission to the intensive care unit (ICU), which explains the between-group differences in esophageal temperature at ICU admission. The median time from venoarterial extracorporeal membrane oxygenation to randomization was 3.0 hours (IQR, 1.7-4.5 hours). The median time from randomization to the first measured temperature was 30 minutes (IQR, 8-82 minutes).
Primary Outcome
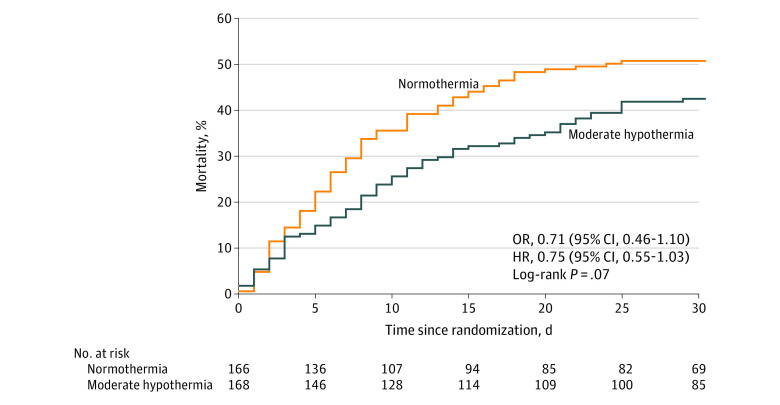
For the primary outcome of mortality at 30 days, 71 patients (42%) in the moderate hypothermia group died compared with 84 patients (51%) in the normothermia group (adjusted OR, 0.71 [95% CI, 0.45 to 1.13], P = .15; risk difference, −8.3% [95% CI, −16.3% to −0.3%]; Table 2 and Figure 3).
Table 2. Analysis of Primary and Secondary Outcomes and Adverse Events.
| Moderate hypothermia (n = 168) |
Normothermia (n = 166) |
Estimate (95% CI)a |
Adjusted OR (95% CI)b |
P valuec | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Mortality at 30 d, No. (%) | 71 (42) | 84 (51) | RD, −8.3 (−16.3 to −0.3) | 0.71 (0.45 to 1.13) | .15 |
| Secondary outcomes d | |||||
| Composite outcome of death, heart transplant, escalation to LVAD implantation, or stroke, No. (%) | |||||
| At 30 de | 88 (52) | 106 (64) | RD, −11.5 (−23.2 to 0.2) | 0.61 (0.39 to 0.96) | .03 |
| At 60 df | 98 (58) | 110 (66) | RD, −8.0 (−21.4 to 5.5) | 0.70 (0.44 to 1.11) | |
| At 180 dg | 102 (61) | 114 (69) | RD, −8.0 (−22.4 to 6.4) | 0.69 (0.43 to 1.11) | |
| Mortality, No. (%) | |||||
| At 48 h | 10 (6) | 11 (7) | RD, −0.7 (−5.3 to 3.9) | 0.98 (0.38 to 2.51) | |
| At 7 d | 29 (17) | 46 (28) | RD, −10.4 (−16.6 to −4.3) | 0.53 (0.31 to 0.93) | |
| At 60 d | 82 (49) | 91 (55) | RD, −6.0 (−16.7 to 4.6) | 0.79 (0.50 to 1.25) | |
| At 180 d | 88 (52) | 96 (58) | RD, −5.5 (−17.5 to 6.5) | 0.81 (0.51 to 1.29) | .38 |
| Venoarterial ECMO duration since randomization, mean (SD), d | 9.3 (9.0) | 8.1 (5.0) | MD, 1.2 (−0.2 to 2.7) | 1.11 (0.76 to 1.61) | |
| 7-d net intake of intravenous fluids, mean (SD), mL | 4042 (5036) | 3692 (5802) | MD, 295 (−409 to 999) | 1.19 (0.82 to 1.73) | |
| Length of use of vasopressors in the ICU, mean (SD), d | (n = 164) 8.4 (8.8) |
(n = 162) 7.2 (6.9) |
MD, 1.2 (−0.7 to 3.1) | 1.18 (0.80 to 1.72) | |
| Time to normalization of lactate level, mean (SD), dh | 2.4 (2.3) | 2.6 (2.4) | MD, −0.2 (−0.6 to 0.3) | 1.08 (0.70 to 1.66) | |
| No. of days alive without failure of organ or organs at 7 d, mean (SD)i | (n = 154) | (n = 156) | |||
| Defined as ≥1 component with a SOFA score >2 | 3.0 (2.5) | 2.5 (2.6) | MD, 0.5 (0 to 1.0) | 1.58 (1.05 to 2.38) | |
| Defined as ≥1 component with a SOFA score >3 | 5.2 (2.6) | 4.3 (2.8) | MD, 0.9 (0.4 to 1.3) | 2.03 (1.31 to 3.15) | |
| Ventilator-free days, mean (SD) | (n = 167) | (n = 165) | |||
| At 30 d | 9.1 (11.2) | 8.3 (11.2) | MD, 0.9 (−2.4 to 4.1) | 1.10 (0.72 to 1.69) | |
| At 60 d | 22.7 (25.1) | 20.8 (24.9) | MD, 1.9 (−4.2 to 7.9) | 1.12 (0.74 to 1.71) | |
| At 180 d | 77.7 (85.9)j | 67.7 (82.9)j | MD, 10.3 (−9.0 to 29.5) | 1.26 (0.83 to 1.93) | |
| Kidney replacement therapy–free days, mean (SD) | (n = 167) | (n = 165) | |||
| At 30 d | 15.2 (15.0) | 11.7 (14.5) | MD, 3.5 (0.6 to 6.5) | 1.55 (1.02 to 2.37) | |
| At 60 d | 29.2 (30.0) | 23.9 (28.4) | MD, 5.3 (−0.2 to 10.8) | 1.40 (0.92 to 2.13) | |
| At 180 d | 84.4 (91.6)j | 71.0 (86.7)j | MD, 13.6 (−5.1 to 32.3) | 1.46 (0.95 to 2.23) | |
| Length of stay, mean (SD), d | |||||
| In the ICU | 18.7 (20.3) | 17.8 (19.1) | MD, 0.9 (−4.0 to 5.7) | 1.16 (0.79 to 1.69) | |
| At the hospital | 32.3 (31.8) | 30.4 (34.0) | MD, 1.9 (−5.7 to 9.5) | 1.33 (0.91 to 1.94) | |
| Adverse events | |||||
| Occurred during venoarterial ECMO | |||||
| Moderate or severe bleeding, No. (%)k | 69 (41) | 70 (42) | RD, −1.0 (−10.1 to 8.1) | 0.94 (0.60 to 1.48) | |
| Received transfusion with only packed red blood cells | |||||
| No. (%) | 53 (32) | 50 (30) | RD, 1.3 (−6.5 to 9.1) | 1.10 (0.68 to 1.79) | |
| No. of units transfused, mean (SD) | 8.3 (9.0) | 5.4 (6.3) | MD, 3.2 (0.6 to 5.8) | 2.84 (1.37 to 5.86) | |
| Had ≥1 of the following 3 infection types, No. (%)l | 87 (52) | 86 (52) | RD, 0 (−12.3 to 12.2) | 0.97 (0.63 to 1.51) | |
| Pulmonary only | 74 (44) | 60 (36) | RD, 7.8 (−2.7 to 18.3) | 1.39 (0.89 to 2.18) | |
| Bacteremia only | 33 (20) | 50 (30) | RD, −10.5 (−22.4 to 1.4) | 0.55 (0.33 to 0.92) | |
| Venoarterial ECMO cannulae only | 14 (8) | 18 (11) | RD, −2.4 (−7.9 to 3.0) | 0.73 (0.35 to 1.54) | |
Abbreviations: ECMO, extracorporeal membrane oxygenation; LVAD, left ventricular assist device; ICU, intensive care unit; MD, mean difference; OR, odds ratio; RD, risk difference; SOFA, sequential organ failure assessment.
The RDs are expressed as percentages and were calculated using a binomial generalized estimating equation (GEE) model with identity link that accounted for intracenter correlation. The MDs were calculated using a gaussian GEE model with identity link that accounted for intracenter correlation.
The ORs were obtained from logistic regression models for binary end points or ordinal logistic regression models for continuous end points and were adjusted for age, prior myocardial infarction, prior cardiac arrest, after cardiac surgery, vasopressor dose, lactate level, and SOFA score at baseline. An OR greater than 1 indicates that the values are increased in the moderate hypothermia group compared with the normothermia group.
The secondary outcomes were analyzed using a hierarchical testing procedure that was implemented until the P value was nonsignificant; therefore, most of the P values were omitted.
Two secondary outcomes were not reported because they were redundant with other outcomes (durations of mechanical ventilation and kidney replacement therapy).
In the moderate hypothermia group, 71 patients died vs 84 in the normothermia group; 3 vs 7, respectively, for heart transplant; 18 vs 15 for escalation to LVAD implantation; and 11 vs 10 for stroke.
In the moderate hypothermia group, 82 patients died vs 91 in the normothermia group; 4 vs 7, respectively, for heart transplant; 20 vs 15 for escalation to LVAD implantation; and 12 vs 12 for stroke.
In the moderate hypothermia group, 88 patients died vs 96 in the normothermia group; 4 vs 8, respectively, for heart transplant; 20 vs 15 for escalation to LVAD implantation; and 12 vs 13 for stroke.
Defined as the first time the lactate level was in the normal range (<2.2 mmol/L measured on days 1-7). If a value was missing, and the previous value was not in the normal range, it was assumed that the patient had not achieved a normal lactate level. The value of 8 days was assigned to all patients who never had a normal level.
The SOFA score is for mortality prediction. The score is based on the degree of dysfunction of 6 organ systems and ranges from 0 to 24. The 4 components (respiratory, liver, coagulation, and kidney; each component scored from 0 to 4) were evaluated on days 1 to 7. Missing values for the 4 components were imputed using the last out carried forward (LOCF) method starting from baseline values (ie, if a value was missing at day 1, this value was replaced with the baseline value). All patients alive on day 1 who had a missing value for at least 1 of the 4 components at day 1 (after the LOCF imputation) were excluded from this analysis. The time alive without organ failure at day 7 was defined as the difference between the number of days alive with organ failure between days 1 and 7 and the number of days alive between days 1 and 7. The value of 0 days was assigned to all patients who died before day 1.
For 180 days, the sample size is the whole sample (168 for moderate hypothermia and 166 for normothermia).
Moderate bleeding was defined as requiring blood transfusion but not resulting in hemodynamic compromise requiring treatment. Severe bleeding was defined as intracerebral or resulting in substantial hemodynamic compromise.
Identified by the clinicians in charge of the patients and selected based on the work of Schmidt et al.18
Figure 3. Kaplan-Meier Survival Estimates for the Primary Outcome of 30-Day Mortality in Patients Treated With Moderate Hypothermia or Normothermia.
For the primary outcome of 30-day mortality, patients were observed for a median of 8 days (IQR, 8-30 days). HR indicates hazard ratio; OR, odds ratio.
Secondary Outcomes
Of the 31 secondary outcomes, 30 were inconclusive. The composite outcome (death, heart transplant, escalation to LVAD implantation, or stroke) was significantly different between groups at day 30 (adjusted OR, 0.61 [95% CI, 0.39 to 0.96], P = .03; risk difference, −11.5% [95% CI, −23.2% to 0.2%]). The risk difference for the composite outcome at day 60 was −8.0% (95% CI, −21.4% to 5.5%) and at day 180 was −8.0% (95% CI, −22.4% to 6.4%) (Table 2).
The risk difference for mortality at day 7 was −10.4% (95% CI, −16.6% to −4.3%) and at day 60 was −6.0% (95% CI, −16.7% to 4.6%) (Table 2 and eFigures 2-3 in Supplement 3). There was no significant between-group difference for mortality at day 180 (adjusted OR, 0.81 [95% CI, 0.51 to 1.29], P = .38; risk difference, −5.5% [95% CI, −17.5% to 6.5%]).
Duration of venoarterial ECMO and time to normalization of lactate level were not different between groups. Compared with the normothermia group, patients in the moderate hypothermia group had more days without organ failure at day 7 when using a SOFA component (respiratory, liver, coagulation, or kidney) threshold of 1 or greater and a SOFA score greater than 2 or 3 (Table 2). For mechanical ventilation, the median duration was 10 days (IQR, 5-19 days) in the moderate hypothermia group vs 7 days (IQR, 4-16 days) in the normothermia group. The number of ventilator-free days at days 30, 60, and 180 was not different between groups. The forest plot suggested a homogenous treatment effect according to baseline demographic characteristics, cardiogenic shock etiology, and cardiogenic shock severity (eFigure 4 in Supplement 3).
Adverse Events
The number of moderate and severe bleeding events was not different between groups, whereas the number of units of packed red blood cells transfused during venoarterial ECMO was higher in the moderate hypothermia group (Table 2). The number of nosocomial infections was not different between groups.
Post Hoc Analysis
All patients who experienced a cardiac arrest had a score of 1-2 (no disability or moderate cerebral disability) on the Cerebral Performance Scale 1 month after inclusion. The range of prior assumptions in the bayesian analysis appear in the eTable in Supplement 3. The posterior probability distributions of the OR for the primary outcome of mortality at 30 days in the various scenarios for prior assumptions (from strongly enthusiastic to strongly skeptical) appear in eFigure 5 in Supplement 3.
With a minimally informative prior, the estimated OR was 0.71 (95% CI, 0.46-1.09) for 30-day mortality in the moderate hypothermia group vs the normothermia group. The posterior probability of mortality benefit with moderate hypothermia (ie, an OR <1) was 94% vs the normothermia group. With the moderately enthusiastic prior, the estimated OR was 0.71 (95% CI, 0.53-0.94) for 30-day mortality in the moderate hypothermia group vs the normothermia group. The posterior probability of mortality benefit with moderate hypothermia was 99% vs the normothermia group. In all other scenarios, the posterior probability of mortality benefit with moderate hypothermia was 85% or greater vs the normothermia group.
Discussion
In this French nationwide randomized clinical trial including adult patients with refractory cardiogenic shock treated with venoarterial ECMO, early application of moderate hypothermia was not significantly associated with lower mortality at 30 days compared with the normothermia group. However, moderate hypothermia was associated with a lower risk of the composite outcome (death, heart transplant, escalation to LVAD implantation, or stroke) at 30 days. The number of patients with serious adverse events (moderate or severe bleeding, infectious events) was not statistically different between groups.
Therapeutic hypothermia has been investigated as a protective intervention in various forms of acute cardiac disease such as myocardial infarction,19 cardiac arrest with or without cardiogenic shock,20,21,22 and cardiogenic shock.3,23,24 Cardiac arrest studies led to the concept of targeted temperature management in which both 33 °C and 36 °C resulted in similar outcomes.14,25 A recent study8 and a network meta-analysis26 found that mild, moderate, or deep hypothermia may not improve survival or functional outcome in patients with cardiac arrest. Compared with the Targeted Hypothermia versus Targeted Normothermia after Out-of-Hospital Cardiac Arrest (TTM2) trial,8 it is difficult to extrapolate the data to the patients enrolled in the current trial because the 2 populations are not comparable in terms of cardiogenic shock incidence (100% in the current trial vs 29%-35% in the TTM2 trial), venoarterial ECMO use (100% vs 0%), and cardiac arrest incidence (50% vs 100%).
In animal models of cardiogenic shock, moderate hypothermia maintained or increased inotropy, decreased oxygen consumption, and improved survival.27 Human data are limited to small groups of patients,28 are mainly centered on hemodynamic status, and are inconclusive regarding effect on mortality. In patients with myocardial infarction and cardiogenic shock, a recent meta-analysis29 found no significant association with hypothermia use; however, among patients assigned to hypothermia, those effectively achieving the protocol-defined target temperature had smaller infarct size. Studies on the mechanisms underlying hypothermia’s protective effects point to 4 key success factors: (1) rapid induction of hypothermia, (2) an optimal duration of 24 hours,30 (3) slow rewarming, and (4) appropriate management of complications.31,32 All of these factors were successfully implemented in the current trial.
Limited data (derived from animal experiments and preliminary results of extracorporeal cardiopulmonary resuscitation33) are available regarding the effect of hypothermia in the most severe forms of cardiogenic shock requiring venoarterial ECMO. Moderate hypothermia was associated with decreased vasopressor use and improved vascular reactivity and cardiac function in a porcine model of cardiogenic shock supported by ECMO.34 Two systematic reviews with meta-analyses21,33 suggested, with a low level of evidence, that therapeutic hypothermia was significantly associated with favorable neurological outcomes and survival during extracorporeal cardiopulmonary resuscitation. In the current trial, the observed reduction of 9% in 30-day mortality in the moderate hypothermia group was not statistically significant (and below the expected reduction of 15% hypothesized during the sample size calculation). A post hoc bayesian analysis demonstrated that across a range of prior assumptions about the probability of benefit, the posterior probability of any mortality benefit (OR <1) with moderate hypothermia ranged from 85% to 100%. The trial likely had insufficient statistical power to detect a moderate treatment effect that may nonetheless be clinically relevant. A sizeable effect with use of moderate hypothermia (a reduction in mortality of 5% to 10%) cannot be ruled out.
Limitations
This trial has several limitations. First, given the use of therapeutic hypothermia, patients were included only if they had been intubated, had been sedated, and required mechanical ventilation, limiting the applicability of the findings to other patients receiving venoarterial ECMO.
Second, patients with all cardiogenic shock etiologies were included (with the exception of cardiac medication poisoning and extracorporeal cardiopulmonary resuscitation), leading to a degree of heterogeneity. Given that cardiogenic shock necessitating treatment with venoarterial ECMO is relatively uncommon, it would be difficult to perform a study focused on a specific indication within the cardiogenic shock population, with the exception of acute myocardial infarction. Ongoing randomized clinical trials in patients after acute myocardial infarction cardiogenic shock will test whether venoarterial ECMO in addition to revascularization and standard therapy will decrease mortality (NCT03813134, NCT03637205, and NCT04184635). In the present study, etiologies of cardiogenic shock were equally distributed. Moreover, the study was pragmatic, and a review of the literature did not provide any preliminary signals favoring a particular etiology.
Third, medium- and long-term outcomes, such as neurocognitive sequelae, which may have been clinically relevant in many patients (considering the cardiac arrest rate of 48% prior to inclusion), were not evaluated. Nevertheless, the observed rate of cardiac arrest was similar to that observed in other studies involving patients with cardiogenic shock.7 The trial design was consistent with the current recommendations concerning temperature control.25 In addition, all the patients who experienced a cardiac arrest had a Cerebral Performance Scale score of 1-2 (no disability or moderate cerebral disability) at 1 month after inclusion.
Fourth, the trial was likely underpowered to statistically detect a survival benefit of 8% to 10% with moderate hypothermia.
Conclusions
In this randomized clinical trial involving patients with refractory cardiogenic shock treated with venoarterial ECMO, early application of moderate hypothermia for 24 hours did not significantly increase survival compared with normothermia. However, because the 95% CI was wide and included a potentially important effect size, these findings should be considered inconclusive.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Trial protocol
Statistical analysis plan
eMethods. Investigators and committees and other information
eFigure 1. HYPO-ECMO study: treatment protocol and follow up
eFigure 2. Kaplan Meier survival estimates during the 60 of VA-ECMO patients treated with moderate hypothermia or normothermia
eFigure 3. Kaplan Meier survival estimates during the 180 days of VA-ECMO patients treated with moderate hypothermia or normothermia
eFigure 4. Primary endpoint - subgroup analysis and interaction terms (forest plot)
eFigure 5. Bayesian analysis: posterior probability distributions for OR for the benefit of moderate hypothermia on mortality at day 30
eTable. Characteristics of reference prior probability distributions representing prior beliefs about mortality benefit from moderate hypothermia and probability of treatment effects estimated by Bayesian analysis according to varying prior beliefs
Nonauthor collaborators
Data sharing statement
References
- 1.Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11(9):e004905. doi: 10.1161/CIRCHEARTFAILURE.118.004905 [DOI] [PubMed] [Google Scholar]
- 2.Chioncel O, Parissis J, Mebazaa A, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(8):1315-1341. doi: 10.1002/ejhf.1922 [DOI] [PubMed] [Google Scholar]
- 3.Combes A, Price S, Slutsky AS, Brodie D. Temporary circulatory support for cardiogenic shock. Lancet. 2020;396(10245):199-212. doi: 10.1016/S0140-6736(20)31047-3 [DOI] [PubMed] [Google Scholar]
- 4.Thiagarajan RR, Barbaro RP, Rycus PT, et al. ; ELSO member centers . Extracorporeal Life Support Organization registry international report 2016. ASAIO J. 2017;63(1):60-67. doi: 10.1097/MAT.0000000000000475 [DOI] [PubMed] [Google Scholar]
- 5.Prondzinsky R, Unverzagt S, Lemm H, et al. Interleukin-6, -7, -8 and -10 predict outcome in acute myocardial infarction complicated by cardiogenic shock. Clin Res Cardiol. 2012;101(5):375-384. doi: 10.1007/s00392-011-0403-3 [DOI] [PubMed] [Google Scholar]
- 6.Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003;107(24):2998-3002. doi: 10.1161/01.CIR.0000075927.67673.F2 [DOI] [PubMed] [Google Scholar]
- 7.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671-2683. doi: 10.1093/eurheartj/ehz363 [DOI] [PubMed] [Google Scholar]
- 8.Dankiewicz J, Cronberg T, Lilja G, et al. ; TTM2 Trial Investigators . Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384(24):2283-2294. doi: 10.1056/NEJMoa2100591 [DOI] [PubMed] [Google Scholar]
- 9.Jacquot A, Lepage X, Merckle L, Girerd N, Levy B. Protocol for a multicentre randomised controlled trial evaluating the effects of moderate hypothermia versus normothermia on mortality in patients with refractory cardiogenic shock rescued by venoarterial extracorporeal membrane oxygenation (VA-ECMO) (HYPO-ECMO study). BMJ Open. 2019;9(10):e031697. doi: 10.1136/bmjopen-2019-031697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rab T, Ratanapo S, Kern KB, et al. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol. 2018;72(16):1972-1980. doi: 10.1016/j.jacc.2018.07.074 [DOI] [PubMed] [Google Scholar]
- 11.Noyes AM, Lundbye JB. Managing the complications of mild therapeutic hypothermia in the cardiac arrest patient. J Intensive Care Med. 2015;30(5):259-269. doi: 10.1177/0885066613516416 [DOI] [PubMed] [Google Scholar]
- 12.Combes A, Leprince P, Luyt CE, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404-1411. doi: 10.1097/CCM.0b013e31816f7cf7 [DOI] [PubMed] [Google Scholar]
- 13.Landoni G, Comis M, Conte M, et al. Mortality in multicenter critical care trials: an analysis of interventions with a significant effect. Crit Care Med. 2015;43(8):1559-1568. doi: 10.1097/CCM.0000000000000974 [DOI] [PubMed] [Google Scholar]
- 14.Nielsen N, Wetterslev J, Cronberg T, et al. ; TTM Trial Investigators . Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197-2206. doi: 10.1056/NEJMoa1310519 [DOI] [PubMed] [Google Scholar]
- 15.Dmitrienko A, Tamhane A, Bretz F. Multiple Testing Problems in Pharmaceutical Statistics. CRC/Taylor & Francis; 2010. [Google Scholar]
- 16.Pedroza C, Truong VT. Performance of models for estimating absolute risk difference in multicenter trials with binary outcome. BMC Med Res Methodol. 2016;16(1):113. doi: 10.1186/s12874-016-0217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goligher EC, Tomlinson G, Hajage D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA. 2018;320(21):2251-2259. doi: 10.1001/jama.2018.14276 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Bréchot N, Hariri S, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55(12):1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villablanca PA, Rao G, Briceno DF, et al. Therapeutic hypothermia in ST elevation myocardial infarction: a systematic review and meta-analysis of randomised control trials. Heart. 2016;102(9):712-719. doi: 10.1136/heartjnl-2015-308559 [DOI] [PubMed] [Google Scholar]
- 20.Annoni F, Peluso L, Fiore M, et al. Impact of therapeutic hypothermia during cardiopulmonary resuscitation on neurologic outcome: a systematic review and meta-analysis. Resuscitation. 2021;162:365-371. doi: 10.1016/j.resuscitation.2021.01.029 [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Zhen Z, Na J, Wang Q, Gao L, Yuan Y. Associations of therapeutic hypothermia with clinical outcomes in patients receiving ECPR after cardiac arrest: systematic review with meta-analysis. Scand J Trauma Resusc Emerg Med. 2020;28(1):3. doi: 10.1186/s13049-019-0698-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stegman B, Aggarwal B, Senapati A, Shao M, Menon V. Serial hemodynamic measurements in post-cardiac arrest cardiogenic shock treated with therapeutic hypothermia. Eur Heart J Acute Cardiovasc Care. 2015;4(3):263-269. doi: 10.1177/2048872614547688 [DOI] [PubMed] [Google Scholar]
- 23.Zobel C, Adler C, Kranz A, et al. Mild therapeutic hypothermia in cardiogenic shock syndrome. Crit Care Med. 2012;40(6):1715-1723. doi: 10.1097/CCM.0b013e318246b820 [DOI] [PubMed] [Google Scholar]
- 24.Stegman BM, Newby LK, Hochman JS, Ohman EM. Post-myocardial infarction cardiogenic shock is a systemic illness in need of systemic treatment: is therapeutic hypothermia one possibility? J Am Coll Cardiol. 2012;59(7):644-647. doi: 10.1016/j.jacc.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 25.Soar J, Berg KM, Andersen LW, et al. ; Adult Advanced Life Support Collaborators . Adult advanced life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2020;156:A80-A119. doi: 10.1016/j.resuscitation.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernando SM, Di Santo P, Sadeghirad B, et al. Targeted temperature management following out-of-hospital cardiac arrest: a systematic review and network meta-analysis of temperature targets. Intensive Care Med. 2021;47(10):1078-1088. doi: 10.1007/s00134-021-06505-z [DOI] [PubMed] [Google Scholar]
- 27.Götberg M, van der Pals J, Olivecrona GK, Götberg M, Koul S, Erlinge D. Mild hypothermia reduces acute mortality and improves hemodynamic outcome in a cardiogenic shock pig model. Resuscitation. 2010;81(9):1190-1196. doi: 10.1016/j.resuscitation.2010.04.033 [DOI] [PubMed] [Google Scholar]
- 28.Fuernau G, Beck J, Desch S, et al. Mild hypothermia in cardiogenic shock complicating myocardial infarction. Circulation. 2019;139(4):448-457. doi: 10.1161/CIRCULATIONAHA.117.032722 [DOI] [PubMed] [Google Scholar]
- 29.Alushi B, Ndrepepa G, Lauten A, et al. Hypothermia in patients with acute myocardial infarction: a meta-analysis of randomized trials. Clin Res Cardiol. 2021;110(1):84-92. doi: 10.1007/s00392-020-01652-7 [DOI] [PubMed] [Google Scholar]
- 30.Kirkegaard H, Søreide E, de Haas I, et al. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2017;318(4):341-350. doi: 10.1001/jama.2017.8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7)(suppl):S186-S202. doi: 10.1097/CCM.0b013e3181aa5241 [DOI] [PubMed] [Google Scholar]
- 32.Polderman KH. Application of therapeutic hypothermia in the intensive care unit: opportunities and pitfalls of a promising treatment modality—part 2: practical aspects and side effects. Intensive Care Med. 2004;30(5):757-769. doi: 10.1007/s00134-003-2151-y [DOI] [PubMed] [Google Scholar]
- 33.Duan J, Ma Q, Zhu C, Shi Y, Duan B. eCPR combined with therapeutic hypothermia could improve survival and neurologic outcomes for patients with cardiac arrest: a meta-analysis. Front Cardiovasc Med. 2021;8:703567. doi: 10.3389/fcvm.2021.703567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanhuyse F, Ducrocq N, Louis H, et al. Moderate hypothermia improves cardiac and vascular function in a pig model of ischemic cardiogenic shock treated with veno-arterial ECMO. Shock. 2017;47(2):236-241. doi: 10.1097/SHK.0000000000000712 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods. Investigators and committees and other information
eFigure 1. HYPO-ECMO study: treatment protocol and follow up
eFigure 2. Kaplan Meier survival estimates during the 60 of VA-ECMO patients treated with moderate hypothermia or normothermia
eFigure 3. Kaplan Meier survival estimates during the 180 days of VA-ECMO patients treated with moderate hypothermia or normothermia
eFigure 4. Primary endpoint - subgroup analysis and interaction terms (forest plot)
eFigure 5. Bayesian analysis: posterior probability distributions for OR for the benefit of moderate hypothermia on mortality at day 30
eTable. Characteristics of reference prior probability distributions representing prior beliefs about mortality benefit from moderate hypothermia and probability of treatment effects estimated by Bayesian analysis according to varying prior beliefs
Nonauthor collaborators
Data sharing statement